Abstract
Endoplasmic reticulum (ER) stress has been implicated in the pathophysiology of human type 2 diabetes (T2DM). Although SIRT1 has a therapeutic effect on metabolic deterioration in T2DM, the precise mechanisms by which SIRT1 improves insulin resistance remain unclear. Here, we demonstrate that adenovirus-mediated overexpression of SIRT1 in the liver of diet-induced insulin-resistant low-density lipoprotein receptor-deficient mice and of genetically obese ob/ob mice attenuates hepatic steatosis and ameliorates systemic insulin resistance. These beneficial effects were associated with decreased mammalian target of rapamycin complex 1 (mTORC1) activity, inhibited the unfolded protein response (UPR), and enhanced insulin receptor signaling in the liver, leading to decreased hepatic gluconeogenesis and improved glucose tolerance. The tunicamycin-induced splicing of X-box binding protein-1 and expression of GRP78 and CHOP were reduced by resveratrol in cultured cells in a SIRT1-dependent manner. Conversely, SIRT1-deficient mouse embryonic fibroblasts challenged with tunicamycin exhibited markedly increased mTORC1 activity and impaired ER homeostasi and insulin signaling. These effects were abolished by mTORC1 inhibition by rapamycin in human HepG2 cells. These studies indicate that SIRT1 serves as a negative regulator of UPR signaling in T2DM and that SIRT1 attenuates hepatic steatosis, ameliorates insulin resistance, and restores glucose homeostasis, largely through the inhibition of mTORC1 and ER stress.—Li, Y., Xu, S., Giles, A., Nakamura, K., Lee, J. W., Hou, X., Donmez, G., Li, J., Luo, Z., Walsh, K., Guarente, L., Zang, M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver.
Keywords: unfolded protein response, XBP-1 splicing, mTORC1, hepatic steatosis, insulin signaling
Nonalcoholic fatty liver disease (NAFLD) is a hallmark of obesity and insulin resistance in patients with poorly controlled type 2 diabetes mellitus (T2DM) (1). The mammalian target of rapamycin complex 1 (mTORC1) and its major target, S6K1, have been implicated in the pathogenesis of insulin resistance (2–4). In obese and NAFLD humans and rodent models, hepatic endoplasmic reticulum (ER) stress plays a central role in disrupting systemic metabolic homeostasis (5–7). Interplay between ER stress and metabolic dysregulation may represent a common pathophysiological mechanism in obesity, metabolic syndrome, and T2DM (8–11). This is of importance to identify the checkpoints of regulatory convergence that can serve as therapeutic targets to relieve ER stress in insulin resistance.
When the ER, an organelle that regulates protein folding (12, 13), is challenged, an adaptive program called the unfolded protein response (UPR) is initiated to mitigate protein folding stress in the ER. During the chronic ER stress that is characteristic of obesity and T2DM, activation of protein kinase-like ER kinase (PERK) and subsequent phosphorylation of eukaryotic initiation factor 2α (elF2α) result in the attenuation of new protein synthesis (9, 12). Activation of inositol-requiring transmembrane kinase and endonuclease 1α (IRE-1α) leads to the splicing and activation of X-box binding protein 1 (XBP-1), a key transcription factor that regulates expression of UPR-target genes, such as GRP78, ultimately facilitating protein folding and degradation (9, 13). On ER stress, 3 arms of the UPR signaling, transcriptional induction mediated by IRE-1α and ATF6 and translational attenuation mediated by PERK, have complementary effects in maintaining ER homeostasis and ensuring cell survival (14). Both PERK and IRE-1α also activate the major stress kinase JNK (8), which, in turn, triggers serine phosphorylation of insulin receptor substrate-1 (IRS-1), leading to the pathogenesis of insulin resistance (8, 9). Chemical chaperones that relieve ER stress can attenuate the liver cell response to ER stress inducers and protect against systemic metabolic dysfunction (15). This suggests that the adaptive capacity of the ER can be enhanced and that such strategies may provide potential new therapeutic avenues.
We have characterized AMPK activation as a critical mechanism by which polyphenols, such as resveratrol and S17834, inhibit hyperlipidemia and atherosclerosis in type 1 diabetic low-density lipoprotein receptor-deficient (LDLR−/−) mice (16). We and others have recently indicated that interdependent activation of SIRT1 and LKB1/AMPK pathways contributes to some of the beneficial effects of polyphenols on hepatocellular lipid metabolism in cultured cells and in vivo (17, 18). Moreover, the small molecular activators of SIRT1, including resveratrol and SRT1720, improve glucose homeostasis and insulin sensitivity in T2DM mice (19–22). Although SIRT1 has been shown to have beneficial metabolic effects in T2DM (23, 24), whether hepatic activation of SIRT1 prevents ER stress and insulin resistance in vivo remains unclear.
Previous studies indicate that compared with wild-type mice, LDLR−/− mice are more susceptible to obesity with moderate insulin resistance and severe hepatic steatosis due to hyperlipidemia after feeding a type 2 diabetogenic diet, such as a high-fat/high-sucrose (HFHS) diet (25–27). This animal model has been widely used for the study of diet-induced obesity, diabetes, and atherosclerosis (28). In this study, we used adenovirus-mediated gene transfer approach to acutely deliver genes to the liver. This approach can specifically target genes to the liver of normal adult animals (29), and avoid potent confounding compensatory developmental effects that commonly occur in response to chronic gene expression. We found that activation of SIRT1 in the liver of HFHS diet-induced obese LDLR−/− mice and genetically obese ob/ob mice attenuated multiple ER stress markers, including phosphorylation of eIF2α and expression of GRP78 and CHOP. SIRT1 overexpression in the liver of both insulin-resistant mouse models exhibited a striking phenotype with the significant improvement in systemic insulin resistance and fatty liver, which was accompanied by the normalization of hyperglycemia, the alleviation of glucose tolerance, and a reduction in hepatic gluconeogenesis and lipid accumulation. In addition, enhancement of insulin sensitivity by SIRT1 was attributed to decreased mTORC1 activity and S6K1-mediated serine phosphorylation of IRS-1 in the liver. Interestingly, SIRT1 activation by resveratrol substantially reduced mTORC1 and XBP-1 splicing in wild-type mouse embryonic fibroblasts (SIRT1+/+ MEFs), but not in SIRT1−/− MEFs. Conversely, the SIRT1 deficiency resulted in increased mTORC1 activity, consequently enhanced ER stress and impaired insulin signaling. These effects of SIRT1 absence were completely abolished by rapamycin. Together, these findings indicate that SIRT1 attenuates obesity-induced ER stress and enhances insulin sensitivity in part through the suppression of mTORC1. Therefore, SIRT1 may be a druggable target to maintain ER homeostasis for the treatment of hepatic steatosis and metabolic disease.
MATERIALS AND METHODS
Reagents and antibodies
Resveratrol (SIRT1 activator) was obtained from Biomol (Plymouth Meeting, PA, USA). Nicotinamide (SIRT1 inhibitor) and tunicamycin (ER stress inducer) were from Sigma (St. Louis, MO, USA), and rapamycin (mTORC1 inhibitor) was from Cell Signaling Technology (Beverly, MA, USA). Rabbit polyclonal phospho-Thr389 S6K1, phospho-Ser235/236 S6, phospho-Thr37/46 4E-BP1, phospho-Ser473 Akt, phospho-Ser636/639, and phospho-Ser1101 IRS-1 and acetyl-Lys382 p53 antibodies, as well as total S6K1, S6, 4E-BP1, Akt, and eIF2α antibodies, were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal SIRT1 and phospho-Ser51 eIF2α antibodies and mouse monoclonal IRS-1 antibody were obtained from Upstate Biotechnology (Lake Placid, NY, USA). Rabbit polyclonal GRP78 (sc-13968) antibody, mouse monoclonal GADPH and p53 (sc-126) antibodies, and horseradish peroxidase-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti-FLAG M2 antibody was from Sigma. Mouse monoclonal β-actin and CHOP (ab-18308-2000) antibodies were from Abcam (Cambridge, MA, USA). All other reagents were of analytical grade.
Obesity and insulin-resistant animal models
Male 8-wk-old LDLR−/− mice on C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME, USA). LDLR−/− mice were rendered insulin-resistant by feeding an HFHS diet consisting of 35.5% fat (primarily lard) and 36.6% carbohydrate (primarily sucrose) and no cholesterol (No. F1850; Booserve, Frenchtown, NJ, USA) for 20 wk, as described previously (26). Genetically obese ob/ob mice at the age of 8–20 wk on C57BL/6 background were obtained from Jackson Laboratory and fed the chow diet. All the mice were kept on a 12-h light-dark cycle and given free access to food and water. All animal use was approved by the University Committee on Use and Care of Animals at Boston University School of Medicine.
In vivo adenovirus-mediated gene transfer
Adenovirus producing Flag-tagged full-length SIRT1 was kindly provided by Dr. Pere Puigserver (Harvard Medical School, Boston, MA, USA; 17, 30) and purified by using Adenovirus Purification Kits (Puresyn, Malvern, PA, USA). Overexpression of SIRT1 in the liver of these two insulin-resistant mouse models was accomplished via jugular vein injection of adenoviruses expressing GFP or Flag-tagged SIRT1 (5×109 pfu). Two weeks postinjection, each group of mice was sacrificed in a postprandial state under isoflurane anesthesia, and tissues were rapidly taken and freshly frozen in liquid nitrogen and stored at −80°C until biochemical analysis. Other parts of tissues were fixed for histology and immunohistochemistry.
Metabolic parameters and glucose-tolerance tests (GTTs) and insulin-tolerance tests (ITTs)
Blood glucose concentrations were measured using a OneTouch Ultra glucometer. Mouse plasma insulin was measured with an ultrasensitive mouse insulin ELISA kit (Mercodia AB, Uppsala, Sweden) with a detection limit of 0.025 μg/L. Mouse plasma samples were mixed with enzyme conjugate solution in a 96-well plate, and incubated on a plate shaker for 2 h at room temperature. After washing 6 times, substrate tetramethylbenzidine was added into each well, followed by incubation at room temperature for 15 min. A 50-μl stop solution was added to each well prior to measurement of optical density at 450 nM. Insulin concentrations were calculated according to the standard curve. The value of the homeostasis model assessment (HOMA)-IR index was calculated by using unfed condition (“fasting”) insulin and glucose values: IR = [insulin (pM) × glucose (mM)]/22.5 as previously described (31). Hepatic triglyceride concentrations were determined as we previously described (16, 32). GTTs were performed with an intraperitoneal injection of glucose solution (2 g/kg body wt) into the mice after 16 h food deprivation. Blood glucose was measured 0, 15, 30, 60, 90, and 120 min after glucose injection. ITTs were performed 10 d after adenovirus injection of ob/ob mice. Short-acting human insulin (0.75 U/kg body wt) was injected intraperitoneally into the mice after 6 h food deprivation, and blood glucose was measured 0, 15, 30, 60, 90, and 120 min after insulin injection.
Liver histology and immunohistochemistry
Liver tissues were fixed in 10% paraformaldehyde and embedded in paraffin. Liver sections (5 μm) were prepared and routinely stained with hematoxylin and eosin (H&E). The unstained vacuoles were visible in the H&E-stained liver sections of ob/ob mice and mice fed the HFHS diet. The degree of lipid infiltration in the liver was semiquantitatively assessed in a masked fashion by using an arbitrary value from 0 to 4, with 0 being normal livers seen in mice fed the normal chow diet and 4 being the worst.
Liver immunohistochemistry was performed as described previously (33). Briefly, paraffin-embedded liver sections were deparaffinized and rehydrated in graded alcohols. A heat-induced epitope retrieval technique was used by boiling liver sections in a solution of 10 mM citrate buffer (pH 6.0) for 2 min in a 700-W microwave 3 times. Slides were blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA, USA) in phosphate-buffered saline (PBS) for 30 min. Sections were incubated with primary antibodies in 1% BSA at 4°C overnight. The specificity of staining was examined by omission of primary antibodies and the use of preimmune serum at the same time. Endogenous peroxidase activity was quenched by the incubation in 3% hydrogen peroxide for 30 min. The biotinylated secondary antibody (Vectastain ABC kit; Vector Laboratories) was incubated, and vector red alkaline phosphatase substrate was used to visualize positive immunoreactivity, according to the manufacturer's instructions. Sections were counterstained with hematoxylin, dehydrated, and mounted. Finally, liver sections were viewed on a digitalized Olympus microscope attached to an Olympus HC5000 digital camera (Olympus, Tokyo, Japan).
Cell culture and treatment
Mouse embryonic fibroblasts derived from wild-type and SIRT1−/− mice were grown in DMEM (5.5 mM glucose) medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (34). Cells at 70–80% confluence were serum starved for 3 h. A pharmacological activator of SIRT1 and inhibitor of mTORC1 or together with tunicamycin, an experimental inducer of ER stress, were gently added to the culture dishes in the DMEM to prevent any environmental stress. Human HepG2 hepatocytes were cultured and treated as described previously (16, 32, 35).
Immunoblotting analysis
Immunoblotting analysis was conducted as described previously (16, 32, 35). Liver tissues or cultured cells were lysed at 4°C in lysis buffer (20 mM Tris-HCl, pH 8.0; 1% (v/v) Nonidet P-40; 150 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM sodium orthovanadate; 25 mM β-glycerolphosphate; 1 mM dithiothreitol; 1 mM phenylmethylsulfonyl fluoride; 2 μg/ml aprotinin; 2 μg/ml leupeptin; and 1 μg/ml pepstatin). Immunoblotting experiments were performed with 100 μg of liver tissues and 50 μg of cell lysates. To assess mTORC1 and UPR activity and IRS-1 function, phosphorylated proteins were quantified by using scanning densitometry with National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij/) and were normalized to the levels of endogenous protein. Phosphorylation intensity was presented relative to the basal or control level. Similarly, the levels of SIRT1 protein and ER stress markers were normalized to those of the β-actin loading control.
RT-PCR and quantitative RT-PCR analysis
Total RNA from mouse livers or cultured cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instruction. Five micrograms of total RNA to cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) and Oligo d (T). Briefly, the reaction mixture was heated to 65°C for 5 min and then quickly chilled on ice. First-strand buffer and DTT were added to the reaction and incubated at 42°C for 2 min. SuperScript II reverse transcriptase was added, and the mixture was incubated at 42°C for 1 h. The reaction was stopped by heating at 70°C for 15 min. The transcripts were quantified with StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) by using the SYBR Green PCR master mix and the ΔΔCT threshold cycle method. Gene expression levels were normalized to those of β-actin and presented relative to the control. Product specificity was verified by the melting curve and running products on an agarose gel. The following primers were used: PEPCK, TTGGAGAGAATGCTCGTGTG (F) and TGGAGAACAGCTGACTGGTG (R); PCK1, GCACAGAGAACAGGCTAGGG (F) and CAGCTAACGGGAAAAACTGG (R); G6Pase, CCTCCTCAGCCTATGTCTGC (F) and AACATCGGAGTGACCTTTGG (R); GCK, TGGTGGATGAGAGCTCAGTG (F) and TGAGCAGCACAAGTCGTACC (R); GRP78, CAGATCTTCTCCACGGCTTC (F) and GCAGGAGGAATTCCAGTCAG (R); CHOP, GCATGAAGGAGAAGGAGCAG (F) and CTTCCGGAGAGACAGACAGG (R); and β-actin, CCACAGCTGAGAGGGAAATC (F) and AAGGAAGGCTGGAAAAGAGC (R).
The splicing of XBP-1 was assessed by RT-PCR from cDNA using Taq DNA polymerase (NEB, Ipswich, MA, USA). PCR primers were designed to flank the 26-bp splicing sequence of mouse XBP-1. PCR reaction was performed as follows: 94°C for 3 min; 32 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. PCR products were separated by electrophoresis on a 2% agarose gel. The following primers were used: XBP-1 splice, AGTTAAGAACACGCTTGGGAAT (F) and AAGATGTTCTGGGGAGGTGAC (R) (36).
Statistical analysis
Data are expressed as means ± se. The significance of the differences in mean values was evaluated by using ANOVA or 2-tailed Student's t test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Adenovirus-mediated overexpression of SIRT1 in the liver ameliorates glucose tolerance and restores glucose homeostasis in LDLR−/− mice fed an HFHS diet
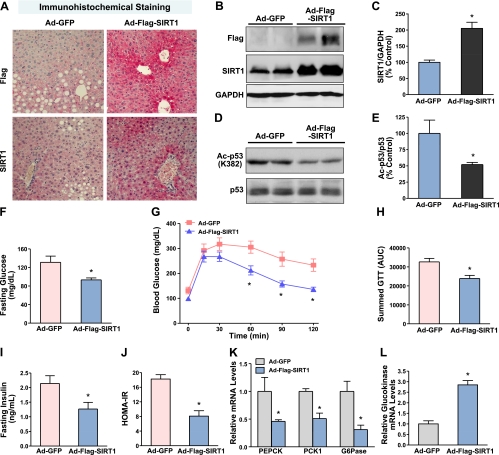
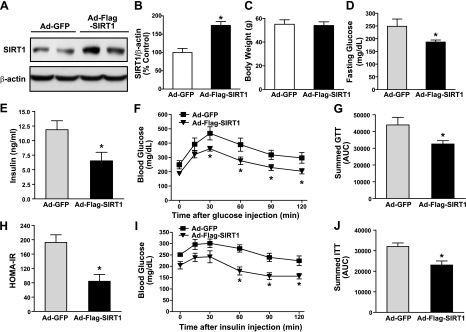
The LDLR−/− mouse model of T2DM induced by feeding an HFHS diet is characterized by obesity, insulin resistance, and hepatic steatosis (25–27). To test the hypothesis that SIRT1 functions as a modulator of obesity-induced ER stress in the liver, an insulin-resistant mouse model was developed in 8-week-old male LDLR−/− mice by feeding the HFHS diet for 22 wk. At 2 wk prior to the end of experiments, the adenoviruses expressing GFP- or Flag-tagged wild-type SIRT1 were delivered into the liver of LDLR−/− mice through jugular vein injections. As shown in Fig. 1A, immunohistochemical analysis of liver sections showed ∼60% of positive staining in the nuclei of hepatocytes around the periportal regions in the liver of mice expressing GFP. Staining signal intensity was significantly enhanced in the liver of SIRT1-injected mice, which was found not only in the nuclei of hepatocytes but also in the cytoplasm of hepatocytes. Immunoblots consistently showed a 2- to 3-fold increase in SIRT1 protein and an ∼50% reduction in deacetylation of p53 at Lys382, a specific deacetylation site of SIRT1 (Fig. 1B–E), confirming increased SIRT1 expression and activity in the liver of SIRT1-injected mice.
Figure 1.
Adenovirus-mediated overexpression of SIRT1 in the liver protects against insulin resistance and restores glucose metabolism in HFHS diet-induced obese LDLR−/− mice. Eight-week-old male LDLR−/− mice were fed an HFHS diet for 20 wk. GFP or Flag-tagged wild-type SIRT1 adenoviruses (5×109 pfu) were delivered into mouse livers via jugular vein injection, and the animals were sacrificed at 2 wk after continuing the HFHS diet. A) Representative immunohistochemical staining of the liver sections with anti-Flag and anti-SIRT1 antibodies. Positive immunostaining for SIRT1 was visualized mainly in the nuclei of the hepatocytes of LDLR−/− mice. Specificity of SIRT1 staining was evidenced by no detectable staining with a nonspecific IgG at the same concentration in the same liver sections (data not shown). Original view ×20. B) Representative immunoblot of Flag-tagged SIRT1 expression from 2 mouse livers in each group. Whole liver cell lysates (100 μg proteins) were resolved by 8% SDS-PAGE and immunoblotted for SIRT1 overexpression. C) Band intensity of SIRT1 in panel B was quantified by densitometry. D, E) Acetylation of p53 at Lys382 is decreased in the liver of mice expressing SIRT1. D) Representative immunoblot with acetylated p53 antibody in the liver lysates. E) Densitometric analysis. Data are represented as means ± se (n=5–6/group). F) Hepatic overexpression of SIRT1 lowers blood glucose levels in diet-induced obesity and insulin-resistant mice following 18 h food deprivation. G, H) SIRT1 overexpression improves glucose intolerance in HFHS diet-fed mice. G) GTTs (2 g/kg i.p.) were performed on mice following 16 h food deprivation at 10 d after adenovirus injection. A small nick was introduced to the tail for blood samples, and glucose levels were determined at 15, 30, 45, 60, 90, and 120 min. H) Area under the curve (AUC) for GTT was estimated by summing the numerical integration values of successive linear segments of the glucose disposal curve for 0–15, 15–30, 30–60, and 60–120 min. I, J) Plasma insulin levels (I) and calculated HOMA-IR values (J) are reduced by SIRT1 treatment. K, L) SIRT1 overexpression suppresses hepatic gluconeogenic gene expression in diet-induced insulin-resistant mice. Mouse liver mRNAs were isolated, and expression levels of gluconeogenic genes (PEPCK, G6Pase, and PCK1; K) and glycolytic gene (glucokinase; L) were evaluated by quantitative RT-PCR. Relative mRNA levels were normalized to those of β-actin and expressed as means ± se (n=5–6/group). *P < 0.05 vs. Ad-GFP group.
To determine in vivo function of hepatic SIRT1 on glucose homeostasis and insulin sensitivity, mice fed the HFHS diet for 20 wk were injected with the SIRT1 adenovirus. At 2 wk after the HFHS feeding, mice expressing SIRT1 displayed an ∼30% reduction in fasting glucose levels, compared with those of control Ad-GFP-injected mice (131.2±13.3 vs. 93.3±4.3 mg/dl; Fig. 1F). Moreover, fasting plasma insulin concentrations were significantly lower in SIRT1-expressing mice than those in GFP-injected mice (2.14±0.27 vs. 1.27±0.23 ng/ml; Fig. 1I). In addition, lower levels of homeostasis model-assessment of insulin resistance (HOMA-IR) were seen in SIRT1-injected mice (Fig. 1J), suggesting that SIRT1 enhances systemic insulin sensitivity. In intraperitoneal GTTs, mice expressing SIRT1 showed significantly lower glucose levels and improved glucose tolerance, compared to Ad-GFP-injected mice. Consistently, integrated glucose concentrations, as calculated by the area under the curve (AUC), were significantly decreased in mice expressing SIRT1 (Fig. 1G, H). These results demonstrate that hepatic overexpression of SIRT1 can significantly reduce the development of insulin resistance associated with dietary obesity.
To identify the physiological mechanisms responsible for SIRT1 to regulate whole-body glucose homeostasis, transcriptional regulation of genes encoding key enzymes of hepatic glucose metabolism was assessed by real-time PCR. The mRNA levels of gluconeogenic genes, including PEPCK, G6Pase, and PCK1, a cytosolic form of PEPCK, were decreased by ∼60% in the liver of SIRT1-injected mice. Conversely, the gene expression of glycolytic enzymes, such as glucokinase, encoded by Gck, was increased ∼3-fold (Fig. 1K, L). Taken together with the reduced hepatic glucose output by the small molecule activator of SIRT1 (SRT1720; ref. 20), our results indicate that hepatic activation of SIRT1 improves glucose tolerance and reduces systemic insulin resistance in large part by decreasing hepatic gluconeogenesis and glucose production.
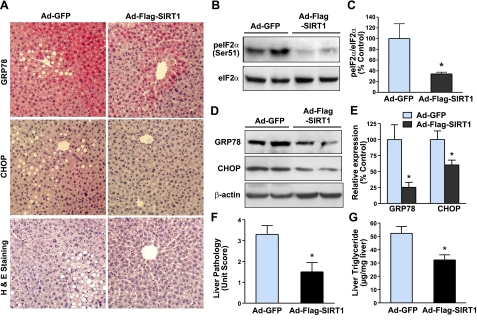
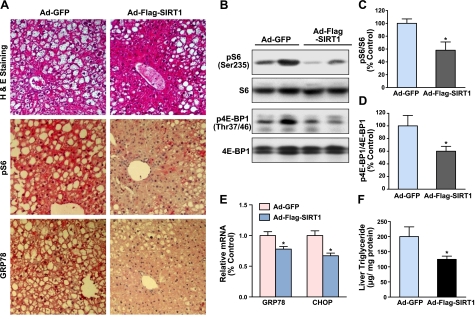
Overexpression of SIRT1 in the liver ameliorates ER stress and hepatic steatosis in diet-induced insulin-resistant LDLR−/− mice
In liver tissues of both obese humans and mice, the most significant and consistent markers of ER stress include phosphorylation of eIF2α at Ser51, a direct target of PERK, the ER stress sensor (13), the splicing of XBP-1, and expression of UPR-targeted genes GRP78 and CHOP (6, 7). To delineate the molecular mechanism by which SIRT1 improves systemic insulin resistance, we determined whether SIRT1 might play a role in regulating ER homeostasis. Compared with GFP control mice, phosphorylation of eIF2α was decreased by ∼60% in the liver of mice expressing SIRT1 (Fig. 2A–C). Immunostaining analysis for expression and distribution of ER stress markers revealed that intense positive staining of GRP78 and CHOP was predominantly located in hepatocytes surrounding the area of lipid droplets and blood vessels in diabetic mice injected with Ad-GFP, whereas the intensity and area of positively staining signals were substantially reduced in SIRT1-expressing mice. Likewise, hepatic expression of GRP78 and CHOP protein was reduced by 75 and 40%, respectively (Fig. 2A, D, E). To determine whether SIRT1-mediated inhibition of UPR activation is of functional relevance, H&E staining showed that hepatic overexpression of SIRT1 largely reduced liver fat infiltration in diet-induced obese LDLR−/− mice (Fig. 2A), although there was no significant difference in body weight between the control and the SIRT1-injected mice (data not shown). Hepatic triglyceride contents were markedly decreased by SIRT1 expression, consistent with its ability to reduce the degree of hepatic steatosis (Fig. 2F, G). These results indicate that SIRT1 alleviates hepatic ER stress and thereby protects against hepatic steatosis and lipid accumulation in diet-induced insulin-resistant mice.
Figure 2.
Hepatic overexpression of SIRT1 alleviates ER stress and ameliorates hepatic steatosis in diet-induced insulin-resistant LDLR−/− mice. A) Representative immunohistochemical staining of liver sections for the expression of ER stress markers, and H&E staining for hepatic steatosis. Positive staining for GRP78 or CHOP in the cytoplasm or nuclei of hepatocytes around the blood vessel area of liver sections was much less in mice expressing SIRT1. Positive staining was specific inasmuch as liver sections of the same mouse were incubated with nonimmuno-IgG substituted for the primary antibody, and no significant staining was detected (data not shown). B) Phosphorylation of eIF2α, a key downstream target of PERK branch of the UPR, is largely decreased in the liver of insulin-resistant mice with Ad-Flag-SIRT1 injection. C) Phosphorylation of eIF2α at Ser51 (peIF2α) was quantified by densitometry and normalized to the level of total eIF2α, and presented as mean ± se relative levels to those of Ad-GFP-injected mice (n=5–6/group). D, E) Immunoblots (D) and densitometric quantification (E) for hepatic ER stress chaperones. Mice overexpressing SIRT1 in the liver exhibit the reduction in UPR-dependent targets, such as GRP78/Bip and CHOP. F, G) Effect of SIRT1 overexpression on liver pathology (F) and triglyceride contents (G) in HFHS diet-fed mice. Degree of lipid infiltration on the liver sections of H&E staining was scored by 3 medical researchers for the overall pathology of fatty liver on a scale of 0–4, with 0 being normal healthy tissue typically seen in normal diet mice and 4 being the worst. Extracted liver lipids were measured, and triglyceride levels were expressed as micrograms of lipid per milligram protein. Data are presented as means ± se (n=5–6/group). *P < 0.05 vs. Ad-GFP group.
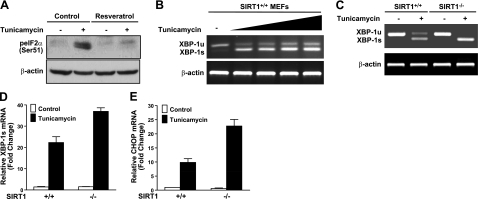
Loss of SIRT1 in cells results in enhanced UPR signaling under ER stress conditions
To seek further evidence for a causal relationship between SIRT1 deficiency and ER stress, pharmacological and genetic approaches for the modulation of SIRT1 activity were used in cultured cell models. As we and others previously demonstrated that resveratrol, a natural polyphenolic product, increases SIRT1 deacetylase activity in vitro (17, 22), we examined whether SIRT1 activation by resveratrol decreases UPR activation in human HepG2 hepatocytes in response to tunicamycin, an ER stress-inducing agent. Because of the limited availability of antibodies that recognize phosphorylated forms of human endogenous PERK and IRE-1α, two major proximal sensors of the UPR pathway (7), phosphorylation of eIF2α at Ser51, which could be mediated by PERK activation, was assessed. As shown in Fig. 3A, exposure of HepG2 cells to tunicamycin stimulated eIF2α phosphorylation. Conversely, resveratrol completely abrogated ER stress-induced phosphorylation of eIF2α. To gain direct evidence for the function of SIRT1 in regulating downstream signaling of IRE-1α, we measured the relative abundance of spliced XBP-1 mRNA in wild-type MEFs, indicative of the kinase and endoribonuclease activity of IRE-1α. As shown in Fig. 3B–D, the spliced XBP-1 form (XBP-1s) was detected in both SIRT1+/+ and SIRT1−/− MEFs challenged with tunicamycin. In contrast, the unspliced form of XBP-1 (XBP-1u) was only detected in MEFs without tunicamycin treatment, similar to earlier observations in fibroblast response to ER stress (37). Treatment of wild-type MEFs with tunicamycin substantially induced the splicing of XBP-1 in a dose-dependent manner. Notably, both XBP-1u and XBP-1s were induced in wild-type MEFs when UPR signaling was activated. It is likely that the UPR activation triggers a positive feedback loop to transcriptionally activate XBP-1, as previous studies reported that XBP-1s activates its own promoter through direct binding to the cis-acting ER stress response element (38). Furthermore, although these ER stress markers were present at very low levels in SIRT1+/+ and SIRT1−/− MEFs under normal circumstances, treatment of SIRT1−/− cells with tunicamycin caused an increase in XBP-1s to a greater extent than that of SIRT1+/+ cells. As expected, CHOP mRNA levels were increased >10-fold by tunicamycin in SIRT1+/+ MEFs. Compared to SIRT1+/+ MEFs, the transcriptional induction of CHOP was further enhanced an additional 2-fold in tunicamycin-treated SIRT1−/− cells. These results suggest that SIRT1-deficient cells are more vulnerable to ER stress through the persistence of XBP-1 splicing and CHOP expression. Together with the in vitro observation, mice with SIRT1 activation in the liver are resistant to obesity-induced ER stress, possibly through the suppression of multiple arms of UPR signaling by decreasing PERK-mediated activation of eIF2α and IRE-1-dependent splicing of XBP-1 and by reducing expression of UPR downstream genes.
Figure 3.
Pharmacological and genetic manipulation of SIRT1 activity regulates UPR signaling in cultured cells in response to ER stress. A) Phosphorylation of eIF2α is stimulated by exposure of HepG2 cells to tunicamycin, an experimental inducer of ER stress, and down-regulated by a polyphenolic SIRT1 activator, resveratrol. Cells were treated with resveratrol (10 μM) in the absence or presence of tunicamycin (2 μg/ml) in DMEM containing 2% FBS for 6 h. Cell lysates were immunoblotted for eIF2α phosphorylation. B) Tunicamycin treatment dose-dependently increases the splicing of XBP-1. Wild-type MEFs were treated with increasing concentrations of tunicamycin (0.5–2 μg/ml) for 6 h. Unsplicing (XBP-1u) and splicing (XBP-1s) forms of XBP-1 were evaluated by RT-PCR. Primers were designed to flank the 26-nt intron excised from the XBP-1 transcript. Resultant PCR products were resolved by 2% agarose gel electrophoresis; β-actin served as the control of total RNA. C, D) Absence of SIRT1 increases XBP-1s levels in cells exposed to tunicamycin. C) SIRT1+/+ and SIRT1−/− MEFs were treated with or without tunicamycin (2 μg/ml) in DMEM containing 2% FBS for 6 h. There was no significant difference in XBP-1s between the cell lines under basal conditions. D) Extent of XBP-1s was quantitated by densitometry, normalized to total XBP-1 transcripts (XBP-1s plus XBP-1u), and graphed. E) mRNA levels of CHOP are determined by quantitative PCR in SIRT1+/+ and in SIRT1−/− MEFs. Data are presented as means ± se (n=3).
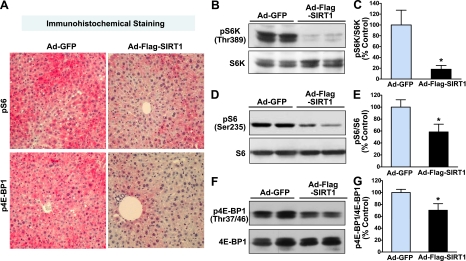
Hepatic overexpression of SIRT1 inhibits mTORC1 signaling in the liver of diet-induced insulin-resistant LDLR−/− mice
ER stress has been identified as a critical component of the pathologies associated with dysregulated mTORC1 activity (39), since the loss of TSC1/2 in cell lines, which constitutively activates mTORC1 (2, 3), triggers ER stress and activates UPR signaling, leading to negative feedback regulation of insulin action (39). To further elucidate the mechanism by which SIRT1 enhances insulin sensitivity, we tested whether that hepatic SIRT1 activation might also inhibit obesity-induced mTORC1 activation in vivo. Thus, we examined the phosphorylation state of two well-characterized substrates of mTORC1, S6K1 and 4E-BP1, which are the frequently used indicators of mTORC1 activity (3). Immunohistochemical analysis for phosphorylation of ribosomal protein S6 at Ser235/236, the downstream target of S6K1, and 4E-BP1, revealed that strong positive staining in the hepatocytes was mainly located in areas of accumulated lipid droplets and blood vessels in GFP-injected mouse livers, but positive staining was much less in SIRT1-injected diabetic mice (Fig. 4A), consistent with reduced hepatic ER stress and improved hepatic steatosis (Fig. 2). Immunoblot analysis confirmed that overexpression of SIRT1 caused ∼70% and 50% decreases in S6K1 and S6 phosphorylation, respectively, which was paralleled by a 60% decrease in 4E-BP1 phosphorylation in the diabetic mouse livers (Fig. 4B–G). These findings indicate that mTORC1-dependent signaling is down-regulated in the liver of SIRT1-expressed diabetic mice, which may explain its protection from hepatic ER stress and insulin resistance.
Figure 4.
Hepatic expression of SIRT1 suppresses mTORC1 activation in the liver of diet-induced insulin-resistant LDLR−/− mice. A) Representative immunohistochemical staining of liver sections for phosphorylation of S6 at Ser235/236 and 4E-BP1 at Thr37/46, two major downstream targets of S6K1 and mTORC1, respectively. Strong positive staining for phosphorylation of S6 (pS6) and 4E-BP1 (p4E-BP1) in hepatocytes was markedly decreased in mice expressing SIRT1. There was no detectable staining with a nonspecific IgG at the same concentration in the same liver sections, indicating the specificity of pS6 and p4E-BP1 staining (data not shown). B–G) Activity of mTORC1 and downstream signaling is reduced in the liver of ob/ob mice overexpressing SIRT1. Representative immunoblots for phosphorylation of S6K (B), S6 (D), and 4E-BP1 (F) in the livers from two mice in each group are shown. Relative phosphorylation levels of S6K (C), S6 (E), and 4E-BP1 (G) were normalized to those of endogenous proteins and presented as the means ± se (n=5–6). *P < 0.05 vs. Ad-GFP group.
Hepatic overexpression of SIRT1 protects against defective glucose homeostasis and insulin resistance in genetically obese ob/ob mice
Ob/ob mice are leptin deficient and become hyperphagic, obese, and insulin resistant (28). To investigate the function of SIRT1 on hepatic ER stress and insulin resistance, we utilized the ob/ob mouse model of severe obesity and hepatic steatosis. As shown in Fig. 5A–C, overexpression of SIRT1 in the liver was achieved in the genetic ob/ob mice by jugular vein injection of SIRT1 adenovirus. The ob/ob mice displayed severe obesity regardless of SIRT1 overexpression, and there was no significant difference in body weights of ob/ob mice expressing either Ad-GFP or Ad-Flag-SIRT1. To determine whether SIRT1-injected ob/ob mice became more sensitive to insulin, we measured fasting blood glucose and plasma insulin concentrations, and calculated HOMA-IR index 2 wk after adenovirus injection. As previously observed, ob/ob animals developed a moderate fasting hyperglycemia and severe hyperinsulinemia (8). The elevation in fasting blood glucose levels was reduced by 25% in the ob/ob mice expressing SIRT1, compared to GFP-treated mice (Fig. 5D). Furthermore, plasma insulin levels were significantly reduced by 50%; therefore, the calculated HOMA-IR index was decreased by 40% in the SIRT1-injected ob/ob mice (Fig. 5E, H). Together, these results indicate that the mice expressing SIRT1 in the liver were protected from the development of obesity-induced hyperglycemia and hyperinsulinemia in HFHS diet-fed LDLR−/− mice and ob/ob mice.
Figure 5.
Adenovirus-mediated hepatic SIRT1 overexpression improves insulin sensitivity and modulates insulin action on glucose metabolism in ob/ob mice. Either Flag-tagged wild-type SIRT1 or control GFP adenoviruses (5×109 pfu) were delivered into the liver of ob/ob mice via jugular vein injection, and the animals were sacrificed 2 wk postinjection. A) Overexpression of SIRT1 in the liver was confirmed by immunoblot analysis. B) Representative immunoblot of total cell lysates from 2 mouse livers in each group and quantitative analysis of SIRT1 overexpression. C) Effect of SIRT1 overexpression on body weight in ob/ob mice. D) Blood glucose levels are lowered in mice injected with Ad-SIRT1 following 18 h food deprivation. E) Plasma insulin levels are reduced in SIRT1-injected mice. F) GTTs (1 g/kg) were performed in ob/ob mice following 18 h food deprivation 5 d after adenovirus injection. G) Summed results from F. H) HOMA-IR values were reduced in SIRT1-injected mice. I) ITTs (0.75 U/kg) were performed in ob/ob mice following 6 h food deprivation at 10 d after adenovirus injection. J) Summed results from I. Data are presented as means ± se (n=5–6/group). *P < 0.05 vs. Ad-GFP group.
To further confirm that SIRT1 overexpression is sufficient to normalize glucose homeostasis and improve insulin sensitivity, we performed intraperitoneal GTTs and ITTs 5 and 10 d after adenovirus injection, respectively. In GTTs, the SIRT1-expressed ob/ob mice displayed less hyperglycemic response after 30–120 min of glucose injection than did the control mice (Fig. 5F, G). The SIRT1-expressing ob/ob mice had greater hypoglycemic response to insulin at 60–120 min than did the control mice (Fig. 5I, J). The findings demonstrate that hepatic activation of SIRT1 restores glucose homeostasis and ameliorates insulin resistance associated with the ob/ob phenotypes.
Hepatic overexpression of SIRT1 suppresses mTORC1 activity and UPR signaling and improves hepatic steatosis in ob/ob mice
We next determined whether increased SIRT1 activity inhibits mTORC1 activation in the liver of ob/ob mice. As shown in Fig. 6A–D, immunostaining analysis for S6 phosphorylation revealed that intense positive staining in hepatocytes was particularly observed around the periportal regions of ob/ob mice, suggesting that obesity promotes the hyperactivation of mTORC1 in the liver. In contrast, less positive staining of hepatocytes was evident in SIRT1-treated ob/ob mice, suggesting that hyperinsulinemia-induced activation of mTORC1 is significantly attenuated by SIRT1. Immunoblots confirmed that, compared with control ob/ob mice, SIRT1 overexpression caused a 40% reduction in phosphorylation of both S6 and 4E-BP-1. The results support the notion that the ability of SIRT1 to improve glucose homeostasis and insulin resistance may be attributed to the inhibition of mTORC1 in the liver of both mild (diet-induced) and severe (genetically induced) mouse models of obesity and insulin resistance.
Figure 6.
Hepatic overexpression of SIRT1 suppresses mTORC1 activation and ER stress and attenuates hepatic steatosis in the liver of ob/ob mice. A) Representative H&E staining of the liver sections for hepatic steatosis and immunohistochemical staining for phosphorylation of S6 at Ser235/236 (pS6) and the ER stress marker. Strong positive staining for phosphorylation of S6 and for expression of GRP78 in the hepatocytes was mainly located around the periportal regions of ob/ob mouse livers that were markedly decreased by hepatic expression of SIRT1. There was no detectable staining with a nonspecific IgG at the same concentration in the same liver sections, indicating the specificity of pS6 and GRP78 staining (data not shown). B–D) Activation of mTORC1 in ob/ob mice is prevented by overexpressing hepatic SIRT1. B) Representative immunoblots for phosphorylation of S6 and 4E-BP1 in mouse livers. C, D) Relative phosphorylation levels of S6 (C) and 4E-BP1 (D) were normalized to those of endogenous proteins. E) Hepatic overexpression of SIRT1 reduced gene expression of ER stress markers, including GRP78 and CHOP, in ob/ob mouse livers. F) Hepatic overexpression of SIRT1 lowers liver triglyceride accumulation in ob/ob mice. Liver triglyceride contents were measured in ob/ob mice. Data are presented as means ± se (n=5–6). *P < 0.05 vs. Ad-GFP group.
ER stress and insulin resistance are progressive disorders in the liver of ob/ob mice (8). As demonstrated in diet-induced insulin-resistant LDLR−/− mice (Fig. 2), our results showed that obesity-induced up-regulation of GRP78 chaperone and CHOP expression was markedly attenuated in SIRT1-expressed ob/ob mouse livers, as compared to GFP control mice (Fig. 6A, E). Furthermore, severe hepatic macrovesicular steatosis in ob/ob mice, as indicated by H&E staining, was partially reversed by SIRT1 treatment, and hepatic triglyceride levels are reduced by 40% (Fig. 6A, F). These results suggest that inhibition of mTORC1 and UPR by SIRT1 contributes to improved hepatic steatosis and liver triglyceride accumulation in ob/ob mice.
SIRT1 activation by resveratrol down-regulates mTORC1 activity and consequently reduces UPR signaling in vitro
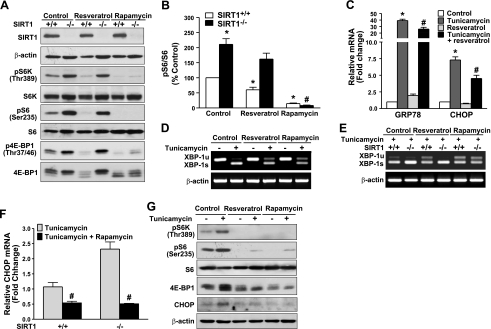
To further examine the role of SIRT1 depletion in the regulation of mTORC1 and its functional outcomes on ER homeostasis, the effect of resveratrol, a SIRT1-activating polyphenol, on mTORC1 activity and UPR signaling was compared between the SIRT1+/+ and SIRT−/− MEFs. As shown in Fig. 7A, B, phosphorylation of S6K1 and S6 was readily detectable in SIRT1+/+ MEFs incubated in the growth cell medium. Likewise, phosphorylation of 4E-BP1, as reflected by a phosphorylation state of 4E-BP1 at Thr37/46 and band mobility, was also detected. Notably, 4E-BP1 had 3 isoforms with varying mobilities on SDS-PAGE due to different extents of phosphorylation, with the hyperphosphorylated form having the slowest mobility. Strikingly, the phosphorylation of S6K1 and 4E-BP1 was much higher in the SIRT1−/− cells than that of SIRT1+/+ cells. On the other hand, resveratrol decreased the phosphorylation of S6K1 and 4E-BP1 in SIRT1+/+ cells. Conversely, the inability of resveratrol to inhibit phosphorylation of both mTORC1 substrates in SIRT1−/− cells indicates that resveratrol inhibits mTORC1 activity through SIRT1. Unlike resveratrol, phosphorylation of S6K1 and 4E-BP1 was markedly reduced by rapamycin, a specific inhibitor of mTORC1, in both cell lines. These results provide evidence that mTORC1-dependent signaling is attenuated by SIRT1 activation and augmented by SIRT1 deficiency.
Figure 7.
Genetic depletion of SIRT1 in cells increases mTORC1 activity and consequently promotes UPR activation and diminishes the effect of resveratrol. A, B) Cells lacking SIRT1 display a marked increase in mTORC1 activity and block the ability of resveratrol to suppress mTORC1 activity. A) SIRT1 MEFs were incubated in DMEM containing 2% FBS and treated with either resveratrol (50 μM) or a specific inhibitor of mTORC1, rapamycin (10 nM) for 1 h. B) Quantitative analysis of phosphorylated S6 is normalized to total S6 protein, and presented as means ± se (n=3). C) Suppression of mTORC1 by resveratrol inhibits the expression of GRP78 and CHOP in SIRT1+/+ MEFs in response to tunicamycin. D) mTORC1 inhibition by either resveratrol or rapamycin strongly decreases XBP-1 splicing in SIRT1+/+ MEFs exposed to tunicamycin. E) Resveratrol suppresses tunicamycin-induced splicing of XBP-1 in SIRT1+/+ MEFs, but not in SIRT1−/− MEFs. F) Rapamycin largely reduces tunicamycin-mediated induction of CHOP mRNA levels in both SIRT1+/+ and SIRT1−/− MEFs. SIRT1+/+ and SIRT1−/− MEFs were cultured quiescently in serum-free medium for 3 h and treated for 6 h with or without resveratrol (10 μM) and rapamycin (10 nM) in the absence or presence of tunicamycin (2 μg/ml) in DMEM containing 2% FBS. Unsplicing and splicing forms of XBP-1 were analyzed by RT-PCR, and mRNA levels of GRP78 and CHOP were evaluated by qRT-PCR. Values represent mean ± se relative induction normalized to β-actin (n=3). G) Resveratrol and rapamycin strongly suppress mTORC1 activity and thereby repress CHOP induction in human HepG2 cells exposed to tunicamycin. Cells were incubated for 6 h with resveratrol (10 μM) and rapamycin (10 nM) in DMEM containing 2% FBS, with or without tunicamycin (2 μg/ml). *P < 0.05 vs. untreated control group; #P < 0.05 vs. tunicamycin treatment group.
To define the functional significance of crosstalk between SIRT1 and mTORC1 nutrient-sensing pathways in maintaining ER homeostasis, the effect of a pharmacological SIRT1 activator and mTORC1 inhibitor on ER stress was examined in cultured MEFs and HepG2 cells. We found that in parallel to inhibition of mTORC1, resveratrol decreased tunicamycin-induced expression of UPR-dependent targets (GRP78 and CHOP) in SIRT1+/+ MEFs (Fig. 7C). Interestingly, the tunicamycin-induced XBP-1 splicing was largely inhibited by resveratrol in SIRT1+/+ MEFs, and the decrease was almost completely abolished in SIRT1−/− MEFs, suggesting that SIRT1 is required for resveratrol to attenuate UPR signaling (Fig. 7D, E). Moreover, the tunicamycin-enhanced splicing of XBP-1 and expression of CHOP mRNA were further potentiated by 2- to 3-fold in SIRT1−/− cells, compared with SIRT1+/+ MEFs (Fig. 7F), suggesting that SIRT1−/−MEFs were more sensitive to ER stress. Moreover, induction of XBP-1s and CHOP by tunicamycin in both cell lines was largely repressed by rapamycin (Fig. 7E, F). Collectively, these results suggest that SIRT1 counteracts the UPR activation in cultured cells in a rapamycin-sensitive manner.
We further determined whether suppression of mTORC1 and UPR signaling by SIRT1 also occurs in hepatocytes. As shown in Fig. 7G, phosphorylation of S6K1 and 4E-BP1 was induced by tunicamycin and abrogated by resveratrol. As a result, transcriptional expression of CHOP was substantially reduced in HepG2 cells. Pharmacological inhibition of mTORC1 by rapamycin decreased CHOP expression in HepG2 cells to the same extent as resveratrol, as was seen in SIRT1+/+ MEFs (Fig. 7A, F). These results further support the hypothesis that mTORC1 inhibition by SIRT1 effectively protects again ER stress in different cell types. Given mTORC1 as a potential downstream of SIRT1 to prevent UPR activation in vitro, these findings provide strong evidence that SIRT1 activation prevents obesity-induced hepatic ER stress and steatosis, at least in part through mTORC1 suppression.
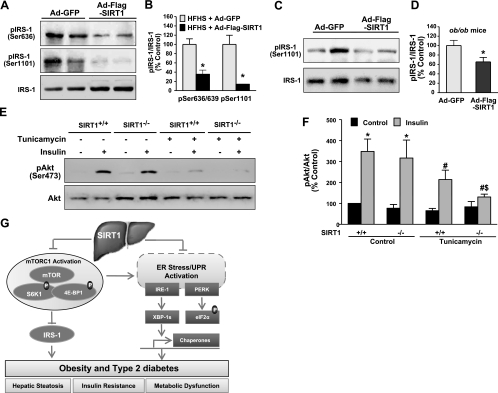
Manipulation of SIRT1 activity alters insulin receptor signaling in diabetic mouse livers in vivo and in vitro
Because of the significant improvement in insulin sensitivity observed in HFHS diet-fed LDLR−/− mice and ob/ob mice that expressed SIRT1 in the liver, we further elucidated the potential signaling mechanisms of SIRT1. Given that high-fat diet-fed mice and ob/ob mice exhibit markedly elevated S6K1 activity and increased serine phosphorylation of insulin receptor substrate 1 (IRS-1), a critical regulator for insulin sensitivity (40, 41), it is important to understand the mechanistic basis of SIRT1 on insulin sensitivity by measuring insulin receptor signaling in liver tissues. As shown in Fig. 8A, B, IRS-1 phosphorylation at Ser636/639 or Ser1101, the direct phosphorylation sites of S6K1 (40, 41), was robustly decreased by 60 and 80% in the liver of Ad-SIRT1-injected diabetic LDLR−/− mice. Furthermore, compared with control GFP-injected ob/ob mice, hepatic overexpression of SIRT1 caused a 35% reduction in IRS-1 phosphorylation at Ser636/639 (Fig. 8C, D), which was less than that of SIRT1 overexpression in HFHS-fed LDLR−/− mice. Collectively, the results support the notion that SIRT1 ameliorates hepatic and systemic insulin resistance through suppression of mTORC1/S6K1-mediated feedback inhibition of insulin receptor signaling in both diet- and obesity-induced insulin-resistant mice.
Figure 8.
Modulated SIRT1 activity regulates insulin signaling in the liver of diet- and obesity-induced insulin-resistant mice and in vitro. A, B) Insulin receptor signaling is enhanced by hepatocellular SIRT1 overexpression in diet-induced insulin-resistant LDLR−/−mice. Phosphorylation of IRS-1 at Ser636/639 and Ser1101, two major specific phosphorylation sites of S6K1, was decreased in the liver of insulin-resistant mice with Ad-Flag-SIRT1 injection. A) Representative immunoblots of phosphorylated IRS-1 and total proteins from 2 mouse livers/group. B) Relative phosphorylation levels were normalized to those of endogenous proteins. C, D) IRS-1 phosphorylation at Ser636/639 in the liver of ob/ob mice is prevented by overexpressing SIRT1. C) Representative immunoblots of phosphorylated IRS-1 and total proteins from 2 mouse livers/group. D) Relative phosphorylation levels were normalized to those of endogenous proteins. Data are presented as means ± se (n=5–6). *P < 0.05 vs. Ad-GFP group. E, F) SIRT1 deficiency impairs insulin-stimulated phosphorylation of Akt under the conditions of ER stress. E) SIRT1+/+ and SIRT1−/− MEFs were cultured quiescently in serum-free medium for 3 h, pretreated with tunicamycin (2 μg/ml) for 6 h, and then stimulated with insulin (100 nM) for 10 min. Cell lysates were subjected to immunoblotting for phosphorylation of Akt. F) Data are presented as means ± se (n=4). *P < 0.05 vs. untreated control group; #P < 0.05 vs. insulin stimulation group; $P < 0.05 vs. tunicamycin treatment and insulin stimulation group. G) Proposed model for SIRT1 activation to attenuate obesity-induced ER stress, hepatic steatosis, and insulin resistance in large part through inhibition of mTORC1. In diet- and obesity-induced T2DM, chronic energy and nutrient excess activate nutrient-sensing pathway of mTORC1 and S6K1, which leads to the feedback inhibition of insulin receptor signaling through serine phosphorylation of IRS-1. Simultaneously, obesity-induced hepatic ER stress activates the UPR that includes PERK-mediated phosphorylation of eIF2α, IRE-1-mediated splicing of XBP-1, and transcription of UPR target genes, GRP78 and CHOP. Hyperactivation of mTORC1 also results in uncontrolled protein synthesis that triggers a vicious cycle to accelerate the development of more severe ER stress in the liver, ultimately culminating in systemic insulin resistance and metabolic dysfunction. Conversely, SIRT1 activation in the liver may counteract the mTORC1/S6K1 activation in diet- and obesity-induced insulin resistance and, in turn, prevent the vicious cycle by restoring hepatocellular ER homeostasis and by blocking S6K1-mediated feedback inhibition of IRS-1—all of which modulates insulin action on hepatic glucose metabolism and enhances systemic insulin sensitivity. The identification of coordinated regulation between nutrient sensing pathways of SIRT1 and mTORC1 provides novel mechanistic insights into potential targets for treatment of ER stress and hepatic steatosis in obesity and T2DM.
To determine whether the absence of SIRT1 in cells can reproduce the causal effects of HFHS diet and obesity on insulin signaling in a cell-autonomous fashion, we assessed a potential role of SIRT1 deficiency in insulin-stimulated phosphorylation of Akt. As compared with the negligible effect of SIRT1 under normal conditions, insulin-stimulated phosphorylation of Akt was reduced by tunicamycin in SIRT1+/+ cells but was further abolished in SIRT1−/− cells (Fig. 8E, F). It is possible that SIRT1−/− cells are more susceptible to ER stress, leading to accelerated mTORC1/S6K1-mediated feedback inhibition of insulin receptor signaling. These data suggest that the SIRT1-dependent changes in hepatic insulin signaling are likely to be direct and cell autonomous. These studies demonstrate that SIRT1 function in the liver increases insulin receptor signaling, likely via inactivating mTORC1 and enhancing IRS-1 function, which, in turn, enhances whole-body and hepatic insulin sensitivity in T2DM mice.
DISCUSSION
The present study provides the first direct evidence that SIRT1-dependent suppression of mTORC1 represents the novel therapeutic mechanism by which SIRT1 prevents obesity-induced ER stress and insulin resistance in the liver. We showed that SIRT1 activation by resveratrol decreased UPR activation by inhibiting mTORC1 in HepG2 cells. Conversely, SIRT1-knockout fibroblasts in response to ER stress showed a marked increase in mTORC1 activity and in UPR signaling and the impairment in insulin signaling, all of which were abolished by mTORC1 inhibition by rapamycin. Studies with the HFHS diet-fed LDLR−/− mouse model and the genetic ob/ob mouse models of obesity and insulin resistance demonstrated that adenovirus-mediated hepatic overexpression of SIRT1 reduced hepatic mTORC1 activation and ER stress, contributing to alleviated fatty liver and enhanced insulin sensitivity. Although other factors, such as PTP1B and PGC-1α, have been shown to contribute to some of the beneficial effects of SIRT1 in T2DM (19, 42), the present study provides a novel and attractive model, in which the suppression of mTORC1 by SIRT1 functionally inhibits hepatocellular ER stress, which, in turn, improves hepatic steatosis and whole body insulin resistance and restores glucose homeostasis (Fig. 8G).
SIRT1 activation attenuates obesity-induced ER stress in the liver
The most important implication of the present study is the identification of a functional connection of SIRT1 nutrient sensor to UPR-dependent pathways. The persistent activation of UPR in the liver and adipose tissue has been implicated in the pathophysiology of obese and insulin-resistant humans and mouse models (6–8, 15, 37). Given the fluctuating environment of the cellular adaptive responses of the ER, one UPR molecule may not be a reliable indicator of ER functional capacity. Therefore, this study assesses different UPR pathways by measuring the proximal sensor activity, their substrate modulation, or the transcription of UPR target genes. Our findings showed that the absence of SIRT1 resulted in greater susceptibility to ER stress and impairment in insulin signaling, as reflected by the increase in XBP-1 splicing and CHOP transcription and the decrease in insulin-stimulated phosphorylation of Akt in SIRT1−/− MEFs under the conditions of ER stress. These results, together with early observation that SIRT1 down-regulates ER stress genes in determining life span in lower organisms (43), indicate that endogenous SIRT1 contributes to the maintenance of ER homeostasis and insulin metabolic action. As demonstrated in cells lacking SIRT1, the ability of resveratrol to suppress the cellular responses to ER stress, such as XBP-1 splicing, was mediated by SIRT1. Likewise, activation of SIRT1 in the liver of both diet- and obesity-induced T2DM mice strongly attenuated UPR activation by decreasing eIF2α phosphorylation, XBP-1 splicing, and CHOP expression. These in vitro and in vivo results are consistent with reduced ER stress and improved insulin sensitivity in obese humans with body weight loss (7). Our data provide evidence that SIRT1 reduces the hepatocellular UPR and consequently enhances insulin sensitivity.
SIRT1 inhibits obesity-induced mTORC1/S6K1 nutrient sensor signaling in the liver
A second finding of the present study is that hepatic overexpression of SIRT1 down-regulates mTORC1 activity, attenuates UPR activation, and enhances insulin sensitivity in vivo. Because cells exhibit a limited adaptive capacity to respond to energy stress, activities of distinct pathways are often coordinately regulated. To our knowledge, our results provide the first evidence that the nutrient and energy sensors, SIRT1 and mTORC1, coordinately regulate ER stress-related adaptive responses and insulin signaling, as well as enhance insulin action on glucose metabolism in vivo.
SIRT1 substantially suppressed mTORC1 activity under the conditions of ER stress, both in vivo and in vitro. First, overexpression and activation of SIRT1 in the liver were sufficient to reduce diet- and obesity-induced mTORC1 activation in vivo. Second, SIRT1-knockout cells exhibited a pronounced increase in mTORC1 signaling that was sensitive to rapamycin for mTORC1 inhibition. These results are consistent with the observation showing that the basal mTORC1 signaling is up-regulated in SIRT1-knockdown HeLa cells (44). Similarly, resveratrol inhibits mammalian S6 kinase in other cells (45). Third, Ozcan et al. (39) demonstrated that the constitutive activation of mTORC1 in TSC-null cells induces UPR signaling, an effect that is completely abrogated by rapamycin. Our data suggest that SIRT1 inhibits hepatocyte ER stress in an mTORC1-dependent manner by showing that the tunicamycin-induced UPR target gene expression was markedly prevented by rapamycin in HepG2 cells and MEFs. It is likely that the protective effect of SIRT1 against hepatocyte ER stress and insulin resistance in vivo is attributable to the same mechanisms by which SIRT1 counteracts the UPR activation through mTORC1 inhibition in cultured cells. Fourth, the conclusion that SIRT1-dependent suppression of mTORC1 is protected from insulin resistance is also supported by our in vitro observation that the ablation of SIRT1 results in impaired insulin signaling under ER stress conditions. Conversely, mTORC1 inhibition by SIRT1 in vivo increased hepatic IRS-1 function and enhanced whole body insulin sensitivity through the inhibition of S6K1-mediated IRS-1 phosphorylation at Ser636/639 or Ser1101 in HFHS-fed LDLR−/− mice and ob/ob mice, as was seen in mice lacking S6K1 (41). Thus, it is conceivable that the ER plays a critical role in the integration of the UPR and insulin metabolic actions in the liver via a mechanism of interdependence of nutrient-sensing pathways of SIRT1 and mTORC1.
In addition to mTORC1, mTOR kinase is also present in another complex designated mTORC2 (3). In our experiments with SIRT1-knockout cells or HepG2 hepatocytes exposed to an ER stress inducer, the increase in mTOR kinase activity and ER stress markers was blocked at subnanomolar concentrations of rapamycin, suggesting that SIRT1 appears to be an upstream regulator of mTORC1, but not of mTORC2, since mTORC1 is much more sensitive to lower concentration of rapamycin than that of mTORC2 (3). Moreover, mTORC1 activation-mediated ER stress induction is known to require the action of TSC1/2, the negative upstream regulators of mTOR (39). Given that the regulation of mTORC1 is very complex and involves multiple interacting proteins (3), whether TSC1/2 and other components of mTORC1 are involved in the regulation of ER homeostasis controlled by SIRT1 remains to be further elucidated.
Therapeutic implication of SIRT1 on insulin resistance and hepatic steatosis
Here, we provide evidence that SIRT1 activation in the liver effectively protects against insulin resistance in at least two different murine models (diet-induced and genetic ob/ob) of obesity. The effect of SIRT1 at alleviating insulin resistance appeared to be greater in dietary obesity than in the ob/ob genetic model of obesity. This might be due to the extreme nature of the ob/ob phenotype compared to that of dietary obesity. Evidence for the regulation of glucose metabolism by SIRT1 in different animal models is controversial. For instance, SIRT1-mediated deacetylation and activation of PGC-1α has been implicated in the induction of hepatic gluconeogenesis in mice under conditions of food deprivation (30, 46). In the current study, hepatic SIRT1 activation in both models lowers fasting glucose and improves glucose tolerance by possibly reducing hepatic gluconeogenesis. Our results are consistent with decreased fasting glucose levels observed in transgenic mice with SIRT1 gain of function (47) and in mouse models of metabolic disease treated with SIRT1 activators, such as resveratrol and similar compounds SRT1720 (20, 21, 42, 48). It is worth noting that activation of SIRT1 by resveratrol leads to the deacetylation and activation of hepatic PGC-1α, but this mechanism cannot account for the glucose-lowering effect of resveratrol in high fat-fed mice (21, 42). We and others recently demonstrate that SIRT1 activation by resveratrol stimulates AMPK activity through deacetylation and activation of LKB1 in cultured cells and in mouse livers (17, 49). LKB1 deficiency in the liver abolishes AMPK activity and consequently up-regulates hepatic gluconeogenesis through activation of CRTC2 (previously TORC2), a key transcriptional coactivator (50). It is possible that SIRT1-mediated activation of LKB1/AMPK may be involved in some of antidiabetic effects of SIRT1 overexpression in T2DM mice.
The current study shows that hepatic SIRT1 activation leads to a reduction in hepatic steatosis in mouse models of dietary and genetic obesity, which supports the notion that SIRT1-dependent suppression of ER stress in the liver is responsible for improved hepatic steatosis. Our data are consistent with the observations that mice lacking DBC1, a negative regulator of SIRT1, exhibit SIRT1 activation and improved hepatic steatosis (51). Our findings indicated that SIRT1 activation in diabetic mouse liver ameliorated hepatic steatosis and insulin resistance, at least partially, by blocking the mTORC1/S6K1-mediated feedback inhibition of IRS-1, as well as by inhibiting ER stress. By contrast, liver-specific SIRT1 knockout mice on a high-fat diet exhibit marked hepatic lipid accumulation and steatosis through impaired PPARα signaling and decreased fatty acid β-oxidation (52). Similarly, the lack of SIRT1 activity in SIRT1+/− mice in response to dietary fat leads to liver steatosis through increased liver inflammation (53). Together, the diverse effects raise an intriguing possibility that SIRT1 may act as a central integrator that prevents hepatic steatosis caused by excess nutrients via distinct and coordinated mechanisms involving decreased hepatic ER stress, inflammation, and lipid accumulation.
In summary, diet- and obesity-induced T2DM mice expressing SIRT1 in the liver are resistant to hepatic ER stress and insulin resistance with lowered fasting glucose and insulin levels and decreased hepatic gluconeogenesis. These effects could be mediated by SIRT1 suppression of mTORC1 and UPR signaling and attenuation of mTORC1/S6K1-mediated feedback inhibition of insulin receptor signaling. These findings highlight the coordinated regulation of the SIRT1-mTORC1 axis in the control of hepatocellular adaptation to ER stress and delineate a novel molecular mechanism by which SIRT1 ameliorates hepatic steatosis and insulin resistance. These studies also suggest that therapeutic approaches, such as polyphenolic SIRT1 activators, may have potential for the treatment of diseases in which the UPR is activated, such as hepatic steatosis, obesity, and T2DM.
Acknowledgments
The authors greatly appreciate Dr. Richard A. Cohen for insightful advice. The authors also thank Dr. Pere Puigserver (Harvard Medical School, Boston, MA, USA) for kindly providing the adenoviral vector encoding Flag-tagged SIRT1 and Dr. Ling Qi (Cornell University, Ithaca, NY, USA) for important suggestions. The authors are grateful to Kimberly Wong and Karlene A. Maitland-Toolan for technical assistance.
This work is supported by National Institutes of Health (NIH) grants DK 076942 and PO1HL068758 and the Robert Dawson Evans Junior Faculty Merit Award to M.Z. and by grants from NIH and the Paul F. Glenn Foundation to L.G.
REFERENCES
- 1. Petersen K. F., Dufour S., Befroy D., Lehrke M., Hendler R. E., Shulman G. I. (2005) Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54, 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapahi P., Chen D., Rogers A. N., Katewa S. D., Li P. W. L., Thomas E. L., Kockel L. (2010) With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 11, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inoki K., Corradetti M. N., Guan K. L. (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 37, 19–24 [DOI] [PubMed] [Google Scholar]
- 4. Krebs M., Brunmair B., Brehm A., Artwohl M., Szendroedi J., Nowotny P., Roth E., Fuernsinn C., Promintzer M., Anderwald C., Bischof M., Roden M. (2007) The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 56, 1600–1607 [DOI] [PubMed] [Google Scholar]
- 5. Nakamura T., Furuhashi M., Li P., Cao H. M., Tuncman G., Sonenberg N., Gorgun C. Z., Hotamisligil G. S. (2010) Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140, 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boden G., Duan X., Homko C., Molina E. J., Song W. W., Perez O., Cheung P., Merali S. (2008) Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gregor M. F., Yang L., Fabbrini E., Mohammed B. S., Eagon J. C., Hotamisligil G. S., Klein S. (2009) Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 9. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang L., Li P., Fu S. N., Calay E. S., Hotamisligil G. S. (2010) Defective hpatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 11, 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birkenfeld A. L., Lee H. Y., Frederick D. W., Jurczak M. J., Jornayvaz F. R., Oyadomari S., Samuel V. T., Ron D., Shulman G. I. (2009) Liver specific inhibition of the elF2 alpha mediated ER stress response pathway improves hepatic insulin action but impairs peripheral insulin sensitivity in mice. Diabetes 58, A387 [Google Scholar]
- 13. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 14. Wu J., Rutkowski D. T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G. D. Y., Kaufman R. J. (2007) ATF6 alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 [DOI] [PubMed] [Google Scholar]
- 15. Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., Hotamisligil G. S. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zang M. W., Xu S. Q., Maitland-Toolan K. A., Zuccollo A., Hou X. Y., Jiang B. B., Wierzbicki M., Verbeuren T. J., Cohen R. A. (2006) Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55, 2180–2191 [DOI] [PubMed] [Google Scholar]
- 17. Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., Cohen R. A., Zang M. (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 283, 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ajmo J. M., Liang X. M., Rogers C. Q., Pennock B., You M. (2008) Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G833–G842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun C., Zhang F., Ge X. J., Yan T. T., Chen X. M., Shi X. L., Zhai Q. W. (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 6, 307–319 [DOI] [PubMed] [Google Scholar]
- 20. Milne J. C., Lambert P. D., Schenk S., Carney D. P., Smith J. J., Gagne D. J., Jin L., Boss O., Perni R. B., Vu C. B., Bemis J. E., Xie R., Disch J. S., Ng P. Y., Nunes J. J., Lynch A. V., Yang H. Y., Galonek H., Israelian K., Choy W., Iffland A., Lavu S., Medvedik O., Sinclair D. A., Olefsky J. M., Jirousek M. R., Elliott P. J., Westphal C. H. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M. Y., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 23. Finkel T., Deng C. X., Mostoslavsky R. (2009) Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guarente L., Picard F. (2005) Calorie restriction—the SIR2 connection. Cell 120, 473–482 [DOI] [PubMed] [Google Scholar]
- 25. Wouters K., van Gorp P. J., Bieghs V., Gijbels M. J., Duimel H., Lutjohann D., Kerksiek A., van Kruchten R., Maeda N., Staels B., van Bilsen M., Shiri-Sverdlov R., Hofker M. H. (2008) Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology 48, 474–486 [DOI] [PubMed] [Google Scholar]
- 26. Schreyer S. A., Vick C., Lystig T. C., Mystkowski P., LeBoeuf R. C. (2002) LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am. J. Physiol. Endocrinol. Metab. 282, E207–E214 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez-Sanabria F., Rull A., Aragones G., Beltran-Debon R., onso-Villaverde C., Camps J., Joven J. (2010) Differential response of two models of genetically modified mice fed with high fat and cholesterol diets: relationship to the study of non-alcoholic steatohepatitis. Mol. Cell. Biochem 343, 59–66 [DOI] [PubMed] [Google Scholar]
- 28. Kennedy A. J., Ellacott K. L. J., King V. L., Hasty A. H. (2010) Mouse models of the metabolic syndrome. Dis. Models Mech. 3, 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M., Pavirani A., Lecocq J. P., Crystal R. G. (1992) Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat. Genet. 1, 372–378 [DOI] [PubMed] [Google Scholar]
- 30. Rodgers J. T., Puigserver P. (2007) Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. U. S. A. 104, 12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., Komuro R., Ouchi N., Kihara S., Tochino Y., Okutomi K., Horie M., Takeda S., Aoyama T., Funahashi T., Matsuzawa Y. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- 32. Zang M. W., Zuccollo A., Hou X. Y., Nagata D., Walsh K., Herscovitz H., Brecher P., Ruderman N. B., Cohen R. A. (2004) AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J. Biol. Chem. 279, 47898–47905 [DOI] [PubMed] [Google Scholar]
- 33. Zuccollo A., Shi C. M., Mastroianni R., Maitland-Toolan K. A., Weisbrod R. M., Zang M. W., Xu S. Q., Jiang B. B., Oliver-Krasinski J. M., Cayatte A. J., Corda S., Lavielle G., Verbeuren T. J., Cohen R. A. (2005) The thromboxane A(2) receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation 112, 3001–3008 [DOI] [PubMed] [Google Scholar]
- 34. Li X., Zhang S., Blander G., Tse J. G., Krieger M., Guarente L. (2007) SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 28, 91–106 [DOI] [PubMed] [Google Scholar]
- 35. Zang M., Gong J., Luo L., Zhou J., Xiang X., Huang W., Huang Q., Luo X., Olbrot M., Peng Y., Chen C., Luo Z. (2008) Characterization of Ser338 phosphorylation for Raf-1 activation. J. Biol. Chem. 283, 31429–31437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sha H. B., He Y., Chen H., Wang C., Zenno A., Shi H., Yang X. Y., Zhang X. M., Qi L. (2009) The IRE1 alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 9, 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winnay J. N., Boucher J., Mori M. A., Ueki K., Kahn C. R. (2010) A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat. Med. 16, 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ozean U., Ozcan L., Yilmaz E., Duvel K., Sahin M., Manning B. D., Hotamisligil G. S. (2008) Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tremblay F., Brule S., Um S. H., Li Y., Masuda K., Roden M., Sun X. J., Krebs M., Polakiewicz R. D., Thomas G., Marette A. (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 104, 14056–14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Um S. H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., Fumagalli S., Allegrini P. R., Kozma S. C., Auwerx J., Thomas G. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 [DOI] [PubMed] [Google Scholar]
- 42. Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 43. Viswanathan M., Kim S. K., Berdichevsky A., Guarente L. (2005) A role for SIR-2.1 regulation of ER stress response genes in determining C-elegans life span. Dev. Cell 9, 605–615 [DOI] [PubMed] [Google Scholar]
- 44. Ghosh H. S., McBurney M., Robbins P. D. (2010) SIRT1 negatively regulates the mammalian target of rapamycin. PLOS One 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armour S. M., Baur J. A., Hsieh S. N., Land-Bracha A., Thomas S. M., Sinclair D. A. (2009) Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging 1, 515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 47. Banks A. S., Kon N., Knight C., Matsumoto M., Gutierrez-Juarez R., Rossetti L., Gu W., Accili D. (2008) SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 8, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feige J. N., Lagouge M., Canto C., Strehle A., Houten S. M., Milne J. C., Lambert P. D., Mataki C., Elliott P. J., Auwerx J. (2008) Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 8, 347–358 [DOI] [PubMed] [Google Scholar]
- 49. Lan F., Cacicedo J. M., Ruderman N., Ido Y. (2008) SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 283, 27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., DePinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Escande C., Chini C. C. S., Nin V., Dykhouse K. M., Novak C. M., Levine J., van Deursen J., Gores G. J., Chen J. J., Lou Z. K., Chini E. N. (2010) Deleted in breast cancer-1 regulates SIRT1 activity and contributes to high-fat diet-induced liver steatosis in mice. J. Clin. Invest. 120, 545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Purushotham A., Schug T. T., Xu Q., Surapureddi S., Guo X. M., Li X. L. (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 9, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu F., Gao Z. G., Zhang J., Rivera C. A., Yin J., Weng J. P., Ye J. P. (2010) Lack of SIRT1 (mammalian sirtuin 1) activity leads to liver steatosis in the SIRT1(+/-) mice: a role of lipid mobilization and inflammation. Endocrinology 151, 2504–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]