Abstract
Chronic kidney disease (CKD) and several other catabolic conditions are characterized by increased circulating inflammatory cytokines, defects in IGF-1 signaling, abnormal muscle protein metabolism, and progressive muscle atrophy. In these conditions, no reliable treatments successfully block the development of muscle atrophy. In mice with CKD, we found a 2- to 3-fold increase in myostatin expression in muscle. Its pharmacological inhibition by subcutaneous injections of an anti-myostatin peptibody into CKD mice (IC50 ∼1.2 nM) reversed the loss of body weight (≈5–7% increase in body mass) and muscle mass (∼10% increase in muscle mass) and suppressed circulating inflammatory cytokines vs. results from CKD mice injected with PBS. Pharmacological myostatin inhibition also decreased the rate of protein degradation (16.38±1.29%; P<0.05), increased protein synthesis in extensor digitorum longus muscles (13.21±1.09%; P<0.05), markedly enhanced satellite cell function, and improved IGF-1 intracellular signaling. In cultured muscle cells, TNF-α increased myostatin expression via a NF-κB-dependent pathway, whereas muscle cells exposed to myostatin stimulated IL-6 production via p38 MAPK and MEK1 pathways. Because IL-6 stimulates muscle protein breakdown, we conclude that CKD increases myostatin through cytokine-activated pathways, leading to muscle atrophy. Myostatin antagonism might become a therapeutic strategy for improving muscle growth in CKD and other conditions with similar characteristics.—Zhang, L., Rajan, V., Lin, E., Hu, Z., Han, H.Q., Zhou, X., Song, Y., Min, H., Wang, X., Du, J., Mitch, W. E. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease.
Keywords: inflammatory cytokines, muscle protein synthesis, muscle protein degradation, muscle wasting
Catabolic conditions including sepsis, inflammation, diabetes, or chronic kidney disease (CKD) cause muscle atrophy by stimulating proteolysis via caspase-3 and the ubiquitin-proteasome system (UPS) and suppressing both protein synthesis and satellite cell function (1–5). Shared changes in gene expression found in muscles of rats with CKD, diabetes, starvation, or cancer (6) suggest that positive therapeutic interventions in one catabolic condition might apply to other conditions with these characteristics. Because there are few reliable treatments for combating CKD-induced muscle atrophy, we explored a new strategy for blocking muscle atrophy, inhibition of myostatin.
Myostatin, a member of the TGF-β superfamily of secreted proteins, is produced primarily in skeletal muscle and functions as a potent negative regulator of muscle mass (7). A pathophysiological role for myostatin is suggested by reports that myostatin expression in muscle increases in rodents with atrophy from hindlimb unloading, disuse atrophy in elderly patients, the presence of infection, thyroid hormone treatment, or exposure to microgravity (8–11). In addition, myostatin infusion into mice led to a 33% decrease in body weight and a 35–50% decrease in muscle mass, whereas gene deletion or loss-of-function mutations in myostatin are associated with a marked increase in the muscle mass of mice, sheep, cattle, or humans (12–16). There also are reports that inhibition of myostatin improved muscle growth and strength in models of muscular dystrophy (17–19). Finally, preliminary results from a clinical trial of the efficacy of a neutralizing antibody to myostatin, MYO-029, in patients with muscular dystrophies indicate that the treatment had an acceptable safety profile (20). Taken together, these reports suggest that interference with myostatin could be used to prevent muscle atrophy in certain catabolic disorders.
It is not known whether suppression of myostatin improves muscle atrophy in a complex condition such as CKD, which is associated with many metabolic defects, including impaired IGF-1 intracellular signaling, increased glucocorticoid production, resistance to growth hormone, and defects in satellite cell function. In these experiments, we assessed how myostatin might influence CKD-induced defects in muscle metabolism. First, we found that myostatin expression is increased in muscle of mice with CKD. Second, we blocked myostatin function by treating CKD mice with a genetically engineered, myostatin-neutralizing peptide fused to Fc (anti-myostatin peptibody). Muscle wasting was prevented, and there was improvement in intracellular phosphorylated (p)-Akt plus increased muscle protein synthesis and reduced protein degradation. There also was an improvement in satellite cell function. Myostatin inhibition suppressed the levels of circulating inflammatory cytokines including TNF-α and IL-6. In exploring the influence of myostatin on these cytokines, we found that TNF-α stimulates myostatin expression in muscle cells, whereas myostatin stimulates IL-6 production. These responses provide support for the hypothesis that inflammation causes the CKD-induced loss of muscle mass (21). Our results suggest that inhibition of myostatin might reverse muscle atrophy associated with CKD and possibly other catabolic conditions that share characteristics of CKD.
MATERIALS AND METHODS
Reagents
Cardiotoxin was obtained from Calbiochem (La Jolla, CA, USA). Antibodies used against p-Akt (Ser473), p-Smad2 (S465/467), p-FoxO3a (Thr32), p-FoxO1 (Thr24), IKKβ, MEK1, and p38 MAPK were from Cell Signaling Technology (Beverly, MA, USA). Antibodies against MyoD were obtained from Vector Laboratories (Burlingame, CA, USA), antibodies against myogenin and types IIA and IIB major histocompatability (MHC) proteins were from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA), antibodies against myostatin or IL-6 were from Abcam (Cambridge, MA, USA), and antibodies against laminin or type I MHC protein were from Sigma-Aldrich (St. Louis, MO, USA). The ELISA kit for IL-6 and the recombinant proteins of myostatin, IL-6, and TNF-α were obtained from R&D Systems (Minneapolis, MN, USA). From Enzo Life Sciences (Plymouth Meeting, PA, USA), we purchased the inhibitors LY294002 (for PI3K), U0126 (for MEK1), SB203580 (for p38 MAPK), QNZ (for NF-κB), and SP600125 (for JNK). N-Acetylcysteine (antioxidant) was from Sigma-Aldrich. Adenoviruses were used as described previously (22, 23).
Cell culture studies
Mouse C2C12 myoblasts (CRL-1772; American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM (Cellgro Mediatech, Manassas, VA, USA), supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. Myoblasts were cultured until they reached 80–95% confluence. The medium was then changed to DMEM supplemented with 2% horse serum (Sigma-Aldrich) to induce differentiation. Myotubes were incubated in serum-free medium for different periods of time with recombinant TNF-α, myostatin, or IL-6. RT-PCR and Western blotting were used to assess the expression of myostatin, IL-6, or TNF-α. To elucidate the signal transduction pathways involved in TNF-α-induced myostatin or myostatin-induced IL-6 production, myotubes were treated with inhibitors for 1 h before addition of TNF-α or myostatin.
Serum protein analyses
Serum proteins were quantified by a multiplex immunoassay (Rules Based Medicine, Inc., Austin, TX, USA). We examined 58 serum proteins using Rodent Multi-Analyte Profile version 2. The expressions of 49 proteins were analyzed, and 10 cytokine proteins were found to be increased in PBS-treated CKD mice compared with values in CKD mice treated with the anti-myostatin peptibody.
Animal studies
All animal experiments and procedures were approved by the Baylor College of Medicine institutional animal care and use committee. Male 12-wk-old C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME, USA) underwent subtotal nephrectomy in 2 stages. In the first stage, ∼70% of the right kidney was removed, and mice were fed 12% protein to minimize mortality from uremia. Seven days later, the left kidney was removed, and 2 wk later, mice were fed a 40% protein diet (Harlan Teklab, Indianapolis, IN, USA) to induce advanced CKD (24, 25). Sham-treated control mice underwent surgery without damaging the kidneys and were fed the same diets. All mice were housed with 12-h light-dark cycles. CKD mice were paired on the basis of body weights (average, 26.25±0.3 g; mean±se) and blood urea nitrogen (BUN) levels (average 80±3 mg/dl). The anti-myostatin peptibody (IC50∼1.2 nM; Amgen, Thousand Oaks, CA, USA) was injected subcutaneously at 5 mg/kg into one of the paired CKD mice every other day for 7–28 d; the other paired mouse was injected with an equal amount of PBS (control). Body weights and food eaten were assessed daily.
Protein synthesis and degradation
Isolated extensor digitorum longus (EDL) muscles were maintained at resting length and incubated in Krebs-Henseleit bicarbonate buffer with 10 mM glucose as described previously (3, 24, 26). Protein synthesis was measured as the rate of incorporation of l-[14C]phenylalanine and protein degradation as the release of tyrosine. These amino acids were measured because they are neither synthesized nor degraded by muscle, and they rapidly equilibrate with the intracellular amino acid pool in muscle.
Real-time PCR
Gastrocnemius muscle RNA was obtained using TRIzol. cDNAs were synthesized using the first-strand cDNA synthesis kit with oligo(dT)12–18 primers (Invitrogen). Real-time PCR was performed with SYBR Green (Bio-Rad Laboratories, Hercules, CA, USA) using Bio-Rad Real-Time PCR System CFX96 at 94°C for 2 min and 40 cycles at 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s, with a final extension at 72°C for 10 min. The relative mRNA expression levels were calculated from cycle threshold (Ct) values using 18S or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control (relative expression=2(sample Ct−18S Ct)). Primer sequences for RT-PCR are listed in Table 1.
Table 1.
RT-PCR primer sequences
| mRNA | RT-PCR primers |
|
|---|---|---|
| Forward | Reverse | |
| IL6 | 5′-GAGGATACCACTCCCAACAGACC-3′ | 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| TNF-α | 5′-CATGAGCACAGAAAGCATGATCCG-3′ | 5′-AAGCAGGAATGAGAAGAGGCTGAG-3′ |
| Myostatin | 5′-TGGCATTACTCAAAAGCAAAAAG-3′ | 5′-CATCAATACTCTGCCAAATACCA-3′ |
| TGF-β1 | 5′-TACCATGCCAACTTCTGTCTGGGA-3′ | 5′-TGTGTTGGTTGTAGAGGGCAAGGA-3′ |
| F4/80 | 5′-AAGCATCCGAGACACACACAGTCT-3′ | 5′-TGACTGTACCCACATGGCTGATGA-3′ |
| Myogenin | 5′-ACAGCATCACGGTGGAGGATATGT-3′ | 5′-CCCTGCTACAGAAGTGATGGCTTT-3′ |
| MyoD | 5′-ACGACTGCTTTCTTCACCACTCCT-3′ | 5′-TCGTCTTAACTTTCTGCCACTCCG-3′ |
| GAPDH | 5′-ACCACCATGGAGAAGGCCGG-3′ | 5′-CTCAGTGTAGCCCAAGATGC-3′ |
| 18S | 5′-GAAACGGCTACCACATCCAAGG-3′ | 5′-GTCCCTCTTAATCATGGCCTCAG-3′ |
In vivo satellite cell function
To evaluate satellite cell function, we used a standard muscle injury model, injection of cardiotoxin (80 μl of 10 μM) into tibiae anterior (TA) muscles of anti-myostatin peptibody or PBS-treated CKD mice (25). Contralateral muscles were injected with the same volume of PBS, serving as controls. Muscles were collected after 3, 7, 14, and 28 d of injury and frozen in liquid nitrogen or O.C.T. medium (Fisher Scientific, Pittsburgh, PA. USA) and then were kept at −80°C.
Imaging of muscle sections and analyses
Images were analyzed using a Nikon 80i microscope (Nikon, Melville, NY, USA); myofiber sizes were measured using NIS-Elements Br 3.0 software (Nikon) after immunostaining with anti-laminin. The distribution of myofiber sizes was calculated on the basis of analysis of 2000 myofibers.
Statistical analysis
Values are presented as means ± se, and results were analyzed using Student's t test when results from 2 experimental groups were compared or using ANOVA when data from ≥3 groups were studied. For ANOVA analyses, pairwise comparisons were made by the Student-Newman-Keuls test.
RESULTS
Myostatin expression is up-regulated in skeletal muscle of CKD mice
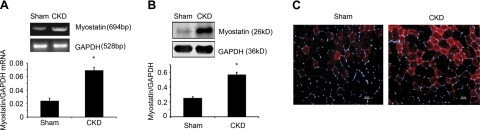
In CKD and sham-operated control mice, serum myostatin concentrations were not statistically different. Myostatin mRNA, however, was 2- to 3-fold greater in muscles of CKD mice vs. levels in muscles of control mice (Fig. 1A). An increase in muscle myostatin was confirmed by Western blots and immunostaining of myostatin protein (Fig. 1B, C). Thus, CKD induces myostatin expression in muscles; myostatin was not significantly changed in serum of CKD mice.
Figure 1.
CKD stimulates myostatin expression in muscle. A) Myostatin mRNA in muscles of CKD mice was significantly higher vs. results in control mice (n=6/group). B) Top panel: representative Western blots of myostatin protein in gastrocnemius muscles of CKD or control mice. Bottom panel: quantification (n=6/group). C) Sections of TA muscles from control and CKD mice were immunostained with an anti-myostatin (red) and DAPI (blue). Scale bars = 20 μm. *P < 0.05.
Myostatin inhibition blocks CKD-induced muscle atrophy
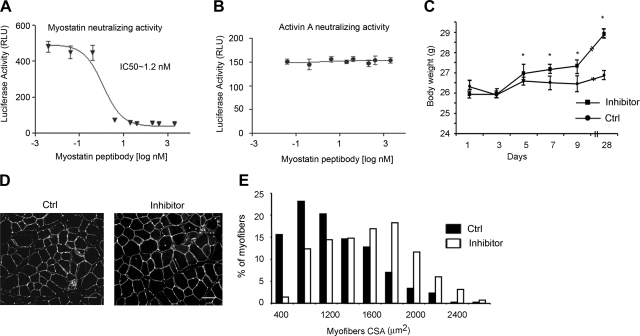
Because muscle growth in genetic models of muscle dystrophy increases when myostatin is inhibited (18), we tested whether injections of the anti-myostatin peptibody would block muscle atrophy induced by a complex metabolic disorder such as CKD. Results of peptibody-treated CKD mice were compared with those from PBS-injected mice that were paired for initial body weights and BUN levels. The peptibody only suppresses myostatin activity rather than activin A (Fig. 2A, B). Of note, after 5 d of peptibody treatment, the body weights of CKD mice increased significantly compared with values in PBS-treated CKD mice, a trend that persisted over 28 d (Fig. 2C). After 7 d of treatment, muscle weights in these mice were significantly greater than those in PBS-treated CKD mice (Table 2). Muscle weights corrected for body weight were also increased, and these responses persisted for 28 d (data not shown). Because food intake was not different, the peptibody treatment also improved the efficiency of food utilization, promoting the growth of skeletal muscle. We also measured myofiber sizes in TA muscles (Fig. 2D) and found a significant (P<0.01) increase in the average cross-sectional areas of myofibers in peptibody-treated (2100±200 μm2) compared with PBS-treated CKD mice (1500±130 μm2). There was a right shift in the myofiber distribution in peptibody vs. PBS treatment (Fig. 2E), confirming that these myofibers were larger.
Figure 2.
Inhibition of myostatin increases body weight and reduces muscle atrophy in CKD mice. A, B) Inhibitory activity of the anti-myostatin peptibody against either myostatin (A) or activin A (B) was measured using a cell-based reporter assay (n=3) as described previously (27). C) CKD mice treated with the peptibody or PBS (n=25/group) had body weights measured repeatedly over 28 d; peptibody-treated CKD mice gained more weight. *P < 0.05. D) Cryosections of TA muscles were immunostained with anti-laminin and DAPI to assess myofiber sizes. Scale bars = 50 μm. E) Distribution of myofiber sizes was shifted to the right (larger size) in peptibody-treated CKD mice (n=3 mice/group; 600 myofibers/muscle measured). RLU, relative light units; Ctrl, control; CSA, cross-sectional area.
Table 2.
Muscle weight after 7 d of anti-myostatin peptibody treatment
| Treatment | Muscle weight (mg) |
|||
|---|---|---|---|---|
| TA | Gastrocnemius | EDL | Soleus | |
| Inhibitor | 61 ± 1.57 | 174.20 ± 5.39 | 13.75 ± 0.78 | 11.08 ± 0.77 |
| PBS | 56 ± 2.37 | 157.80 ± 2.88 | 11.67 ± 0.17 | 9.33 ± 1.01 |
| P value | 0.037* | 0.027* | 0.038* | 0.016* |
Values are means ± se.
P < 0.05.
In addition to controlling muscle mass, myostatin could regulate muscle fiber-type composition postnatally (28, 29). To determine whether there is a change in the fiber type distribution associated with the anti-myostatin peptibody, we treated CKD mice for 2 wk with the peptibody before obtaining cryosections of TA muscles and immunostaining them for types 1, IIA, and IIB MHC antibodies as described previously (2). In comparison with results from CKD mice treated with PBS, there was a slight decrease in the percentage of type I and type IIA fiber plus an increase in type IIB fiber in TA muscles of peptibody-treated CKD mice; these differences did not reach statistical significance (Table 3). We conclude that myostatin inhibition does not significantly induce change in muscle fiber types. These results differ from those obtained from a myostatin knockout mouse (29) but are consistent with a report in which mice were treated with a myostatin-neutralizing antibody (28). Such differences in responses could be related to a longer duration of myostatin inhibition in the knockout mice.
Table 3.
Fiber type composition of TA muscle of anti-myostatin peptibody-treated or PBS-treated CKD mice
| Treatment | Fiber type (%) |
||
|---|---|---|---|
| I | IIA | IIB | |
| Inhibitor | 2.13 ± 1.04 | 7.16 ± 1.77 | 90.69 ± 2.47 |
| PBS | 2.43 ± 1.09 | 9.47 ± 1.34 | 88.09 ± 1.01 |
| P value | 0.449 | 0.245 | 0.261 |
Values are means ± se.
Myostatin inhibition improves muscle protein metabolism in CKD mice
CKD induces muscle atrophy by impairing both protein synthesis and degradation (2, 30). We examined how myostatin inhibition increases muscle mass by measuring muscle protein synthesis and degradation. After 7 d of peptibody administration, there was a significant improvement in muscle protein synthesis (13.21±1.09%, P<0.05, n=9) and a decrease in protein degradation (16.38±1.29%, P<0.05, n=9) compared with results from CKD mice treated with PBS (Table 4). These responses could contribute to the increase in muscle mass caused by the peptibody treatment (Table 2 and Fig. 2D, E).
Table 4.
Protein synthesis and degradation measured in EDL muscle after treatment of CKD mice with anti-myostatin peptibody or PBS for 7 d
| Treatment | Synthesis (nmol Phe/g/h) | Degradation (nmol Tyr/g/h) |
|---|---|---|
| PBS | 53 ± 4.32 | 147.7 ± 8.88 |
| Inhibitor | 60 ± 5.51 | 123.5 ± 5.33 |
| P value | 0.0307 | 0.0233 |
Values are means ± se; n= 9 mice/group.
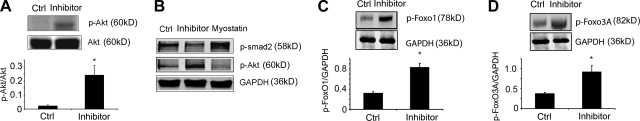
Potential mechanisms for the improvement in muscle protein metabolism were found by assessing changes in Akt phosphorylation as an index of IGF-1 intracellular signaling. Administration of the peptibody significantly increased p-Akt levels in muscle compared with results from PBS-treated CKD mice (Fig. 3A). To test whether this was a direct or indirect response, we treated C2C12 myotubes with recombinant myostatin and found that p-Akt decreased. In contrast, the peptibody increased the p-Akt level in cultured muscle cells (Fig. 3B). The relevance of an increase in p-Akt is that it would stimulate the phosphorylation of forkhead transcription factors (FoxO) and prevent their translocation into nuclei (31–33). This, in turn, could decrease expression of the E3 ubiquitin ligases, atrogin-1/MAFbx or MuRF1, and suppress muscle protein degradation (31, 34). Administration of the peptibody to CKD mice for 7 d did increase the phosphorylation of FoxO3A and FoxO1 coincident with suppression of protein degradation in muscle (Fig. 3C, D; Table 4). Peptibody treatment also reduced the muscle mRNA levels of atrogin-1 and MuRF1, but these differences were not statistically significant.
Figure 3.
Myostatin inhibition increased insulin/IGF-1 intracellular signaling pathway in muscle. A) Top panel: representative Western blot of p-Akt in muscle. Bottom panel: quantification of p-Akt/Akt ratio (n=7/group). B) Recombinant myostatin decreased Akt hosphorylation in C2C12 myotubes, and the anti-myostatin peptibody increased p-Akt. Western blot of p-Smad2 was used to document myostatin activity (n=3 repeated experiments). C, D) Myostatin inhibition increased FoxO1 (C) and FoxO3A (D) phosphorylation (n=7/group). Ctrl, control. *P < 0.05.
Myostatin inhibition improves satellite cell function in CKD mice
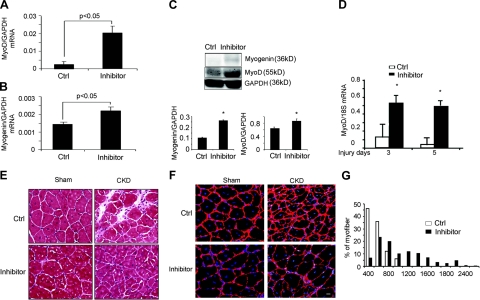
In muscles of CKD mice treated with the peptibody, there was an increase in the number of myofibers with central nuclei, implying that the peptibody had activated satellite cells (Fig. 2D). This result was unexpected because CKD suppresses satellite cell function (25). Therefore, we examined whether satellite cells were activated by measuring the mRNA and protein levels of MyoD and myogenin (markers of satellite cell proliferation and differentiation, respectively). In peptibody-treated vs. PBS-treated CKD mice, both markers were significantly increased after 7 d of peptibody treatment (Fig. 4A–C).
Figure 4.
Myostatin inhibition improves satellite cell function. A, B) The mRNAs of MyoD (A) and myogenin (B) in gastrocnemius muscles of CKD mice were increased by the peptibody (n=9/group). C) Top panel: representative Western blots of MyoD and myogenin proteins in muscles of CKD mice treated with the anti-myostatin peptibody (inhibitor) or PBS (Ctrl). Bottom panel: ratio of MyoD or myogenin proteins to GAPDH (n=9). D) MyoD mRNA expression was sharply increased in muscles of anti-myostatin peptibody-treated CKD mice after muscle injury at 3 or 5 d compared with injured muscle of CKD mice-treated with PBS (n=4). E) Improved myofiber formation in anti-myostatin peptibody-treated sham or CKD mice 14 d after TA muscle injury was detected by hematoxylin and eosin staining. Newly formed myofibers (recognized by central nuclei) were larger in peptibody-treated control mice. There was less interstitial space with inflammatory cells in peptibody-treated CKD mice compared with results in PBS-treated sham or CKD (Ctrl) mice. F) Cryosections of TA muscles (from inhibitor- or PBS-treated CKD mice at 1 mo after injury) were immunostained with anti-laminin (red) and DAPI (blue) to assess myofiber sizes. G) In CKD mice treated with the myostatin inhibitor, sizes of newly formed myofibers were shifted to the right vs. results from PBS-treated (Ctrl) CKD mice (n=4 mice/group; 300 myofibers/muscle measured). Scale bars = 20 μm. *P < 0.05.
Peptibody treatment also influenced satellite cell function in vivo. Using a standard, cardiotoxin model of muscle injury (25, 35), we found that mice with CKD treated with the peptibody had a significant increase in the mRNA of MyoD at 3 or 5 d after muscle injury (Fig. 4D). At 14 d after muscle injury, the sizes of newly formed myofibers containing central nuclei were greater in peptibody-treated mice vs. PBS-treated mice. The recovery of injured myofibers in peptibody-treated CKD mice improved compared with responses in PBS-treated CKD mice; with the peptibody, there were fewer infiltrating cells and a decrease in the interstitial space of muscle (Fig. 4E). One month after injury, there was a 3-fold increase in the average cross-sectional areas of newly formed myofibers (1500±25 μm2; peptibody-treated) compared with PBS-treated, CKD mice (500±33 μm2; n=3, P<0.05; Fig. 4F). There also was a rightward shift in the size distribution of newly formed myofibers (Fig. 4G).
Myostatin inhibition suppresses inflammation in CKD mice
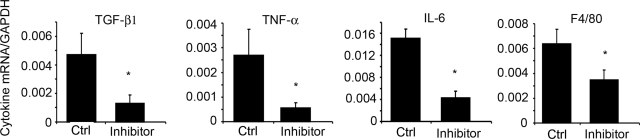
Patients with CKD frequently have increased levels of circulating inflammatory markers and inflammation can interfere with insulin/IGF-1 signaling (21, 36). In CKD mice, we found that circulating levels of 10 cytokines, including TNF-α, IL6, INF-γ, and macrophage colony-stimulating factor-1, were increased compared with results from control mice (Table 5). Peptibody treatment for 7 d decreased circulating levels of these cytokines, suggesting that myostatin inhibition blocks CKD-induced systemic inflammation. We also examined mRNAs in muscle by quantitative RT-PCR and found that peptibody treatment caused a 45–79% decrease in the gene expression of TNF-α, IL-6, F4/80, and TGF-β compared with values in PBS-treated CKD mice (Fig. 5).
Table 5.
Increased inflammatory cytokines induced by CKD are suppressed by treatment with the anti-myostatin peptibody
| Cytokine | Treatment |
P value |
|||
|---|---|---|---|---|---|
| Sham | CKD-PBS | CKD-peptibody | CKD vs. sham | CKD-peptibody vs. CDK-PBS | |
| CD40 (pg/ml) | 101.5 ± 33.70 | 204.5 ± 74.30 | 181 ± 9.59 | 0.045* | 0.554 |
| Fibrinogen (μg/ml) | 156.75 ± 34.87 | 2877.5 ± 1007.68 | 323.25 ± 306.50 | 0.0016* | 0.003* |
| KC (ng/ml) | 0.065 ± 0.03 | 0.086 ± 0.04 | 0.081 ± 0.03 | 0.407 | 0.849 |
| IFN-γ (pg/ml) | 16.15 ± 5.04 | 17.55 ± 2.58 | 12.57 ± 2.66 | 0.638 | 0.036* |
| IL-7 (ng/ml) | 0.26 ± 0.10 | 0.37 ± 0.17 | 0.26 ± 0.10 | 0.322 | 0.314 |
| M-CSF-1 (ng/ml) | 7.31 ± 2.51 | 11.61 ± 2.08 | 7.48 ± 1.0 | 0.039* | 0.012* |
| MIP-1β (pg/ml) | 224 ± 58.76 | 255.5 ± 41.4 | 240.75 ± 73.56 | 0.414 | 0.739 |
| MPO (ng/ml) | 135.9 ± 53.89 | 208.5 ± 135.66 | 168.5 ± 38.79 | 0.358 | 0.591 |
| TNF-α (ng/ml) | 0.1 ± 0.06 | 0.151 ± 0.03 | 0.075 ± 0.04 | 0.189 | 0.033* |
| IL-6 (pg/ml) | 5.8 ± 0.48 | 10.48 ± 2.23 | 3.05 ± 0.73 | 0.041* | 0.036* |
Values are means ± se. M-CSF-1, macrophage colony-stimulating factor-1; MIP-1β, macrophage inflammatory protein-1β; MPO, myeloperoxidase.
P < 0.05.
Figure 5.
Myostatin inhibition decreases the mRNAs of inflammatory cytokines in muscles of CKD mice. Gastrocnemius muscles from CKD mice obtained 7 d after treatment with the peptibody or PBS (Ctrl) were obtained, and cytokine mRNAs were measured by RT-PCR using GAPDH as the internal control (n=3/group). *P < 0.05.
Interactions between myostatin and cytokines
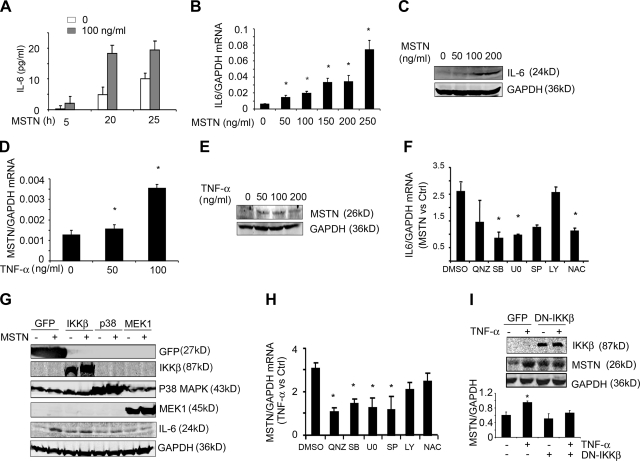
To assess how myostatin and cytokines interact, we incubated C2C12 myotubes without or with recombinant myostatin for 5 h. The presence of myostatin caused a significant release of IL-6 into the cell culture medium; the pattern persisted over 25 h (Fig. 6A). Myostatin increased IL-6 mRNA and protein in C2C12 myotubes in a dose-dependent fashion (Fig. 6B, C). Of note, TNF-α did not significantly change in cells exposed to myostatin. However, when C2C12 myotubes were incubated with TNF-α, both myostatin mRNA and protein significantly increased (Fig. 6D, E). In contrast, myostatin did not increase in C2C12 myotubes exposed to IL-6.
Figure 6.
Inflammatory cytokines interact with myostatin. A) C2C12 myotubes were treated with myostatin (MSTN) for 5, 20, or 25 h, and the IL-6 concentration in the culture medium was measured by ELISA (n=3 separate experiments). B) C2C12 myotubes were treated with different concentrations of myostatin for 24 h; IL-6 mRNA was determined by RT-PCR (n=3 separate experiments). C) C2C12 myotubes were treated with different concentrations of myostatin for 48 h, and IL-6 expression was examined by Western blot (n=3 repeats). D) C2C12 myotubes were treated with different concentrations of TNF-α for 24 h, and myostatin mRNA was examined by RT-PCR (n=3 separate experiments). E) C2C12 myotubes were treated with different concentrations of TNF-α for 48 h, and myostatin protein was assessed by Western blot (n=3 repeats). F) C2C12 myotubes were preincubated for 1 h with inhibitors of different signaling pathways before myostatin (200 ng/ml) was added to the medium. After 24 h, the IL-6 mRNA was measured by RT-PCR, using GAPDH as the internal control. Ratio of IL-6 mRNA in cells treated with myostatin compared with the IL-6 mRNA found in cells not treated with myostatin is shown (n=3 independent experiments). Inhibitors were SB203580 (SB; for p38 MAPK), U0126 (U0; for MEK1), SP600125 (SP; for JNK), LY294002(LY; for PI3K), QNZ (for NF-κB), and NAC (antioxidant). G) C2C12 myotubes were infected with an adenovirus expressing GFP or the DN-p38MAPK, DN-IKKβ, or DN-MEK1 for 24 h before addition of myostatin. After 48 h, the expressions of GFP, IKKβ, p38 MAPK, MEK1, IL-6, or GAPDH were examined by Western blot (n=3 repeated experiments). H) C2C12 myotubes were preincubated for 1 h with different inhibitors before TNF-α (100 ng/ml) was added. After 24 h, myostatin mRNA was measured by RT-PCR; columns represent fold change induced by TNF-α plus the inhibitor vs. results with the inhibitor only (n=3 independent experiments). I) C2C12 myotubes infected with an adenovirus expressing DN-IKKβ for 24 h before addition of TNF-α. After 48 h, the expressions of myostatin, IKKβ, and GAPDH were examined by Western blot. Ratios of density of myostatin to GAPDH are shown (bottom panel; n=3). *P < 0.05.
To identify the signaling pathway by which myostatin stimulated IL-6 in muscle cells, we treated myotubes with selective inhibitors of different signaling pathways and 1 h later added myostatin. Analysis by RT-PCR indicated that SB203580 or U0126 significantly suppressed the myostatin-induced increase in IL-6 mRNA (Fig. 6F). N-Acetylcysteine, QNZ, or SP600125 partially inhibited the myostatin-induced increase in IL-6, but LY294002 did not interfere with myostatin-stimulated IL-6 mRNA. To verify these results with more specific inhibitors, we infected C2C12 myotubes with adenoviruses expressing dominant-negative (DN) p38 MAPK or MEK1 or IKKβ and then added myostatin. As shown in Fig. 6G, expression of mutant p38 MAPK partially suppressed myostatin-stimulated IL-6 mRNA. Expression of the mutant MEK1, however, completely blocked myostatin-induced IL-6 expression. These results implicate MEK1/2 and p38 MAPK as important regulators of myostatin-induced IL-6 production in muscle cells.
We also explored the signaling pathway by which TNF-α stimulates myostatin expression. With the same approaches we used to study the myostatin-IL-6 interaction, myotubes were incubated in the presence or absence of selective inhibitors and then stimulated by TNF-α. Inhibition of NF-κB blocked TNF-α-stimulated myostatin expression, whereas p38 MAPK, JNK, or MEK inhibitors only partially blocked it, and PI3K or reactive oxygen species inhibitors did not impair TNF-α-stimulated myostatin expression (Fig. 6H). To verify that the NF-κB pathway is responsible for TNF-α-stimulated myostatin, we infected myotubes with an adenovirus expressing mutant IKKβ. The ability of TNF-α to increase myostatin expression in muscle cells was blocked (Fig. 6I). We note that myostatin expression was increased in muscle cells infected with the DN IKKβ adenovirus compared with cells infected with the control GFP adenovirus. This observation might reflect responses to IKKβ. Regardless, TNF-α did not stimulate myostatin expression in cells infected with the DN IKKβ.
DISCUSSION
Muscle wasting is a well-established complication of CKD, and like certain other catabolic conditions, it is characterized by defects in insulin/IGF-1 signaling in muscle, increased circulating cytokines, increased glucocorticoid production, and changes in muscle protein metabolism (4, 25, 37, 38). Our goal was to identify a method of preventing CKD-induced muscle atrophy. Our results demonstrate that subcutaneous injections of the anti-myostatin peptibody blocks muscle atrophy. We also demonstrated that the improvement in muscle mass was due to an increase in muscle protein synthesis and suppression of protein degradation in muscle, and we uncovered novel interactions among myostatin and the inflammatory cytokines, TNF-α and IL-6. These interactions provide new insights into mechanisms of muscle wasting. In particular, we found that TNF-α triggers myostatin production in muscle, which stimulates IL-6 expression and its release. This result is relevant because we have found that IL-6 impairs insulin/IGF-1 signaling and inhibition of this pathway will decrease muscle protein synthesis and increase protein degradation (2, 5).
Evidence that myostatin acts as a key mediator of muscle atrophy in CKD includes the finding that myostatin mRNA and protein expression are increased in muscles of CKD mice plus the finding that abnormalities in muscle protein turnover and satellite cell function due to CKD were blocked by subcutaneous injections of the anti-myostatin peptibody. The importance of myostatin was further established when the losses of body weight and muscle mass were blocked by the peptibody despite the presence of CKD (Fig. 2 and Table 2). One mechanism for these positive responses is the increase in muscle p-Akt in CKD mice treated with the peptibody because it could stimulate muscle protein synthesis and suppress protein degradation while improving satellite cell activation (Figs. 3 and 4 and Table 4; refs. 2, 5, 25).
How could myostatin inhibition block muscle atrophy in this complex metabolic disorder? One possibility is that CKD increases circulating cytokines, a response that has been epidemiologically closely associated with the loss of protein stores in patients with kidney disease (21). In CKD mice, circulating levels of cytokines (including TNF-α and IL-6) were increased, and, interestingly, both cytokines were suppressed by peptibody treatment (Table 5 and Fig. 5). There is precedence for a link between myostatin and circulating inflammatory cytokines. When myostatin-knockout mice were fed a fed a high-fat diet, the circulating TNF-α and IL-6 levels were lower than those in wild-type mice fed the same diet (39). We determined that myostatin interacts with inflammatory cytokines in muscle cells. C2C12 myotubes treated with TNF-α significantly increased myostatin expression, but exposure of muscle cells to IL-6 did not change myostatin expression. Second, we treated myotubes with myostatin and found a significant increase in IL-6 mRNA and protein plus release of IL-6 from muscle cells (Fig. 6). These IL-6 responses are relevant because we have shown that an increase in IL-6 stimulates SOCS3 activity, leading to degradation of IRS-1 and suppression of p-Akt and p-FoxO, which results in stimulation of muscle proteolysis (2).
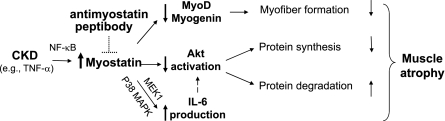
The mechanisms by which TNF-α increases myostatin in muscle involve a NF-κB-dependent pathway. This result is consistent with the report that there is a NF-κB consensus sequence in the myostatin promoter (40). However, the pathway by which myostatin stimulates IL-6 involves MEK and p38 MAPK (Fig. 6) and, hence, is similar to the report that myostatin induces growth inhibition via a p38 MAPK pathway in muscle cells (41). These relationships provide new insights into mechanisms by which inflammatory cytokines are postulated to cause loss of muscle mass in CKD (ref. 21 and Fig. 7).
Figure 7.
Proposed scheme by which the CKD-induced increase in TNF-α stimulates myostatin in muscle, leading to suppression of satellite cell function and p-Akt while raising IL-6 production. The increase in IL-6 potentiates the decrease in Akt activation, resulting in a decrease in muscle protein synthesis and an increase in protein degradation. The anti-myostatin peptibody blocks these pathways to prevent muscle atrophy.
Mechanisms by which myostatin influences muscle mass have been investigated in myostatin knockout mice (42). In these mice, muscle protein synthesis increased by a fraction similar to the change we measured in CKD mice treated with the anti-myostatin peptibody (Table 4). The authors also concluded that myostatin gene knockout did not change muscle protein degradation. In contrast, we found that inhibition of myostatin with the peptibody blunted the muscle proteolysis of CKD (Table 4). The difference in these conclusions could reflect the fact that Welle et al. (42) assessed protein half-lives and did not measure protein degradation. In a recent study, Zhou et al. (27) reported that administering a decoy for the activin receptor IIB (sActRIIB) suppressed muscle wasting, increased grip strength, and prolonged survival in mouse models of cancer cachexia. In that report, the mechanisms of muscle protein wasting, protein synthesis, and degradation were not characterized. In addition, the relative contribution to an inhibition of myostatin was not defined because sActRIIB administration will also block activin A and other ligands. The present results only depend on inhibition of myostatin (Fig. 2A, B).
How could myostatin inhibition improve defects in muscle protein metabolism that are stimulated by a catabolic condition? One possibility is that myostatin inhibition improved insulin/IGF-1 intracellular signaling, leading to an increase in Akt phosphorylation (5). As expected from studies of mouse models of diabetes or cancer (27, 39), there was a close relationship between myostatin and p-Akt: it decreased when myotubes were exposed to myostatin but myostatin inhibition increased p-Akt (Fig. 3A, B). An increase in p-Akt is relevant because it will improve muscle protein synthesis (Table 4) by raising muscle levels of S6K and the mammalian target of rapamycin (5). A higher p-Akt will also prevent muscle protein degradation by increasing forkhead phosphorylation, which suppresses the expression of the E3 ubiquitin ligases, atrogin-1/MAFbx and MuRF1, leading to reduced proteolysis in the UPS (Fig. 3C, D; refs 5, 34). Treatment with the peptibody did lower atrogin-1/MAFbx or MuRF-1 mRNAs expression in muscles of CKD mice, but the difference was not statistically significant possibly because of variability in the degrees of CKD and/or in vivo responses to the anti-myostatin peptibody. As an alternative, it may represent a contribution of another proteolytic activity (5).
Myostatin inhibition could also prevent muscle atrophy by improving the function of skeletal muscle progenitor or satellite cells (43, 44). We have found that CKD reduces expression of the myogenic genes, MyoD and myogenin, and impairs satellite cell functions in vivo (25). Both abnormalities were largely corrected by treating CKD mice with the anti-myostatin peptibody (Fig. 4). Therefore, inhibiting myostatin in CKD improves satellite cell function, consistent with previous reports (44).
Myostatin inhibition has elicited beneficial responses in models of muscular dystrophies (45). However, myostatin inhibition did not correct severe spinal muscular atrophy (46), and there was no improvement in muscle strength or function in the clinical trial of MYO-029 in patients with muscular dystrophies (20). Whether the variability in responses obtained in these studies was due to the types of muscle disease or an inability to inhibit myostatin more completely is not known. Our results show that myostatin inhibition can suppress inflammation and ameliorate the muscle wasting in a catabolic condition that is characterized by inflammation, impaired IGF-1 intracellular signaling, and excess glucocorticoids. These results are encouraging because they suggest a strategy that could prove to be beneficial for treating patients with CKD. It is also possible that such a strategy might prevent muscle atrophy arising from other catabolic conditions that are characterized by increased inflammatory cytokines, defects in insulin/IGF-1 signaling, and muscle protein metabolism.
Acknowledgments
The authors thank Dr. Huiling Wang and Limei Ran for their assistance with care of mice with CKD.
This study was sponsored by Satellite Healthcare (L.Z.), the American Diabetes Association (1-11-BS-194; L.Z.), the National Institutes of Health (grants R37-DK37175, R01-DK62828, and P50-DK64233), and a grant from Dr. and Mrs. Harold Selzman. Portions of these experiments were supported by a grant from Amgen Inc. L.Z., V.R., E.L., Z.H., X.W., J.D., and W.E.M declare no conflicts of interest. H.Q.H., X.Z., Y.S., and H.M. are current or former employees of Amgen Inc.
REFERENCES
- 1. Hasselgren P.-O., Warner B. W., James H., Takehara H., Fischer J. E. (1987) Effect of insulin on amino acid uptake and protein turnover in skeletal muscle from septic rats: evidence for insulin resistance of protein degradation. Arch. Surg. 122, 228–233 [DOI] [PubMed] [Google Scholar]
- 2. Zhang L., Du J., Hu Z., Han G., Delafontaine P., Garcia G., Mitch W. E. (2009) IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 20, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Z., Wang H., Lee I. H., Du J., Mitch W. E. (2009) Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Invest. 119, 7650–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Du J., Wang X., Meireles C. L., Bailey J. L., Debigare R., Zheng B., Price S. R., Mitch W. E. (2004) Activation of caspase 3 is an initial step triggering muscle proteolysis in catabolic conditions. J. Clin. Invest. 113, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lecker S. H., Goldberg A. L., Mitch W. E. (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 17, 1807–1819 [DOI] [PubMed] [Google Scholar]
- 6. Lecker S. H., Jagoe R. T., Gomes M., Baracos V., Bailey J. L., Price S. R., Mitch W. E., Goldberg A. L. (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 [DOI] [PubMed] [Google Scholar]
- 7. Lee S. J. (2004) Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61–86 [DOI] [PubMed] [Google Scholar]
- 8. Carlson C. J., Booth F. W., Gordon S. E. (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am. J. Physiol. 277, R601–R606 [DOI] [PubMed] [Google Scholar]
- 9. Zachwieja J. J., Smith S. R., Sinha-Hikim I., Gonzalez-Cadavid N., Bhasin S. (1999) Plasma myostatin-immunoreactive protein is increased after prolonged bed rest with low-dose T3 administration. J. Gravit. Physiol. 6, 11–15 [PubMed] [Google Scholar]
- 10. Gonzalez-Cadavid N. F., Taylor W. E., Yarasheski K., Sinha-Hikim I., Ma K., Ezzat S., Shen R., Lalani R., Asa S., Mamita M., Nair G., Arver S., Bhasin S. (1998) Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc. Natl. Acad. Sci. U. S. A. 95, 14938–14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lalani R., Bhasin S., Byhower F., Tarnuzzer R., Grant M., Shen R., Asa S., Ezzat S., Gonzalez-Cadavid N. F. (2000) Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 167, 417–428 [DOI] [PubMed] [Google Scholar]
- 12. Zimmers T. A., Davies M. V., Koniaris L. G., Haynes P., Esquela A. F., Tomkinson K. N., McPherron A. C., Wolfman N. M., Lee S.-J. (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296, 1486–1488 [DOI] [PubMed] [Google Scholar]
- 13. McPherron A. C., Lawler A. M., Lee S. J. (1997) Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 [DOI] [PubMed] [Google Scholar]
- 14. Clop A., Marcq F., Takeda H., Pirottin D., Tordoir X., Bibe B., Bouix J., Caiment F., Elsen J. M., Eychenne F., Larzul C., Laville E., Meish F., Milenkovic D., Tobin J., Charlier C., Georges M. (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 38, 813–818 [DOI] [PubMed] [Google Scholar]
- 15. Grobet L., Martin L. J., Poncelet D., Pirottin D., Brouwers B., Riquet J., Schoeberlein A., Dunner S., Menissier F., Massabanda J., Fries R., Hanset R., Georges M. (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 17, 71–74 [DOI] [PubMed] [Google Scholar]
- 16. Schuelke M., Wagner K. R., Stolz L. E., Hubner C., Riebel T., Komen W., Braun T., Tobin J. F., Lee S. J. (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N. Engl. J. Med. 350, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 17. Wagner K. R., McPherron A. C., Winik N., Lee S. J. (2002) Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann. Neurol. 52, 832–836 [DOI] [PubMed] [Google Scholar]
- 18. Bogdanovich S., Krag T. O., Barton E. R., Morris L. D., Whittemore L. A., Ahima R. S., Khurana T. S. (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature 420, 418–421 [DOI] [PubMed] [Google Scholar]
- 19. Bogdanovich S., Perkins K. J., Krag T. O., Whittemore L. A., Khurana T. S. (2005) Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 19, 543–549 [DOI] [PubMed] [Google Scholar]
- 20. Wagner K. R., Fleckenstein J. L., Amato A. A., Barohn R. J., Bushby K., Escolar D. M., Flanigan K. M., Pestronk A., Tawil R., Wolfe G. I., Eagle M., Florence J. M., King W. M., Pandya S., Straub V., Juneau P., Meyers K., Csimma C., Araujo T., Allen R., Parsons S. A., Wozney J. M., Lavallie E. R., Mendell J. R. (2008) A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann. Neurol. 63, 561–571 [DOI] [PubMed] [Google Scholar]
- 21. Stenvinkel P., Ketteler M., Johnson R. J., Lindholm B., Pecoits-Filho R., Riella M., Heimburger O., Cederholm T., Girndt M. (2005) IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 67, 1216–1233 [DOI] [PubMed] [Google Scholar]
- 22. Zhang L., Cui R., Cheng X., Du J. (2005) Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by a novel mechanism activating IkB kinase. Cancer Res. 65, 457–464 [PubMed] [Google Scholar]
- 23. Zhang L., Cheng J., Ma Y., Thomas W., Zhang J., Du J. (2005) Dual pathways for nuclear factor κB activation by angiotensin II in vascular smooth muscle: phosphorylation of p65 by IκB kinase and ribosomal kinase. Circ. Res. 97, 975–982 [DOI] [PubMed] [Google Scholar]
- 24. May R. C., Kelly R. A., Mitch W. E. (1987) Mechanisms for defects in muscle protein metabolism in rats with chronic uremia: the influence of metabolic acidosis. J. Clin. Invest. 79, 1099–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang L., Wang X. H., Wang H., Hu Z., Du J., Mitch W. E. (2010) Satellite cell dysfunction and impaired IGF-1 signaling contribute to muscle atrophy in chronic kidney disease. J. Am. Soc. Nephrol. 21, 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey J. L., Wang X., England B. K., Price S. R., Ding X., Mitch W. E. (1996) The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent, ubiquitin-proteasome pathway. J. Clin. Invest. 97, 1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou X., Wang J. L., Lu J., Song Y., Kwak K. S., Jiao Q., Rosenfeld R., Chen Q., Boone T., Simonet W. S., Lacey D. L., Goldberg A. L., Han H. Q. (2010) Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 [DOI] [PubMed] [Google Scholar]
- 28. Girgenrath S., Song K., Whittemore L. A. (2005) Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31, 34–40 [DOI] [PubMed] [Google Scholar]
- 29. McFarlane C., Plummer E., Thomas M., Hennebry A., Ashby M., Ling N., Smith H., Sharma M., Kambadur R. (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-κB-independent, FoxO1-dependent mechanism. J. Cell. Physiol. 209, 501–514 [DOI] [PubMed] [Google Scholar]
- 30. Brunskill N. J., Stuart J., Tobin A. B., Walls J., Nahorski S. (1998) Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. J. Clin. Invest. 101, 2140–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandri M., Sandri C., Gilbert A., Skuck C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S. H., Goldberg A. L. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stitt T. N., Drujan D., Clarke B. A., Panaro F., Timofeyva Y., Klinenber J. R., Gonzalez M., Yancopoulos G. D., Glass D. J. (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 [DOI] [PubMed] [Google Scholar]
- 33. Lee S. W., Dai G., Hu Z., Wang X., Du J., Mitch W. E. (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J. Am. Soc. Nephrol. 15, 1537–1545 [DOI] [PubMed] [Google Scholar]
- 34. Sacheck J. M., Ohtsuka A., McLary S. C., Goldberg A. L. (2004) IGF-1 stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 287, E591–E601 [DOI] [PubMed] [Google Scholar]
- 35. Musaro A., Giacinti C., Borsellino G., Dobrowolny G., Pelosi L., Cairns L., Ottolenghi S., Cossu G., Bernardi G., Battistini L., Molinaro M., Rosenthal N. (2004) Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc. Natl. Acad. Sci. U. S. A. 101, 1206–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai D., Yuan M., Frantz D. F., Melendez P. A., Hansen L., Lee J., Shoelson S. E. (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bailey J. L., Price S. R., Zheng B., Hu Z., Mitch W. E. (2006) Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J. Am. Soc. Nephrol. 17, 1388–1394 [DOI] [PubMed] [Google Scholar]
- 38. Wang X. H., Zhang L., Mitch W. E., LeDoux J. M., Hu J., Du J. (2010) Caspase-3 cleaves specific proteasome subunits in skeletal muscle stimulating proteasome activity. J. Biol. Chem. 285, 3527–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkes J. J., Lloyd D. J., Gekakis N. (2009) Loss-of-function mutation in myostatin reduces tumor necrosis factor α production and protects liver against obesity-induced insulin resistance. Diabetes 58, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma K., Mallidis C., Artaza J., Taylor W., Gonzalez-Cadavid N., Bhasin S. (2001) Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am. J. Physiol. Endocrinol. Metab. 281, E1128–E1136 [DOI] [PubMed] [Google Scholar]
- 41. Philip B., Lu Z., Gao Y. (2005) Regulation of GDF-8 signaling by the p38 MAPK. Cell. Signal. 17, 365–375 [DOI] [PubMed] [Google Scholar]
- 42. Welle S., Bhatt K., Pinkert C. A. (2006) Myofibrillar protein synthesis in myostatin-deficient mice. Am. J. Physiol. Endocrinol. Metab. 290, E409–E415 [DOI] [PubMed] [Google Scholar]
- 43. Shortreed K., Johnston A., Hawke T. J. (2008) Satellite cells and muscle repair. In Skeletal Muscle Damage and Repair (Tiidus P. M. ed) pp. 77–88, Human Kinetics, Champaign, IL, USA [Google Scholar]
- 44. McCroskery S., Thomas M., Maxwell L., Sharma M., Kambadur R. (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bartoli M., Poupiot J., Vulin A., Fougerousse F., Arandel L., Daniele N., Roudaut C., Noulet F., Garcia L., Danos O., Richard I. (2007) AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not α-sarcoglycan deficiency. Gene Ther. 14, 733–740 [DOI] [PubMed] [Google Scholar]
- 46. Sumner C. J., Wee C. D., Warsing L. C., Choe D. W., Ng A. S., Lutz C., Wagner K. R. (2009) Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Hum. Mol. Genet. 18, 3145–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]