Abstract
BACKGROUND
About 20% of women with ovarian cancer have low concentrations of serum cancer antigen 125 (CA125), and this important tumor marker cannot be used to monitor their disease. The measured concentration for mucin 1 (MUC1), or CA15–3, another tumor marker, can be lowered in breast and ovarian cancer patients when circulating immune complexes (CICs) containing antibodies bound to the free antigen are present. Because CA125 and MUC1 are related members of the mucin family, we sought to determine whether CICs might also exist for CA125 and interfere with its clinical assay.
METHODS
We developed an antigen capture–based assay to determine the presence of CICs for CA125. We spotted mouse antibodies to CA125 onto nanoparticle slides, incubated them with patient serum, and added Cy5-tagged goat antihuman IgG antibodies. Fluorescence intensities were read and normalized to the intensities for glutathione S-transferase A1 as a control.
RESULTS
Assay results for 23 ovarian cancer cases with high CA125 concentrations, 43 cases with low CA125 concentrations, and 19 controls (mean CA125 concentrations, 2706, 23, and 11 kilounits/L, respectively) revealed mean fluorescence intensities for CA125 CIC of 2.30, 2.72, and 1.99 intensity units (iu), respectively. A generalized linear model adjusted for batch and age showed higher CA125 CIC fluorescence intensities in low-CA125 cases than in high-CA125 cases (P = 0.03) and controls (P = 0.0005). Four ovarian cancer patients who had recurrent disease and always had low CA125 values had a mean CA125 CIC value of 3.06 iu (95% CI, 2.34–4.01 iu).
CONCLUSIONS
These preliminary results suggest the existence of CICs involving CA125, which may help explain some ovarian cancer cases with low CA125 concentrations.
In 1983, cancer antigen 125 (CA125)6 was proposed as a serum biomarker for ovarian cancer (1) and in 2001 was recognized as a member of the mucin family of cell-surface or secreted glycoproteins (2). Clinical CA125 concentrations are increased to >35 kilounits/L in about 80% of women with epithelial ovarian cancer, and CA125 concentration is correlated directly with disease stage and inversely with survival (3, 4). The kinetics of this marker during chemotherapy predict disease status and survival (5, 6), and concentrations after therapy are used to monitor disease recurrence (7). CA125 has been suggested for distinguishing benign from malignant pelvic masses (8) and in combination with ultrasound as a potential screening tool for ovarian cancer (9, 10). For all these uses, the ovarian cancer case with low CA125 concentrations is problematic.
One determinant of CA125 concentration is the degree of its production in tumors. Høgdall et al. (11) found the percentage of CA125-positive tumors to be highest for serous tumors (85%–90%), lowest for mucinous tumors (6%–12%), and intermediate for clear cell and endometrioid tumors (40%–65%). Although the serum CA125 concentration generally tracks with tissue production, some patients have a low serum CA125 concentration despite high tissue production. These authors wondered whether detection was reduced because of an altered form of CA125. Our observations related to another mucin [mucin 1 (MUC1)] and its assay (CA15–3) prompted us to study this idea.
Similarities that MUC1 shares with CA125 (also known as MUC16) include a cytoplasmic tail, a trans-membrane component, and a surface component consisting of heavily glycosylated peptide repeats that is shed during carcinogenesis (12). Because shed MUC1 is less glycosylated, it can elicit anti-MUC1 antibodies, which are found in association with various tumors (13) as well as with certain inflammatory conditions, such as ulcerative colitis (14). These antibodies can form circulating immune complexes (CICs) with MUC1. An inverse correlation observed between MUC1 CICs and CA15–3 in patients with breast and ovarian cancers indicates that such complexes can interfere with the free-antigen assay (15). That CICs may also exist for CA125 is suggested by the results of testing of a murine anti-CA125 antibody as an imaging agent, which revealed that the antibody formed CICs with CA125 after injection (16). Thus, we sought to determine whether “natural” CA125 CICs also exist and, if so, whether they help explain the ovarian cancer case with a low CA125 value.
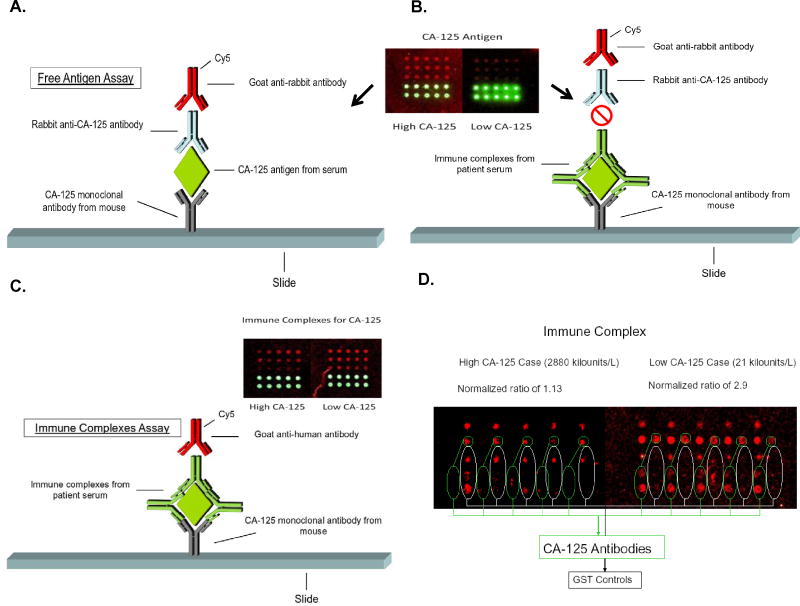
We developed an antigen-capture approach to measure CA125 CICs. The platform uses mouse monoclonal antibodies to CA125 purified from human ovarian carcinoma (clone ×75 from Novus Biologicals) spotted onto nanoparticle slides (Inanovate) to capture CA125 antigen and immune complexes. Antibodies are printed at 3 different amounts (400, 200, and 100 μg per spot), with each amount spotted 5 times in a given array. To measure CA125 antigen, we added 100 μL of undiluted serum to the microarray, incubated the slides for 1 h at room temperature with gentle rocking, and then washed the slides with 10% I-wash microarray buffer (Inanovate). We added rabbit anti-MUC16 antibody (Novus Biologicals) and then Cy5-tagged goat antirabbit antibody (Novus Biologicals) to detect free CA125 (see Fig. 1A). If CA125 is in an immune complex, the rabbit anti-CA125 antibodies may be blocked (Fig. 1B).
Fig. 1. Illustration of antigen capture–based assays for CA125 free antigen and CA125 in immune complexes.
Mouse monoclonal antibodies to CA125 are spotted onto a nanoparticle slide and patient serum is added. After washing, rabbit anti-CA125 antibody is added, followed by Cy5- tagged goat antirabbit antibody (A). When CA125 is bound in an immune complex with human anti-CA125, the rabbit anti-CA125 antibodies may be blocked (B). CA125 immune complexes are measured by adding Cy5-tagged goat antihuman IgG antibodies immediately following incubation with the sera (C). The inserts of panels A-C illustrate the array-based antigen and immune complex reactions in an invasive serous case with a CA125 concentration of 3331 kilounits/L, compared with an invasive serous case with a CA125 concentration of 26.7 kilounits/L. The free-antigen assay is strongly positive for the high-CA125 case and weakly positive for the low-CA125 case. The opposite occurs for the CA125 CIC assay. Green spots are BSA controls as orientation markers. We subsequently introduced an array that included a mouse monoclonal antibody to GSTA1, which served as a negative control and allowed calculation of a "normalized" intensity for the CA125-CIC (D). A pair of cases from Table 1— one with a CA125 concentration of 21 kilounits/L and a normalized fluorescence intensity of 2.9 iu (right) and a second with a CA125 of 2880 kilounits/L and normalized fluorescence intensity of 1.1 iu (left).
To test for a CA125 CIC, we diluted serum samples from cases and controls to 1 volume in 1000 with I-wash microarray buffers, incubated the sera (without the addition of rabbit anti-MUC16 antibody), and added Cy5-tagged goat antihuman IgG antibodies (Novus Biologicals) immediately after incubation (Fig. 1C). Fig. 1D illustrates results from the latest prototype of the assay, which uses a glutathione S-transferase A1 (GSTA1) control to distinguish nonspecific binding and to calculate a “normalized” intensity. We used a ScanArray microarray scanner and software system (PerkinElmer) to scan slides for fluorescence intensities and to normalize them to the fluorescence intensities of mouse monoclonal antibodies to GSTA1 (clone 2F7 from Novus Biologicals), which served as a negative control. The GSTA1 antibodies were similarly spotted at 400, 200, and 100 μg per spot, and each concentration was spotted 5 times in a given array. For a given spot at a given antibody concentration (i.e., 400, 200, or 100 μg), normalized values for the CA125 CIC were calculated as the fluorescence intensity of Cy5-tagged goat antihuman IgG antibodies bound to CA125 CIC, divided by the fluorescence intensity of Cy5-tagged goat antihuman IgG antibodies to GSTA1. Because we spotted each antibody 5 times for each amount, we were able to calculate the between-spot CV for each amount.
To test the CA125 CIC assay, we used preoperative blood samples collected from ovarian cancer patients and control samples from individuals in the general population. Individuals in both groups were enrolled under protocols approved by the Brigham and Women’s Hospital and Massachusetts General Hospital Institutional Review Board. Patients were selected on the basis of their pretherapy CA125 concentration, as measured in the clinical laboratories of these 2 institutions. Controls were enrolled as part of an ovarian cancer case–control study and were age-matched to the cases. The assays were performed in 2 batches, each of which had high-CA125 and low-CA125 cases and controls.
Readings obtained with the 200-μg amount produced the lowest CVs and were chosen for this report. Within-sample (between-spot) CVs averaged 17% for the cases and 14% for the controls, whereas between-replicate CVs averaged 14% for cases and 11% for the controls. Twenty-three high-CA125 cancer cases (mean CA125 concentration, 2706 kilounits/L) had a mean CA125 CIC fluorescence intensity of 2.3 intensity units (iu) (95% CI, 2.02–2.62 iu) (Table 1). Forty-three low-CA125 cases (mean CA125 concentration, 23 kilounits/L) had a mean CA125 CIC fluorescence intensity of 2.72 iu (95% CI, 2.48–2.98 iu). Nineteen controls (mean CA125 concentration, 11 kilounits/L) had a mean CIC fluorescence intensity of 1.99 iu (95% CI, 1.73–2.29 iu). Analysis of a generalized linear model that adjusted for batch and age showed that CA125 CIC values for low-CA125 cases were significantly higher than for high-CA125 cases (P = 0.03) and controls (P = 0.0005). Similarly significant P values for differences in CA125 CICs were observed when only the subset of serous low-CA125 cases was compared with the latter 2 groups (P =0.05, and P = 0.003, respectively). There was no significant difference in CA125 CIC values between the high-CA125 cases and controls (P = 0.18). Four ovarian cancer patients who had recurrent disease and always had low CA125 concentrations had a mean fluorescence intensity for CA125 CIC of 3.06 iu (95% CI, 2.34–4.01 iu). Tissue staining for CA125 in samples from a small number of high-CA125 cases (n = 5) and low-CA125 cases (n = 3) revealed strong staining in both groups.
Table 1.
Description of cases and controls tested for CA125 immune complexes.
| Group | n | Age, yearsa | CA125, kilounits/Lb | Immune complex, iub | GLM Pc |
|---|---|---|---|---|---|
| High-CA125 cases | |||||
| Serous invasive | 23 | 64 (46–84) | 2706 (2071–3536) | 2.30 (2.02–2.62) | 0.03 |
| Stage I–II | 2 | 59 (59–59) | 2815 (888–8925) | 2.38 (1.61–3.51) | |
| Stage III–IV | 20 | 64 (46–84) | 2851 (1979–4107) | 2.04 (1.82–2.29) | |
| Low-CA125 cases | 43 | 59 (25–82) | 23 (19–28) | 2.72 (2.48–2.99) | Referent |
| Serous borderline | 10 | 53 (28–68) | 16 (11–21) | 2.87 (2.31–3.55) | |
| Serous invasived | 21 | 65 (49–80) | 32 (26–40) | 2.82 (2.45–3.26) | |
| Mucinous invasive | 3 | 43 (25–53) | 16 (9–28) | 2.75 (1.88–4.01) | |
| Other invasive | 9 | 58 (44–82) | 20 (14–28) | 2.53 (2.05–3.13) | |
| Stage I–II | 25 | 55 (25–82) | 19 (16–23) | 2.76 (2.45–3.12) | |
| Stage III–IV | 18 | 66 (49–77) | 33 (26–41) | 2.78 (2.41–3.20) | |
| Controls | 19 | 59 (46–74) | 11 (8–14) | 1.99 (1.73–2.29) | 0.0005 |
Data are presented as the mean (range).
Data are presented as the mean (95% CI).
Generalized linear model (GLM) adjusted for batch and age.
When the low-CA125 cases were restricted to only serous invasive cases, P values for high-CA125 cases and controls vs these low-CA125 cases were 0.05 and 0.003, respectively.
Although our CA125 CIC assay must be further refined and validated with larger sample sets, results from this experiment provide preliminary evidence that immune complexes for CA125 exist in patients with ovarian cancer and may affect conventional assay readings. The fact that CA125 is the most important biomarker for ovarian cancer underscores the need to better understand this marker and to explain why CA125 concentrations are not increased in some cases of ovarian cancer. A reliable assay for detecting and quantifying CA125 CICs could have important implications for the current use of CA125 in disease monitoring and may have a future use in screening.
Acknowledgments
The assistance of Dale Edwards, Mary Ellen Fitzgerald, and Emily Kantoff in sample processing and collection is gratefully acknowledged. D.J. O’Rourke received a First Prize Award at the Students Oral Presentation Contest at the 2010 Annual Meeting of the AACC for a report on part of this work.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: CA125, cancer carbohydrate antigen 125; MUC1, mucin 1; CIC, circulating immune complex; GSTA1, glutathione S-transferase A1; iu, intensity units.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: D.W. Cramer, Inanovate; D.J. O'Rourke, Inanovate; B.C.-S. Liu, Inanovate.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: D.W. Cramer, National Cancer Institute grants P50 CA105009 (Ovarian Cancer SPORE), R01 CA54419, and 5U01 CA86381 (Early Detection Research Network).
Expert Testimony: None declared.
References
- 1.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–7. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 2.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–5. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 3.Cooper BC, Sood AK, Davis CS, Ritchie JM, Sorosky JI, Anderson B, Buller RE. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol. 2002;100:59–64. doi: 10.1016/s0029-7844(02)02057-4. [DOI] [PubMed] [Google Scholar]
- 4.Geisler JP, Miller GA, Lee TH, Harwood RM, Wiemann MC, Geisler HE. Relationship of preoperative serum CA-125 to survival in epithelial ovarian carcinoma. J Reprod Med. 1996;41:140–2. [PubMed] [Google Scholar]
- 5.Buller RE, Berman ML, Bloss JD, Manetta A, DiSaia PJ. Serum CA125 regression in epithelial ovarian cancer: correlation with reassessment findings and survival. Gynecol Oncol. 1992;47:87–92. doi: 10.1016/0090-8258(92)90082-t. [DOI] [PubMed] [Google Scholar]
- 6.Gadducci A, Zola P, Landoni F, Maggino T, Sartori E, Bergamino T, Cristofani R. Serum half-life of CA 125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: results of a multicentric Italian study. Gynecol Oncol. 1995;58:42–7. doi: 10.1006/gyno.1995.1181. [DOI] [PubMed] [Google Scholar]
- 7.Schilthuis MS, Aalders JG, Bouma J, Kooi H, Fleuren GJ, Willemse PH, De Bruijn HW. Serum CA 125 levels in epithelial ovarian cancer: relation with findings at second-look operations and their role in the detection of tumour recurrence. Br J Obstet Gynaecol. 1987;94:202–7. doi: 10.1111/j.1471-0528.1987.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 8.Malkasian GD, Jr, Knapp RC, Lavin PT, Zurawski VR, Jr, Podratz KC, Stanhope CR, et al. Preoperative evaluation of serum CA 125 levels in pre-menopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341–6. doi: 10.1016/s0002-9378(88)80081-4. [DOI] [PubMed] [Google Scholar]
- 9.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–40. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 10.Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–82. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish “MALOVA” Ovarian Cancer Study. Gynecol Oncol. 2007;104:508–15. doi: 10.1016/j.ygyno.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 13.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–60. [PubMed] [Google Scholar]
- 14.Hinoda Y, Nakagawa N, Nakamura H, Makiguchi Y, Itoh F, Adachi M, et al. Detection of a circulating antibody against a peptide epitope on a mucin core protein, MUC1, in ulcerative colitis. Immunol Lett. 1993;35:163–8. doi: 10.1016/0165-2478(93)90086-h. [DOI] [PubMed] [Google Scholar]
- 15.Gourevitch MM, von Mensdorff-Pouilly S, Litvinov SV, Kenemans P, van Kamp GJ, Verstraeten AA, Hilgers J. Polymorphic epithelial mucin (MUC-1)-containing circulating immune complexes in carcinoma patients. Br J Cancer. 1995;72:934–8. doi: 10.1038/bjc.1995.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13—evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm. 2001;16:187–203. doi: 10.1089/10849780152389384. [DOI] [PubMed] [Google Scholar]