Abstract
Fingolimod (FTY720) is a sphingosine 1-phosphate (S1P) receptor modulator that regulates lymphocyte trafficking and exerts pleiotropic actions on oligodendrocytes (OLGs) and other neural cells. The purpose of this study was to investigate the role of S1P receptors in a non-T-cell model of demyelination, the cuprizone (cupr) model in C57BL/6 mice. Treatment with FTY720 (1 mg/kg) led to attenuated injury to OLGs, myelin, and axons in the corpus callosum (percentage of myelinated fibers was 44.7% in cupr-water and 63% in cupr-FTY720). Reactive astrogliosis and microgliosis were ameliorated when FTY720 was given from d 1, but astrogliosis was augmented when FTY720 was given from wk 4–9. FTY720 did not promote remyelination in this model. The protective effect of FTY720 was associated with decreased interleukin-1β and CCL2 transcripts in the corpus callosum, as well as altered S1P1 expression. Targeted deletion of S1P1 in OLG lineage cells did not lead to obvious clinical phenotype, but resulted in subtle abnormalities in myelin and an increased susceptibility to cupr-induced demyelination. We conclude that S1P receptors expressed by neuroglia are involved in regulating the response to injury, and CNS effects of FTY720 could contribute to its favorable therapeutic response in multiple sclerosis.—Kim, H. J., Miron, V. E., Dukala, D., Proia, R. L., Ludwin, S. K., Traka, M., Antel, J. P., Soliven, B. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model.
Keywords: FTY720, fingolimod, oligodendrocytes, multiple sclerosis, demyelination, remyelination
Sphingosine 1-phosphate (SIP) and its G-protein-coupled receptors constitute an autocrine and paracrine network of signaling that regulates cellular responses to diverse external stimuli, and have emerged as promising targets for drug development (1). Insights from S1P receptor biology and preclinical studies in experimental autoimmune encephalomyelitis (EAE) have led to clinical trials and recent FDA approval of a nonselective S1P receptor modulator fingolimod (FTY720) for treatment in multiple sclerosis (MS) (2–7). FTY720P, the active form of FTY720, binds to 4 of 5 receptors. Using GTPγS binding assay, the EC50 (expressed as −log molar) for FTY720P was found to be 8.2 at S1P1, 8.4 at S1P3, 7.2 at S1P4, and 8.2 at S1P5 (2). In vivo, a bolus injection of 1 mg/kg of FTY720 or FTY720P reduced circulating T cells by ∼70% in rats (2). The efficacy of these compounds is largely attributed to lymphocyte sequestration in lymph nodes as a result of functional antagonism of lymphocyte S1P1 receptors and possibly functional agonism of endothelial S1P1 receptors (8–12).
Aside from its immunomodulatory actions, there is evidence that FTY720 and related compounds could exert direct effects on the CNS. FTY720 crosses the blood-brain barrier, and is rapidly phosphorylated by sphingosine kinase 2 to its active form, FTY720P, resulting in higher concentrations of both forms in the brain relative to the blood (13, 14). It is also known that glial cells express S1P receptors, albeit differing in the predominant subtypes. Astrocytes express predominantly S1P1 and S1P3 (15–18). Cultured rat oligodendrocytes (OLGs) express mRNAs encoding S1P5 > S1P1 = S1P2 > S1P3, whereas OLG progenitor cells (OPCs) express S1P1 ≥ S1P2 = S1P5 > S1P3 (19, 20). In vitro studies have demonstrated the pleiotropic actions of S1P and FTY720P in OLG lineage cells, which include regulation of cell survival, mitogenesis, migration, and cytoskeletal dynamics via G-protein-dependent signaling pathways (19–26). The effect of FTY720P on OPC differentiation and cytoskeletal dynamics is concentration- and treatment duration-dependent (24, 27). Together, these findings support the concept that FTY720 may be glioprotective or may influence remyelination.
The goal of this study was to investigate the role of S1P receptors expressed by neuroglial cells during demyelination and remyelination in a non-T-cell model of demyelination, the cuprizone (cupr) model. In this model, demyelination results from injury to OLGs, and the release of myelin debris is followed by activation and recruitment of astrocytes and microglia (28–30). Cupr-induced OLG apoptosis is mediated by poly (ADP-ribose) polymerase (PARP) and apoptosis-inducing factor (AIF), and is preceded by down-regulation of myelin protein genes (31–33). This model allows one to study the CNS effects of FTY720 that are independent of its action on lymphocyte trafficking. Given the expression of S1P receptors by glial cell subtypes and neurons, we have also generated S1P1 conditional knockout (CKO) mice to study the effect of S1P1 deletion in OLG lineage cells on the susceptibility to cupr-mediated injury.
MATERIALS AND METHODS
Induction of demyelination and treatment
Adult male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) at 6–7 wk of age were housed a in pathogen-free barrier facility and allowed access to food and water ad libitum. All animal use procedures were conducted in strict accordance with the guidelines from the U.S. National Institutes of Health (NIH) and the University of Chicago. To induce demyelination, 8-wk-old animals were fed a diet containing 0.2% cupr (bis-cyclohexanone oxaldihydrazone; Sigma, St. Louis, MO, USA) mixed into ground mouse chow (2918; Harlan Teklad, Madison, WI, USA) for 6 wk. Remyelination was achieved by resuming a normal food (NF) diet of rodent chow for 3 wk following the demyelination period.
To study the effect on demyelination, FTY720 was reconstituted in distilled water and given orally 1×/d by gavage at 1 mg/kg body weight from d 1 of cupr diet for 6 wk. The dose of FTY720 chosen was based on our recent study in an animal model of spontaneous autoimmune polyneuropathy (12). For remyelination studies, animals were treated with FTY720 (0.3–1 mg/kg) by gavage from wk 4–6 of cupr diet through wk 7–9 of normal diet. For comparison, animals fed a normal diet and cupr diet were gavaged with the same volume of water. In some experiments, we included animals that did not receive any gavage as additional controls. FTY720 was generously provided by Novartis (Basel, Switzerland).
Generation and analysis of S1P1-CKO mice
S1P1 f/f mice (generated in R.L.P.'s laboratory) were bred with C57BL/6 mice expressing Cre recombinase under the control of 2′3′-cyclic nucleotide phosphodiesterase 1 (CNPWT/Cre; a generous gift from K. Nave, Max Planck Institute of Experimental Medicine, Göttingen, Germany; refs. 34, 35). To detect the Cre allele, primers CNP E3 sense (5′-GCCTTCAAACTGTCCATCTC), CNP 5′-EcoIN2 (5′-GATGGGGCTTACTCTTGC), and puro3 (5′-CATAGCCTGAAGAACGAGA) were used. To detect the S1P1 alleles, primers P1 (5′-GAGCGGAGGAAGTTAAAAGTG), P2 (5′-CCTCCTAAGAGATTGCAGCAA), and P3 (5′-GATCCTAAGGCAATGTCCTAGAATGGGACA) were used. The PCR products were digested with SacI before electrophoresis, giving a 250-bp fragment for the S1P1 allele containing loxP sites (S1Ploxp/loxp), a 200-bp band for the wild-type (WT) S1P1 allele, and a 180-bp band for the Cre-recombined allele S1P1ΔEx2. The specificity of CNP/Cre has been demonstrated in previous studies (35).
Histological studies and immunohistochemistry
Animals underwent intracardiac perfusion under deep anesthesia (pentobarbital, 50 mg/kg i.p.) with cold heparinized PBS followed by 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). Brains were removed, postfixed in the same fixative at 4°C overnight, and cryoprotected in 30% sucrose in 0.1 M PBS for 48–72 h.
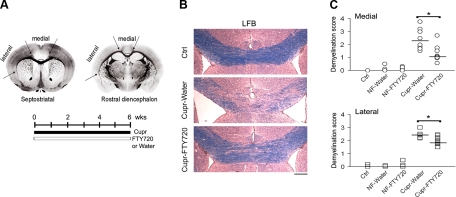
At least 3 sections (10 μm) from each animal were stained with luxol fast blue (LFB) overnight at 56°C, and rinsed with 95% ethanol, lithium carbonate, 70% ethanol, and water. Sections were then counterstained with hematoxylin and eosin and subsequently dehydrated. LFB-stained septostriatal and rostral diencephalon sections were assessed for demyelination by 2 observers in a masked procedure, as described previously, with some modifications (36). Demyelination scores ranged from 0 to 4 for the medial corpus callosum [0, fully myelinated; 1, mild demyelination (≤1/3) in the center (see Fig. 1A; arrows); 2, moderate demyelination (≤2/3); 3, no myelin in the center; and 4, demyelination extending to the arch]. For the lateral (callosal) projections, scores from 0 to 3 were used, with 0 as normal and 3 as no myelin.
Figure 1.
Amelioration of cupr-induced demyelination by FTY720. A) Levels of brain sections (septostriatal and rostral diencephalon) sampled for LFB staining, areas analyzed, and treatment schedule to induce demyelination. Arrows delineate medial and lateral corpus callosum; boxed area delineates center of medial corpus callosum. B) Examples of LFB-stained medial corpus callosum. Scale bar = 200 μm. C) Summary of demyelination scores in the corpus callosum. Data were from n = 3 for control (ctrl) group; n = 5 each for NF-water and NF-FTY720 groups; n = 8–9 for cupr-water and cupr-FTY720 groups. Horizontal bars indicate median values. Ctrl, C57BL/6 mice fed normal diet without gavage; NF, normal food diet; cupr, cuprizone treatment. Both medial and lateral parts of the corpus callosum were similarly affected. *P < 0.01; Mann-Whitney test.
For immunohistochemistry, the following monoclonal antibodies (mAbs) were used: CC1 (1:50; Calbiochem, Gibbstown, NJ, USA), anti-glial fibrillary acidic protein (GFAP; 1:100), anti-phosphorylated neurofilament heavy chain (pNF-H; 1:40; Sigma), Nkx2.2 (1:100; clone 74.5A5; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA); anti-proliferating cell nuclear antigen (PCNA; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Polyclonal Abs included anti-myelin basic protein (MBP; 1:1000; Dako, Carpinteria, CA, USA), anti-Iba1 (1:100; Wako Chemicals, Richmond, VA, USA), anti-β-amyloid precursor protein (β-APP; 1:200; Abcam, Cambridge, MA, USA), anti-Olig2 (1:200; Millipore, Temecula, CA, USA), and anti-NG2 (1:100; Millipore). Appropriate alkaline phosphatase-labeled or peroxidase-labeled secondary antibodies (1:200) and corresponding substrates were used for detection. For Nkx2.2, immunoreactivity was detected using the Ultravision LP detection system (LabVision; Thermo Fisher Scientific, Fremont, CA, USA). In some experiments, Alexa 488-conjugated and Alexa 594-conjugated secondary antibodies were used (1:500; Molecular Probes, Eugene, OR, USA). No staining was observed in tissue sections when primary antibodies were omitted (Dako). For quantitative analysis, the number of immunoreactive cells was counted at ×20 with tissue areas measured by image analysis (Image J software; NIH, Bethesda, MD, USA). Some immunostaining results were analyzed as integrated density/intensity and normalized to values from controls of each experiment. Confocal microscopy (×40) was used for analysis of axonal degeneration (pNF-H and β-APP immunofluorescence). For evaluation of apoptosis, TUNEL assay (Millipore) was performed according to the manufacturer's instructions.
Electron microscopy (EM)
Animals were perfused with 4% PFA and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. Coronal slices (1 mm thick) through the corpus callosum were cut in midsagittal plane and embedded in epon, oriented to visualize the entire cross-section of the midsagittal corpus callosum. Septostriatal sections were stained with uranyl acetate and lead acetate, and photographed as described previously (37). The number of myelinated axons in multiple nonoverlapping fields (5 areas/section) for 3 mice in each group was counted with results expressed as a percentage of myelinated axons (number of myelinated axons/total axons × 100). The g ratio was determined by calculating the ratio of the inner axonal diameter to the total outer diameter (inner axonal diameter plus surrounding myelin). At least 300 fibers were analyzed.
Real-time RT-PCR studies
Total RNA was isolated from the corpus callosum using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The purity and concentrations were determined using Gene Spec III (MiraiBio, South San Francisco, CA, USA). Reverse transcription was performed from 1 μg total RNA in 20 μl of diethylpyrocarbonate-treated water. The complementary DNA (cDNA) at 1:10 dilution was used for SYBR Green real-time RT-PCR (Applied Biosystems, Foster City, CA, USA). Primers designed using the Primer3 software (http://frodo.wi.mit.edu/primer3/) were used for amplification for transcripts of MBP, proteolipid protein (PLP), platelet-derived growth factor (PDGFA), insulin-like growth factor-1 (IGF-1), tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), CCL2 (MCP-1), CCL5 (RANTES), S1P1, S1P5, and GAPDH (Supplemental Table S1). The expression of each gene was normalized by the corresponding amount of GAPDH mRNA for each condition. Relative amounts of each product were expressed as (2−ΔCT) relative to GAPDH or as 2−ΔΔCT relative to controls, as described in the ABI Prism 7300 Sequence Detection System user manual (Applied Biosystems). At least 3 independent experiments were done, with each test condition in triplicates.
Western blot analysis
Corpus callosum was homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1% TritonX, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM PMSF, and 2 μg/ml leupeptin. Samples (30 μg/lane) were resolved by 12% SDS-PAGE and electroblotted to 0.45-μm PVDF membranes. After blocking with 5% nonfat milk in 0.1% Tween 20 for 1 h, blots were incubated overnight at 4°C with polyclonal Ab against S1P1 (1:500; Abcam), AIF (1:500; Cell Signaling, Danvers, MA, USA), or ERK2 (1:500; Upstate Biotechnology, Lake Placid, NY, USA); washed 3 times; and then incubated for 1–2 h at room temperature with goat anti-rabbit horseradish peroxidase-conjugated secondary Abs (1:1000–1:5000; Calbiochem, San Diego, CA, USA). Immunoreactive proteins were visualized by the enhanced chemiluminescence detection method (ECL; GE Healthcare, Little Chalfont, UK).
Data analysis
Demyelination scores from septostriatal section and rostral diencephalon were combined and analyzed by nonparametric methods, Kruskal-Wallis or Mann-Whitney tests. Results from quantitative histological analysis, real-time RT-PCR analysis, grip strength measurements, and rotarod treadmill testing were expressed as means ± se (n=number of animals). Unless otherwise specified, statistical analysis was performed by the Student's t test, or ANOVA followed by Bonferroni method for multiple comparisons. For data with unequal variance, Dunnett's T3 was used. For simplification, statistical analysis to confirm that cupr had worked as expected (e.g., cupr-water vs. NF-water) is not shown in figures, unless otherwise specified.
RESULTS
Effect of FTY720 on cupr-induced demyelination and remyelination
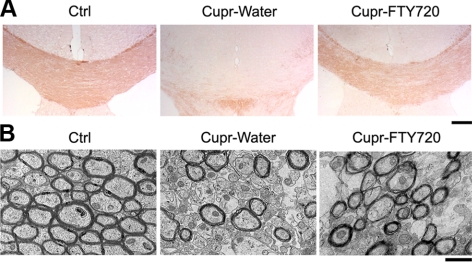
Figure 1A shows levels of brain sections used, areas analyzed, and treatment schedule to induce demyelination in the cupr model. Sections from both septostriatal and rostral diencephalon (38) were sampled for LFB staining. In addition, demyelination in both the medial and lateral corpus callosum was scored separately. Representative images of LFB-stained medial corpus callosum are shown in Fig. 1B. As expected, demyelination was observed in the corpus callosum of cupr-fed animals. In addition, the severity of demyelination was attenuated by treatment of cupr-fed animals with FTY720 (Fig. 1C). Results from LFB staining were confirmed by MBP immunohistochemistry and by EM in a subset of animals (Fig. 2A, B). For MBP immunoreactivity, the normalized integrated density was 104.8 ± 6.3% in the NF-FTY720 group, 34.4 ± 7.1% in cupr-water and 67.8 ± 4.8% in cupr-FTY720 (P<0.002 for cupr-water vs. cupr-FTY720, n=4–6 each). EM analysis showed that percentage of myelinated fibers was 79.7 ± 1.3% in the NF-water group, 85.3 ± 0.1% in NF-FTY720, 44.7 ± 1.2% in cupr-water, and 63.5 ± 2.9% in cupr-FTY720 (P<0.0001 for NF-water vs. cupr-water; P<0.002 for cupr-water vs. cupr-FTY720, n=3 each).
Figure 2.
Immunohistochemical and ultrastructural assessment of cupr-induced demyelination in the corpus callosum. A) Images illustrating the MBP immunoreactivity in brain sections from animals in control (ctrl), cupr-water, and cupr-FTY720 treatment groups. B) Representative electron micrographs showing demyelination in the corpus callosum in sections from animals in control, cupr-water, and cupr-FTY720 groups. Scale bars = 200 μm (A); 1 μm (B).
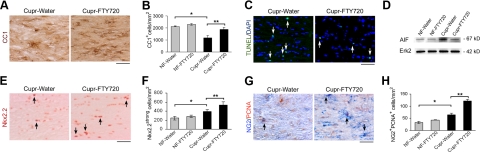
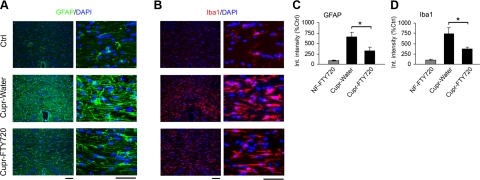
To examine the effect of FTY720 on various glial cell types, OLGs were identified in the corpus callosum using clone CC1 (APC-7). Late OPCs were identified as Nkx2.2strong cells, which represent a subset of Olig2+ cells (data not shown), while proliferating OPCs were identified as NG2+PCNA+ cells. We confirmed that cupr exposure for 6 wk led to a decrease in the number of OLGs, and increased number of OPCs. FTY720 treatment of cupr-fed mice led to increased number of OLGs at 6 wk, and attenuated apoptosis and reduced AIF protein levels at 3 wk (Fig. 3A–D). The number of TUNEL+ nuclei was 161.8 ± 32.5 in the cupr-water group and 85.7 ± 7.1 in cupr-FTY (n=3 each, P<0.05). We also observed an increase in the number of late OPCs (Nkx2.2+) and NG2+PCNA+ cells in sections from FTY720-treated cupr-fed animals (Fig. 3E–H). FTY720 had no effect on OLG lineage cells in animals fed NF diet. Cupr-induced demyelination was accompanied by accumulation of microglia (Iba1+ cells) and astrocytes (GFAP+ cells). Integrated intensity measurements revealed that both GFAP and Iba1 immunoreactivity in the corpus callosum were attenuated by FTY720 treatment in cupr-fed animals (Fig. 4). Analysis by counting showed that the number of astrocytes per square millimeter was 266.7 ± 26.5 in the NF-water group, 307.1 ± 35.7 in NF-FTY720, 1125 ± 58.7 in cupr-water, and 782.8 ± 76 in cupr-FTY720 (P<0.00004 for NF-water vs. cupr-water; P<0.006 for cupr-water vs. cupr-FTY720, n=4–5 each). The number of microglia in cupr-fed animals was also attenuated by FTY720 treatment, albeit to a lesser extent compared to that of astrocytes (n=4–5, data not shown).
Figure 3.
Effect of FTY720 on OLGs and OPCs in the cupr model. A, C, E, G) Representative images of stained sections. B, F, H) Data summary (n=4–5 each). A, B) OLGs. *P < 0.001; **P < 0.013. C) TUNEL labeling. Arrows indicate apoptotic nuclei (TUNEL+DAPI+). D) Western blots of corpus callosum homogenates demonstrating the effect of FTY720 treatment on AIF protein levels (n=2). E, F) Late OPCs. Arrows indicate examples of Nkx2.2strong cells. *P < 0.02; **P < 0.04. G, H) Proliferating OPCs. Arrows indicate examples of NG2+PCNA+ cells. *P < 0.01; **P < 0.0003. C, D) Data from cupr-fed animals treated with water or FTY720 for 3 wk; the rest are data from treatment schedule outlined in Fig. 1. Scale bars = 50 μm.
Figure 4.
Decreased accumulation of astrocytes (GFAP+) and microglia/ macrophages (Iba1+) in brain sections from FTY720-treated cupr-fed animals. A, B) Images of brain sections showing the GFAP (green; A) and Iba1 (red; B) immunoreactivity. Nuclei were counterstained with DAPI (blue). Scale bars = 50 μm. C, D) Quantitative analysis of astrogliosis (C) and microgliosis (D) (integrated intensity). Data were normalized to values from control group (ctrl; NF-water) in each set (n=4 or 5 each). *P < 0.03 (C); *P < 0.016 (D).
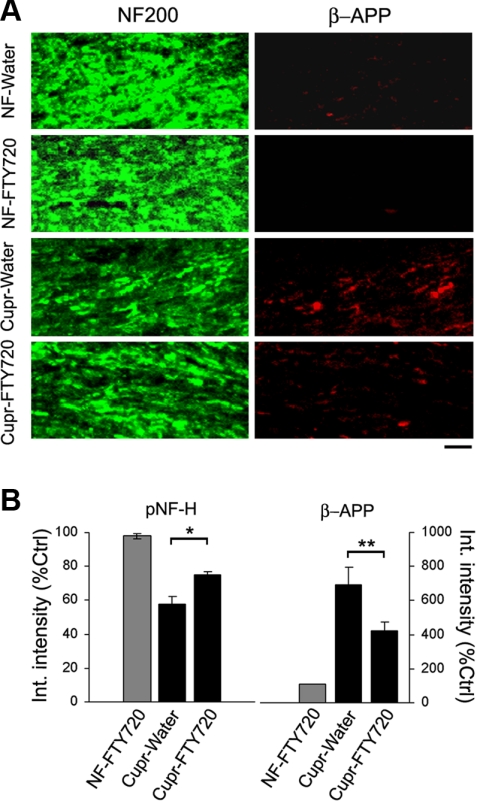
Next, we examined the effect of FTY720 treatment on axonal health or integrity. Axonal damage occurs in the cupr model during both the acute (3–6 wk of cupr) and chronic demyelination paradigm (12 wk of cupr) and can be detected by loss of phosphorylated neurofilament (pNF-H) staining, axonal tortuosities, or accumulation of β-APP (39–41). As shown in Fig. 5, brain sections from FTY720-treated cupr-fed animals had more pNF-H staining and less β-APP accumulation in the corpus callosum compared to those from water-treated ones. Thus, FTY720 exerts a protective action on OLGs, myelin, and axons during cupr-induced demyelination.
Figure 5.
Attenuation of axonal degeneration by FTY720 in the cupr model. A) Representative images showing immunoreactivity against pNF-H (green) and β-amyloid precursor protein (β-APP; red) in the corpus callosum. Scale bar = 10 μm. B) Quantitative analysis of pNF-H and β-APP immunoreactivity (integrated intensity). Data were normalized to values from control group (ctrl; NF-water; n=4–5/group). *P < 0.004; **P < 0.02.
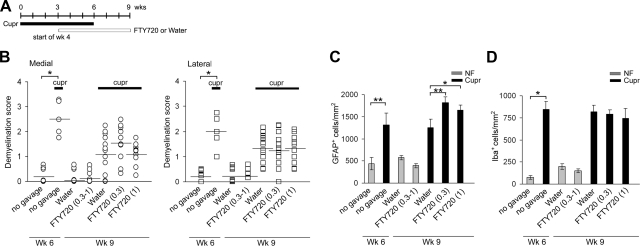
To study the effect of FTY720 on remyelination, animals were gavaged with the drug at 0.3 or 1 mg/kg, in view of its concentration-dependent effects on OPC differentiation and cytoskeletal dynamics in vitro (24, 26). Treatment schedule is shown in Fig. 6A. The number of animals in these experiments was increased to detect subtle differences in remyelination. We confirmed that cupr-induced demyelination was observed at wk 6, with improvement on cupr withdrawal when assessed at wk 9. However, LFB staining showed no difference in the extent of remyelination in sections from cupr-FTY720 vs. cupr-water (Fig. 6B). There was also no difference in the MBP immunoreactivity or in the number of OLGs and OPCs between these groups (data not shown, n=4–5 each). Unexpectedly, treatment with FTY720 in this paradigm led to increased number of astrocytes but no changes in the number of microglia (Fig. 6C, D).
Figure 6.
Effect of FTY720 on remyelination and astrogliosis (recovery phase). A) Treatment schedule to induce demyelination and remyelination in these studies. B) Lack of effect of FTY720 on remyelination in the corpus callosum. Presence of demyelination at wk 6 was confirmed for NF vs. cupr without gavage (n=5 or 6). For mice sacrificed at wk 9, n = 6–9 for NF-water and NF-FTY720 groups; n = 11–15 for cupr-water and cupr-FTY720 (0.3 and 1 mg/kg) groups. *P<0.001. C) Cupr-induced astrogliosis was augmented by FTY720 treatment when given from wk 4 to 9; n values similar to B. *P < 0.05; **P < 0.01. D) FTY720 had no effect on cupr-induced accumulation of microglia/macrophages during remyelination; n values similar to B. *P<0.03.
Possible mechanisms underlying the protective action of FTY720
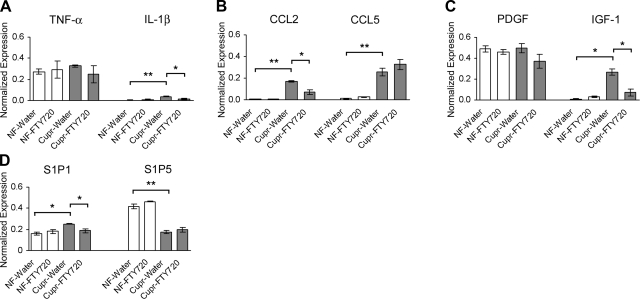
Real-time RT-PCR studies were performed to examine the expression of TNF-α, IL-1β, CCL2, CCL-5, PDGF, and IGF-1 in the corpus callosum. These cytokines, chemokines, and growth factors have been shown to be up-regulated in the cupr model or to play an important role in demyelination and remyelination (42–46). In these experiments, animals were treated with water or FTY720 for 5 wk from d 1. Exposure to cupr led to a significant increase in IL-1β, CCL2, CCL5, and IGF-1 but not in TNF-α or PDGF expression. FTY720 treatment led to attenuation of cupr-induced increase in IL-1β, CCL2, and IGF-1 transcripts. Cupr-induced demyelination was also associated with an increase in S1P1 and a decrease in S1P5 transcript levels; only S1P1 was reduced by FTY720 treatment of cupr-fed animals (Fig. 7).
Figure 7.
Effect of FTY720 on the expression of selected cytokines, chemokines, growth factors, and S1P receptors in the corpus callosum during toxic demyelination. At least 3 independent experiments were done with each condition in triplicates. Because of extremely low levels of expression of IL-1β, CCL2, CCL5, and IGF-1 under control conditions, data were normalized to GAPDH mRNA (r=2−ΔCT) instead of the analysis by comparative method 2−ΔΔCT. A) TNF-α and IL-1β transcripts. *P < 0.01; **P < 0.0001. B) CCL2 and CCL5 transcripts. *P < 0.002; **P < 0.0002. C) PDGF and IGF-1 transcripts. *P < 0.0005. D) S1P1 and S1P5 transcripts. *P < 0.002 for cupr-water vs. NF-water; *P < 0.01 for cupr-water vs. cupr-FTY720; **P < 0.0004.
S1P1 is expressed not only in OLG lineage cells, but also in other glial cells (16, 17, 47). Therefore, we generated mutants with targeted ablation of S1P1 in OLG lineage cells by crossing CNPWT/Cre mice with S1P1f/f mice. Cre recombination was confirmed in brain tissues of S1P-CKO mice, which resulted in decreased S1P1 transcript and protein levels in the corpus callosum (Supplemental Fig. S1A–C). These S1P1 CKO mice (S1P1f/f; CNP WT/Cre), which are on C57BL/6 background, had no obvious clinical phenotype, and no deficits in motor coordination (rotarod treadmill test) or in grip strength measurements (Supplemental Table S2). A decrease in MBP and PLP transcript levels was observed in the corpus callosum from S1P1-CKO mice by real-time RT-PCR analysis (Supplemental Fig. S1C). Yet, LFB staining of brain sections from S1P1-CKO mice at 3 mo appeared normal. Ultrastructural studies of the corpus callosum at 3 wk and 3 mo revealed no difference in the axon diameter or the percentage of myelinated axons between S1P1-CKO and WT mice (Supplemental Fig. S1D). However, there was a decrease in myelin thickness in sections from S1P1-CKO mice, which resulted in a subtle increase in g ratio, compared to WT mice (Supplemental Table S3).
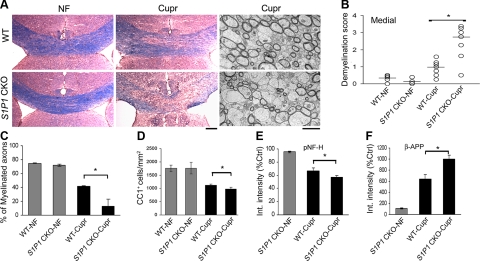
Concurrent with studies on myelination, we also determined whether targeted S1P1 deletion in cells of OLG lineage would lead to an increase in the susceptibility to detrimental stimuli. To facilitate the detection of earlier or more severe demyelination, WT and S1P1-CKO mice were fed with cupr for 3 wk instead of 6 wk. LFB staining revealed that the severity of demyelination in the corpus callosum was enhanced in brain sections from S1P1-CKO mice compared to WT mice (Fig. 8A, B). EM analysis revealed that the percentage of myelinated fibers was markedly decreased in S1P1-CKO-cupr mice when compared to WT-cupr mice (Fig. 8A, C). In addition, cupr induced a greater loss of OLGs in S1P1-CKO mice than WT mice, although the difference was not as dramatic as that observed for demyelination score (Fig. 8D). The enhanced severity of demyelination in cupr-fed S1P1-CKO mice was accompanied by an increase in the extent of axonal damage (Fig. 8E, F). These results indicate that S1P1-CKO mice exhibit increased susceptibility to cupr-induced injury, even though no clinical phenotype is detectable at baseline.
Figure 8.
Targeted deletion of S1P1 in OLG lineage cells increases the susceptibility to cupr-induced demyelination. A) Examples of LFB-stained septostriatal sections (color) and electron micrographs (grayscale) of the corpus callosum from WT and S1P1-CKO mice after 3 wk of cupr diet. Scale bars = 200 μm (LFB); 2 μm (EM). B) Demyelination scores in the medial corpus callosum (n=4 or 5 each for WT-NF and S1P1-CKO-NF; n=7 each for WT-cupr and S1P1-CKO-cupr). *P < 0.005; Mann-Whitney test. C) Cupr-induced loss of myelinated axons at 3 wk (EM analysis; n=3 each). *P < 0.03. D) Data summary on the number of OLGs (CC1+ cells; n=6–7 each). *P < 0.05. E) Cupr-induced axonal damage (decreased pNF-H integrated intensity; n=5 each). *P < 0.04. F) Cupr-induced axonal damage (increased β-APP integrated intensity; n=5 each). *P < 0.007. Data were normalized to the integrated intensity of NF-water.
DISCUSSION
We describe here the actions of FTY720 and the role of S1P1 in cupr-induced demyelination. Bearing in mind the potential caveats associated with the cupr model, we stringently monitored readouts for pathology and treatment effects with LFB staining, MBP immunoreactivity, EM analysis, and number of OLGs (CC1+ cells). These analyses revealed that treatment of cupr-fed animals with FTY720 led to decreased severity of demyelination, decreased AIF protein levels, and attenuated loss of OLGs, while promoting OPC proliferation. These findings are in agreement with our previous work showing that FTY720P promotes OLG survival and enhances PDGF-induced proliferation (24). The attenuation of axonal damage in FTY720-treated cupr-fed animals could reflect the consequence of less myelin damage, or imply direct protection of axons against injury. In the cupr model, there is a critical window beyond which axonal damage precludes a complete functional recovery. Reversal of conduction deficit on cupr withdrawal is complete in animals exposed to cupr for 1.5 wk, but only partial in those with cupr exposure of ≥3 wk (41).
We found that FTY720 treatment did not promote remyelination in the cupr model, in contrast to positive results in neonatal mouse cerebellar slice cultures following lysolecithin-induced demyelination (48). This discrepancy may denote the limitation of the cupr model for studying remyelination, or simply reflect the confounding variables when comparing these two approaches, such as method of demyelination, brain regions analyzed, treatment schedule, FTY720 concentrations, and the degree of astroglial and microglial activation. Additional studies on the chronic cupr model (12 wk of cupr diet followed by recovery) may clarify this issue. In dark Agouti rats with MOG-induced EAE, marked improvement in disease severity and electrophysiological parameters was observed even after a delayed initiation of FTY720 therapy from d 25 to 45, although a similar extent of remyelination was seen in untreated and FTY720-treated EAE rats by d 53 (5). FTY720 has also been reported to reduce T-cell infiltration and promote recovery in a spinal cord injury model (49).
In contrast to EAE and spinal cord injury, the contribution of T cells to the pathology of cupr-induced demyelination is thought to be insignificant. Although T cells are recruited to the demyelinated corpus callosum, they do not exhibit an activated phenotype (50). Rag−/− mice that are deficient in T and B cells exhibit no difference in the severity of cupr-induced demyelination when compared to control mice (51). However, there is evidence of local inflammatory response during cupr-induced injury, such as prominent activation and proliferation of microglia and astrocytes, and expression of cytokines and chemokines in the CNS (29, 44, 50). We found that FTY720 treatment of cupr-fed animals led to a decrease in astroglial and microglial accumulation at 6 wk, but resulted in enhanced astrogliosis with no effect on microglia when given from wk 4 to 9. The effect of timing of treatment on glial responses to FTY720 may be explained by the complex interplay of factors regulating the expression of S1P receptors, such as state of activation or cytokine milieu. For example, acutely dissociated microglia express S1P1 > S1P3 > S1P2 > S1P5, while LPS-activated microglia express S1P2 > S1P1 (47). Up-regulation of S1P1 and S1P3 in astrocytes can be induced by TNF-α, and is also observed in MS lesions (17, 18). Both S1P1 and S1P3 can contribute to astrogliosis, while microgliosis is mediated by S1P1 and possibly S1P5 (48, 52, 53). Aside from proliferation, FTY720 or S1P also stimulates astroglial migration and regulates the expression of growth factors and cytokine release in vitro (16–18, 54, 55). S1P stimulates the production of TNF-α or IL-1β in a microglial cell line (47, 56). These proinflammatory cytokines have complex effects on demyelination and remyelination in the cupr model (36, 57). We found that FTY720 treatment of cupr-fed animals for 5 wk led to decreased IL-1β and CCL2 transcripts in the corpus callosum, which would imply a diminished activation of astrocytes and microglia in these animals. Transient changes in TNF-α expression at an earlier time point may have been missed in our study. Taken together, a diminished accumulation and activation of astrocytes and microglia during demyelination phase may reflect attenuated myelin injury in FTY720-treated animals, or indicate anti-inflammatory actions of FTY720 on astrocytes and microglia.
FTY720 could also act indirectly by regulating the synthesis of trophic factors by astrocytes, as observed with S1P in vitro (55, 58). Among growth factors implicated in OLG survival and/or OPC proliferation are PDGF and IGF-1. Studies in hPDGF-A transgenic mice revealed that PDGF promotes OPC proliferation and remyelination following cupr-mediated injury (42, 59). IGF-1 is primarily expressed by astrocytes and by a subpopulation of microglia. There is less OLG apoptosis and more rapid recovery following cupr injury in IGF-1 transgenic mice than in WT mice (43). We found that the protective effect of FTY720 on OLGs and increased OPC proliferation in our study is not due to an increased expression of PDGF or IGF-1 in the corpus callosum. On the contrary, IGF-1 expression is decreased in FTY720-treated animals, which correlates with attenuated astrogliosis.
That cupr-induced injury is accompanied by increased S1P1 and decreased S1P5 transcripts in the corpus callosum may be due to loss of OLGs, the predominant cell type expressing S1P5; accumulation of other glial cells that express S1P1; and increased proliferation of OPCs, which express S1P1 > S1P5. S1P1 is involved in PDGF-induced OPC mitogenesis and at the same time is up-regulated by PDGF (24). FTY720 treatment led to decreased S1P1 expression in cupr-fed animals, which may reflect a reduction in astroglial and microglial accumulation, or due to treatment-induced cycling of S1P1 receptors, as reported in human OPCs and OLGs (26, 27).
We examined the effect of targeted disruption of S1P1 in OLG lineage cells on myelination and demyelination. Because of the redundancy of lysosphingolipid receptors, it is perhaps not surprising that S1P1-CKO mice have no obvious clinical phenotype, similar to previous observations on S1P5-KO mice (23). However, myelination is not totally normal in S1P1-CKO mice, as shown by a decrease in myelin protein gene transcripts, and a subtle increase in the g ratio, albeit still within the range of typical values for a myelinated axon (0.6 to 0.8) (60). S1P1-CKO mice exhibited increased susceptibility to cupr-induced demyelination compared to WT mice. A logical explanation would be that a decrease in MBP and PLP transcripts leads to suboptimal myelin thickness and/or stability, which predisposes the S1P1-CKO mice to further injury. Furthermore, there may be down-regulation of some S1P1-dependent gene targets or pathways that are important in glioprotection under pathological conditions. The apparent contradictory outcome from the pharmacologic approach and S1P1-CKO experiments would argue against a simple model of FTY720-induced down-regulation of S1P1 in OLG lineage cells. FTY720 induces internalization and degradation of S1P1 receptors, but there is also evidence for persistent agonism by internalized ligand-bound S1P1 (61, 62). Aside from S1P1 signaling, other factors could contribute to the beneficial effect of FTY720 in the cupr model, including glioprotection via S1P5 signaling in OLGs and indirect or anti-inflammatory actions of FTY720 on astrocytes and microglia.
In summary, we have provided evidence for a protective effect of FTY720 in the cupr model, which is independent of its action on lymphocyte trafficking; and a potential role of S1P1 in OLG lineage cells in regulating myelination and the response to injury. Compared to other immunomodulatory agents in MS therapy, FTY720 is unique in its neurobiological effects, which may translate to slowing of disease progression. Indeed, FTY720 not only reduces relapse rate, but also decreases the cumulative probability of disability progression in MS (6). A solely anti-inflammatory action of FTY720 would have led to the so called pseudoatrophy in brain MRI during the first year of treatment due to a reduction of water content, but this was not observed in the case of FTY720. Our results suggest that the best strategy for initiating treatment with FTY720 would be prior to or at the onset of insult (e.g., in between MS exacerbations or during disease quiescence). During MS relapse, FTY720 may augment astrogliosis once an injury response has occurred. Overall, our data support the concept that S1P receptors expressed by neuroglia are potential target for drug development that may have a wide therapeutic applications.
Supplementary Material
Acknowledgments
The authors thank Dr. B. Popko, Dr. N. Turgut, Dr. T. Johnson, and B. Durafourt for their assistance or advice in some experiments.
This work was supported by the U.S. National Institute of Neurological Disorders and Stroke (R21 NS049014), the National MS Society (RG3951A7/1), and a gift from M. P. Miller (B.S.); the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R.L.P.); the National MS Society (TR3762-A-1), a grant from the MS Society of Canada, and a grant from Novartis (J.P.A.); and a Canadian Institutes of Health Research studentship (V.E.M.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Spiegel S., Milstien S. (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Mol. Cell. Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 2. Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., Foster C. A., Zollinger M., Lynch K. R. (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457 [DOI] [PubMed] [Google Scholar]
- 3. Fujino M., Funeshima N., Kitazawa Y., Kimura H., Amemiya H., Suzuki S., Li X. K. (2003) Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J. Pharmacol. Exp. Ther. 305, 70–77 [DOI] [PubMed] [Google Scholar]
- 4. Webb M., Tham C. S., Lin F. F., Lariosa-Willingham K., Yu N., Hale J., Mandala S., Chun J., Rao T. S. (2004) Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J. Neuroimmunol. 153, 108–121 [DOI] [PubMed] [Google Scholar]
- 5. Balatoni B., Storch M. K., Swoboda E. M., Schonborn V., Koziel A., Lambrou G. N., Hiestand P. C., Weissert R., Foster C. A. (2007) FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain. Res. Bull. 74, 307–316 [DOI] [PubMed] [Google Scholar]
- 6. Kappos L., Radue E. W., O'Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., Burtin P. (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 [DOI] [PubMed] [Google Scholar]
- 7. Cohen J. A., Barkhof F., Comi G., Hartung H. P., Khatri B. O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., Tiel-Wilck K., de Vera A., Jin J., Stites T., Wu S., Aradhye S., Kappos L. (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 [DOI] [PubMed] [Google Scholar]
- 8. Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G. J., Card D., Keohane C., Rosenbach M., Hale J., Lynch C. L., Rupprecht K., Parsons W., Rosen H. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 [DOI] [PubMed] [Google Scholar]
- 9. Graler M. H., Goetzl E. J. (2004) The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18, 551–553 [DOI] [PubMed] [Google Scholar]
- 10. Brinkmann V., Lynch K. R. (2002) FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr. Opin. Immunol. 14, 569–575 [DOI] [PubMed] [Google Scholar]
- 11. Wei S. H., Rosen H., Matheu M. P., Sanna M. G., Wang S. K., Jo E., Wong C. H., Parker I., Cahalan M. D. (2005) Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat. Immunol. 6, 1228–1235 [DOI] [PubMed] [Google Scholar]
- 12. Kim H. J., Jung C. G., Dukala D., Bae H., Kakazu R., Wollmann R., Soliven B. (2009) Fingolimod and related compounds in a spontaneous autoimmune polyneuropathy. J. Neuroimmunol. 214, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Billich A., Bornancin F., Devay P., Mechtcheriakova D., Urtz N., Baumruker T. (2003) Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 278, 47408–47415 [DOI] [PubMed] [Google Scholar]
- 14. Foster C. A., Howard L. M., Schweitzer A., Persohn E., Hiestand P. C., Balatoni B., Reuschel R., Beerli C., Schwartz M., Billich A. (2007) Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J. Pharmacol. Exp. Ther. 323, 469–475 [DOI] [PubMed] [Google Scholar]
- 15. Rao T. S., Lariosa-Willingham K. D., Lin F. F., Palfreyman E. L., Yu N., Chun J., Webb M. (2003) Pharmacological characterization of lysophospholipid receptor signal transduction pathways in rat cerebrocortical astrocytes. Brain Res. 990, 182–194 [DOI] [PubMed] [Google Scholar]
- 16. Pebay A., Toutant M., Premont J., Calvo C. F., Venance L., Cordier J., Glowinski J., Tence M. (2001) Sphingosine-1-phosphate induces proliferation of astrocytes: regulation by intracellular signalling cascades. Eur. J. Neurosci. 13, 2067–2076 [PubMed] [Google Scholar]
- 17. Mullershausen F., Craveiro L. M., Shin Y., Cortes-Cros M., Bassilana F., Osinde M., Wishart W. L., Guerini D., Thallmair M., Schwab M. E., Sivasankaran R., Seuwen K., Dev K. K. (2007) Phosphorylated FTY720 promotes astrocyte migration through sphingosine-1-phosphate receptors. J. Neurochem. 102, 1151–1161 [DOI] [PubMed] [Google Scholar]
- 18. Van Doorn R., Van Horssen J., Verzijl D., Witte M., Ronken E., Van Het Hof B., Lakeman K., Dijkstra C. D., Van Der Valk P., Reijerkerk A., Alewijnse A. E., Peters S. L., De Vries H. E. (2010) Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 58, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 19. Yu N., Lariosa-Willingham K. D., Lin F. F., Webb M., Rao T. S. (2004) Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia 45, 17–27 [DOI] [PubMed] [Google Scholar]
- 20. Novgorodov A. S., El-Alwani M., Bielawski J., Obeid L. M., Gudz T. I. (2007) Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 21, 1503–1514 [DOI] [PubMed] [Google Scholar]
- 21. Hida H., Nagano S., Takeda M., Soliven B. (1999) Regulation of mitogen-activated protein kinases by sphingolipid products in oligodendrocytes. J. Neurosci. 19, 7458–7467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soliven B., Ma L., Bae H., Attali B., Sobko A., Iwase T. (2003) PDGF upregulates delayed rectifier via Src family kinases and sphingosine kinase in oligodendroglial progenitors. Am. J. Physiol. Cell Physiol. 284, C85–C93 [DOI] [PubMed] [Google Scholar]
- 23. Jaillard C., Harrison S., Stankoff B., Aigrot M. S., Calver A. R., Duddy G., Walsh F. S., Pangalos M. N., Arimura N., Kaibuchi K., Zalc B., Lubetzki C. (2005) Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 25, 1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung C. G., Kim H. J., Miron V. E., Cook S., Kennedy T. E., Foster C. A., Antel J. P., Soliven B. (2007) Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia 55, 1656–1667 [DOI] [PubMed] [Google Scholar]
- 25. Coelho R. P., Payne S. G., Bittman R., Spiegel S., Sato-Bigbee C. (2007) The immunomodulator FTY720 has a direct cytoprotective effect in oligodendrocyte progenitors. J. Pharmacol. Exp. Ther. 323, 626–635 [DOI] [PubMed] [Google Scholar]
- 26. Miron V. E., Jung C. G., Kim H. J., Kennedy T. E., Soliven B., Antel J. P. (2008) FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 63, 61–71 [DOI] [PubMed] [Google Scholar]
- 27. Miron V. E., Hall J. A., Kennedy T. E., Soliven B., Antel J. P. (2008) Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod. Am. J. Pathol. 173, 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludwin S. K. (1978) Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Lab. Invest. 39, 597–612 [PubMed] [Google Scholar]
- 29. Hiremath M., Saito Y., Knapp G., Ting J.-Y., Suzuki K., Matsushima G. (1998) Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol. 92, 38–49 [DOI] [PubMed] [Google Scholar]
- 30. Mason J., Jones J., Taniike M., Morell P., Suzuki K., Matsushima G. (2000) Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J. Neurosci. Res. 61, 251–262 [DOI] [PubMed] [Google Scholar]
- 31. Morell P., Barrett C. V., Mason J. L., Toews A. D., Hostettler J. D., Knapp G. W., Matsushima G. K. (1998) Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol. Cell. Neurosci. 12, 220–227 [DOI] [PubMed] [Google Scholar]
- 32. Lindner M., Heine S., Haastert K., Garde N., Fokuhl J., Linsmeier F., Grothe C., Baumgartner W., Stangel M. (2008) Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol. Appl. Neurobiol. 34, 105–114 [DOI] [PubMed] [Google Scholar]
- 33. Veto S., Acs P., Bauer J., Lassmann H., Berente Z., Setalo G., Jr., Borgulya G., Sumegi B., Komoly S., Gallyas F., Jr., Illes Z. (2010) Inhibiting poly(ADP-ribose) polymerase: a potential therapy against oligodendrocyte death. Brain 133, 822–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allende M. L., Yamashita T., Proia R. L. (2003) G protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665–3667 [DOI] [PubMed] [Google Scholar]
- 35. Lappe-Siefke C., Goebbels S., Gravel M., Nicksch E., Lee J., Braun P. E., Griffiths I. R., Nave K. A. (2003) Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374 [DOI] [PubMed] [Google Scholar]
- 36. Arnett H. A., Mason J., Marino M., Suzuki K., Matsushima G. K., Ting J. P. (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 4, 1116–1122 [DOI] [PubMed] [Google Scholar]
- 37. Bacia A., Wollmann R., Soliven B. (2004) K+ channel blockade impairs remyelination in the cuprizone model. Glia 48, 156–165 [DOI] [PubMed] [Google Scholar]
- 38. Sidman R. L., Kosaras B., Misra B. M., Senft S. L. (1999) High-resolution mouse brain atlas. http://www.hms.harvard.edu/research/brain
- 39. Harsan L. A., Steibel J., Zaremba A., Agin A., Sapin R., Poulet P., Guignard B., Parizel N., Grucker D., Boehm N., Miller R. H., Ghandour M. S. (2008) Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 28, 14189–14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lindner M., Fokuhl J., Linsmeier F., Trebst C., Stangel M. (2009) Chronic toxic demyelination in the central nervous system leads to axonal damage despite remyelination. Neurosci. Lett. 453, 120–125 [DOI] [PubMed] [Google Scholar]
- 41. Crawford D. K., Mangiardi M., Xia X., Lopez-Valdes H. E., Tiwari-Woodruff S. K. (2009) Functional recovery of callosal axons following demyelination: a critical window. Neuroscience 164, 1407–1421 [DOI] [PubMed] [Google Scholar]
- 42. Woodruff R. H., Fruttiger M., Richardson W. D., Franklin R. J. (2004) Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 25, 252–262 [DOI] [PubMed] [Google Scholar]
- 43. Mason J. L., Ye P., Suzuki K., D'Ercole A. J., Matsushima G. K. (2000) Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J. Neurosci. 20, 5703–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Biancotti J. C., Kumar S., de Vellis J. (2008) Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem. Res. 33, 2615–2628 [DOI] [PubMed] [Google Scholar]
- 45. Komoly S., Hudson L., Webster H., Bondy C. (1992) Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc. Natl. Acad. Sci. U. S. A. 89, 1894–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang D. R., Wang J., Kivisakk P., Rollins B. J., Ransohoff R. M. (2001) Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 193, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tham C. S., Lin F. F., Rao T. S., Yu N., Webb M. (2003) Microglial activation state and lysophospholipid acid receptor expression. Int. J. Dev. Neurosci. 21, 431–443 [DOI] [PubMed] [Google Scholar]
- 48. Miron V. E., Ludwin S. K., Darlington P. J., Jarjour A. A., Soliven B., Kennedy T. E., Antel J. P. (2010) Fingolimod (FTY720) Enhances remyelination following demyelination of organotypic cerebellar slices. Am. J. Pathol. 176, 2682–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee K. D., Chow W. N., Sato-Bigbee C., Graf M. R., Graham R. S., Colello R. J., Young H. F., Mathern B. E. (2009) FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J. Neurotrauma 26, 2335–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Remington L. T., Babcock A. A., Zehntner S. P., Owens T. (2007) Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol. 170, 1713–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hiremath M. M., Chen V. S., Suzuki K., Ting J. P., Matsushima G. K. (2008) MHC class II exacerbates demyelination in vivo independently of T cells. J. Neuroimmunol. 203, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu Y. P., Mizugishi K., Bektas M., Sandhoff R., Proia R. L. (2008) Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum. Mol. Genet. 17, 2257–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choi J. W., Gardell S. E., Herr D. R., Rivera R., Lee C. W., Noguchi K., Teo S. T., Yung Y. C., Lu M., Kennedy G., Chun J. (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosin 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. U. S. A. 108, 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bassi R., Anelli V., Giussani P., Tettamanti G., Viani P., Riboni L. (2006) Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia 53, 621–630 [DOI] [PubMed] [Google Scholar]
- 55. Yamagata K., Tagami M., Torii Y., Takenaga F., Tsumagari S., Itoh S., Yamori Y., Nara Y. (2003) Sphingosine 1-phosphate induces the production of glial cell line-derived neurotrophic factor and cellular proliferation in astrocytes. Glia 41, 199–206 [DOI] [PubMed] [Google Scholar]
- 56. Nayak D., Huo Y., Kwang W. X., Pushparaj P. N., Kumar S. D., Ling E. A., Dheen S. T. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience 166, 132–144 [DOI] [PubMed] [Google Scholar]
- 57. Mason J. L., Suzuki K., Chaplin D. D., Matsushima G. K. (2001) Interleukin-1beta promotes repair of the CNS. J. Neurosci. 21, 7046–7052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sato K., Ishikawa K., Ui M., Okajima F. (1999) Sphingosine 1-phosphate induces expression of early growth response-1 and fibroblast growth factor-2 through mechanism involving extracellular signal-regulated kinase in astroglial cells. Brain Res. Mol. Brain Res. 74, 182–189 [DOI] [PubMed] [Google Scholar]
- 59. Vana A. C., Flint N. C., Harwood N. E., Le T. Q., Fruttiger M., Armstrong R. C. (2007) Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J. Neuropathol. Exp. Neurol. 66, 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coetzee T., Fujita N., Dupree J., Shi R., Blight A., Suzuki K., Popko B. (1996) Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell 86, 209–219 [DOI] [PubMed] [Google Scholar]
- 61. Oo M. L., Thangada S., Wu M. T., Liu C. H., Macdonald T. L., Lynch K. R., Lin C. Y., Hla T. (2007) Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089 [DOI] [PubMed] [Google Scholar]
- 62. Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.