Abstract
Because early detection of pancreatic cancer is the best way to cure this disease, investigators continue to try and identify accurate markers of early pancreatic cancer. Since early-stage pancreatic cancer is generally asymptomatic, the only reliable way to detect it is by targeting individuals at increased risk for pancreatic screening.
In this issue of Clinical Cancer Research, Brand et al, report on their evaluation of the diagnostic performance of measuring 83 circulating proteins in sera of patients with pancreatic ductal adenocarcinoma (n=333) compared to those with benign pancreatic conditions (n=144, benign pancreatic cysts, pancreatitis), and healthy controls (n=227)(1). The markers selected required having commercially-available antibodies, and were detected in multiplex fashion with the Luminex platform. The markers included those previously reported to have potential diagnostic utility for pancreatic cancer (CA19-9, CEA, osteopontin, MIC-1, TIMP-1, HIP (REG3), osteoprotegerin, ICAM-1, SAA). The remaining markers were mostly cytokines, chemokines, hormones and apolipoproteins. Samples were split randomly into training and blinded validation sets prior to analysis. The best 3-marker panel identified for discriminating patients with pancreatic cancer from healthy controls (CA19-9, ICAM-1, osteoprotegerin) yielded a sensitivity/specificity (SN/SP) of 78%/94% in the validation set. The best 3-marker panel identified for discriminating patients with pancreatic cancer from disease controls (CA19-9, CEA, TIMP-1) yielded a SN/SP of 71%/90%, superior to the performance of CA19-9 alone (SN/SP, 51%/90%). Several other marker combinations had similar diagnostic utility.
The strengths of this multi-center study include the large number of cases, disease controls and healthy controls enrolled; the use antibody-based assays, as well as standardized sample processing and rigorous data analysis. The study provided insight into the performance of many markers and identified marker combinations with improved performance over serum CA19-9 measurements alone. One important limitation was the inclusion of patients with all stages of pancreatic cancer, ~half of whom had stage IV disease. As pancreatic cancer progresses and spreads beyond the pancreas, abnormalities that are not specific to pancreatic cancer accumulate.
As a result, marker behavior is likely to be significantly different among patients with early- vs. late-stage pancreatic cancer (see Figure). Although the use of disease controls can help identify non-specific alterations, advanced pancreatic cancer is associated with many secondary changes including pancreatic injury, inflammation and fibrosis, obstructive jaundice, diabetes, weight loss, cachexia, tumor invasion into the duodenal wall and other surrounding organs and metastases to the liver, peritoneum and elsewhere, and it is difficult to account for all of the non-specific abnormalities associated with advanced pancreatic cancer using disease controls. Even CA19-9, a relatively specific marker of pancreatic ductal adenocarcinomas reaches higher levels and achieves greater diagnostic accuracy when measured in patients with advanced compared to early-stage pancreatic cancer.
Figure 1. The production of tumor markers at different stages in the natural history of pancreatic cancer development.
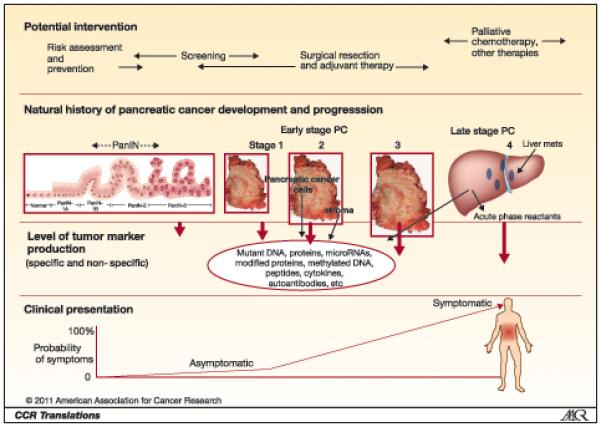
Markers that become readily detectable only advanced stages of pancreatic ductal adenocarcinoma after the onset of clinical symptoms will not provide opportunities for early detection and cure. Detecting pancreatic ductal adenocarcinoma and its precursors at a curable stage requires screening asymptomatic individuals with markers that can reliably detect early stage disease such as PanIN-3 or Stage 1 pancreatic cancer.
Many of the elevated markers evaluated by Brand et al in pancreatic cancer patients were acute phase reactants (SAA, ICAM-1, CRP, osteoprotegerin) whose expression is regulated by inflammatory cytokines and whose primary source is probably not pancreatic cancer cells. These markers are elevated in many inflammatory conditions and have limited diagnostic utility. For example, elevations of ICAM-1 and/or osteoprotegerin are observed not only in chronic inflammatory conditions but are also in patients with conditions common to a pancreatic cancer population including diabetes, hypercholesterolemia, atherosclerotic disease, obesity and hypertension. This likely explains why markers that performed best in the Brand study were proteins thought to arise predominantly from pancreatic cancer cells (CA19-9, CEA and TIMP-1). Although some of the acute phase reactant markers tested did show some ability to discriminate between pancreatic cancers and the benign pancreatic conditions, their diagnostic performance may not be as useful in real world settings where many patients with suspected pancreatic cancer have co-morbidities such as diabetes, atherosclerosis, etc. For these reasons, investigating marker behavior in patients with advanced pancreatic cancer may not be the best strategy for identifying specific markers of early pancreatic cancer.
In current clinical practice, markers have a limited role in diagnosing pancreatic cancer. The best initial diagnostic test for suspected pancreatic cancer is a pancreatic-protocol CT scan. Endoscopic ultrasound is also highly accurate for detecting pancreatic neoplasms. Only highly accurate marker(s) will supplant pancreatic imaging tests as initial tests for pancreatic cancer. For this reason, research efforts are continuing to try and identify highly accurate markers with better performance than the panel identified by Brand et al. Circulating mutant DNA levels reflect tumor burden in patients with colorectal cancer (2), and could prove to be useful for diagnosing pancreatic cancers. Currently, assays measuring circulating mutant DNA are research tools but could become part of clinical practice in the near future. Efforts are underway to develop newer antibody-based tests for proteins overexpressed in pancreatic cancer (3). In addition to ongoing proteomics research for protein markers, other markers are under investigation for their diagnostic utility including aberrantly methylated DNA (4), autoantibodies, aberrantly glycosylated molecules (5) and microRNAs (6).
When evaluating an early detection marker, it is important to determine the goal of early detection. Although recent estimates of cancer evolution suggests that pancreatic cancers can reside in the pancreas for several years before metastasis (7), anecdotal evidence from pancreatic screening studies suggests that some patients can progress from apparently non-invasive pancreatic disease to metastatic pancreatic cancer between short screening intervals. And since cure of invasive pancreatic cancer is rarely achieved even for patients with early-stage pancreatic cancer, the primary goal of pancreatic screening programs for high-risk individuals has been to prevent pancreatic cancer developing by detecting and resecting pancreatic precursor lesions. These precursor lesions include PanINs (Pancreatic Intraepithelial Neoplasias), and IPMNs (Intraductal Papillary Mucinous Neoplasms) (8). Although low-grade PanINs are common, high-grade PanINs (PanIN-3, carcinoma-in-situ) are usually found in pancreata with an invasive pancreatic cancer and in high-risk individuals screened for pancreatic neoplasia. PanINs are too small to be detected by pancreatic imaging, but thanks to better pancreatic imaging IPMNs are increasingly diagnosed and treated. Removing IPMNs or widespread PanIN by pancreatic resection in patients with a strong family history of pancreatic cancer appears to prevent the development of pancreatic cancer (9)(10). The prevalence of detectable neoplasia identified by pancreatic screening depends on the risk of those being screened. Most screening programs target individuals age ≥50 with multiple first-degree relative with pancreatic cancer or BRCA2/p16 and other germline mutation carriers with a family history of pancreatic cancer. Using EUS as a screening tool ~10% of those screened have prevalent IPMNs (>1cm in diameter), and many also have suspected PanIN (10). Risk estimates and recent experience of screening indicate that individuals with less extensive family histories of pancreatic cancer probably have a sufficiently increased risk of pancreatic cancer to justify screening (11)(Kurtz et al, unpublished).
What is the best early detection strategy? Initial results of the CAPS3 multicenter screening trial (clinicaltrials.gov NCT00438906) indicate that pancreatic cystic lesions are detected more often using EUS and MRI than with CT (12). If the goal of early detection is the accurate detection of pre-invasive disease, then marker research should focus on markers of pre-invasive disease. Research is attempting to identify markers in pancreatic fluid that could reliably identify high-grade PanIN (4). Since screening brings with it the risk of overtreatment more controlled trials are needed to better determine the risks, benefits and optimal approaches to pancreatic screening.
The available evidence indicates that the best way to prevent the development of pancreatic cancer and to identify early pancreatic cancer is to follow high-risk individuals with screening protocols. Investigating marker behavior in these high-risk groups is likely to be the best way to identify accurate markers of early pancreatic cancer that can improve the accuracy of pancreatic screening.
Acknowledgments
This work was supported by the National Cancer Institute grants (CA62924, CA120432, RC2CA148346), and the Michael Rolfe Foundation.
References
- 1.Brand RE, Nolen BM, Zeh HJ, Allen PJ, Eloubeidi MA, Goldberg M, et al. Serum Biomarker Panels for the Detection of Pancreatic Cancer. Clin Cancer Res. 2011;17 doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. Epub 2007 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsubayashi H, Canto M, Sato N, Klein A, Abe T, Yamashita K, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–17. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 5.Yue T, Goldstein IJ, Hollingsworth MA, Kaul K, Brand RE, Haab BB. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol Cell Proteomics. 2009;8:1697–707. doi: 10.1074/mcp.M900135-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–37. doi: 10.1158/0008-5472.CAN-09-4227. Epub 2010 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hruban RH, Takaori K, Klimstra DS, Adsay NV, A-S J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Kloppel G, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Shimizu M, Yonezawa S. An Illustrated Consensus on the Classification of Pancreatic Intraepithelial Neoplasia and Intraductal Papillary Mucinous Neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 9.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 10.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 12.Canto M, Schulick R, Kamel RI, Fishman E, Topazian M, Takahashi N, et al. Screening for Familial Pancreatic Neoplasia: A Prospective, Multicenter Blinded Study of EUS, CT, and Secretin-MRCP (The NCI-SPORE/Lustgarten Foundation Cancer of the Pancreas “CAPS3” Study. 2010 [Google Scholar]


