Abstract
Receptor interacting protein 140 (RIP140) is a nuclear receptor coregulator that affects a wide spectrum of biological processes. It is unclear whether and how the expression level of RIP140 can be modulated and whether RIP140 is involved in inflammatory diseases. Here, we examine how intracellular cholesterol regulates RIP140 expression, and we evaluate the effect of RIP140 expression on macrophage proinflammatory potential. Macrophages treated with modified low-density lipoprotein express higher RIP140 mRNA and protein levels. Consistently, simvastatin reduces RIP140 levels, which can be reversed by mevalonate. Moreover, a high-fat diet elevates RIP140 but lowers miR-33 levels in peritoneal macrophages, and increases the production of IL-1β and TNF-α in macrophages. Mechanistically, miR-33 targets RIP140 mRNA by recognizing its target located in a highly conserved sequence of the 3′-untranslated region (3′-UTR) of RIP140 mRNA. Consequentially, miR-33 reduces RIP140 coactivator activity for NF-κB, which is supported by the reduction in NF-κB reporter activity and the inflammatory potential in macrophages. This study uncovers a cholesterol-miR-33-RIP140 regulatory pathway that modulates the proinflammatory potential in macrophages in response to an alteration in the intracellular cholesterol status, and identifies RIP140 as a direct target of miR-33 that mediates simvastatin-triggered anti-inflammation.—Ho, P.-C.; Chang, K.-C., Chuang, Y.-S., Wei, L.-N. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production.
Keywords: 3′-UTR, simvastatin, high-fat diet, sepsis, NF-κB
Macrophages are central components of the innate immune system and important players in maintaining cholesterol homeostasis (1–3). Resident and circulating macrophages can recognize invading pathogens and sense tissue damage mediated by Toll-like receptors (TLRs), and produce proinflammatory cytokines that evoke inflammation. However, massive production of TNF-α and IL-1β by activated macrophages can also lead to septic shock, causing tissue damage, multiple organ failure, and death. Clinically, both the susceptibility to, and mortality from, septic shock are dramatically higher in obese patients (4–6). Studies over the past decade revealed that nutrient status (especially lipid and cholesterol) could be sensed by macrophages to modulate inflammation in metabolic diseases (7, 8).
Although free fatty acids have been shown to promote proinflammatory cytokines production via TLRs, the role of cholesterol in modulating macrophage activity remains unclear. Modified forms of low-density lipoprotein (LDL) are engulfed by macrophages, and the cholesterol is either stored in lipid droplets or transported out by cholesterol efflux. Defects in cholesterol efflux result in cholesterol accumulation in the macrophages; this has been implicated in inflammation-related diseases, such as atherosclerosis (7, 9, 10). Interestingly, the HMG-CoA reductase inhibitor statin (which is used to lower plasma cholesterol) has been shown to possess anti-inflammatory properties that act by both cholesterol-dependent and -independent mechanisms (11–13). Although these studies suggest that cholesterol is important to the proinflammatory potential of macrophages and might play a role in related diseases, the exact mechanisms by which cholesterol modulates macrophage activity remain elusive (9, 10, 14, 15).
Receptor-interacting protein 140 (RIP140) is a master coregulator for a variety of transcription factors (16–19) and affects gene expression in ovary and metabolic tissues, including liver, muscle, and adipocytes. In addition to being infertile, RIP140-null mice are lean and resistant to diet-induced diabetes (17, 20–24). Recent proteomic analyses identified several post-translational modifications (PTMs) of RIP140 that play important roles in modulating the function and cellular localization of RIP140 (25–29). Furthermore, changes in these PTMs in response to nutrient status in adipocytes trigger different signaling pathways that modulate the functions of RIP140 (20, 30, 31). In addition to its role in metabolic tissues, RIP140 can associate with NF-κB in macrophages to coactivate proinflammatory cytokine production (32). Specifically, RIP140 is essential for TLR2-, TLR3-, and TLR4-mediated production of inflammatory cytokines. However, it remains unclear whether the expression of RIP140 in macrophages is altered in response to changes in cellular lipid contents, and whether this underlines the regulation of macrophage inflammatory potential.
MicroRNAs (miRNAs) are single-stranded noncoding RNAs 21–23 nucleotides in length. These molecules regulate gene expression by recognizing targets in either the 5′- or 3′-untranslated region (UTR). Binding to 3′-UTR is the major mechanism by which miRNAs form miRNA-RNA-induced silencing complexes, thus promoting the degradation of target mRNAs and reducing protein expression (33). Unique miRNA expression profiles have been identified in both innate and adaptive immune systems and are believed to control their development and functions. For example, several miRNAs modulate macrophage inflammatory responses by negative or positive feedback (33–36). These miRNAs could play roles in the progression of inflammation-related diseases, such as atherosclerosis, Alzheimer's disease, and rheumatoid arthritis (33). Recent studies have revealed that cholesterol content within macrophages can affect miRNA-33 (miR-33) expression, modulating cholesterol efflux (37–41).
This study examines RIP140 expression levels in macrophages in response to alterations in cholesterol content, both in vitro and in vivo. The results show that miR-33 mediates the regulation of RIP140 expression by intracellular cholesterol. Specifically, high-fat-diet (HFD) feeding reduces miR-33, but elevates RIP140, levels in peritoneal macrophages, and promotes an inflammatory response that manifests as acute septic shock. Consistently, simvastatin treatment (to block cholesterol synthesis) reduces RIP140 expression by increasing miR-33 expression. Further investigations reveal that miR-33 affects RIP140 by recognizing a highly conserved sequence in its 3′-UTR. These findings demonstrate the importance of the levels of the transcription coregulator RIP140 in the macrophage inflammatory response and establish that its expression in macrophages can be modulated by cholesterol content. This study also identifies miR-33 as an anti-inflammatory miRNA that acts in response to cholesterol depletion.
MATERIALS AND METHODS
Materials
Male C56BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Raw264.7 murine macrophage and 293T cell lines were from American Type Culture Collection (Manassas, VA, USA). Modified LDLs, oxidized LDL (OxLDL) and acetylated LDL (AcLDL), were purchased from Intracel (Frederick, MD, USA). Simvastatin, mevalonate, Sandoz 58–035, LPS and d-galactosamine were from Sigma-Aldrich (St. Louis, MO, USA). Cholesterol assay kit was obtained from Cayman Chemicals (Ann Arbor, MI, USA). ELISA kits for TNF-α and IL-1β were from BD Biosciences (San Jose, CA, USA) and RayBiotech (Norcross, GA, USA), respectively. Anti-β-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for RIP140 and F4/80 were obtained from Abcam (Cambridge, MA, USA). Hiperfect, miScript reverse transcription kit, miScript SYBR PCR kit, miR-33 PCR primer, siRNAs, miR-33 and miR-33 inhibitor were obtained from Qiagen (Valencia, CA, USA). Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA).
Cell culture and transfection
Raw 264.7 murine macrophages, BV2 microglia cell line, and 293T cells were maintained in DMEM with 10% FBS and 1% antibiotics. For modified LDL treatment, Raw264.7 cells were treated with 50 μg/ml OxLDL or 50 μg/ml AcLDL for 8 h. For simvastatin treatment, Raw264.7 cells were treated with 5 μM simvastatin alone, 100 μM mevalonate alone, or 5 μM simvastatin plus 100 μM mevalonate for 24 h. Plasmid transfection was performed using Lipofectamine 2000 and siRNAs, and miRNAs transfection was performed using Hiperfect according to the manufacturer's instructions. For proinflammatory cytokine production, Raw264.7 macrophages or peritoneal macrophages were treated with vehicle or 20 ng/ml LPS for 4 h. Media were collected to determine TNF-α and IL-1β levels by ELISA kits.
Plasmids and luciferase reporter assays
RIP140 3′-UTR was amplified from cDNA of 3T3-L1 cell by primers as follows: forward 5′-gagtcatctagaatgtgtacctgccataccac-3′; reverse 5′-gagtcatctagactggtgaagttgtgcattttaatg-3′. The amplified 3′-UTR was cloned into pGL3-promoter vector (Promega, Madison, WI, USA) with the XbaI site. The mutant with point mutations in the seed sequence of miR-33 was cloned by a different reverse primer with point mutations: 5′-gagtcatctagactggtgaagttgtcgattttaatg-3′.
For RIP140 3′-UTR luciferase reporter assay, 293T cells were transfected with 0.03 μg reporter plasmid, 0.2 μg LacZ plasmid, and 100 nM miRNA by Lipofectamine 2000, according to manufacturer's instruction. After 24 h, luciferase and LacZ activities were determined, as described previously (42). For reporter assay with simvastatin, BV2 microglia cells were transfected with 0.03 μg reporter plasmid and 0.2 μg LacZ plasmid using Lipofectamine 2000. At 24 h after transfection, cells were treated with or without 50 μM simvastatin for another 24 h. For NF-κB reporter assay, 293T cells were transfected with 0.6 μg NF-κB reporter (43) and 0.2 μg Flag-RIP140 containing different 3′-UTR or control vector.
Animal studies and peritoneal macrophage isolation
Five-week-old male C56BL/6J mice were fed a normal diet (ND) containing 18% calories from fat and undetectable cholesterol (2018; Harlan Teklad, Madison, WI, USA) or an HFD containing 60% calories from fat and 345 mg cholesterol/kg (F3282; Bio-Serv, West Chester, PA, USA). After 2 wk, mice were analyzed for acute septic shock and peritoneal macrophage isolation. For primary peritoneal macrophage isolation, mice were injected intraperitoneally with 3 ml thioglycolate. After 3 d, mice were sacrificed to isolate peritoneal macrophages, as described previously. Peritoneal macrophages were plated within RPMI 1640 medium containing 0.2% fatty acid-free BSA. After 2 h, cells were washed with the same medium once and then used for indicated analysis.
Acute septic shock animal model
Acute septic shock was performed as previously reported (44). Briefly, mice were injected intraperitoneally with LPS (0.1 mg/25 g body weight) plus d-galactosamine (0.5 mg/g body weight). Survival was monitored every hour for the next 18 h.
Flow cytometry
Macrophages derived from mice were cultured for 2 h for adhesion. Macrophage surface antigens were stained with rat-anti-mouse F4/80 antibody for 30 min in staining buffer (1% heat-inactivated FCS and 0.09%, w/v, sodium azide in DPBS) at 4°C and fixed as well as permeabilized with fixation/permeabilization solution (BD Biosciences) for 30 min at 4°C. Cells were further stained with rabbit-anti RIP140 antibody for 30 min in staining buffer. PE F(ab′)2 donkey anti-rabbit IgG (BD Biosciences) and goat F(ab′)2 anti-rat IgG-PerCP (R&D Systems, Minneapolis, MN, USA) in staining buffer were incubated with cells at 4°C 30 min in the dark. The stained cells were analyzed with fluorescence-activated cell sorting (FACS; BD Biosciences) according to the manufacturer's instructions. Fluorescence of F8/40 was collected in the FL3 detector, and fluorescence of RIP140 was collected in the FL2 detector.
Semiquantitative real-time PCR and quantitative miRNA real-time PCR
mRNA from cells were extracted by TRIzol (Invitrogen, Carlsbad, CA, USA) and then converted into cDNA by Omniscript RT kit or miScript reverse transcription kit (Qiagen). Real-time PCR for gene expression was performed with specific primer sets by Brilliant II Fast SYBR Green QPCR reagent (Agilent, Santa Clara, CA, USA) in Mx3005P QPCR system (Agilent). Primers for QPCR are as follows: mouse RIP140: forward 5′-GGCAGCAAACCTGAATTCGGC-3′, reverse 5′-CTCACCGGGCACGGAACATC-3′; mouse TNF-α: forward 5′-ATGAGCACAGAAAGCATGATCCGC-3′, reverse 5′-CCAAAGTAGACCTGCCCGGACTC-3′; mouse IL-1β: forward 5′-TCAGGCAGGCAGTATCACTCA-3′, reverse 5′-GGAAGGTCCACGGGAAAGAC-3′.
For miR-33 expression, real-time PCR was performed by miScript SYBR PCR kit (Qiagen) with miR-33 specific primer.
Statistical analysis
Results are presented as means ± sd. Statistical analysis was performed by Student's t test, and values of P < 0.05 were considered significant. For survival rate, the result was analyzed by Kaplan-Meier analysis.
RESULTS
HFD up-regulates RIP140 expression in macrophages
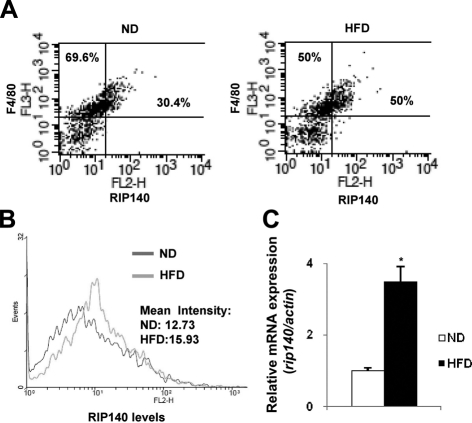
Hypercholesterolemia is a risk factor contributing to various inflammatory diseases, and RIP140 has been shown to be involved in inflammatory cytokine production (32). We asked whether an HFD that drastically elevates cholesterol levels in the animals could affect RIP140 expression in macrophages, through which their proinflammatory potential might be modulated. We compared the RIP140 expression levels in peritoneal macrophages from male C56BL/6J mice fed either an ND or HFD for 2 wk. We costained peritoneal macrophages with anti-RIP140 and an antibody against the macrophage surface marker, F4/80, then analyzed them by flow cytometry. Approximately 30.4% of macrophages from ND mice expressed high levels of RIP140; whereas HFD dramatically expanded the population expressing high-RIP140 levels to ∼50% (Fig. 1A). Notably, although the profile of macrophage RIP140 levels was altered in HFD mice, there was no change in the F4/80 expression profile. We compared the histograms of RIP140 expression between the F4/80-positive macrophage populations from ND and HFD mice, and found a substantial increase in the mean of RIP140 expression levels for the HFD mice (Fig. 1B). This was further supported by real-time qPCR analyses of RIP140 mRNA in peritoneal macrophages from ND and HFD mice, which detected approximately a 3-fold increase in RIP140 mRNA for the HFD mice (Fig. 1C). Taken together, these results show that HFD feeding can increase RIP140 mRNA and protein levels in macrophages.
Figure 1.
Effect of high-fat diet on RIP140 expression in peritoneal macrophages. A) Primary peritoneal macrophages were obtained from C56BL/6J mice fed either a normal diet (ND) or a high-fat diet (HFD) for 2 wk. RIP140 and F4/80 expression profiles were determined by flow cytometry. The y axis shows the staining intensity of macrophage marker F4/80. The x axis shows the staining intensity of RIP140. B) Histogram showing RIP140 expression from F4/80-positive peritoneal macrophages. Geological means for ND and HFD were indicated. C) Real-time analysis of RIP140 mRNA levels in peritoneal macrophages from animals fed the indicated diets. All values represent means ± sd; n = 3. *P < 0.05 vs. normal diet.
Intracellular cholesterol content modulates RIP140 levels
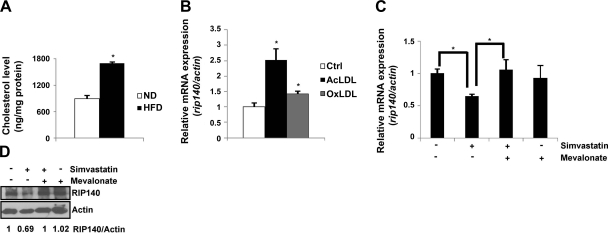
Under an HFD, circulating LDL can be acetylated and oxidized, which can then be recognized and engulfed by macrophages. These cholesterol-loaded macrophages become foam cells (2, 9), increasing their proinflammatory potential (9, 10). Given that a short-term HFD feeding in animals substantially increased their RIP140 expression in macrophages (Fig. 1) and intracellular cholesterol content in peritoneal macrophages (Fig. 2A), we asked whether intracellular cholesterol accumulation could promote RIP140 expression in Raw264.7 mouse macrophage cell line. It appeared that both AcLDL and OxLDL significantly enhanced RIP140 mRNA levels (Fig. 2B), suggesting that intracellular cholesterol content might be an important regulator for RIP140 expression in macrophages. We then applied an HMG-CoA reductase inhibitor, simvastatin, to block cholesterol synthesis (mimicking a cholesterol-depletion state) in these macrophages (38, 45) and found that simvastatin treatment for 24 h, indeed, significantly decreased RIP140 mRNA levels. Further, in the presence of mevalonate, the product of HMG-CoA reductase, simvastatin failed to reduce RIP140 mRNA levels (Fig. 2C). Consistently, the reduction in RIP140 protein level after simvastatin treatment was also abolished by mevalonate treatment (Fig. 2D). These results show that indeed, RIP140 expression in macrophages, at both mRNA and protein levels, is negatively regulated by intracellular cholesterol accumulation.
Figure 2.
Effect of intracellular cholesterol level on RIP140 expression. A) Intracellular cholesterol contents of primary peritoneal macrophages from ND or HFD. *P < 0.05 vs. normal diet. B) Raw264.7 murine macrophages were treated with vehicle, AcLDL, or OxLDL in the medium for 8 h. mRNA levels of RIP140 were determined by semiquantitative real-time PCR. *P < 0.05 vs. normal diet. C, D) mRNA (C) and protein (D) levels of RIP140 from Raw264.7 murine macrophages treated as indicated for 24 h. Quantified results are shown at bottom of image. mRNA levels were determined by semiquantitative real-time PCR. *P < 0.05. Values represent means ± sd; n = 3.
Cholesterol up-regulates RIP140 expression via repressing miR-33 that down-regulates RIP140 by targeting its conserved 3′-UTR
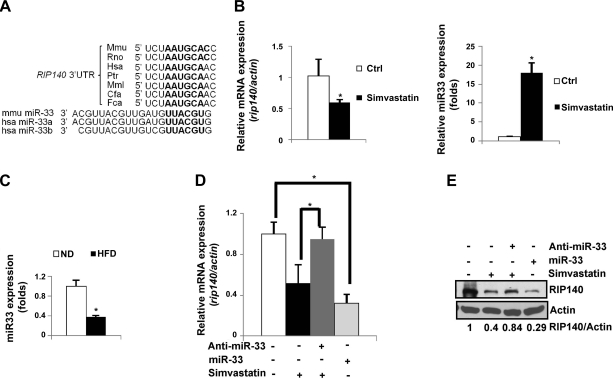
Recent studies have shown that miR-33 is produced from an intron of SREBP-2 in response to intracellular cholesterol accumulation, which inhibits cholesterol efflux by down-regulating ABCA1 and ABCG1 expression at the post-transcriptional level (37–41). On the basis of the reverse correlation between cholesterol content and RIP140 mRNA level shown above, we suspected a potential role for miR-33 in RIP140 expression. Interestingly, miR-33 was predicted to recognize a conserved 3′-UTR of RIP140 mRNA (Fig. 3A). We then compared RIP140 mRNA and mature miR-33 levels in Raw264.7 macrophages treated with or without simvastatin. It appeared that simvastatin not only reproducibly reduced RIP140 mRNA level but also increased miR-33 level (Fig. 3B). We further compared miR-33 levels in primary peritoneal macrophages from ND- and HFD-fed animals, and found that peritoneal macrophages from HFD-fed mice expressed a much lower level of mature miR-33 as compared to the ND group (Fig. 3C). To obtain direct evidence for a functional role of miR-33, we used Raw264.7 cells to assess the effects of gain and loss of function of miR-33 on RIP140 expression. Figure 3D, E shows that RIP140 mRNA and protein levels were both reduced in Raw264.7 cells transfected with miR-33 mimic even without depleting cholesterol (by simvastatin treatment), and simvastatin-triggered reduction in RIP140 was abolished by a miR-33 inhibitor (anti-miR-33).
Figure 3.
Simvastatin reduces RIP140 expression, mediated by miR-33. A) Predicted target site of miR-33 located in a conserved 3′-UTR of RIP140 mRNA, conducted by TargetScan. B) Left panel: RIP140 mRNA level. Right panel: mature miR-33 level. Samples were from Raw264.7 macrophages under control or simvastatin treatment for 24 h. C) Real-time analysis of mature miR-33 levels in peritoneal macrophages from mice fed either ND or HFD for 2 wk. D) RIP140 mRNA levels from Raw264.7 macrophages treated with control vehicle or simvastatin in the presence of miR-33 or miR-33 inhibitor. Anti-miR-33:miR-33 inhibitor. E) RIP140 protein levels from Raw264.7 macrophages treated with control vehicle or simvastatin in the presence of miR-33 or miR-33 inhibitor (anti-miR-33). Quantified data are shown at bottom of image. All values show means ± sd; n = 3. *P < 0.05.
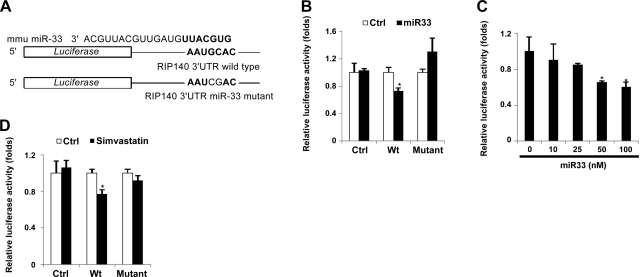
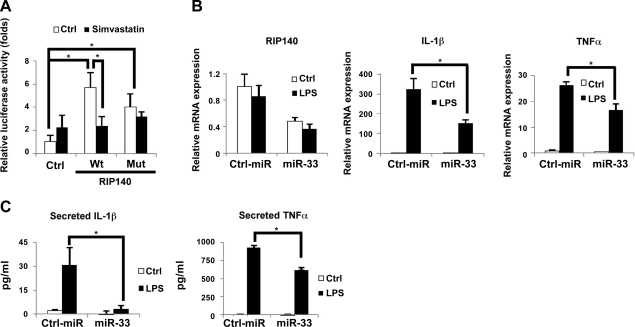
To further confirm the effect and target of miR-33 on RIP140 mRNA, we generated luciferase reporter constructs containing either the wild-type or miR-33 target mutated 3′-UTR of RIP140 mRNA (Fig. 4A) to assess the effects in 293T cells. In the presence of miR-33 mimic, the wild type, but not the mutated, reporter activity was significantly lowered as compared to the control reporter activity (Fig. 4B). Further, miR-33 mimic repressed the reporter activity in a dose-dependent manner (Fig. 4C). These results confirm that miR-33, indeed, acts by targeting the conserved 3′-UTR of RIP140 mRNA to suppress its expression. To further evaluate whether this conserved miR-33 target sequence in the 3′-UTR of RIP140 was important for simvastatin-triggered reduction in RIP140 expression, we used this reporter system to assess the effect of simvastatin. Indeed, simvastatin inhibited only the wild-type, but not the mutated or control reporter (Fig. 4D). Together, these experiments identify RIP140 as a target of miR-33, and delineate the mechanism of cholesterol action in elevating RIP140 expression, which is mediated by down-regulating miR-33 that targets the 3′-UTR of RIP140 mRNA.
Figure 4.
miR-33 targets a conserved 3′-UTR of RIP140 mRNA to repress RIP140. A) Schematic diagram of reporter constructs containing the wild-type or mutated 3′-UTR of RIP140 mRNA. B) miR-33 represses the luciferase activity of the wild-type reporter but not the mutant reporter in 293T cells. C) Dose-dependent repressive effects of miR-33 mimic on the wild-type reporter in 293T cells. *P < 0.05 vs. 0 nM. D) Simvastatin suppresses luciferase activity of the wild-type reporter but not the mutant reporter in BV2 cells. Ctrl, control vector; Wt, wild-type 3′-UTR reporter; mutant, 3′-UTR reporter with mutations in the miR-33 target site. *P < 0.05 vs. control treatment. All values show means ± sd; n = 3.
MiR-33 decreases RIP140 expression to down-regulate inflammatory cytokines production
Simvastatin has been shown to possess anti-inflammatory activities by blocking NF-κB activity, thereby reducing TNF-α and IL-1β expression (46). Since RIP140 could function as coactivator for NF-κB to activate TNF-α and IL-1β expression, we hypothesized that reducing cholesterol content, such as by simvastatin treatment, may modulate NF-κB activity by at least partially controlling the level of RIP140. We used an NF-κB reporter in 293T cells to assess the effects of reducing cholesterol content (by simvastatin treatment) and expressing an RIP140 expression vector containing either the wild-type or miR-33 target mutated 3′-UTR. As shown in Fig. 5A, cotransfection with either the wild type, or 3′-UTR mutated, RIP140 vector with NF-κB increased the NF-κB reporter activity in cells without simvastatin (Ctrl), supporting the hypothesis that RIP140 can coactivate NF-κB activity. However, simvastatin treatment significantly inhibited the NF-κB reporter activity in cells cotransfected with the wild-type, but not the miR-33 target mutated, RIP140 vector. This result indicates that reducing cholesterol content by simvastatin treatment can repress NF-κB activity by reducing its coactivator RIP140 via elevating miR-33 that targets the RIP140 3′-UTR, and suggests that miR-33 can be anti-inflammatory and reduce inflammatory cytokines production in macrophages. Indeed, transfection of Raw264.7 macrophages with miR-33 not only reduced RIP140 mRNA level but also suppressed LPS-stimulated production of TNF-α and IL-1β mRNA (Fig. 5B). Consistently, the secreted levels of TNF-α and IL-1β in the cell culture media were also decreased (Fig. 5C). These experiments support our hypothesis that miR-33 can be anti-inflammatory in macrophages by inhibiting RIP140 expression. These results delineate a new mechanism by which simvastatin can exert an anti-inflammatory effect, and identify miR-33 as a new miRNA involved in the regulation of macrophage inflammatory response.
Figure 5.
miR-33 modulates inflammatory cytokine production by controlling RIP140 expression. A) Simvastatin suppresses the effect of RIP140 on promoting NF-κB-driven reporter activity in 293T cells. After transfection with plasmids for 12 h, 293T cells were treated with vehicle (Ctrl) or simvastatin for another 24 h, followed by assays of luciferase activity. B) Real-time analysis of mRNA levels of IL-1β and TNF-α expression in Raw264.7 murine macrophages transfected with the control miR or miR-33 in the absence or presence of LPS for 4 h. C) miR-33 reduces IL-1β and TNF-α contents in the culture supernatant of Raw264.7 macrophages in the absence or presence of LPS. Cytokine production was determined by ELISA. All values show means ± sd; n = 3. *P < 0.05.
RIP140 up-regulates inflammatory cytokines production and increases the potential of acute septic shock
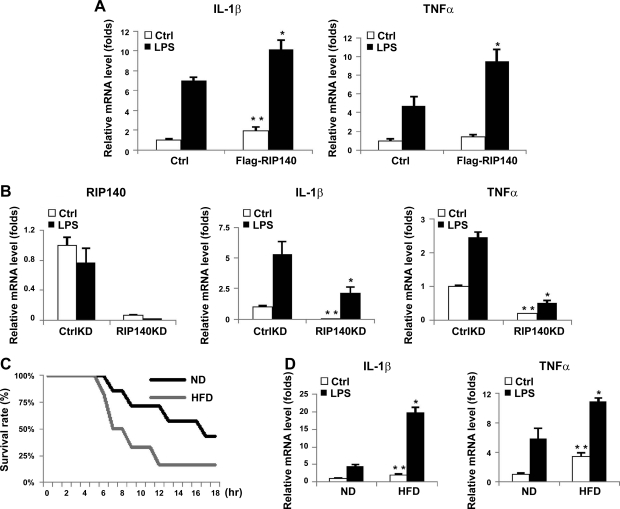
To provide direct gain-of-function evidence for the role of RIP140 in TNF-α and IL-1β expression, we overexpressed RIP140 in Raw264.7 macrophages and determined the mRNA levels of TNF-α and IL-1β. Figure 6A shows that overexpressing RIP140 in Raw264.7 macrophages significantly elevated both TNF-α and IL-1β mRNA levels, with or without LPS stimulation. In contrast, silencing RIP140 in macrophages reduced the basal and LPS-stimulated levels of TNF-α and IL-1β (Fig. 6B). These results further support the hypothesis that RIP140 can positively regulate proinflammatory cytokine production, and suggest that HFD might affect inflammatory potential, at least partially, through increasing RIP140 expression in macrophages.
Figure 6.
Expression of RIP140 in macrophages modulates inflammatory cytokines production and acute septic shock. A) Overexpression of RIP140 in Raw264.7 cells up-regulated basal and LPS-induced levels of IL-1β and TNF-α. B) Silencing RIP140 in Raw264.7 macrophages reduced the levels of IL-1β and TNF-α. mRNA, determined by real-time qPCR; n = 3. *P < 0.05 vs. control vector LPS or control siRNA LPS group; **P < 0.05 vs. control vector or control siRNA group. C) HFD reduced the animal survival rate in the acute septic shock model. Difference was significant between ND and HFD in Kaplan-Meier analysis. D) Real-time analysis of mRNA levels of proinflammatory cytokines, including IL-1β and TNF-α, in the absence or presence of LPS in peritoneal macrophages; n = 3–4. Ctrl, control treatment. *P < 0.05 vs. ND LPS group; **P < 0.05 vs. ND control group. All values show means ± sd.
To address this mechanism in the context of whole animals, we compared the immune responses of mice fed either ND or HFD, using acute septic shock as the indicator. Mice fed ND or HFD for 2 wk were challenged with LPS and d-galactosamine, and their survival rates within 18 h were monitored. It appeared that the HFD-fed mice had a higher mortality rate in response to LPS challenge, and they also died earlier, as compared to the ND-fed mice (Fig. 6C). In this septic shock model, robust elevation in circulating TNF-α and IL-1β is responsible for multiple organ failure, tissue damage, and death, and macrophages are the central players in the production and secretion of these cytokines (4, 47). Accordingly, we predicted that HFD would elevate TNF-α and IL-1β levels in macrophages and enhance their inflammatory potential. As expected, the basal and LPS-stimulated mRNA levels of TNF-α and IL-1β in peritoneal macrophages of HFD-fed mice, indeed, were much higher as compared to that of ND-fed mice (Fig. 6D). The results are consistent with a contributory role for RIP140 in the response of HFD-animals to septic shock. Together with data shown in Fig. 1, the results support the hypothesis that HFD elevates RIP140 expression in peritoneal macrophages, which activates TNF-α and IL-1β expression and enhances macrophages' inflammatory potential, thereby contributing to increased susceptibility to septic shock in certain conditions such as obesity.
DISCUSSION
RIP140 is known to be involved in diet-induced diabetes and can be an important player in Toll-like receptor-mediated inflammatory response. Because of its significantly altered subcellular distribution following a HFD, at least in adipocytes, it has also been proposed as a disease marker for the progression of metabolic diseases (20). However, whether and how its expression level may alter in response to diet/nutritional factors or as a result of disease progression, remains elusive. Here, we identify intracellular cholesterol accumulation as an important trigger to elevate RIP140 expression in macrophages through decreasing a specific miRNA that negatively regulates RIP140 expression post-transcriptionally. Because RIP140 can function as a coactivator for NF-κB, its increased expression in macrophages would enhance their inflammatory responses. Interestingly, the key to this regulatory mechanism relies, at least partially, on the expression of a recently discovered miRNA, miR-33, derived from an intron of SREBP-2 and reported to regulate several important genes in homeostatic control of cholesterol (37–41). This current study further extends the finding to provide a potential mechanistic explanation for certain clinically important inflammatory diseases, such as acute septic shock, that can be more severe under the obese condition. Finally, this study also demonstrates miR-33 as an anti-inflammatory miRNA in macrophages.
ABCA1- and ABCG1-knockout mice aberrantly accumulated cholesterol and exhibited a higher inflammatory potential, i.e., they had increased inflammatory cytokines (9, 10). It would be interesting to examine RIP140 expression in peritoneal macrophages from ABCA1- or ABCG1-knockout mice, which may provide a potentially new mechanism different from TLR activation. It is quite likely that macrophages utilize multiple mechanisms/pathways to elicit inflammatory responses. Five groups recently reported miR-33 and proposed the therapeutic potential of anti-miR-33 in enhancing high-density lipoprotein level (37–41). On the basis of data presented in this current study, we propose that miR-33 may also be a potential anti-inflammatory therapeutic.
The mouse miR-33 is produced from an intron of SREBP-2. Humans express miR-33a and miR-33b from introns of SREBP-2 and SREBP-1, respectively. Cholesterol depletion caused by HMG-CoA reductase inhibitor, such as simvastatin, results in the induction of miR-33 expression along with SREBP-2 expression (38). SREBP-2 elevation can trigger reactions replenishing depleted intracellular cholesterol pools. Increased miR-33 in a cholesterol-depleted state not only blocks cholesterol efflux by targeting ABCA1 and ABCG1 to facilitate the accumulation of intracellular cholesterol but also reduces RIP140 level in order to prevent potential inflammation caused by overaccumulation of cholesterol. In contrast, when macrophages engulf a massive amount of modified LDL, accumulation of cholesterol signals cells to reduce the expression of miR-33 along with SREBP-2. As a result, ABCA1 and ABCG1 are elevated to promote cholesterol efflux, and RIP140 is also increased to promote inflammatory responses. The enhancement in macrophage inflammatory response may be needed to react to tissue damage in the pathophysiological condition caused by hypercholesterolemia. Under other pathophysiological conditions, macrophages may infiltrate into damaged tissues and increase their intracellular cholesterol contents by taking up damaged or apoptotic cells via phagocytosis. This newly uncovered mechanism could also be in operation to modulate macrophage inflammatory potential in these circumstances. This would allow fine-tuning the functions of macrophages in response to changes in the cholesterol status, and may also be involved in other metabolic disorders.
This study shows miR-33 as a novel anti-inflammatory miRNA by targeting the 3′-UTR of RIP140 mRNA. We previously reported another miRNA, miR-346, that could enhance RIP140 translation by associating with its 5′-UTR (48). We also demonstrated that coregulators could modulate transcription factor activity via competitive interaction and the level of their expression can be critical to specific gene regulation (49, 50). In future studies, it would be interesting and important to dissect the relationship of these miRNAs and the expression level of RIP140 in particular physiological conditions or during disease progression. It would also be interesting to explore the therapeutic potential of specific miRNAs to target RIP140.
Finally, simvastatin is the most potent cholesterol-lowering statin. We show its anti-inflammatory and RIP140-suppressing properties in macrophages in this current study, which is partially attributed to a cholesterol-dependent mechanism (11, 12). However, the effects of simvastatin on RIP140 expression in other metabolic tissues remain to be examined. It is also important to investigate the effects of simvastatin on the expression of RIP140 in other types of cells. Further, HFD could potentially affect not only cholesterol levels but also fat contents; therefore, the effects observed in this HFD mouse model may also be contributed by alteration in fat content.
Acknowledgments
The authors thank Y.-C. Tsui for help with the septic shock model and animal handling. The authors also thank F. A. Beyan and A. Smith for technical help.
This study was supported in part by U.S. National Institutes of Health grants DK60521, DK060521-07S1, DK54733, DK054733-09S1, DA11190, and K02-DA13926; a Distinguished McKnight University Professorship (L.-N.W.); and an American Heart Association predoctoral fellowship (P.-C.H.).
REFERENCES
- 1. Chawla A. (2010) Control of macrophage activation and function by PPARs. Circ. Res. 106, 1559–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maxfield F. R., Tabas I. (2005) Role of cholesterol and lipid organization in disease. Nature 438, 612–621 [DOI] [PubMed] [Google Scholar]
- 3. Chen G. Y., Nunez G. (2010) Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russell J. A. (2006) Management of sepsis. N. Engl. J. Med. 355, 1699–1713 [DOI] [PubMed] [Google Scholar]
- 5. Vachharajani V., Vital S. (2006) Obesity and sepsis. J. Intens. Care Med. 21, 287–295 [DOI] [PubMed] [Google Scholar]
- 6. Rivera C. A., Gaskin L., Singer G., Houghton J., Allman M. (2010) Western diet enhances hepatic inflammation in mice exposed to cecal ligation and puncture. BMC Physiol. 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotamisligil G. S., Erbay E. (2008) Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naura A. S., Hans C. P., Zerfaoui M., Errami Y., Ju J., Kim H., Matrougui K., Kim J. G., Boulares A. H. (2009) High-fat diet induces lung remodeling in ApoE-deficient mice: an association with an increase in circulatory and lung inflammatory factors. Lab. Invest. 89, 1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yvan-Charvet L., Wang N., Tall A. R. (2010) Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., Tall A. R. (2008) Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118, 1837–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schonbeck U., Libby P. (2004) Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation 109, II18–26 [DOI] [PubMed] [Google Scholar]
- 12. Gao F., Linhartova L., Johnston A. M., Thickett D. R. (2008) Statins and sepsis. Br. J. Anaesth. 100, 288–298 [DOI] [PubMed] [Google Scholar]
- 13. Gilroy D. W., Lawrence T., Perretti M., Rossi A. G. (2004) Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3, 401–416 [DOI] [PubMed] [Google Scholar]
- 14. Lefevre L., Gales A., Olagnier D., Bernad J., Perez L., Burcelin R., Valentin A., Auwerx J., Pipy B., Coste A. (2010) PPARγ ligands switched high fat diet-induced macrophage M2b polarization toward M2a thereby improving intestinal Candida elimination. PLoS One 5, e12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strandberg L., Verdrengh M., Enge M., Andersson N., Amu S., Onnheim K., Benrick A., Brisslert M., Bylund J., Bokarewa M., Nilsson S., Jansson J. O. (2009) Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One 4, e7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White R., Morganstein D., Christian M., Seth A., Herzog B., Parker M. G. (2008) Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett. 582, 39–45 [DOI] [PubMed] [Google Scholar]
- 17. Fritah A., Christian M., Parker M. G. (2010) The metabolic coregulator RIP140: an update. Am. J. Physiol. Endocrinol. Metab. 299, E335–E340 [DOI] [PubMed] [Google Scholar]
- 18. Lee C. H., Chinpaisal C., Wei L. N. (1998) Cloning and characterization of mouse RIP140, a corepressor for nuclear orphan receptor TR2. Mol. Cell. Biol. 18, 6745–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cavailles V., Dauvois S., L'Horset F., Lopez G., Hoare S., Kushner P. J., Parker M. G. (1995) Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 14, 3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho P. C., Lin Y. W., Tsui Y. C., Gupta P., Wei L. N. (2009) A negative regulatory pathway of GLUT4 trafficking in adipocyte: new function of RIP140 in the cytoplasm via AS160. Cell. Metab. 10, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. White R., Leonardsson G., Rosewell I., Ann Jacobs M., Milligan S., Parker M. (2000) The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat. Med. 6, 1368–1374 [DOI] [PubMed] [Google Scholar]
- 22. Leonardsson G., Steel J. H., Christian M., Pocock V., Milligan S., Bell J., So P. W., Medina-Gomez G., Vidal-Puig A., White R., Parker M. G. (2004) Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc. Natl. Acad. Sci. U. S. A. 101, 8437–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Christian M., Kiskinis E., Debevec D., Leonardsson G., White R., Parker M. G. (2005) RIP140-targeted repression of gene expression in adipocytes. Mol. Cell. Biol. 25, 9383–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powelka A. M., Seth A., Virbasius J. V., Kiskinis E., Nicoloro S. M., Guilherme A., Tang X., Straubhaar J., Cherniack A. D., Parker M. G., Czech M. P. (2006) Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J. Clin. Invest. 116, 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mostaqul Huq M. D., Gupta P., Wei L. N. (2008) Post-translational modifications of nuclear co-repressor RIP140: a therapeutic target for metabolic diseases. Curr. Med. Chem. 15, 386–392 [DOI] [PubMed] [Google Scholar]
- 26. Mostaqul Huq M. D., Gupta P., Tsai N. P., White R., Parker M. G., Wei L. N. (2006) Suppression of receptor interacting protein 140 repressive activity by protein arginine methylation. EMBO J. 25, 5094–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta P., Huq M. D., Khan S. A., Tsai N. P., Wei L. N. (2005) Regulation of co-repressive activity of and HDAC recruitment to RIP140 by site-specific phosphorylation. Mol. Cell. Proteomics 4, 1776–1784 [DOI] [PubMed] [Google Scholar]
- 28. Huq M. D., Khan S. A., Park S. W., Wei L. N. (2005) Mapping of phosphorylation sites of nuclear corepressor receptor interacting protein 140 by liquid chromatography-tandem mass spectroscopy. Proteomics 5, 2157–2166 [DOI] [PubMed] [Google Scholar]
- 29. Huq M. D., Ha S. G., Barcelona H., Wei L. N. (2009) Lysine methylation of nuclear co-repressor receptor interacting protein 140. J. Proteome Res. 8, 1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huq M. D., Tsai N. P., Lin Y. P., Higgins L., Wei L. N. (2007) Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat. Chem. Biol. 3, 161–165 [DOI] [PubMed] [Google Scholar]
- 31. Gupta P., Ho P. C., Huq M. D., Khan A. A., Tsai N. P., Wei L. N. (2008) PKCepsilon stimulated arginine methylation of RIP140 for its nuclear-cytoplasmic export in adipocyte differentiation. PLoS One 3, e2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zschiedrich I., Hardeland U., Krones-Herzig A., Berriel Diaz M., Vegiopoulos A., Muggenburg J., Sombroek D., Hofmann T. G., Zawatzky R., Yu X., Gretz N., Christian M., White R., Parker M. G., Herzig S. (2008) Coactivator function of RIP140 for NFκB/RelA-dependent cytokine gene expression. Blood 112, 264–276 [DOI] [PubMed] [Google Scholar]
- 33. O'Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 [DOI] [PubMed] [Google Scholar]
- 34. O'Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tili E., Michaille J. J., Cimino A., Costinean S., Dumitru C. D., Adair B., Fabbri M., Alder H., Liu C. G., Calin G. A., Croce C. M. (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 179, 5082–5089 [DOI] [PubMed] [Google Scholar]
- 36. El Gazzar M., McCall C. E. (2010) MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J. Biol. Chem. 285, 20940–20951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Najafi-Shoushtari S. H., Kristo F., Li Y., Shioda T., Cohen D. E., Gerszten R. E., Naar A. M. (2010) MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 328, 1566–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rayner K. J., Suarez Y., Davalos A., Parathath S., Fitzgerald M. L., Tamehiro N., Fisher E. A., Moore K. J., Fernandez-Hernando C. (2010) MiR-33 contributes to the regulation of cholesterol homeostasis. Science 328, 1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horie T., Ono K., Horiguchi M., Nishi H., Nakamura T., Nagao K., Kinoshita M., Kuwabara Y., Marusawa H., Iwanaga Y., Hasegawa K., Yokode M., Kimura T., Kita T. (2010) MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc. Natl. Acad. Sci. U. S. A. 107, 17321–17326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marquart T. J., Allen R. M., Ory D. S., Baldan A. (2010) miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. U. S. A. 107, 12228–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gerin I., Clerbaux L. A., Haumont O., Lanthier N., Das A. K., Burant C. F., Leclercq I. A., MacDougald O. A., Bommer G. T. (2010) Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J. Biol. Chem. 285, 33652–33661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho P. C., Gupta P., Tsui Y. C., Ha S. G., Huq M., Wei L. N. (2008) Modulation of lysine acetylation-stimulated repressive activity by Erk2-mediated phosphorylation of RIP140 in adipocyte differentiation. Cell. Signal. 20, 1911–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park S. W., Huq M. D., Hu X., Wei L. N. (2005) Tyrosine nitration on p65: a novel mechanism to rapidly inactivate nuclear factor-κB. Mol. Cell. Proteomics 4, 300–309 [DOI] [PubMed] [Google Scholar]
- 44. Chang M., Jin W., Sun S. C. (2009) Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat. Immunol. 10, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tabas I. (2010) Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hernandez-Presa M. A., Ortego M., Tunon J., Martin-Ventura J. L., Mas S., Blanco-Colio L. M., Aparicio C., Ortega L., Gomez-Gerique J., Vivanco F., Egido J. (2003) Simvastatin reduces NF-κB activity in peripheral mononuclear and in plaque cells of rabbit atheroma more markedly than lipid lowering diet. Cardiovasc. Res. 57, 168–177 [DOI] [PubMed] [Google Scholar]
- 47. Redmond H. P., Chavin K. D., Bromberg J. S., Daly J. M. (1991) Inhibition of macrophage-activating cytokines is beneficial in the acute septic response. Ann Surg 214, 502–508; discussion 508–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsai N. P., Lin Y. L., Wei L. N. (2009) MicroRNA mir-346 targets the 5′-untranslated region of receptor-interacting protein 140 (RIP140) mRNA and up-regulates its protein expression. Biochem. J. 424, 411–418 [DOI] [PubMed] [Google Scholar]
- 49. Chen Y., Kerimo A., Khan S., Wei L. N. (2002) Real-time analysis of molecular interaction of retinoid receptors and receptor-interacting protein 140 (RIP140). Mol. Endocrinol. 16, 2528–2537 [DOI] [PubMed] [Google Scholar]
- 50. Chen Y., Hu X., Wei L. N. (2004) Molecular interaction of retinoic acid receptors with coregulators PCAF and RIP140. Mol. Cell. Endocrinol. 226, 43–50 [DOI] [PubMed] [Google Scholar]