Abstract
This study aimed to determine whether aging negatively affects MSC replication and osteogenesis and whether these features could be altered by exposure to an extracellular matrix (ECM) generated by marrow cells from young or old mice. A cell-free ECM was prepared from cultured femoral marrow cells from either 3- or 18-mo-old C57BL/6 mice (young-ECM or old-ECM, respectively). The replication and osteogenesis of young or old MSCs maintained on young-ECM vs. old-ECM as well as plastic were examined in vitro and in vivo. We found that the frequency of MSCs in marrow from old mice, measured by colony-forming cells, was only marginally lower than that of young mice. In contrast, defects in the self-renewal and bone formation capacity of old MSCs were remarkable. These defects were corrected by provision of a young-ECM but not old-ECM. In parallel cultures maintained on a young-ECM, the intracellular levels of reactive oxygen species from both old and young mice were reduced 30–50% compared to those maintained on old-ECM or plastic. We concluded that aging negatively affects the formation of an ECM that normally preserves MSC function, and aged MSCs can be rejuvenated by culture on a young-ECM.—Sun, Y., Li, W., Lu, Z., Chen, Z., Ling, J., Ran, O., Jilka, R. L., Chen, X. D. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix.
Keywords: reactive oxygen species, tissue regeneration, niche
Mesenchymal stem cells (MSCs) mainly reside in bone marrow. They can self-renew and differentiate into multiple cell lineages, including osteoblasts, chondrocytes, adipocytes, muscle cells, neurons, and hepatocytes (1–5). As a result of these capabilities, MSCs play an important role in continuous maintenance and repair of most tissue types. In general, it is accepted that the quantity and quality of MSCs decrease with aging (6), which is associated with the progressive failure of function of tissues and organs. However, the precise phenotype of aged MSCs is unclear. Relevant literature is very inconsistent and appears to be in conflict (reviewed by Sethe et al., ref. 7). Apparently, the various methods for MSC isolation can lead to enrichment of different subsets of MSCs with different biological properties, which might explain the discrepancies in the literature.
Recently, we reported that in both mice and humans a native extracellular matrix (ECM) generated by bone marrow cells dramatically promoted MSC proliferation, preserved the stem cell properties, and enhanced their capacity for skeletogenesis (8, 9). This led us to investigate whether culture of aged MSCs on an ECM could improve their number and quality. In particular, we want to take advantage of this established model to address the question of whether the effect of age on MSCs themselves (intrinsic theory), or changes to MSCs by the surrounding ECM (extrinsic theory) occurs, or both. Due to evidence that aging of C57BL/6 mice is associated with decreased bone mineral density (BMD), osteoblast numbers, and bone formation (10, 11), we examined whether aging negatively affected the replication of MSCs as well as the capacity of MSCs for bone formation, by comparing femoral marrow cells isolated from 3-mo-old (young) vs. 18-mo-old (old) female C57BL/6 mice, and whether such features of young or old MSCs would be altered by exposure to an ECM made by marrow stromal cells from young or old mice. The present study suggests that the number of MSCs in marrow from old mice, measured by their ability to generate a colony-forming unit of osteoblasts (CFU-OB), was only marginally lower than that of young mice. However, defects in the self-renewal and bone formation capacity of aged MSCs were remarkable. Strikingly, these defects were corrected by the provision of an ECM made by marrow stromal cells from young animals.
MATERIALS AND METHODS
Animals
C57BL6 female mice, 3 mo (young) and 18 mo old (old), were obtained from the National Institute on Aging (NIA; Bethesda, MD, USA). The generation of glutathione peroxidase 4 (Gpx4) transgenic mice [Tg(GPX4)+/o] was previously reported (12). Tg(GPX4)+/o mice were generated using a human endogenous GPX4 gene and showed overexpression of Gpx4 in all tissues (12, 13). It has been reported that Tg(GPX4)+/o mice are resistant to the administration of diquat that induces hepatotoxicity and apoptosis, as compared to wild-type (WT) mice (13). In the present study, 3-mo-old C57BL6 female Tg(GPX4)+/o mice were used. All animal procedures were approved by the University of Texas Health Science Center Animal Care and Use Committee.
Preparation of cell-free ECM generated by cultured bone marrow cells from either young or old mice
A standard procedure based on our previous studies was utilized (8). Briefly, freshly isolated bone marrow cells from either young or old mice were cultured in 6-well plates (Corning Inc, Corning, NY, USA) at 3 × 106 cells/10 cm2 well in 4 ml of a standard culture medium comprising α-MEM (Life Technologies, Grand Island, NY, USA) supplemented with glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml) (Sigma Chemical Company, St. Louis, MO, USA), and 20% preselected fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA, USA). After 7 d of culture, nonadherent cells were removed by rinsing with PBS. The adherent stromal cell layer was dispersed with PBS containing 400 U/ml type II collagenase (Worthington Biochemical Inc, Lakewood, NJ, USA) for 10 min at 37°C, then 1 × 105 adherent cells were seeded into a 10-cm2 well of a 6-well plate containing a 24- × 30-mm Thermanox plastic coverslip (Nalge Nunc International, Rochester, NY, USA), and cultured for an additional 15 d. The medium was changed every 3–4 d; ascorbic acid (50 μM; Sigma) was added during the final 8 d of culture. After extensive washing with PBS, cells were removed from the ECM by incubation with 0.5% Triton X-100 containing 20 mM NH4OH in PBS for 5 min at 37°C, similar to a previously described procedure (14). The ECM was washed with PBS 3 times and stored in 2.0 ml of PBS containing penicillin (100 U/ml), streptomycin (100 μg/ml), and fungizone (0.25 μg/ml) at 4°C for up to 4 mo.
Measurement of the total amount of protein extracted from either young- or old-ECM
After rinsing with PBS 2 times, cell-free ECM proteins prepared from young or old cells were extracted using lysis buffer containing 7 M urea, 2 M thiourea, 2% CHAPS, 50 mM DTT, and 40 mM Tris (pH 8.8), 0.5 ml/100-mm culture dish, followed by sonication. The total protein concentration was measured with the use of the RC DC protein assay (Bio-Rad Laboratories, Richmond, CA, USA) according to the manufacturer's instructions.
One-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1-D SDS-PAGE)
SDS-PAGE was performed as described by Bio-Rad protocol using protein Criterion cells (Bio-Rad Laboratories). ECM protein sample was diluted at 1:2 with SDS reducing sample buffer, heated at 95°C for 5 min and resolved by 1-D SDS-PAGE (1 mm thickness of 4–20% gradient acrylamide/bis-acrylamide gel). Bio-Rad recombinant prestained Precision Plus protein standards were run to calculate the apparent molecular weight of protein bands. For quantification of relative protein abundances, gel was visualized with Coomassie blue staining. The images of gel bands were captured, digitized, and analyzed with the use of Kodak Molecular Imaging software (Carestream Health, Inc., Rochester, NY, USA).
Determination of CFU-OB replication capacity
Replication of CFU-OBs (Table 1) was determined by comparing the number present in the initial femoral marrow cell isolate to the number present after 7 d of culture on the various matrices, as described previously (8). Freshly isolated bone marrow cells were pooled from 3 to 6 mice, and an aliquot was used to determine CFU-OB number. The total number of CFU-OBs present in the initial isolate was calculated by multiplying the number of CFU-OBs per cell seeded by the number of cells present in the isolate. Portions of the remaining freshly isolated bone marrow cells were cultured in standard culture medium in 6-well plates at 7 × 106 cells/10-cm2 well on either tissue culture plastic or ECMs prepared from either young or old mice. After 7 d of culture to allow replication, nonadherent cells were removed; adherent cells were then detached with collagenase. The cells were then counted and replated for quantification of CFU-OBs. The same number of cells was seeded onto plastic for determination of CFU-OB number regardless of the substratum used for expansion. The total number of CFU-OBs after expansion (had the entire femoral marrow isolate been cultured on plastic or a particular ECM) was calculated by multiplying the number of CFU-OBs obtained per cell seeded by the number of cells obtained after expansion and then dividing the result by the fraction of the initial marrow isolate used for expansion (Table 1).
Table 1.
Analysis of CFU-OBs after culture on plastic, young-ECM, or old-ECM
| Initial marrow cell isolate | ||
|---|---|---|
| Parameter | 3 mo | 18 mo |
| Frequency of CFU-OBs (n/106 cells) | 64 ± 4 | 57 ± 3 |
| Total CFU-OBs per femur (n×103/femur)a | 0.887 ± 0.049 | 1.038 ± 0.453 |
| After expansion | ||||||
|---|---|---|---|---|---|---|
| Parameter | Plastic |
Young-ECM |
Old-ECM |
|||
| 3 mo | 18 mo | 3 mo | 18 mo | 3 mo | 18 mo | |
| Average cells (n×106/well) | 0.5 | 0.25 | 2.2 | 1.9 | 0.8 | 1.1 |
| CFU-OBs (n×103/106 cells) | 2.0 ± 0.1 | 0.8 ± 0.2 | 3.1 ± 0.1 | 2.7 ± 0.5 | 1.7 ± 0.6 | 1.3 ± 0.3 |
| Total CFU-OBs (n×103)b | 1.0 ± 0.1 | 0.2 ± 0.1 | 6.9 ± 0.3 | 5.1 ± 0.9 | 1.3 ± 0.5 | 1.4 ± 0.3 |
| Total CFU-OBs per femur (n×103)c | 2.0 ± 0.1 | 0.5 ± 0.1 | 13.8 ± 0.6 | 13.0 ± 2.2 | 2.7 ± 1.0 | 3.6 ± 1.0 |
| Fold changed | 2.3 ± 0.1* | 0.5 ± 0.2 | 15.6 ± 1.1# | 12.6 ± 4.4# | 3.0 ± 1.0 | 3.4 ± 1.7 |
Number of CFU-OBs per 106 cells multiplied by average number of BMNCs per femur (3 mo, 1.4×107 BMNC/femur; 18 mo, 1.8×107 BMNC/femur).
Number of CFU-OBs per 106 cells multiplied by average number of cells obtained per well after expansion.
Total number of CFU-OBs after expansion divided by fraction of cells used for expansion (3 mo, 0.5; 18 mo, 0.39).
Total CFU-OBs after expansion of marrow cell isolate per femur divided by total number of CFU-OBs present in initial isolate per femur.
P < 0.05 vs. 18 mo.
P < 0.05 vs. plastic and old-ECM; ANOVA.
The replication of MSCs expanded on the various substrata was presented by fold changes, as described previously (8), which was determined by dividing the calculated total number of CFU-OBs after expansion by the total number of CFU-OBs present in the initial femoral marrow cell isolate (Table 1).
The CFU-OB assay has been described previously (8). Cells were placed into 6-well plates at 1 × 106 cells/10-cm2 well for primary CFU-OBs (before expansion) or at 5 × 104 cells/10 cm2 well for secondary CFU-OBs (after expansion), incubated for 4 h at 37°C to allow attachment of adherent cells, and washed twice with PBS to remove nonadherent cells. Then, 3 × 106 irradiated guinea pig feeder cells were added immediately in 4 ml of standard culture medium containing 1 mM l-ascorbate-2-phosphate (Wako Chemicals, Richmond, VA, USA). Half of the medium was replaced every 5 d. After 25 d of culture, CFU-OB colonies were visualized with Von Kossa staining.
Measurements of intracellular reactive oxygen species (ROS)
Intracellular ROS generation was measured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) using a ROS Assay Kit (Invitrogen, Eugene, OR, USA) following the manufacturer's recommendations. ROS levels were expressed as arbitrary units (AU) of DCF fluorescence per 105 cells.
Measurements of intracellular telomerase activity and ATP concentration
Intracellular telomerase activity was measured using the quantitative telomerase detection kit (Allied Biotech, Inc., Twinsburg, OH, USA) according to the manufacturer's instructions. Briefly, freshly isolated bone marrow cells from either young or old mice were seeded at 7 × 106 cells per 10 cm2 well onto tissue culture plastic, or onto young- or old-ECM, and cultured for 7 d. After rinsing with PBS to remove nonadherent cells, adherent cells were detached with collagenase. The precultured cells (1×106) from the various matrices were resuspended in 200 μl of lysis buffer, and incubated on ice for 30 min. The protein samples were centrifuged at 12,000 g for 30 min at 4°C. After the protein concentration was determined, the aliquots were quick-frozen and stored at −80°C for assay. The heat-inactivated cell extract was used as a negative control. Experiments were performed in triplicate, and telomerase levels were expressed as attomoles per 106 cells. ATP levels were measured using an assay from HemoGenix, Inc.(Colorado Springs, CO, USA) according to the manufacturer's instructions. Precultured cells (1×106) were collected from the various matrices. Experiments were performed in triplicate, and ATP levels were expressed as micromoles per 106 cells.
Flow cytometry
Anti-SSEA-4 antibodies were purchased from R&D Systems (Minneapolis, MN, USA). Anti-CD44, CD90, and Sca-1 antibodies were purchased from eBioscience (San Diego, CA, USA). Anti-CD45 antibodies were purchased from BD Bioscience (San Jose, CA, USA). Single-cell suspensions (1×106) were incubated in 100 μl of tested antibodies (10 μg/ml) for 30 min at 4°C. The stained cells were washed twice in staining buffer (PBS containing 5% FCS and 0.01% sodium azide) and incubated in 20 μg/ml of FITC-conjugated goat anti-mouse IgG for 20 min at 4°C. The cells were then washed twice with staining buffer and either immediately analyzed or fixed with 1% paraformaldehyde in PBS and analyzed within 96 h using a Becton Dickinson FACStarplus flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), with 10,000 events collected for each sample. The percentage of positively stained cells was determined from fluorescence-activated cell sorting (FACS). Cells were stained with isotype IgG as a negative control.
In vivo bone formation
Freshly isolated marrow cells from either young or old mice, pooled from 3 to 6 mice for each age, were seeded at 7 × 106 cells/10-cm2 well onto tissue culture plastic or marrow cell-derived ECM prepared from either young or old mice, and cultured for 7 d. After rinsing with PBS to remove nonadherent cells, adherent cells were detached with collagenase. The cells (1×106) were loaded onto Gelfoam (Pharmacia & Upjohn Co., Kalamazoo, MI, USA), and implanted subcutaneously into the dorsal surface of 10-wk-old immunodeficient beige mice (NIH-bg-nu-xid; Harlan Sprague Dawley, Indianapolis, IN, USA), as described previously (15, 16). Cells precultured on tissue culture plastic were implanted on the left side, and cells precultured on marrow derived-ECM were implanted on the right side of each animal. As a negative control, a Gelfoam vehicle without cells was implanted into the mouse. The implants were harvested after 8 wk and scanned using an eXplore Locus RS Small Animal MicroCT (μCT) scanner (GE Healthcare, London, Ontario). The data obtained were quantitatively analyzed for bone content using software with optional bone analysis plug-ins (MicroView@ 2.1.2; GE Healthcare; http://microview.sourceforge.net).
For histological analysis, implants were fixed in 10% phosphate-buffered formalin at 4°C for 24 h, decalcified with 5% EDTA at room temperature for 1–2 wk, and embedded in paraffin. Each ossicle was bisected, and 3 sections (10 μm thick) were cut, starting at the bisection point of each half-ossicle at 50-μm intervals to yield a total of 12 sections for each ossicle. Sections were stained with H&E.
To determine the capacity of MSCs from Tg(Gpx4)+/0 mice to generate skeletal tissue in vivo, the same procedure was followed, except that cells were only expanded on tissue culture plastic for 7 d.
Measurement of the Raman spectra of old-ECM vs. young-ECM
Cell-free ECMs generated on a plastic coverslip were carefully scraped off, collected, and stored in PBS at 4°C until analyzed. The Raman spectrum of the ECM in the fingerprint region between the wavenumber of 200 and 1800 cm−1 was acquired with a Renishaw 2000 Raman microscope (Renishaw, Wotton-under-Edge, UK). Five randomly selected areas were imaged in each sample, and 6 samples were examined for either young- or old-ECMs prepared from the independent experiments. The spectra from young- or old-ECMs were averaged, respectively.
Analysis of BMD in the femur of Tg(Gpx4)+/0 mice vs. WT littermates
Femora were dissected from 3-mo-old female Tg(Gpx4)+/0 mice or WT littermates. After removal of soft tissue, the femora were stored in 70% ethanol until analyzed. The femora were scanned on volumetric μCT at 27 μm3 voxel resolution using the eXplore Locus μCT scanner for 10 frames/view for a total of 125 min of image acquisition time. Images were reconstructed with the manufacturer's proprietary EVSBeam software (GE Healthcare) and calibrated to standard CT number, measured in Hounsfield units (HU), and further calibrated to permit determination of equivalent mass of hydroxyapatite.
The bone analysis was performed on MicroView (http://microview.sourceforge.net.). Two-dimensional transfer function (2DTF) visualizations were performed on the obtained datasets with software from the University of Utah Scientific Computing Institute (Salt Lake City, UT, USA; Imagevis3D; http://www.sci.utah.edu/cibc/software).
Statistical analysis
All data are presented as means ± sd, with n = 3 or 6, depending on the experiment. Statistical analyses were done using Student's t test or 1-way ANOVA with significance at values of P < 0.05. All results were reproduced in ≥3 independent experiments.
RESULTS
Defective replication of aged MSCs is restored by exposure to a young-ECM
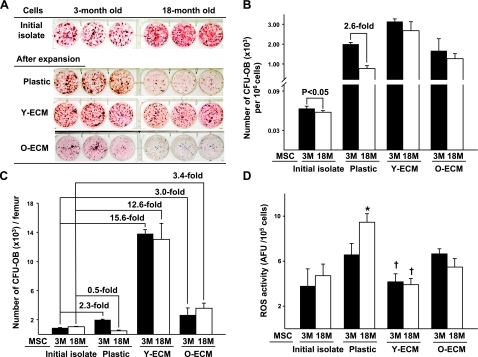
We first examined whether aging negatively affected the number and ex vivo replication of MSCs by comparing femoral marrow cells isolated from 3- to 18-mo-old female C57BL/6 mice. In this experiment, MSCs and osteoblast progenitors were defined by their ability to form a CFU-OB. Freshly isolated bone marrow cells obtained from either young or old mice were divided into aliquots for the determination of CFU-OBs present in the initial isolate as well as for culture on plastic, young-ECM, or old-ECM. After the 7 d culture period, nonadherent cells were removed, and adherent cells were detached from the various matrices and reseeded onto plastic for colony assay. These procedures have been used previously for determination of CFU replication (8, 9, 17). Figure 1A, B shows that the frequency of CFU-OBs in initial isolates from old mice was ∼57 colonies/106 mononuclear cells, which was only 5–10% less than those from young mice (P<0.05; Fig. 1B). However, most MSCs and osteoblast progenitors from old mice were depleted, showing fewer CFU-OBs compared to those from young mice, after the 7 d culture on ordinary tissue culture plastic. Notably, the decreased number of CFU-OBs from old mice was restored when they were cultured on a young-ECM (Fig. 1A, B). Interestingly, the numbers of CFU-OBs from both young and old mice were decreased significantly after culture on old-ECM, as compared to those cultured on young-ECM (Fig. 1A, B). The replication of MSCs or colony-forming cells during 7 d of culture on plastic, young-ECM, or old-ECM was determined by measuring the fold increase in CFU-OBs, shown in Table 1. The number of CFU-OBs in initial isolate did not differ significantly between young and old mice (Table 1 and Fig. 1C). After 7 d of culture on plastic, the numbers of CFU-OBs from young mice increased 2.0-fold, whereas those from old mice decreased (0.5-fold; Fig. 1C). Parallel experiments were performed with MSCs cultured for 7 d on either young-ECM or old-ECM. Under the former condition, the replication of MSCs from both young and old mice increased indistinguishably (15.6- and 12.6-fold, respectively; Table 1 and Fig. 1C). Under the latter condition, the replication of MSCs from young and old mice increased only 3.0- and 3.4-fold, respectively, a dramatic decrease when compared to MSCs cultured on young-ECM (Fig. 1C).
Figure 1.
Correction of a defect in the replication of MSCs from old mice by exposure to an ECM made by marrow stromal cells from young mice. Aliquots of freshly isolated bone marrow cells from mice aged either 3 mo (3M) or 18 mo (18M) were used to determine the numbers of CFU-OBs in initial isolate, and portions of the remaining cells were seeded onto tissue culture plastic, or tissue culture plastic coated with young-ECM (Y-ECM) or old-ECM (O-ECM). After 7 d of culture, adherent cells were detached from the various substrata and then reseeded on plastic separately for determination of CFU-OBs by visualization with Von Kossa stain, which appears dark. Replication of CFU-OBs was determined by comparing the number present in the initial femoral marrow cell isolate to the number present after 7 d of culture on the various matrices, as described previously (8). A) Appearance of CFU-OBs assayed before (initial isolate) and after 7 d of culture on plastic, young-ECM, or old-ECM. B) Frequency (number of CFU-OBs per 106 cells) in initial isolate and after culture. C) Replication of MSCs cultured on the various substrata. Replication is represented by fold change in CFU-OBs during expansion. See Table 1 for cell yields and calculation of CFU values. D) Comparison of ROS activity between young and aged MSCs before (initial isolate) and after 7 d of culture on tissue culture plastic or the ECMs. Intracellular level of ROS was quantified using H2DCFDA (described in Materials and Methods). ROS levels are expressed as arbitrary fluorescence units (AFU) of DCF fluorescence per 105 cells. *P < 0.05 vs. 3M. †P < 0.05 vs. plastic and O-ECM.
To determine whether the restoration of age-related MSC replication was associated with the reduction of oxidative stress, the intracellular level of ROS was also measured in the above experiments. It was found that ROS was 20% higher in cultured bone marrow cells from old mice than young mice when cultures were performed on plastic (P<0.05; Fig. 1D). In parallel cultures maintained on the young-ECM, ROS levels in bone marrow-cultured cells from both young and old mice were dramatically reduced 30 to 50% when compared to those maintained on plastic as well as the old-ECM (Fig. 1D).
Young-ECM enriches bone marrow adherent cells that exhibit high levels of telomerase and ATP activities
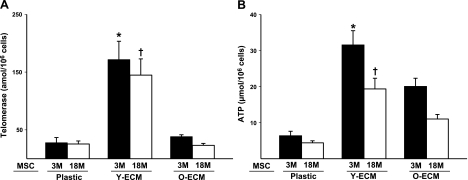
Since telomerase is required for the extension of telomere length associated with cellular life span, and there is evidence that MSCs maintained on the ECM retain a high level of telomerase activity (9, 18), we measured intracellular telomerase activity of cultured cells on the various matrices. Indeed, cells from both young and old mice exhibited significantly higher levels of telomerase activity when they were cultured on a young-ECM vs. those cultured on an old-ECM or on plastic (P<0.05; Fig. 2A). There was no significant difference in the levels of telomerase activity between young and old cells when both were cultured on young-ECM. In view of a significant correlation between ATP activity and number of highly functional stem cells (19), the intracellular ATP activity of cultured cells was measured in the parallel experiments. Overall, both young and old cells cultured on ECM showed significantly increased ATP levels as compared to plastic (P<0.05; Fig. 2B). However, the increase in the levels of ATP was ∼1.5 to 2-fold greater when cultured on young-ECM compared to old-ECM, regardless of age (Fig. 2B). To further determine whether these results were related to the alteration of cellular composition caused by aging and/or the various culture conditions, we also examined a series of MSC-related markers, including SSEA-4, CD44, CD90, and Sca-1 (20, 21), and a hematopoietic cell marker, CD45. The results indicated that levels of all markers expressed by young cells were higher than those expressed by old cells in initial cell isolate (Table 2). After culture on the various matrices, cells maintained on either young-ECM or old-ECM contained ∼23% SSEA-4+ cells, which was significantly higher than when these cells were maintained on plastic (P<0.05; Table 2). Unexpectedly, we found that there were no significant differences in the proportions of those positive cells after culture on young-ECM vs. old-ECM.
Figure 2.
Bone marrow cells cultured on young-ECM increased the levels of intracellular telomerase and ATP. Freshly isolated bone marrow cells from mice aged either 3 mo (3M) or 18 mo (18M) were seeded onto tissue culture plastic, young-ECM (Y-ECM), or old-ECM (O-ECM), and cultured for 7 d. After rinsing with PBS to remove nonadherent cells, adherent cells were detached with collagenase. Adherent bone marrow cells (1×106) collected from the various substrates were used for the measurements of telomerase or ATP levels. A) Intracellular telomerase activity (amol/106 cells) was measured using the quantitative telomerase detection kit (Allied Biotech) according to manufacturer's instructions. Experiments were performed in triplicate. B) Intracellular ATP levels (μmol/106 cells) were measured according to the manufacturer's instructions (HemoGenix). Experiments were performed in triplicate. *P < 0.05 vs. plastic and O-ECM. †P < 0.05 vs. plastic and O-ECM.
Table 2.
FACS analysis before and after bone marrow cells cultured on the various matrices
| Marker (%) | Initial marrow cell isolate |
Plastic |
Young-ECM |
Old-ECM |
||||
|---|---|---|---|---|---|---|---|---|
| 3 mo* | 18 mo | 3 mo | 18 mo | 3 mo | 18 mo | 3 mo | 18 mo | |
| SSEA-4+ | 37 ± 4 | 18 ± 3 | 12 ± 2# | 15 ± 2# | 22 ± 3 | 24 ± 2 | 24 ± 2 | 25 ± 3 |
| CD44+ | 67 ± 5 | 45 ± 4 | 84 ± 8 | 79 ± 6 | 88 ± 7 | 88 ± 6 | 86 ± 8 | 85 ± 7 |
| CD90+ | 18 ± 3 | 11 ± 2 | 20 ± 3 | 25 ± 3 | 11 ± 2 | 13 ± 2 | 17 ± 3 | 12 ± 3 |
| Sca-1+ | 37 ± 3 | 26 ± 3 | 50 ± 4 | 48 ± 3 | 50 ± 3 | 49 ± 4 | 63 ± 7 | 54 ± 5 |
| CD45+ | 63 ± 7 | 46 ± 5 | 72 ± 8# | 76 ± 6# | 89 ± 8 | 90 ± 9 | 86 ± 7 | 89 ± 9 |
P < 0.05 vs. 18 mo.
P < 0.05 vs. young-ECM and old-ECM.
ECM promotes bone-forming capacity of MSCs from both young and old mice
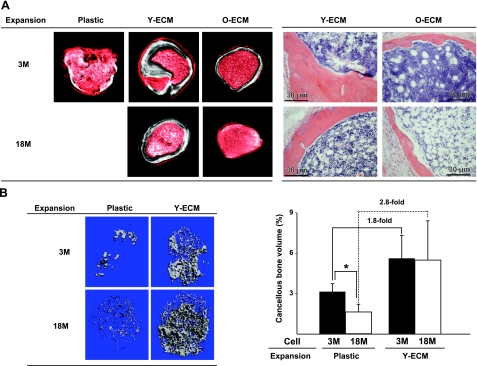
Next, we compared the influence of culture on young- vs. old-ECM on the capacity of old or young MSCs to form bone in vivo using an implantation assay, as described previously (8). After 7 d of culture of either young or old bone marrow cells on plastic, young-ECM, or old-ECM, 1 × 106 cells were loaded onto Gelfoam and implanted subcutaneously into the dorsal surface of immunodeficient mice. The implants were harvested 8 wk after implantation. Bone formed was quantified using μCT. As shown in Fig. 3A and Table 3, no or less bone was generated from old MSCs precultured on plastic or old-ECM, respectively. In contrast, old MSCs as well as young MSCs precultured on young-ECM formed the same amount of bone as determined by BMD, which was ∼2 to 3 times more than that formed by young MSCs precultured on plastic or old-ECM. Histological analysis showed that either young cells or old cells precultured on old-ECM generated skeletal tissues with many large pale spaces, formerly filled by large fat droplets, suggesting formation of more adipose tissue than with cells precultured on young-ECM (Fig. 3A, right panels). In a second experiment (Fig. 3B), old MSCs formed less bone than young MSCs when both were cultured on plastic. Consistent with the previous experiment, increased skeletal tissue formation by MSCs (from either young or old mice) expanded on young-ECM was determined by the percentage of bone volume in the total area of ossicle. Implantation of cells precultured on a young-ECM generated 1.8–2.8 times more cancellous bone than did cells precultured on plastic (Fig. 3B, right panel). These findings suggested that culture of old MSCs on a young-ECM improved their quantity and quality.
Figure 3.
Increased skeletal tissue formation by MSCs, from mice aged either 3 mo (3M) or 18 mo (18M), cultured on young-ECM. Freshly isolated marrow cells from either young or old mice were seeded at 7 × 106 cells/10-cm2 well onto tissue culture plastic, or onto tissue culture plastic coated with ECMs prepared from either young or old mice, and cultured for 7 d. Then precultured cells (1×106) were loaded onto Gelfoam and implanted subcutaneously into the dorsal surface of 10-wk-old immunodeficient mice. Implants were harvested 8 wk following transplantation. Bone content was determined by μCT and histological analysis. A) Experiment 1. Left panels show μCT images from the middle section of implants; white color indicates skeletal tissue. Quantification is shown in Table 3. Right panels show histological analysis of sections from ossicles stained with H&E to visualize bone. B) Experiment 2. Left panels show high-resolution μCT images of whole implants. Right panel shows quantification. *P < 0.05.
Table 3.
Measurement of BMD
| BMD (mg/cc) | Plastic |
Young-ECM |
Old-ECM |
|||
|---|---|---|---|---|---|---|
| 3 mo | 18 mo | 3 mo | 18 mo | 3 mo | 18 mo | |
| Implant 1 | 13 | ND | 43 | 29 | 20 | 1.2 |
| Implant 2 | 15 | ND | 32 | 67 | 22 | ND |
| Implant 3 | 9 | ND | 46 | 34 | 24 | ND |
| Mean ± sd | 12.3 ± 3.1 | 40.3 ± 7.3* | 43.3 ± 20.0 | 22.0 ± 2.0# | ||
ND, not determined.
P < 0.05 vs. plastic and old-ECM.
P < 0.05 vs. plastic.
Differential composition of ECM elaborated by young vs. old cells
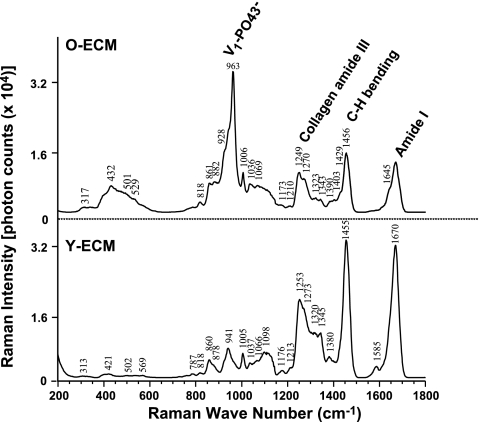
Due to the differential features of MSCs when exposed to young- vs. old-ECM, we compared total amount of protein per well (10 cm2) and separated proteins with different molecular mass using 1-D SDS-PAGE between young- and old-ECMs. The results showed no significant difference in amount of total protein generated by young cells compared to old cells (98.1±15.67 vs. 91.17±8.04 μg/well, n=6). However, SDS-PAGE showed that the old-ECM proteins around 140 and 40 kDa were significantly less than those from young-ECM (data not shown). In addition, we compared the composition of these two ECMs using confocal Raman microscopy (Fig. 4). Compared to young-ECM, old-ECM exhibited a distinct, sharp Raman peak at 960 cm−1, consistent with symmetric stretching vibrations of phosphate ions (V1-PO43−), suggesting that mineral was deposited on the ECM. The presence of a broad bump around 430 cm−1 (hydroxyapatite V2-PO43−) and the absence of a well-defined characteristic peak of bone phosphate at 589 cm−1 (V4-PO43−) indicated that the mineral deposited in ECM may not be as well organized as in bone. In contrast, no evidence of mineral phosphate was observed in young-ECM. In addition, young-ECM showed high peaks at 1249 and 1270 cm−1, ∼1455 cm−1, and ∼1670 cm−1, corresponding to collagen amide III, C-H bending, and amide I, respectively, which suggested that young-ECM contained more collagens. Apparently, the ratio of mineral to collagen was higher in the old-ECM than in the young-ECM.
Figure 4.
Raman spectrum: differential composition of young-ECM vs. old-ECM. Five randomly selected areas were imaged in each sample, and 6 samples were examined for either young- or old-ECMs prepared from the independent experiments. Spectra from young- or old-ECMs were averaged, respectively. Graph represents an ensemble average of Raman spectrum. Old-ECM exhibited a sharp peak at ∼960 cm−1, related to mineral phosphate (V1-PO43−), and a few smaller peaks at 1249 and 1270, ∼1455, and ∼1670 cm−1, corresponding to collagen amide III, C-H bending, and amide I, respectively, as compared to the young-ECM.
Enhancement of antioxidant defenses reduces ROS production and promotes CFU-OB replication while increasing bone formation
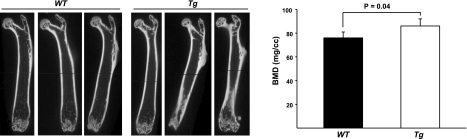
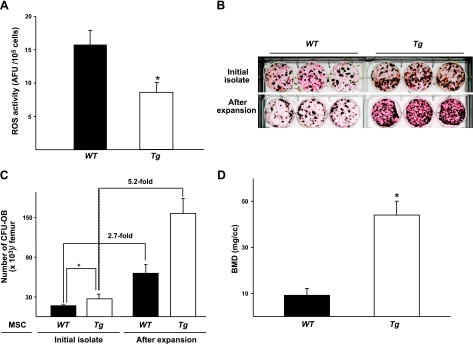
Since the improvement of MSC self-renewal by exposure to a young-ECM was associated with the reduction of ROS, it was necessary to assess further whether a decrease in ROS level helped facilitate MSC self-renewal. Therefore, we examined the replication of MSCs from transgenic C57BL6 mice overexpressing Gpx4, which has been reported to reduce oxidative stress-induced apoptosis (13). In the present study, we found that bone mass, measured with BMD, in the femur of 3-mo-old Tg(Gpx4)+/0 mice was significantly higher than that of WT littermates (P<0.05; Fig. 5). Indeed, ROS levels from freshly isolated bone marrow cells from Tg(Gpx4)+/0 mice were ∼50% less than those from WT littermates (Fig. 6A). Consistent with decreased ROS levels, the initial number of MSCs from Tg(Gpx4)+/0 mice was significantly higher than that of MSCs from WT littermates (P<0.05; Fig. 6B, C). Notably, the replication of MSCs from Tg(Gpx4)+/0 mice during 7 d of culture on plastic was increased markedly, as compared to that of MSCs from the WT (5.2- vs. 2.7-fold, respectively; Fig. 6B, C). To show the capacity of MSCs from Tg(Gpx4)+/0 mice to generate skeletal tissue, we used an implantation assay. We found that BMD in bone ossicles generated by MSCs or osteoblast progenitors from Tg(Gpx4)+/0 mice was remarkably increased, as compared to that generated by cells from WT littermates (Fig. 6D).
Figure 5.
Tg(Gpx4)+/0 mice exhibit increased BMD in the femur compared to WT mice. Femoral BMD in 3-mo-old female Tg(Gpx4)+/0 mice and WT littermates was measured using an eXplore Locus RS Small Animal μCT scanner (GE Healthcare). A) Images of μCT of femora from Tg(Gpx4)+/0 mice and WT littermates. B) Femoral BMD in Tg(Gpx4)+/0 mice and WT littermates, n = 3.
Figure 6.
MSCs from Tg(Gpx4)+/0 mice exhibit increased replication as well as skeletal tissue formation. A) Comparison of ROS levels between Tg(Gpx4)+/0 mice and WT littermates. Bone marrow cells were harvested from femora obtained from 3-mo-old female Tg(Gpx4)+/0 mice and WT mice. Intracellular levels of ROS in these cells were quantified using H2DCFDA (described in Materials and Methods). ROS levels are expressed as arbitrary fluorescence units (AFU) of DCF fluorescence per 105 cells. *P < 0.05 vs. WT. B) Appearance of CFU-OBs assayed after 7 d of culture on plastic. CFU-OBs were determined by Von Kossa stain, which appears dark. C) Comparison of MSC replication between Tg(Gpx4)+/0 mice and WT littermates. In the same experiments, replication of CFU-OBs was determined by measuring numbers before and after expansion, as described in Fig. 1. D) Increased skeletal tissue formation in vivo by MSCs from Tg(Gpx4)+/0 mice compared to WT littermates. Freshly isolated marrow cells from either Tg(Gpx4)+/0 mice or WT littermates were seeded at 7 × 106 cells/10-cm2 well onto tissue culture plastic and cultured for 7 d. Cultured adherent cells (1×106) were loaded onto Gelfoam and implanted subcutaneously into the dorsal surface of immunodeficient mice. Implants were harvested 8 wk following transplantation. BMD in ossicles was determined by μCT analysis; n = 6. *P < 0.05.
DISCUSSION
In this study, we showed that the deleterious effect of aging on the replication of MSCs was remarkable and reproducible in comparison with the initial number of MSCs defined by CFUs, suggesting that aging changes the quality of MSCs rather than the quantity of MSCs.
MSCs are surrounded by an ECM composed of collagens, adhesion proteins, proteoglycans, and growth factors, which form a unique microenvironment or niche (22, 23). MSCs living in the ECM not only receive cues from the ECM but also influence the ECM by secreting ECM components and by proteolytic modification of proteins and growth factors in the ECM. The end result is a give and take relationship between MSCs and ECM, which defines MSC behavior (24). Strong evidence indicates that the relative abundance of senescent MSCs in vivo increases with aging. The accumulation of such cells has negative implications for the integrity of the ECM (25), which might further influence MSC adhesion, migration, proliferation, differentiation, and survival. An example of how the ECM might alter the features of stem cells is the recent finding that human embryonic stem cells (hESCs), when exposed to aged ECM, lose their regenerative capacity (26).
Recently, we reported that in both mice and humans, ECM prepared from marrow stromal cells significantly promoted proliferation of MSCs, preserved their stem cell properties, and enhanced their capacity for skeletogenesis (8, 9). This finding led us to investigate whether culture of aged MSCs on ECM could improve their number and quality. Specifically, we want to use this model to address whether the effect of aging on MSC themselves (intrinsic theory), or changes in MSCs by the surrounding ECM (extrinsic theory) occurs or both. Indeed, our data revealed that defects in the replication of aged MSC were completely restored by exposure to an ECM made by marrow stromal cells from young animals. Under this condition, both number and replication of MSCs were increased dramatically regardless of age. More important, such improvement was extremely diminished when cells (from either young or old mice) were cultured on ECM made by marrow stromal cells from old animals. Consistent with the results from the in vitro studies, increased skeletal tissue formation occurred by MSCs (from either young or old mice) expanded on young-ECM, but not on old-ECM. In particular, both young and old MSCs precultured on old-ECM generated more adipose tissue in vivo, suggesting that old-ECM may accelerate aging of MSCs. Taken together, the uniqueness of the present study is to provide strong evidence that the aging of ECM as surrounding tissue is the major determinant driving MSCs to age. Moreover, aged MSCs themselves can also alter the composition of the ECM. Evidence obtained from the present study suggested that the amount ECM protein elaborated by young vs. old cells was the same, but their compositions were different. SDS-PAGE showed that the old-ECM proteins around 140 and 40 kDa were significantly less than those from young-ECM (data not shown). Interestingly, collagen type I α-1 and α-2 chains are 139 and 129 kDa, respectively (27), while the core proteins of small leucine-rich proteoglycans are ∼40 kDa (28). The alteration of these components might change the architecture of ECM, affecting the ability of ECM to interact with growth factors, resulting in an effect on MSC behavior. To confirm this, comparative proteomic analysis will be performed. The candidates that contribute to the integrity of ECM will be dissected further by the modification of ECM made by cells treated with siRNA to silence the tested protein.
In the present study, we compared the composition of these two ECMs using confocal Raman microscopy. Clearly, the data suggested that ECM prepared from cultured bone marrow stromal cells from old animals contained more mineral phosphate and less collagen than those from young animals. It has been known that calcium phosphate particles impair osteoblast progenitor viability and proliferation (29), which could explain that the capacity of young MSCs to self-renew and generate skeletal tissue was diminished after exposure to old-ECM.
To demonstrate the functional potential of progenitors further, we compared intracellular telomerase and ATP activities from cells cultured on the various matrices since the former is associated with cellular life span, and the latter is directly correlated to the proliferation status of stem cells (18, 19). Based on the levels of telomerase and ATP activities, it was suggested that a high quality of stem cells was enriched from bone marrow cells by exposure to a young-ECM, supporting the observation that young-ECM promoted MSCs for osteoblastogenesis in vitro and in vivo. To test the related probability that cellular compositions varied with age and that a different cell population could be selectively enriched by exposure to the different matrices, we measured several MSC related markers such as SSEA-4, CD44, CD90, and Sca-1. Unexpectedly, we found no significant difference in the proportion of those positive cells when cells were maintained on young- vs. an old-ECM. Although SSEA-4 originally identified as an early embryonic glycolipid antigen, has been utilized to identify MSCs from bone marrow (20, 30), our previous studies indicate that SSEA-4 is expressed mainly by dividing cells that do not necessarily represent pluripotent MSCs (9). Thus, our findings suggest that these markers might not be specific enough to define highly functional MSCs.
Increasing evidence indicates that the continuous production of intracellular ROS, including superoxide anions, hydroxyl radicals, and hydrogen peroxide, are a major determinant of life span (31). Although the mechanisms underlying the influence of life span are not completely understood, increased ROS is thought to cause cell death and accelerate the aging process by, at least in part, stimulation of stem cells or progenitors into a state of replicative senescence in which their growth is arrested (32). Recent studies in hematopoietic stem cells have shown that a high level of ROS is associated with loss of stem cell self-renewal and increased differentiation as well as their apoptosis (33). Moreover, culture of MSCs under low oxygen tension (3%) to mimic the microenvironment of the bone marrow enhances MSC “stemness” (34). Evidence obtained from our present studies showed that intracellular level of ROS was higher with a decrease in the number of CFU-OBs, and vice versa. Fascinatingly, in cultures maintained on young-ECM, ROS levels from both old and young mice were reduced 50 and 30%, respectively. Under this condition, the number of CFU-OBs from old and young mice increased 13- and 16-fold, respectively. In contrast, ROS levels were elevated in cultured MSCs (from either young or old animals) on old-ECM, which was accompanied by a decrease in the number of CFU-OBs.
To further confirm whether ECM restored the replication of aged MSCs by means of reducing ROS, we tried the alternative approach of attempting to enhance antioxidant defenses through genetic modification—mouse overexpression of Gpx4 [Tg(Gpx4)+/0 mice]. Antioxidant enzymes mainly include superoxide dismutase (SOD), catalase, and Gpx, which act to remove ROS production by free radical reactions (35, 36). Based on differential tissue-specific distribution, the Gpx family has been classified into at least 4 types (reviewed by Brigelius-Flohe, ref. 37). It is considered that Gpx4 plays an important role in protecting against oxidative stress-induced apoptosis via the stabilization or the repair of mitochondrial membranes as well as cellular membranes (37). The present studies showed that intracellular ROS levels in bone marrow cells from Tg(Gpx4)+/0 mice were reduced 40–50%, and the capacity for replication as well as bone generation of MSCs from Tg(Gpx4)+/0 mice was enhanced markedly, as compared to WT mice. These findings indicate that increased oxidative stress is associated with defects in the self-renewal of aged MSCs and osteoblast progenitors and that such defects might be corrected by reducing ROS. Although Tg(Gpx4)+/0 mice had only a marginally increased BMD compared to WT mice at 3 mo of age, we speculate that Tg(Gpx4)+/0 mice might delay their bone loss during aging.
In the present study, we propose for the first time a unique model to study the roles of MSC aging (cell intrinsic) and ECM or niche aging (cell extrinsic). Our studies revealed that defects in replication (in vitro) and bone formation capacity (in vivo) of aged MSCs were very remarkable and reproducible. Moreover, the increased oxidative stress associated with old age exhausts a limited pool of MSC or osteoblast progenitors, and the old-ECM itself, and/or factors embedded in it, contributes by increasing ROS or reducing defenses against oxidative stress. More important, this study indicates that aging negatively affects the formation of an ECM that normally preserves MSC function, and MSCs from aged animals can be improved by culture on ECM made by stromal cells from young mice. Taken together, we suggest that culture of aged MSCs on a young-ECM may improve their number and quality, thereby optimizing the effectiveness of autologous MSC administration for future therapeutic applications.
Acknowledgments
The authors thank Dr. Valerie A. Lee [University of Texas Health Science Center at San Antonio (UTHSCSA)] for her careful review of the manuscript, and Dr. Sergey Leikin [U.S. National Instiute of Child Health and Human Development/National Institutes of Health (NIH)] for his critical discussion about the analysis of Raman spectrum.
This work was supported by a grant from the University Research Council Grants Program at UTHSCSA (X.D.C.), and supported by the National Institutes of Health (R21 AG025466; X.D.C.). Analysis of μCT was performed in the Core Small Animal Imaging Facility, Greehey Children Cancer Research Institute of UTHSCSA.
REFERENCES
- 1. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 2. Smith J. R., Pochampally R., Perry A., Hsu S. C., Prockop D. J. (2004) Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22, 823–831 [DOI] [PubMed] [Google Scholar]
- 3. Deng J., Petersen B. E., Steindler D. A., Jorgensen M. L., Laywell E. D. (2006) Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 24, 1054–1064 [DOI] [PubMed] [Google Scholar]
- 4. Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 [DOI] [PubMed] [Google Scholar]
- 5. Petersen B. E., Bowen W. C., Patrene K. D., Mars W. M., Sullivan A. K., Murase N., Boggs S. S., Greenberger J. S., Goff J. P. (1999) Bone marrow as a potential source of hepatic oval cells. Science 284, 1168–1170 [DOI] [PubMed] [Google Scholar]
- 6. Blazsek I., Chagraoui J., Peault B. (2000) Ontogenic emergence of the hematon, a morphogenetic stromal unit that supports multipotential hematopoietic progenitors in mouse bone marrow. Blood 96, 3763–3771 [PubMed] [Google Scholar]
- 7. Sethe S., Scutt A., Stolzing A. (2005) Aging of mesenchymal stem cells. Ageing Res. Rev. 5, 91–116 [DOI] [PubMed] [Google Scholar]
- 8. Chen X. D., Dusevich V., Feng J. Q., Manolagas S. C., Jilka R. L. (2007) Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner Res. 22, 1943–1956 [DOI] [PubMed] [Google Scholar]
- 9. Lai Y., Sun Y., Skinner C. M., Son E. L., Lu Z., Tuan R., Jilka R. L., Ling J., Chen X. D. (2010) Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 19, 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T. L. (2004) Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone 35, 83–95 [DOI] [PubMed] [Google Scholar]
- 11. Knopp E., Troiano N., Bouxsein M., Sun B. H., Lostritto K., Gundberg C., Dziura J., Insogna K. (2005) The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146, 1983–1990 [DOI] [PubMed] [Google Scholar]
- 12. Yant L. J., Ran Q., Rao L., Van R. H., Shibatani T., Belter J. G., Motta L., Richardson A., Prolla T. A. (2003) The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 [DOI] [PubMed] [Google Scholar]
- 13. Ran Q., Liang H., Gu M., Qi W., Walter C. A., Roberts L. J., Herman B., Richardson A., Van R. H. (2004) Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J. Biol. Chem. 279, 55137–55146 [DOI] [PubMed] [Google Scholar]
- 14. Vlodavsky I. (1999) Preparation of extracellular matrices produced by cultured corneal endothelial and PF-HR9 endodermal cells. In Current Protocols in Cell Biology, pp. 10.4.1–10.4.14, John Wiley & Sons, New York: [DOI] [PubMed] [Google Scholar]
- 15. Krebsbach P. H., Kuznetsov S. A., Satomura K., Emmons R. V. B., Rowe D. W., Robey P. G. (1997) Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63, 1059–1069 [DOI] [PubMed] [Google Scholar]
- 16. Bi Y., Stuelten C. H., Kilts T., Wadhwa S., Iozzo R. V., Robey P. G., Chen X. D., Young M. F. (2005) Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J. Biol. Chem. 280, 30481–30489 [DOI] [PubMed] [Google Scholar]
- 17. Di Gregorio G. B., Yamamoto M., Ali A. A., Abe E., Roberson P., Manolagas S. C., Jilka R. L. (2001) Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J. Clin. Invest. 107, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cong Y., Shay J. W. (2008) Actions of human telomerase beyond telomeres. Cell Res. 18, 725–732 [DOI] [PubMed] [Google Scholar]
- 19. Reems J. A., Hall K. M., Gebru L. H., Taber G., Rich I. N. (2008) Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion 48, 620–628 [DOI] [PubMed] [Google Scholar]
- 20. Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. (1983) Stage-specific embryonic antigens (SSEA-3 and −4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 2, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adewumi O., Aflatoonian B., Ahrlund-Richter L., Amit M., Andrews P. W., Beighton G., Bello P. A., Benvenisty N., Berry L. S., Bevan S., Blum B., Brooking J., Chen K. G., Choo A. B., Churchill G. A., Corbel M., Damjanov I., Draper J. S., Dvorak P., Emanuelsson K., Fleck R. A., Ford A., Gertow K., Gertsenstein M., Gokhale P. J., Hamilton R. S., Hampl A., Healy L. E., Hovatta O., Hyllner J., Imreh M. P., Itskovitz-Eldor J., Jackson J., Johnson J. L., Jones M., Kee K., King B. L., Knowles B. B., Lako M., Lebrin F., Mallon B. S., Manning D., Mayshar Y., McKay R. D., Michalska A. E., Mikkola M., Mileikovsky M., Minger S. L., Moore H. D., Mummery C. L., Nagy A., Nakatsuji N., O'Brien C. M., Oh S. K., Olsson C., Otonkoski T., Park K. Y., Passier R., Patel H., Patel M., Pedersen R., Pera M. F., Piekarczyk M. S., Pera R. A., Reubinoff B. E., Robins A. J., Rossant J., Rugg-Gunn P., Schulz T. C., Semb H., Sherrer E. S., Siemen H., Stacey G. N., Stojkovic M., Suemori H., Szatkiewicz J., Turetsky T., Tuuri T., van den B. S., Vintersten K., Vuoristo S., Ward D., Weaver T. A., Young L. A., Zhang W. (2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 25, 803–816 [DOI] [PubMed] [Google Scholar]
- 22. Fuchs E., Tumbar T., Guasch G. (2004) Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 [DOI] [PubMed] [Google Scholar]
- 23. Moore K. A., Lemischka I. R. (2006) Stem cells and their niches. Science 311, 1880–1885 [DOI] [PubMed] [Google Scholar]
- 24. Behonick D. J., Werb Z. (2003) A bit of give and take: the relationship between the extracellular matrix and the developing chondrocyte. Mech. Dev. 120, 1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campisi J. (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 [DOI] [PubMed] [Google Scholar]
- 26. Carlson M. E., Conboy I. M. (2007) Loss of stem cell regenerative capacity within aged niches. Aging Cell 6, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lareu R. R., Arsianti I., Subramhanya H. K., Yanxian P., Raghunath M. (2007) In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 13, 385–390 [DOI] [PubMed] [Google Scholar]
- 28. Hocking A. M., Shinomura T., McQuillan D. J. (1998) Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 17, 1–19 [DOI] [PubMed] [Google Scholar]
- 29. Pioletti D. P., Takei H., Lin T., Van L. P., Ma Q. J., Kwon S. Y., Sung K. L. (2000) The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 21, 1103–1114 [DOI] [PubMed] [Google Scholar]
- 30. Gang E. J., Bosnakovski D., Figueiredo C. A., Visser J. W., Perlingeiro R. C. (2007) SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 109, 1743–1751 [DOI] [PubMed] [Google Scholar]
- 31. Balaban R. S., Nemoto S., Finkel T. (2005) Mitochondria, oxidants, and aging. Cell 120, 483–495 [DOI] [PubMed] [Google Scholar]
- 32. Kirkwood T. B. (2005) Understanding the odd science of aging. Cell 120, 437–447 [DOI] [PubMed] [Google Scholar]
- 33. Tothova Z., Kollipara R., Huntly B. J., Lee B. H., Castrillon D. H., Cullen D. E., McDowell E. P., Lazo-Kallanian S., Williams I. R., Sears C., Armstrong S. A., Passegue E., DePinho R. A., Gilliland D. G. (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 [DOI] [PubMed] [Google Scholar]
- 34. D'Ippolito G., Diabira S., Howard G. A., Roos B. A., Schiller P. C. (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39, 513–522 [DOI] [PubMed] [Google Scholar]
- 35. McCord J. M., Fridovich I. (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 [PubMed] [Google Scholar]
- 36. McCord J. M., Fridovich I. (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 244, 6056–6063 [PubMed] [Google Scholar]
- 37. Brigelius-Flohe R. (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 27, 951–965 [DOI] [PubMed] [Google Scholar]