Abstract
Cartilage tissue engineering based on cultivation of immature chondrocytes in agarose hydrogel can yield tissue constructs with biomechanical properties comparable to native cartilage. However, agarose is immunogenic and non-degradable, and our capability to modify the structure, composition, and mechanical properties of this material is rather limited. In contrast, silk hydrogel is biocompatible and biodegradable, and it can be produced using a water-based method without organic solvents that enables precise control of structural and mechanical properties in a range of interest for cartilage tissue engineering. We observed that one particular preparation of silk hydrogel yielded cartilaginous constructs with biochemical content and mechanical properties matching constructs based on agarose. This finding and the possibility to vary the properties of silk hydrogel motivated this study of the factors underlying the suitability of hydrogels for cartilage tissue engineering. We present data resulting from a systematic variation of silk hydrogel properties, silk extraction method, gel concentration, and gel structure. Data suggest that silk hydrogel can be used as a tool for studies of the hydrogel-related factors and mechanisms involved in cartilage formation, as well as a tailorable and fully degradable scaffold for cartilage tissue engineering.
Keywords: cartilage, hydrogel, tissue engineering, chondrocyte, silk, agarose
INTRODUCTION
The clinical need for improved treatment options for patients with cartilage injuries has motivated tissue engineering studies aimed at the in vitro generation of cell-based replacement tissues (or implants) with functional properties: the ability for load bearing and capacity for integration with the host tissues.1 Functional tissue engineering can also involve the application of physical loading during the in vitro cultivation (mimicking the in vivo environment), to foster the development of tissue constructs that can meet the mechanical demands at the time of implantation.1 To achieve tissue functionality, agarose has been a particularly successful material for cartilage, yielding constructs with functional properties that approached native articular cartilage by 6 weeks of culture.2
Agarose is a clear, thermoreversible polysaccharide hydrogel3–5 that has been used extensively for maintaining long-term chondrocyte cultures, and it is currently under clinical trials in Europe as a composite with alginate for cartilage repair.6 By encapsulating dedifferentiated chondrocytes in agarose, Benya and Shaffer4 demonstrated recovery of chondrocytic phenotype following long monolayer culture, as evidenced by increases in synthesis rates of type II collagen and proteoglycan. The lack of attachment sites, hydrophilicity, and electrical neutrality are thought to contribute to the efficacy of agarose. However, agarose can be immunogenic and is nondegradable. Agarose structure, composition, and mechanical properties cannot be customized, limiting our ability to explore the mechanisms underlying cartilage formation in agarose.
Silk fibroin materials in various structural forms (fiber, porous, thin film) have been successfully used as tissue engineering scaffolds7–9 because of their versatility, biodegradation, and biocompatibility. We recently demonstrated long-term stability and biocompatibility of silk scaffolds in vivo.10 Subsequently, silk and silk-composite materials have been examined in vivo for many systems, including bone11–13 and soft tissue.14,15 Silk materials consistently initiate little to no inflammatory or immune response in these animal studies. In addition, silk scaffolds were recently approved by FDA for soft tissue repair and marketed by Serica.16 Silk also biodegrades by ubiquitous proteases in vivo, enzymes that degrade silk over periods of time ranging from days to years, depending on the formulation of silk protein.17,18 Purified native silk fibroin forms a cross-linked hydrogel rich in β-sheets. Several environmental parameters were found to influence the gelation process and gel properties. The original method for preparing silk hydrogels from aqueous solutions of native silk protein involved conditions that are not suitable for incorporation of live cells, such as high temperature, low pH, and long gelation times (days).19
Recently, we developed a new technique for allowing rapid cell encapsulation in silk hydrogels with the full maintenance of cell viability.20 The process allows for manipulation of hydrogel hydrophilicity, electrical charge, and mechanical properties by modifying the parameters during silk extraction and processing.21–24 Primary calf chondrocytes were encapsulated into various formulations of the silk hydrogel by systematically changing silk material concentration and extraction method, or cultured in porous silk scaffolds. One particular silk hydrogel preparation resulted in excellent mechanical properties of engineered cartilage. For the first time, another material—silk hydrogel—produced functional properties of engineered cartilage similar to those achieved with agarose. On the basis of the ability to modify silk hydrogel properties, this finding enables mechanistic study of the efficacy of hydrogels in cartilage development.
MATERIALS AND METHODS
Silk fibroin purification
Two methods were used to purify silk fibroin. In the first method, referred to as salt extraction method, cocoons of B. mori were boiled for 40 min in an aqueous solution of 0.02M sodium carbonate (Na2CO3), and then rinsed thoroughly with pure water. In the second, detergent method, cocoons were boiled for 30 min in an aqueous solution containing 0.05M sodium carbonate and 0.01 wt % Triton X-100 (detergent), and then rinsed thoroughly with pure water. This boiling and washing were repeated once with freshly prepared solution.25 After drying, the extracted silk fibroin was dissolved in 9.3M LiBr solution at 60°C for 4 h, to obtain a 20% (w/v) solution, that was dialyzed against distilled water in Slide-a-Lyzer dialysis cassettes (MWCO 3500, Pierce) for 2 days to remove the salt. The final concentration of the aqueous solution of silk fibroin was 8% (w/v). Silk solutions with lower concentrations were prepared by diluting the 8% solution with water. All solutions were stored at 4°C.
Chondrocyte isolation
Articular cartilage was harvested from fresh bovine carpometacarpal joints obtained from 4- to 6-month-old calves. Cartilage was rinsed and digested in Dulbecco’s Modified Essential Medium (DMEM) with 0.5 mg/mL collagenase type V (Sigma Chemicals, St. Louis, MO) for 10 h at 37°C with stirring. The resulting cell suspension was filtered through a 70 μm pore size mesh to isolate individual cells.26 After rinsing the pellets, the chondrocytes were plated at high density (>1 × 105 cells/cm2) in chondrocyte culture medium (hgDMEM supplemented with 10% FBS, 10 mM HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL Amphotericin B).
Preparation of cell-hydrogel constructs
For silk hydrogel material, sterile DMEM powder was added to the sterilized silk fibroin solution (via autoclaving) at desired concentration. The solution was sonicated (Branson 450 ultrasonicator) to initiate gel formation. Before gelation (10 min after sonication), the silk solution was mixed with the cell suspension. For the preparation of cell/hydrogel constructs, one volume of cell suspension (at 40 × 106 cells/mL in culture medium) was mixed with an equal volume of either silk hydrogel or 4% low-melt agarose (Type VII, Sigma) in phosphate buffered saline (PBS) at 37°C to yield a final cell concentration of 20 × 106 cells/mL in hydrogel. After mixing, the cell/hydrogel mixtures were poured into sterile molds made of two glass plates and plastic spacers that were clamped together to form a uniform rectangular slab (2.5 mm × 70 mm × 80 mm). In all groups, cylindrical disks (4 mm in diameter × 2.5 mm thick) were cored out using a biopsy punch as in our previous studies,26 resulting in 6.2 × 105 cells per scaffold.
Preparation of cell-porous silk constructs
Porous silk scaffolds were prepared as in our previous studies. 27 Briefly, silk fibroin was prepared using the detergent method as described earlier, and the silk solution were mixed with NaCl particles in Teflon cylinder containers and incubated for 24 h at room temperature. NaCl particles were then leached out in distilled water for 2 days, leading to porous silk scaffolds with pore size of 500–600 μm. The porous scaffolds were then sterilized via autoclaving and hydrated by incubation in culture medium (overnight). Similar to the hydrogel constructs, disks (4 mm in diameter × 2.5 mm thick) were cored out using a biopsy punch from the porous silk scaffold stock. Chondrocytes (6.2 × 105 cells per scaffold) were suspended in 25 mL of culture medium and slowly loaded into the hydrated scaffold. Constructs were incubated at 37°C for 2 h for complete attachment. The culture medium was then switched to chondrogenic growth medium for the duration of the culture period.
Construct cultivation
Experimental variables included: (i) silk structure (hydrogel and porous scaffold), (ii) silk protein extraction methods (salt and detergent), (iii) silk concentration in solution (2% and 4%), and (iv) type of hydrogel (silk and agarose). Constructs from all groups were maintained in culture for up to 42 days, with the twice weekly change of chondrogenic growth medium (hgDMEM supplemented with 5 mg/mL proline, 1% ITS+, 100 nM dexamethasone, 50 μg/mL ascorbate, and 10 ng/mL TGF-b3 for the first 2 weeks28).
Biochemical composition
Tissue constructs were blotted dry, weighed, and lyophilized overnight. Dry samples were weighed again and digested with proteinase K overnight at 56°C, as described previously. 2 For glycosaminoglycan (GAG) content, aliquots of digest were analyzed using the 1,9-dimethylmethylene blue dye binding (DMMB) assay.29 For DNA content, additional aliquots were analyzed using the PicoGreen assay (Invitrogen). For total collagen content, aliquots were acid hydrolyzed in 12N HCl at 110°C for 16 h, dried over NaOH, and resuspended in assay buffer (24 mM citric acid monohydrate, 0.012% v/v glacial acetic acid, 85 mM sodium acetate trihydrate, 85 mM sodium hydroxide, pH 6.0). Ortho-hydroxyproline (OHP) content was determined via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde, 30 that was scaled down for microplates. OHP content was converted to total collagen content using the 1:7.64 ratio of OHP to collagen.31 Each biochemical constituent (DNA, GAG, and collagen) was normalized to the tissue wet weight to obtain the actual concentration.
Mechanical properties
Constructs were tested in unconfined compression using our established custom designed testing system.32 Briefly, after equilibration under a tare load of 0.5 g, stress-relaxation tests were conducted at a ramp rate of 1 μm/s up to the 10% strain. The equilibrium Young’s modulus (EY) was calculated from the equilibrium stress and the cross-sectional area of the construct. Unconfined dynamic modulus (G*) was performed after a stress-relaxation to 10% strain at equilibrium, for which a 2% strain was superimposed, at a frequency of 1 Hz.
Histology
Staining for sulfated GAG, total collagen, and overall histomorphology was performed using the protocols described later. Samples were fixed overnight at 4°C in acid formalin ethanol, dehydrated with a graded series of ethanol, embedded in paraffin blocks and cut in cross-section to 7 μm. Once on slides, specimens were deparaffinized with Citri-Solv, rehydrated, and stained with either alcian blue (to view proteoglycan distribution), or picrosirius red (to view bulk collagen), or hematoxylin/counterstained with eosin Y (H&E) (to determine cell distribution). For immunohistochemical staining, tissue sections were first incubated for 1 h with mouse blocking serum to eliminate nonspecific staining, and then with monoclonal antibody against type I or type II collagen, for 16 h at 4°C. The monoclonal antibodies CIIC1 developed by Holmdahl and Rubin33 were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Biotinylated secondary antibody (Vector) was applied to the sections, and the antibody binding was detected with a Vectastain ABC alkaline phosphatase Kit (Vector). The samples were imaged using a color CCD camera mounted onto an inverted microscope (Olympus IX-81) and analyzed using MetaMorph (Molecular Devices, PA).
Statistical analysis
Statistics were performed with Statistica or SPSS Statistics software. Each data point represents the Average ± SD of n = 3–6 samples. Each group was examined for significant differences by analysis of variance (a = 0.05), with EY, GAG, hydroxyproline or DNA as the dependent variable using Tukey’s Honest Significant Difference Test.
RESULTS
Silk hydrogel and porous scaffolds
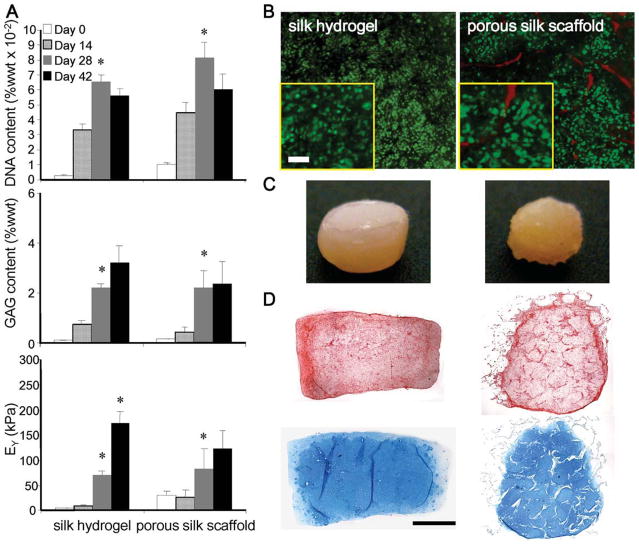
Constructs from both the silk hydrogel and porous silk scaffold groups demonstrated significant growth in vitro, as shown in Figure 1. Cell proliferation and GAG accumulation were comparable for the two groups up to day 28. A decrease of the DNA content (% wet weight) observed for both groups on day 42 was associated with an increase in wet weight. Specifically, there was a 29 and 42% increase of wet weight from day 28 to 42 for the hydrogel and porous scaffold groups, respectively. Furthermore, in the porous scaffold group, the GAG content reached a plateau on day 42, while in the hydrogel group GAG content continued to increase although not significantly. The compressive modulus of the cultured constructs also reached a plateau for the porous scaffold group, and increased continuously and significantly in the silk hydrogel group [Figure 1(A)].
FIGURE 1.
Silk hydrogel and porous scaffolds. (A) DNA, GAG content, and compressive modulus (*p < 0.05 vs. the previous time point, n = 4–5). DNA and GAG contents are expressed per unit wet weight. (B) Live-dead staining on day 42 (insert-magnified for cell morphology, bar = 50 mm). (C) Construct gross appearance after 42 days of culture. (D) Picrosirius red (top) and alcian blue (bottom) staining for collagen and GAG content, respectively. Bar = 1 mm. In (C) and (D), images at the left are for silk hydrogel constructs, and the images at the right are for porous silk scaffold constructs. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Chondrocytes encapsulated in the silk hydrogel exhibited spherical morphology, in contrast to a fibroblastic, spindlelike morphology of cells in porous scaffolds [Figure 1(B)]. In addition, cells appeared smaller in size in hydrogel than in the porous scaffolds. Figure 1(C) illustrates the gross appearance of the constructs after 42 days of culture. The silk hydrogel group maintained the cylindrical disk shape with a smooth surface while the porous scaffolds appeared to be more irregular. Histological staining of collagen and GAG revealed uniform matrix distribution in the hydrogel construct with lacunae formations [Figure 1(D)]. Further-more, immunohistochemical labeling revealed faint type I collagen staining (mostly on edges) and stronger and more uniform type II collagen staining throughout the constructs (Figure 2).
FIGURE 2.
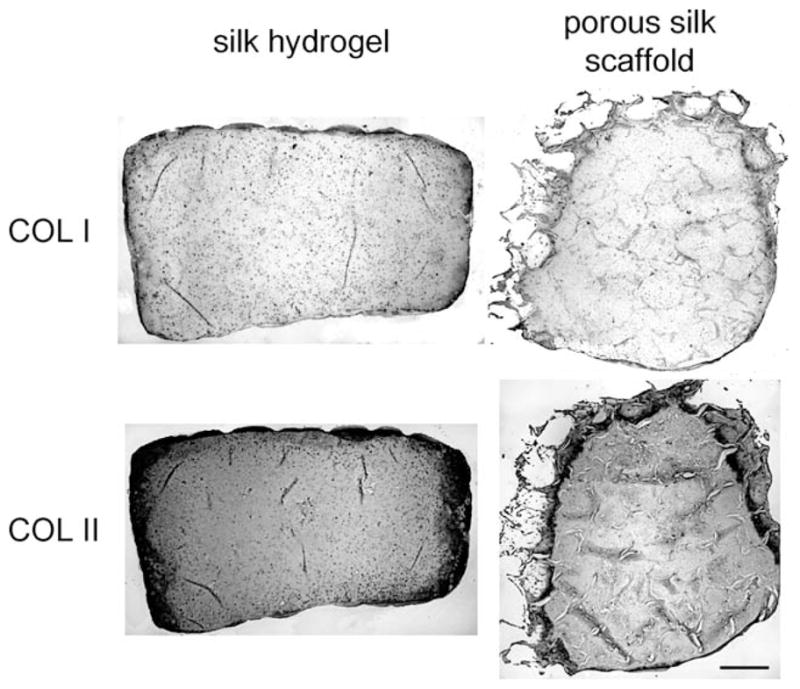
Immunostaining of type specific collagen in silk hydrogel and porous silk scaffolds on day 42. Bar = 0.5 mm.
Formulation of silk hydrogel
As shown in Figure 3(A), 4% hydrogels made by both the salt and detergent extraction methods promoted chondrocyte proliferation and proteoglycan synthesis throughout the duration of culture. Interestingly, while hydrogels made by the salt method promoted cell proliferation more than those made by the detergent method (p = 0.01 on day 14), significantly more GAG were synthesized or retained in the hydrogels made by the detergent method (p < 0.05 on days 14 and 28). We also tested the effect of silk fibroin concentration on cartilage tissue development [Figure 3(B)]. Significant cell proliferation and GAG accumulation were observed in both 2 and 4% gels. Again, more cell proliferation was observed with the 2% group, while more matrix accumulation was observed in the 4% group (p < 0.05 on days 14 and 28 for DNA and GAG contents). Additional parameters of silk formulation such as dialysis time and sonication power were also tested, but without any significant differences between the groups (data not shown).
FIGURE 3.
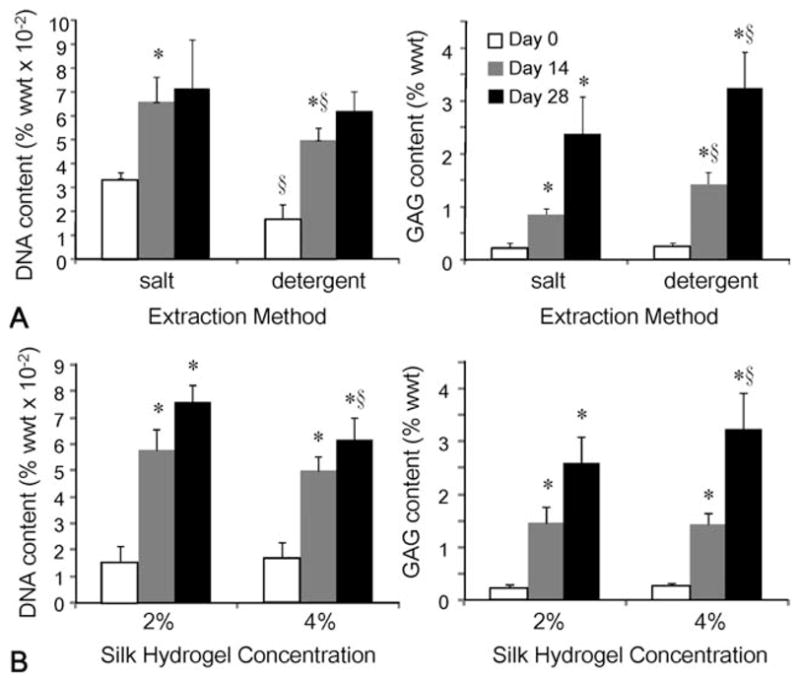
Effects of silk fibroin extraction method (A) and concentration (B) on cartilage tissue development. (*p < 0.05 compared with the previous time point, §p < 0.05 compared with the other group at the same time point, n = 6 for all groups).
FIGURE 4.
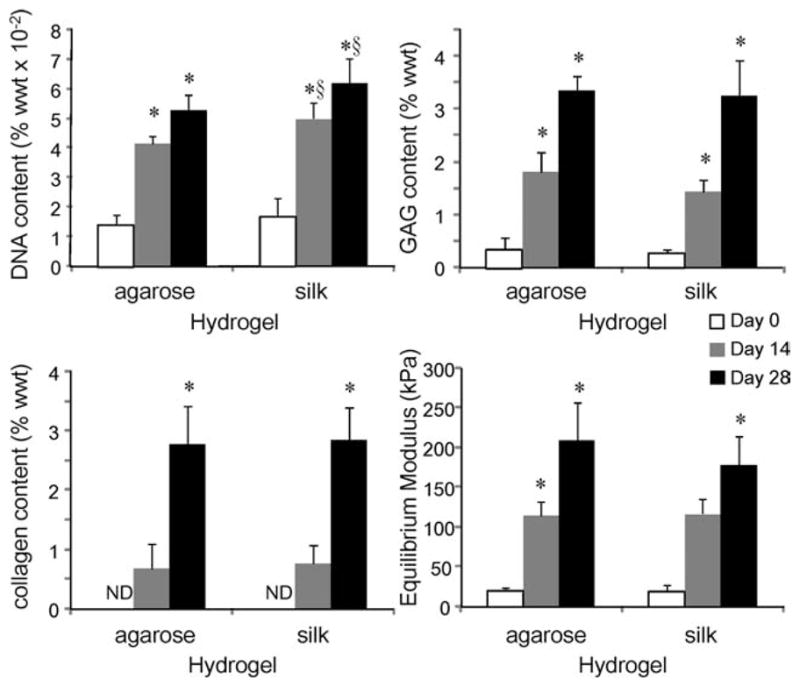
Agarose (2%) and silk (4%) hydrogels. (ND, not detectable; *p < 0.05 compared with the previous time point, §p < 0.05 compared with the agarose group at the same time point, n = 3–6).
Silk and agarose hydrogel
Four percent silk hydrogel constructs extracted with the detergent method were compared to 2% agarose constructs under the same culture conditions (Figure 4). Both hydrogels supported cartilage tissue development, with increases in cell number, GAG and collagen content, and mechanical strength. Silk hydrogels promoted cell proliferation significantly more than agarose hydrogels (p < 0.02 on days 14 and 28). With all other parameters tested, no significant differences were found between the agarose and silk hydrogel groups (at day 28, p = 0.63, 0.79, and 0.13 for GAG content, collagen content, and equilibrium Young’s modulus, respectively).
Intrinsic mechanical properties of silk and agarose hydrogels
To examine if the intrinsic mechanical properties could be a factor in cartilage development, equilibrium and dynamic (at 1 Hz) compressive moduli of the hydrogels and the porous scaffolds were compared (Figure 5). Silk hydrogels made by detergent extraction had similar equilibrium modulus to agarose hydrogel. Silk hydrogel made by salt extraction had significantly lower equilibrium and dynamic moduli. Porous silk scaffold, in contrast, had significantly higher moduli compared to agarose.
FIGURE 5.
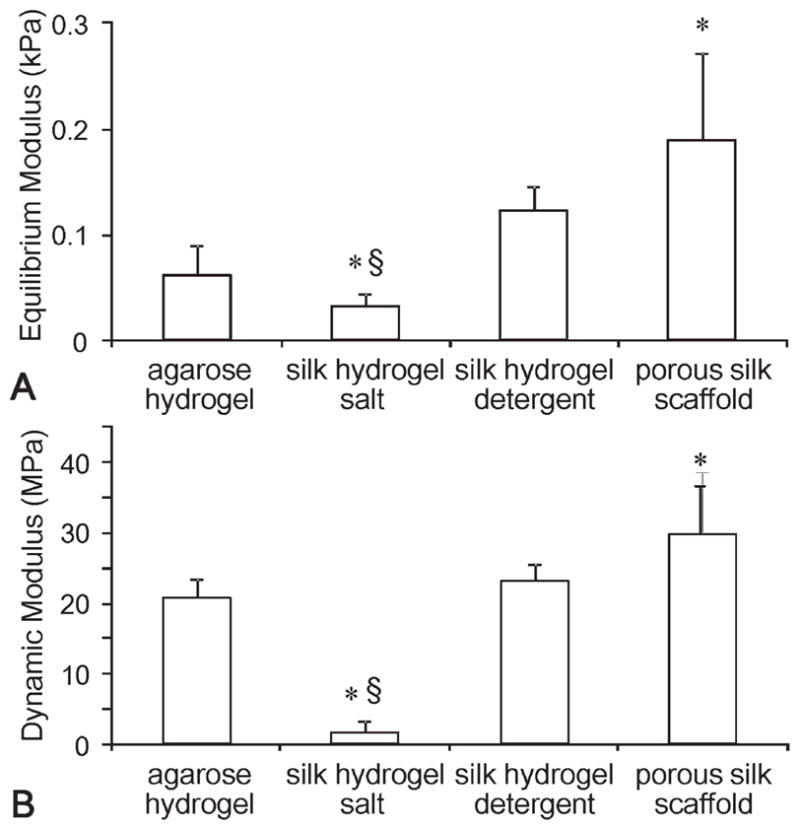
Intrinsic biomechanical properties of the materials. Equilibrium (A) and dynamic (B) modulus (at 1 Hz) (*p < 0.05 compared with the agarose hydrogel group, §p < 0.05 compared with the silk hydrogel detergent.
DISCUSSION
Agarose has been a “gold standard” for tissue engineering of cartilage, yielding constructs with functional properties approaching those of native articular cartilage.2 However, agarose is not biodegradable and can cause immunogenic responses when implanted. To mitigate this effect, and to better understand the efficacy of the agarose hydrogel, we developed a novel silk hydrogel for cartilage tissue engineering using primary calf chondrocytes for their robustness. One formulation of silk hydrogel with primary calf chondrocytes produced engineered cartilage with functional properties similar to those achieved with agarose. On the basis of this finding, several parameters were tested to optimize matrix development, and in particular GAG accumulation.
Silk hydrogel is unique in its capability to provide a neutral and hydrophilic environment that supports the spherical chondrocyte phenotype, similar to agarose. A number of other hydrogels—alginate, collagen, chitosan, and PEG—have been investigated for cartilage tissue engineering; however, none of these materials have resulted in mechanical functionality approaching that of native cartilage.34–36
While hydrogel has been shown to maintain spherical morphology of chondrocytes and promote chondrocytic phenotype,4 many studies have used fibrous scaffolds for cartilage tissue engineering.37,38 Porous and filamentous silk scaffolds have also been used in other tissue engineering applications.8,39 With the new silk hydrogel formulation, we were able to compare silk hydrogels with porous silk scaffolds. It has been proposed that agarose hydrogel promotes the chondrocytic phenotype by maintenance of the chondrocytic morphology.4 To the best of our knowledge, there were no studies to assess the effects of different structures of the same biomaterial on cartilage development.
Both scaffolds (silk hydrogel and porous silk) supported chondrocyte proliferation and phenotype (type II collagen expression and GAG synthesis, Figures 1 and 2). However, by day 42, DNA, GAG, and mechanical properties of the porous scaffold group has reached a plateau. At the same time, silk hydrogel has supported continuous increase of mechanical properties of engineered cartilage. Interestingly, the initial growth profiles for the groups were not significantly different, even though there were observed differences in cell morphology [Figure 1(B)]. Studies have demonstrated decreasing chondrocytic phenotype with 2D culture and serial passaging of primary chondrocytes.4 In our porous scaffolds, while the chondrocytes exhibited a more fibroblastic morphology, the porous structure and minimal passaging may provide some 3D support and resulted in less dedifferentiation.
Variations of silk material and concentration were tested to optimize silk hydrogel for cartilage development. Silk fibroin extractions using salt or detergent were found to be significantly different. Silk extraction using salt has been reported to degrade the fibroin protein and result in smaller molecular weights, whereas the addition of detergents maintained the integrity of silk fibroin protein.25 Figure 3(A) demonstrates that detergent treated silk generated constructs with significantly higher GAG content than salt-treated silk. Silk concentrations were also tested, as a factor shown to influence the secondary structure of fibroin during gelation.40 While higher gel concentration may lead to impeded nutrient transfer,41 the 4% silk hydrogel was found to support cartilage development in terms of GAG content [Figure 3(B)].
Substrate biomechanics is another important factor controlling cell differentiation, proliferation, and behavior.42–44 Equilibrium and dynamic compressive moduli were investigated for all groups. Porous silk scaffolds and silk hydrogel made by the salt method were found to be significantly different from the other hydrogels. Interestingly, the groups with the mechanical properties most similar to agarose initially (day 0) were also the groups that generated similar tissue structures (Figure 5). While many other parameters could contribute to this finding, the intrinsic material mechanical properties may also be an “instructive” cue for tissue development. A more systematic characterization of the relationship between the material physical properties and tissue growth could lead to mechanistic understanding and optimization of tissue engineering materials.
When comparing silk hydrogel parameters, an interesting trend was observed where the group with less DNA had more GAG accumulation (Figure 3). One possible explanation would be the loss of chondrocytic phenotype because of excessive cell proliferation. However, in both circumstances, the number of chondrocytes increased by two to five times over the time of culture, whereas the GAG content demonstrated at least a 9-fold increase. Therefore, it is unlikely that the chondrocytic phenotype was lost. An alternative mechanism may be that while one formulation promotes more cell proliferation, it reduces cellular abilities to organize or retain matrix molecules, resulting in a lesser GAG content. Matrix porosity and permeability have been shown to change the partition coefficient of macro-molecules. 41 Stimulations such as mechanical loading can influence matrix production and organization, as well as proteoglycan release into the medium.45–47
Versatility of the silk fibroin protein allowed for several formulations of silk hydrogel to be tested for cartilage tissue engineering. For the first time, another material generated an engineered cartilage construct that is comparable with those made with agarose, a nondegradable material that has generated constructs with the most physiologic properties.2 The ability to manipulate these parameters using the same material provides a valuable tool for systematic studies of mechanisms underlying its efficacy in supporting chondrogenesis, and to optimize silk hydrogels for engineering human grafts.
Silk is biodegradable and has been FDA approved as an implant material for soft tissue repair.16 In addition, silk can be made into various structural forms—fibers, membranes, mineralized porous scaffolds, and hydrogels.9,17,39 Combining the hydrogel with mineralized scaffolds used for bone tissue engineering, an all-silk osteochondral construct can be built with spatial control of tissue development. The silk system thus provides a creative approach to repair joint damage by utilizing state-of-the-art techniques in tissue engineering.
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant numbers: DE016525, EB002520, EB011869.
Contract grant sponsor: National Taiwan University Development Plan for Excellence; contract grant number: 98R0702
Contract grant sponsor: National Science Council of Taiwan; contract grant numbers: NSC 97-2221-E-002-214, 98-2221-E-002-158
Contract grant sponsor: The Royal Thai Government
References
- 1.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: The role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 2.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J Biomed Mater Res. 1995;29:663–671. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- 4.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 5.Smetana K. Cell biology of hydrogels. Biomaterials. 1993;14:1046–1050. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 6.Selmi TAS, Verdonk P, Chambat P, Dubrana F, Potel J-F, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate- agarose hydrogel: Outcome at two years. J Bone Joint Surg Br. 2008;90B:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann S, Knecht S, Langer R, Kaplan DL, Vunjak-Novakovic G, Merkle HP, Meinel L. Cartilage-like tissue engineering using silk scaffolds and mesenchymal stem cells. Tissue Eng. 2006;12:2729–2738. doi: 10.1089/ten.2006.12.2729. [DOI] [PubMed] [Google Scholar]
- 8.Marolt D, Augst A, Freed LE, Vepari C, Fajardo R, Patel N, Gray M, Farley M, Kaplan D, Vunjak-Novakovic G. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–6149. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Meinel L, Betz O, Fajardo R, Hofmann S, Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic G, Merkle HP, Rechenberg B, Kaplan DL, Kirker-Head C. Silk based biomaterials to heal critical sized femur defects. Bone. 2006;39:922–931. doi: 10.1016/j.bone.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessa PCBE, Hartinger J, Zanoni G, Dopler D, Meinl A, Banerjee A, Casal M, Redl H, Reis RL, van Griensven M. Silk fibroin micro-particles as carriers for delivery of human recombinant bone morphogenetic protein-2: In vitro and in vivo bioactivity. Tissue Eng Part C Methods. doi: 10.1089/ten.TEC.2009.0486. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 12.Park SY, Ki CS, Park YH, Jung HM, Woo KM, Kim HJ. Electrospun silk fibroin scaffolds with macropores for bone regeneration: An in vitro and in vivo study. Tissue Engineering Part A. 2010;16:1271–1279. doi: 10.1089/ten.TEA.2009.0328. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YWC, Friis T, Xiao Y. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials. 2010;31:2848–2856. doi: 10.1016/j.biomaterials.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Etienne OSA, Kluge JA, Bellemin-Laponnaz C, Polidori C, Leisk GG, Kaplan DL, Garlick JA, Egles C. Soft tissue augmentation using silk gels: An in vitro and in vivo study. J Periodontol. 2009;80:852–858. doi: 10.1902/jop.2009.090231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She Z, Liu W, Feng Q. Self-assembly model, hepatocytes attachment and inflammatory response for silk fibroin/chitosan scaffolds. Biomed Mater. 2009;4:45014. doi: 10.1088/1748-6041/4/4/045014. [DOI] [PubMed] [Google Scholar]
- 16.Serica Technologies I. Serica Technologies Receives FDA 510(k) Clearance for SeriScaffold™ Technology for Soft Tissue Repair. Available at: http://www.sericainc.com/en-us/news/2009.
- 17.Minoura N, Tsukada M, Nagura M. Physico-chemical properties of silk fibroin membrane as a biomaterial. Biomaterials. 1990;11:430–434. doi: 10.1016/0142-9612(90)90100-5. [DOI] [PubMed] [Google Scholar]
- 18.Postlethwait RW. Long-term comparative study of nonabsorbable sutures. Ann Surg. 1970;171:892–898. doi: 10.1097/00000658-197006010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim U-J, Park J, Li C, Jin H-J, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5:786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto A, Lindsay A, Abedian B, Kaplan DL. Silk fibroin solution properties related to assembly and structure. Macromol Biosci. 2008;8:1006–1018. doi: 10.1002/mabi.200800020. [DOI] [PubMed] [Google Scholar]
- 23.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Hu X, Daley A, Rabotyagova O, Cebe P, Kaplan DL. Nanolayer biomaterial coatings of silk fibroin for controlled release. J Controlled Release. 2007;121:190–199. doi: 10.1016/j.jconrel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada H, Nakao H, Takasu Y, Tsubouchi K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater Sci Eng C. 2001;14:41–46. [Google Scholar]
- 26.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. The influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 27.Kim U-J, Park J, Joo Kim H, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissueengineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethyl-methylene blue. BiochimBiophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 30.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 31.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 32.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 33.Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- 34.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao P-HG, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 35.Concaro S, Nicklasson E, Ellowsson L, Lindahl A, Brittberg M, Gatenholm P. Effect of cell seeding concentration on the quality of tissue engineered constructs loaded with adult human articular chondrocytes. J Tissue Eng Regen Med. 2008;2:14–21. doi: 10.1002/term.60. [DOI] [PubMed] [Google Scholar]
- 36.Mouw JK, Case ND, Guldberg RE, Plaas AHK, Levenston ME. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257–264. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 38.Carver SE, Heath CA. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5:1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- 39.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto A, Chen J, Collette AL, Kim U-J, Altman GH, Cebe P, Kaplan DL. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006;110:21630–21638. doi: 10.1021/jp056350v. [DOI] [PubMed] [Google Scholar]
- 41.Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152–3157. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Boonen KJM, Rosaria-Chak KY, Baaijens FPT, van der Schaft DWJ, Post MJ. Essential environmental cues from the satellite cell niche: Optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:1338–1345. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- 44.Hadjipanayi E, Mudera V, Brown RA. Guiding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motility and the Cytoskeleton. 2009;66:121–128. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 45.Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 46.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Kelly TN, Ng KW, Wang CC-B, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]