Abstract
Objective/Hypothesis
Determine whether low-frequency rTMS improves tinnitus by decreasing neural activity in auditory processing regions of the temporal cortex and the utility of PET for targeting treatment.
Study Design
Randomized, sham-controlled crossover.
Methods
Patients received a 5-day course of active and sham 1-Hz rTMS (1800 pulses at 110% of motor threshold) to the temporal cortex, with a week separating active and sham treatment. Visual analogue ratings of tinnitus loudness (VARL) were assessed at baseline and the end of each treatment week; regional brain blood flow (rBBF) and glucose metabolism (via PET) were measured before and after treatment in regions of interest (ROI) beneath the stimulating coil and control sites.
Results
The VARL for both ears significantly decreased after active but not sham treatment. Responders comprised 43% of patients, experiencing at least a 33% drop in tinnitus loudness. The site most consistently associated with a positive response was the secondary auditory cortex (Brodmann Area 22) in either hemisphere. PET asymmetries were variable across patients and not always accessible to rTMS. Whereas PET activity decreased significantly beneath the stimulating coil following active treatment, similar changes occurred at control sites and after sham stimulation. Change in tinnitus perception did not correlate significantly with change in PET activity at the treatment site ROI.
Conclusions
Active TMS led to a significant reduction in tinnitus loudness, but PET scans failed to support the hypothesis that low-frequency rTMS improves tinnitus by reducing cortical activation at the stimulation site, questioning the utility of PET for targeting rTMS.
Keywords: Transcranial Magnetic Stimulation, Tinnitus, PET Imaging
INTRODUCTION
Tinnitus, the perception of sound in the absence of external stimulation, affects 17% of the general population in the United States, roughly 51 million people.1 Approximately one-fourth of these individuals seek professional help due to associated mood, sleep, and concentration disturbances. The exact mechanisms of tinnitus generation are uncertain, making diagnosis and treatment difficult and often empirical. Current theories of tinnitus propose both peripheral and central mechanisms.2–5 Sensory deafferentiation due to peripheral injury may incite tinnitus, but central components, such as thalamocortical dysrhythmia6 and cortical reorganization,4 appear to promote and maintain tinnitus. These processes may promote excessive neural activity in auditory processing regions of the temporal cortex, which has been reported in several different types of imaging studies.4,7–14 Reviews of these studies14,15 concluded that tinnitus is indeed associated with changes in brain activity that are characterized by asymmetries between the right and left cortical areas related to auditory processing. Although these reports converge in finding excessive neural activation in the central auditory system consistent with asymmetric excitability, they differ in the exact location and laterality of these changes and none of them directly link this activation to tinnitus perception.
Transcranial Magnetic Stimulation (TMS) has emerged as a potential viable treatment option for tinnitus.12,16–21 TMS induces electrical stimulation of cortical neurons by creating a brief, focused magnetic field over the surface of the brain.22 When magnetic pulses are delivered repetitively and rhythmically, the process is called repetitive TMS (rTMS). The magnetic field induced by TMS is brief (microseconds), relatively weak (except directly under the coil),23 and declines rapidly with distance away from the coil (falling off sharply after 2 cm).22,24 Pulse trains can be delivered at low (≤1 Hz) or high (>3 Hz) frequencies, which tend to decrease or increase neural activity beneath the coil, respectively. Studies of the motor cortex indicate that low-frequency stimulation produces a temporary inhibitory effect25 whereas high-frequency stimulation (>5 Hz) produces an excitatory effect.26,27 Standard TMS coils are only able to directly stimulate the superficial cortex, but deeper brain structures and structures in the opposite cerebral hemisphere may be affected by TMS via connecting neural pathways.28,29 Relatively less is known about the neurobiological mechanisms involved in treatment studies, where the goal is to use rTMS to effect change over days, weeks, and months.
Many studies have used rTMS either to investigate the parameters and locations of stimulation that can disrupt tinnitus transiently or to learn how trains of stimulation delivered over consecutive days can decrease tinnitus chronically.21 Whereas the investigative studies often used high-frequency rTMS (10–20 Hz) to interrupt tinnitus perception, treatment studies have largely relied on applications of low-frequency rTMS. This trend seems to have resulted from the theory that tinnitus is associated with increased neural activity in auditory processing areas within the temporal cortex and that low-frequency rTMS can decrease neural activity.
Several studies used PET scans to visualize areas of excessive asymmetric cortical activity in the left or right temporal cortex and to target 1-Hz rTMS over these areas.12,13,17,19 Changes in neural activity and differences in activity between regions can be seen by PET because they are tightly coupled to associated changes in both regional brain blood flow (rBBF) and glucose metabolism.30 Somewhat surprisingly, however, using PET imaging to guide rTMS has not been shown to be more effective for treating tinnitus than simply placing the stimulating coil over the left temporal cortex without any prior knowledge of whether cortical asymmetries are present or where they may be located.14 One group study used [15O]H2O PET to measure rBBF changes associated with intravenous lidocaine administration.10 Lidocaine can both increase and decrease tinnitus loudness in patients with tinnitus and it can induce tinnitus in persons without tinnitus. Changes in loudness were positively associated with changes in neural activity in the right temporal, auditory association cortex. Substantially larger changes in rBBF were observed following lidocaine-induced decreases rather than increases in tinnitus loudness. No study, to our knowledge, has used follow-up PET scans in a large series of tinnitus patients to determine whether rTMS actually decreases neural activity beneath the stimulating coil and whether change in neural activity is associated with change in tinnitus perception.
The current study was designed to test the hypothesis that tinnitus is associated with increased asymmetric neural activity in auditory processing areas within the temporal cortex and that low-frequency rTMS could be used to improve tinnitus by decreasing neural activity in these regions. Our focus in this manuscript is on assessment immediately following treatment. Baseline PET studies were obtained at rest and before treatment to identify asymmetric areas of increased activity and to guide the placement of the standard, figure-of-eight stimulation coil. Follow-up PET scans were obtained at rest and after a week of active or sham treatment to determine if PET activity decreased beneath the coil and if change in tinnitus perception was associated with change in brain activity detected by PET. Preliminary data from the first 5 participants in this study were published previously.18,19 The protocol was then modified as follows: 1) the original method of sham stimulation—a 45-degree tilt of the active coil—was replaced with a method using a sham coil and electrical stimulation of the scalp; 2) subjects rated tinnitus loudness independently for each ear rather than providing one rating of tinnitus loudness for both ears; and 3) the Tinnitus Handicap Questionnaire (THQ) was added as a baseline assessment and the Beck Depression Inventory (BDI) was added as an outcome measure in addition to the Tinnitus Severity Index (TSI). Methods of rTMS delivery and PET acquisition were not modified.
MATERIALS AND METHODS
Study Subjects
A total of 21 patients (28 to 75 years of age) with chronic bilateral tinnitus of more than 6-month duration were enrolled in this treatment trial. All participants were seen in the Hearing and Balance Clinic (HBC) by the coauthor (J.D.) and diagnosed as having subjective, bilateral tinnitus. Audiograms obtained upon entry showed that 5% of subjects had normal hearing, 15% had mild sensorineural hearing loss (SNHL), 40% had moderate SNHL, 35% had severe SNHL, and 5% had profound SNHL (at 8 kHz). SNHL was observed in the 4-kHz range for 37% of subjects, in the 6 kHz-range for 21%, and in the 8-kHz range for 42%.
Participants completed either the THQ (n=16) or the TSI (n=13) at baseline (8 patients completed both measures). The THQ yields a total score and three factor scores. Subjects taking the THQ obtained a mean percent total score of 50.1 (SE=6.4), which corresponds to the 70th percentile of a large normative sample of patients seen at audiology clinics.31 A mean percent score of 48.7 (SE=7.3) was obtained for factor one of the THQ (i.e., social, emotional, and behavioral effects of tinnitus), which corresponds to the 70th percentile; a mean percent score of 51.2 (SE=7.9) was obtained for factor two (i.e., hearing loss), which corresponds to the 72nd percentile; and a mean percent score of 53.2 (SE=4.9) was obtained for factor three (i.e., patient’s view of tinnitus), which corresponds to the 55th percentile. Subjects completing the TSI obtained a mean score of 29.5 (SE=2.6), which corresponds to a low average score. Subjects completing the BDI (n=16) obtained a mean score of 7.3 (SE=1.9), which indicates few or no significant depressive symptoms for the sample as a whole.
All subjects met inclusion criteria as follows: 1) completing an informed consent process and signing a written informed consent to participate in the study, which was approved by the Institutional Review Board governing the use of human subjects in biomedical research; 2) completing and passing the Transcranial Magnetic Stimulation Adult Safety Screen (TASS); 3) having a CT or MRI scan showing no brain abnormality. Individuals taking selective serotonin reuptake inhibitors (SSRIs) for depression related to tinnitus had to be stable on doses of these medications for 3 months and could not change medications during the course of the study. No subjects met any of the exclusion criteria, which were as follows: 1) a history of epilepsy (or a first-degree relative diagnosed with epilepsy), head injury resulting in loss of consciousness for >10 minutes, aneurysm, stroke, previous cranial neurosurgery, acoustic neuroma, or glomus tumor; 2) active Meniere’s disease; 3) diagnosis of a neurological or major psychiatric disorder (excluding depression or anxiety related to tinnitus); 4) metal implants in the head or neck or a pacemaker (because of possible interference with the magnetic field); 5) pregnancy or the possibility of becoming pregnant during the study; or 6) currently taking medications that lower seizure threshold or reduce cortical excitation (e.g., tricyclic antidepressants, benzodiazepines, bupropion, or anticonvulsants).
Apparati
PET imaging
Both a baseline FDG-PET/CT scan and post–rTMS treatment FDG-PET scan were performed using a Biograph 6 PET/CT scanner (Siemens Medical Systems, Malvern, PA). The CT portion was a 6-slice Siemens Sensation helical CT scanner, and the PET portion had “Hi-Rez”® LSO (lutetium silicate oxime) 4-mm crystals arranged in a full-ring gantry with high-speed Pico Electronics.™ Images were acquired 30 min after the intravenous administration of 12 mCi (444 MBq) FDG (fluorodeoxyglucose). Automated analysis of the PET brain studies was performed by the NeuroQ™ Display and Analysis Program (Cardinal Health, Dublin, Ohio, Version 2.0). This system expresses activity within predefined regions of the participant’s brain image as standard deviations of the mean activity, in the same predefined regions, obtained from a normal PET brain database of 50 patients. The registration algorithm for fitting the patient’s brain scans to the normal template was a robust spatial transformation method.32
Active and sham TMS
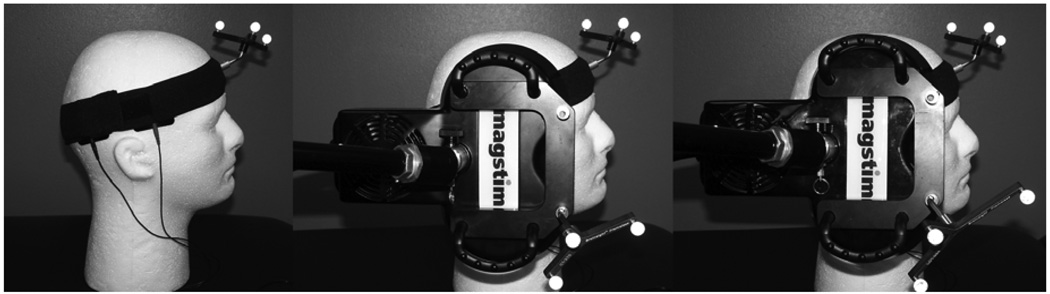
A Magstim Super Rapid stimulator and two Magstim air-film, figure-of-eight, 70-mm stimulating coils were used to deliver stimulation (Figure 1). One coil is active and the other is a sham coil (Magstim Company, Whitland, Wales, UK). A neuronavigational system (Brainsight Frameless Steriotaxy, Rouge Research) was used to place the stimulating coil over a predetermined site in the temporal cortex on the coregistered CT image and to track coil location across treatment sessions. Active stimulation was delivered via an active stimulating coil, and sham stimulation was delivered using a visually identical sham coil that produces clicking sounds but delivers only 5% of the maximum stimulator output. In a method developed in our laboratory33 two large rubber electrodes are placed on the scalp over the temporal lobe and deliver electrical stimulation from a DS3 Isolated Stimulator (Digitimer Ltd., Welwyn Garden City, Hertfordshire, U.K.) sufficient to cause scalp muscle twitching (i.e., between 9 and 15 mA). Electrical pulses, of 500-µsec width, are triggered by the Magstim unit and coincide exactly with each rTMS pulse. Electrical stimulation is on when the sham coil is in use but is off when the active coil is in use.
Figure 1. rTMS Apparatus.
Figure shows electrode placement (left panel) as well as sham (center) and active rTMS coils (right).
Procedure
Trial design
Each patient entered a randomized, sham-controlled clinical trial with treatment crossover that used 1-Hz rTMS to treat chronic tinnitus. Subjects were randomly assigned to receive either active or sham treatment first, with one week of no treatment intervening between the sham and active weeks.
Targeting rTMS
The original plan for targeting rTMS delivery was to visualize areas of increased, asymmetric neuronal activity over the temporal cortex using the baseline PET scans obtained from each subject. Areas of asymmetry were to be marked on the co-registered CT image and then used to guide coil placement over this area within the temporal lobe. A revised plan for targeting rTMS had to be adopted, however, when it became apparent that baseline PET asymmetries did not always yield reliable targets for rTMS. Whereas 61% of subjects exhibited asymmetries at baseline that could be targeted with rTMS (i.e., at least a 10% difference between the left and right temporal cortex), 14% of subjects had asymmetries deep within the temporal lobe and 24% had asymmetries in the inferior temporal lobe curving inward and away from the ear and jaw that were not accessible to rTMS.
A revised targeting algorithm was developed as follows: 1) the temporal lobe PET asymmetry was targeted when it was both clear and accessible to rTMS (61%), 2) the posterior one-third portion of the superior temporal gyrus that lies opposite to the ear with loudest tinnitus was targeted if PET asymmetry was not accessible to rTMS (33%), and 3) the same location in the left hemisphere was targeted when no PET asymmetry was accessible and when tinnitus perception could not be lateralized (5%) (targeting the left temporal lobe is a standard approach in the literature21). The right temporal lobe was targeted for treatment in 62% of subjects, and the left temporal lobe was targeted in 38%. Other factors affecting coil location were repositioning of the coil if a subject complained of discomfort during rTMS and drifting of the coil, which occurs due to either movement of the coil or the subject’s head during rTMS delivery.
Each treatment site was tracked in BrainSight on the participant’s CT image. These sites were transferred to a standard template of a brain image using a program called MRICroN. The location of each stimulation site was defined by a circular “region of interest” (ROI) with a 3-mm radius. A subtraction procedure (described below) was used to identify sites common among participants who responded to rTMS but not those who failed to respond to rTMS. Sites in the treatment responders were designated “positive” and those in nonresponders “negative”.
Delivering stimulation
During the week of active treatment, rTMS was applied one time per day at an intensity of 110% of the motor threshold (MT) measured that day and at a rate of 1 Hz for a total of 30 minutes (i.e., 1800 pulses per session). Treatment was delivered for 5 consecutive days (9000 pulses total). The first five subjects in our series were tested in a protocol that used a 45-degree coil-tilt method of sham stimulation.18,19 The remaining subjects were tested using the sham stimulation described above.33
Measuring change in tinnitus and comorbidities following rTMS
The primary outcome measure of tinnitus perception was the VARL, or ear-specific visual analogue rating of tinnitus loudness (0=tinnitus absent; 100=extremely or painfully loud tinnitus), which was assessed at baseline and before and after each day of treatment. Additionally, both the TSI and BDI were assessed at baseline and on the last day of each treatment week (active and sham). Tests of neuropsychological function (i.e., the Digit Symbol Test, the three-words-at-five-minutes test, and the finger tapping test34) were also administered before and after each rTMS session as measures of safety rather than as primary outcome measures.
Measuring change in PET
Follow-up resting PET scans were obtained on the last day of the active treatment week, except for four subjects who had PET scans on the last day of the sham week as a control. Subjects were informed that they would have active and sham treatment and that the PET scan could follow either treatment week. They were not informed; however, which treatment was active or sham or which would occur before the follow-up PET scan. PET analyses were performed using the NeuroQ™ Display and Analysis Program (Cardinal Health, Dublin, Ohio, Version 2.0). The primary, predefined ROI for data analysis was an area over the superior lateral temporal cortex of the right and left hemispheres where rTMS was applied. Data for control ROIs over the 1) sensori-motor, 2) primary visual, 3) mid-frontal, and 4) inferior lateral posterior temporal cortex of each hemisphere were also analyzed. PET data for each ROI in the baseline and post-treatment scans was expressed as a standard deviation of the mean value for the corresponding ROI from the NeuroQ™ database of 50 normal subjects (Cardinal Health, Dublin, Ohio, Version 2.0).
RESULTS
Behavioral Effects of rTMS
Consistent with most rTMS studies of tinnitus,21 no detrimental effects of active or sham rTMS were observed on any of the neuropsychological tests, and there were no reported changes in hearing or in available follow-up audiological studies at the end of treatment. Neither the TSI nor the BDI changed significantly from baseline as a result of either active or sham treatment.
The effect of rTMS on the VARL for each ear was evaluated using a 2 (treatment type: sham or active) × 2 (side of rating: ipsilateral or contralateral to rTMS) × 2 (treatment order: active or sham first) mixed model, three-way ANOVA. The dependent measure was change in the VARL from pre- to post-active and sham treatment, expressed as a percentage of the pretreatment score (i.e., pre-treatment VARL minus the VARL obtained on the last day of the active and sham treatment weeks, respectively, divided by the pretreatment score multiplied by 100). Results of the ANOVA revealed a significant three-way interaction between treatment type, side of rating, and treatment order (F (1, 18) = 6.10, p<.03). There was also a significant main effect for the between subjects factor of treatment order (F (1, 18) = 6.77, p<.02.) Follow-up analysis of the interaction using simple effects revealed a significant order effect observed primarily for the ear contralateral to treatment. When active treatment came first, a significant carryover effect was observed into the sham treatment week for the ear contralateral to treatment (F=11.5, df=18,1, p<.003), i.e., the VARL was decreased from baseline by 31% (SE=8) at the end of the sham treatment week. A similar but marginal effect was observed for the ear ipsilateral to treatment (F=3.89, df=18,1, p<.064), which was decreased by 12% (SE=9) from baseline. In contrast, when sham treatment came first, ratings of tinnitus loudness were not significantly different from pretreatment either for the ear contralateral or ipsilateral to treatment (i.e., a mean increase of 9.7% [SE=8.7] for the contralateral ear and a mean decrease of 2.3% [SE=9.9] for the ipsilateral ear).
Because the main effect comparing sham and active treatment was contaminated by carryover of active treatment into the sham treatment week, a paired t-test was used to compare the VARL obtained at baseline to that at the end of the active treatment week. The mean VARL was significantly decreased from baseline for both the contralateral [t=3.06, df=20, p<.006; baseline mean (SE) = 58.8 (21.2); active treatment mean (SE) = 47.7 (27.4)] and ipsilateral ear [t=2.3, df=20, p<.03; baseline mean (SE) = 32.9 (25.7); active treatment mean (SE) = 27.9 (26.2)].
Classification as Treatment Responder Versus Nonresponder
Subjects were classified as either responders or nonresponders to rTMS based on the percentage of change in the VARL from pre- to post–active treatment (Table 1). The percent difference scores were converted to z scores. A treatment responder was defined as someone who had a negative z score for one or both ears. A negative z score corresponded to a minimum of a 33% decrease in the VARL from baseline following active treatment. This classification resulted in 9 treatment responders (43%) and 12 nonresponders (57%).
Table 1.
Percent change in VARL rating following active stimulation
| Ispsilateral | Contralateral | ||||
|---|---|---|---|---|---|
| Order | % Diff | Z Score | % Diff | Z Score | Class |
| 8 | −36 | −.43 | −92 | −2.23 | R |
| 11 | −50 | −.80 | −90 | −2.17 | R |
| 17 | −63 | −1.16 | −70 | −1.51 | R |
| 10 | −89 | −1.83 | −46 | .75 | R |
| 16 | −14 | .14 | −44 | −.69 | R |
| 1 | −42 | −.60 | −42 | −.64 | R |
| 7 | −33 | −.36 | −33 | −.34 | R |
| 14 | −87 | −1.79 | −21 | .06 | R |
| 13 | 0 | .52 | −20 | .07 | R |
| 9 | −19 | .02 | −16 | .20 | NR |
| 4 | −14 | .15 | −14 | .27 | NR |
| 20 | 82 | 2.68 | −7 | .49 | NR |
| 15 | 1 | .55 | −7 | .50 | NR |
| 19 | 0 | .52 | −4 | .58 | NR |
| 21 | −17 | .06 | 0 | .72 | NR |
| 6 | 0 | .52 | 0 | .72 | NR |
| 18 | −50 | *−.80 | 3 | .82 | NR |
| 5 | 0 | .52 | 5 | .89 | NR |
| 12 | 0 | .52 | 6 | .90 | NR |
| 3 | 10 | .79 | 10 | 1.05 | NR |
| 2 | 11 | .80 | 11 | 1.06 | NR |
Ipsilateral = ear ipsilateral to stimulation, Contralateral = ear contralateral to stimulation.
Order refers to the order in which subjects were enrolled (Patients are listed in order of z score for the contralateral side).
% Diff = the percentage of change in VARL score from baseline to the last day of active treatment. The z score is the standardized z score calculated from each % Diff distribution.
Class is classification of treatment outcome: R=treatment responder (negative z score following active stimulation for either ear, corresponding to at least a 33% decrease in VARL from baseline); NR=nonresponder (positive z score after active stimulation).
Subject was classified as NR because VARL changed from 2 to 1, which is not clinically relevant.
Targeting
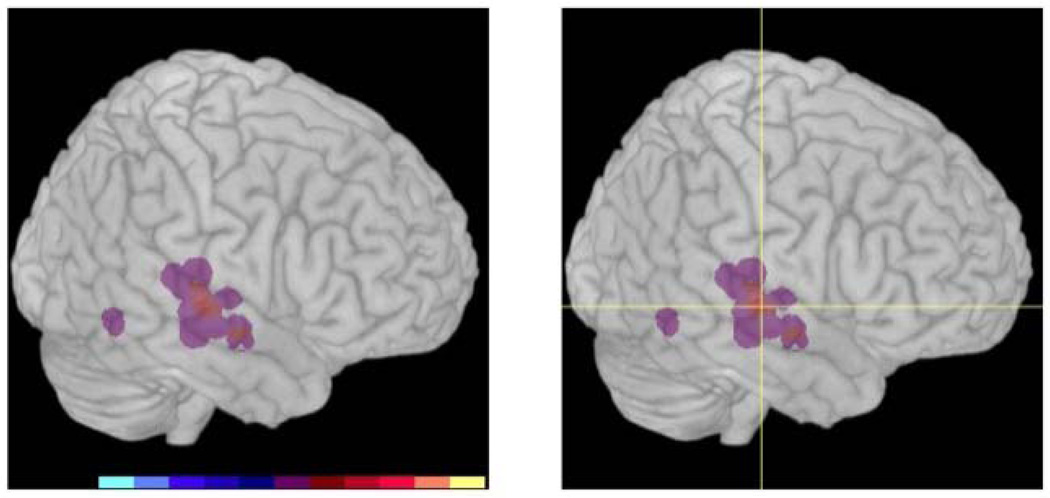
A positive response to rTMS was associated with targeting treatment over the secondary auditory cortex, or Brodmann area 22. Sites in the right hemisphere provided the clearest distinction and are displayed in Figure 2. The 3D brain image shows where the positive areas overlap and are indicated by the brighter red colors (image on the left).
Figure 2. Sites of rTMS delivery.
Left image: Shows overlap of areas stimulated in the right hemisphere. Right image: Crosshair shows the location of the site that was common among subjects who responded positively to rTMS but not common to subjects who failed to respond to rTMS. The site corresponds to Brodmann area 22.
Change in PET following rTMS
Pre- and post-treatment PET scans were available for 18 patients (7 treatment responders and 11 nonresponders). Fourteen subjects had PET scans before and after active stimulation. Four subjects had PET scans before and after sham stimulation. The dependent measure was the patient’s standard deviation score relative to the database of 50 normal subjects for the following ROIs. The primary ROI was the superior lateral temporal cortex (sLT), where rTMS was delivered in all subjects. Control sites included ROIs for the sensory motor cortex (SM), inferior lateral parietal temporal cortex (iLPT), and primary visual cortex (PVC), where rTMS was not delivered. Data were analyzed using a 2 × 2 × 2 mixed model ANOVA. The factors were treatment site (i.e., ROIs ipsilateral versus contralateral to rTMS), time of PET acquisition (i.e., pre- versus post-rTMS), and treatment response (i.e., responder versus nonresponder to rTMS). Separate analyses were conducted for active and sham stimulation owing to the large differences in sample size. Power analyses were conducted for significant interactions involving treatment site to understand how sample size influenced results. Analyses for active stimulation are presented first.
Interactions involving treatment site
Analysis of the primary ROI (sLT) revealed a significant interaction of treatment site and time, F (1, 12) = 7.01, p <.05. Paired t-tests showed that this effect was accounted for primarily by a decrease in activity post-rTMS for the ROI located ipsilateral to stimulation (pre: M = .001, SD =.84; post: M = −.41, SD= 1.06; t (13) = 1.96, p=.07). The statistical power for this effect was low (.44) and the effect size was small to medium (Cohen's d = .31).
Analysis of one of the control ROIs (SM) similarly showed a significant interaction of treatment site and time, F (1, 12) = 5.08, p<.05. A paired t-test revealed the effect was also accounted for by a decrease in activity post-rTMS in the ROI located ipsilateral to stimulation (pre: M = .30, SD=.40; post: M = .12, SD= .28; t (13)=2.27, p<.05). Power for this effect was low (.56) and the effect size was medium (.61).
Main effects
A significant main effect of treatment site was observed for one of the control ROIs (iLPT), F (1, 12) = 5.44, p<.05, such that activity was greater for the ipsilateral (M = −.45, SE=.09) than contralateral ROI (M= −.67, SE=.10) both before and after rTMS, suggesting that this baseline asymmetry was not altered by rTMS.
A significant main effect of time was observed for one of the control ROIs (PVC), F (1, 12) = 8.19, p<.05), such that activity increased in this region of both cerebral hemispheres post-rTMS (pre: M= .04, SE=.11; post: M = .35, SE= .11).
The effect of treatment response was not significant in any of the ANOVA models. Further, change in tinnitus loudness following active rTMS did not correlate significantly with change in PET activity at the site of TMS (r = .33 for the ipsilateral sLT ROI and r =.27 for the contralateral sLT ROI, p>.05).
Effect of sham stimulation
A series of paired samples t-tests was conducted to determine whether sham stimulation influenced PET activity at each ROI. None of these comparisons reached significance. Whereas, statistical power was uniformly low (ranging from .05 to .19) due to the small sample size (n=4); medium effect sizes were observed for several comparisons. The effect size for pre–post comparisons of the ipsilateral sLT reached .54 with increased activity post stimulation, the ipsilateral PVC reached .75 with increased activity post stimulation, and the contralateral SM reached .67 with decreased activity post stimulation.
DISCUSSION
This study tested the hypothesis that tinnitus relates to increased, asymmetric activity in auditory processing areas of the temporal cortex. It is unique among other studies in its attempt to compare PET scans obtained before and after active and sham 1-Hz rTMS treatment. We discuss first the effect of rTMS on perception of tinnitus loudness and then turn to comparisons of PET scans before and after stimulation. A week-long course of active, low-frequency rTMS led to a significant reduction in VARL ratings for both ears by the end of the treatment week. Sham stimulation had no effect on tinnitus loudness ratings for either ear; however, a significant carryover effect of active stimulation was observed into the sham treatment week for those subjects who received active treatment first. This carryover effect was most pronounced for the ear contralateral to treatment, where, in general, treatment effects were observed to be the strongest. Because the carryover effect of active stimulation was assessed at the end of the week of sham stimulation, it is apparent that tinnitus remained significantly reduced for a minimum of two weeks in those subjects who received active stimulation first.
Individual subjects could be classified as either treatment responders or nonresponders. Forty-three percent of subjects were classified as responders, experiencing at least a 33% reduction in tinnitus loudness from baseline. The remaining 57% of subjects did not report marked changes in tinnitus perception following rTMS. More patients in this sample received treatment over the right than left temporal lobe, but active treatment of either hemisphere could produce a treatment responder. These results converge with previous studies of tinnitus, which show that rTMS improves tinnitus in approximately 50% of patients, across studies and laboratories, following stimulation of either the left or right hemisphere.21 The rate of improvement in this study might have been lower than 50% simply because of sampling or because our method of defining a treatment responder is conservative. We think this classification scheme has advantages over questionnaires that assess tinnitus generally because it focuses directly on changes in perception of tinnitus loudness and this is what we aimed to achieve in applying 1-Hz rTMS as a treatment. Although scores improved on the TSI following treatment, the change was not significant despite rather large improvements in tinnitus loudness. Therefore the VARL appears to have better sensitivity for detecting rTMS-induced perceptual changes in tinnitus than a questionnaire like the TSI.
PET asymmetries were of limited use in targeting rTMS. Asymmetries were accessible to rTMS in only 61% of subjects. As a result, our initial attempt to target rTMS using PET scans evolved into a decision flow chart that targeted the superior posterior temporal lobe opposite the ear with loudest tinnitus when PET asymmetries were not accessible and to target the left temporal lobe as a default. Our experience is apparently consistent with that of other studies that used PET to guide rTMS treatment for tinnitus. A recent review concluded that PET-guided targeting is no more effective than simply placing the coil over the left temporal cortex.14 In fact, it may not even be advantageous to target the left rather than the right temporal lobe as we observed a positive response to rTMS associated with targeting treatment over the secondary auditory cortex (i.e., Brodmann area 22) in either hemisphere.
A comparison of PET scans before and after treatment did not support the hypothesis that 1-Hz rTMS improves tinnitus by decreasing neural activity in auditory processing areas of the treated hemisphere. Comparing activity in the ROIs of the patient’s scans before and after treatment lacked a specific relationship either to the site of rTMS delivery, to treatment outcome, or even to active stimulation. Whereas a significant decrease in activity beneath the site of stimulation was observed following active rTMS, similar changes could be observed in control ROIs that did not receive stimulation. Further, change in tinnitus perception was not significantly correlated with change in PET activity at the site of stimulation or any other site. Finally, effect sizes observed for three ROIs following sham stimulation were larger than those observed in association with active stimulation. It is important to note that one of these four subjects received active stimulation the week prior to sham stimulation, and so carryover effects of active treatment are likely for this person; however, the remaining three subjects received sham stimulation first and none was classified as a treatment responder.
Limitations in our method might have hindered our ability to find changes in brain activation associated with changes in tinnitus. For example, the NeuroQ program limited our analysis to predefined ROIs. We could not, for example, examine ROIs involving limbic structures that might influence emotional aspects of tinnitus. A more powerful and targeted analysis of the PET data might have yielded different results. Additionally, we compared PET scans obtained at rest, which can be influenced by factors other than rTMS-induced changes in tinnitus. Also, acquisition of the second PET scan was timed to coincide with the last day of treatment. This strategy; however, may not be optimal because in some patients there may be a delay between the end of treatment and the time before tinnitus perception improves.35 In the remainder of the discussion, we compare our study to the only other treatment study of tinnitus, to our knowledge, that used functional imaging as an outcome measure following sham and active rTMS.
Marcondes et al.35 examined the effects of 1-Hz rTMS over the left temporo-parietal cortex in patients with tinnitus with no hearing impairment using single-photon emission computed tomography (SPECT) obtained at baseline and two weeks following treatment. Sham (n=9) and active rTMS (n=10) were compared using a parallel treatment design. Active stimulation improved tinnitus as measured with the Tinnitus Handicap Inventory and a VARL. Sham did not. Analysis of SPECT scans showed reductions of neural activity in the inferior temporal lobes of both hemispheres (below the site of stimulation) and increased activity in the right uncus and cingulate gyrus in association with active rTMS. Increased activity in the left middle temporal gyrus, the cingulate gyrus bilaterally, and in the right insula was observed after sham stimulation.
Even though the Marcondes study differs from the current study in subject selection, design, imaging technique, and delay of the follow-up scan; the two studies are similar in failing to support a connection between rTMS-induced change in tinnitus perception and change in neural activity within auditory processing regions of the temporal cortex. Instead, after active stimulation, Marcondes et al. found changes in ROIs with an unclear relationship to tinnitus perception and, after sham stimulation, changes that were perhaps even harder to interpret. In light of the observation that neuroimaging does not improve the efficacy of rTMS for tinnitus over non-guided coil placement,14 one has to question how sensitive these imaging techniques are to states of cortical activity associated with tinnitus perception. If one accepts that they are sensitive, then the simple hypothesis that rTMS decreases tinnitus by inhibiting excessive neural activity within auditory processing regions of the temporal lobe is probably incorrect.
CONCLUSIONS
In agreement with other published work regarding rTMS as a treatment for chronic bilateral tinnitus, a marked reduction of tinnitus loudness was seen in 43% of subjects, with a positive response seen most frequently when the secondary auditory cortex (Brodmann area 22) was targeted, regardless of side. Variability in the presence and location of pretreatment asymmetries on PET combined with a poor correlation between change in the post-treatment scan and change in tinnitus perception after treatment raises questions about the sensitivity of this technique for targeting rTMS and for detecting rTMS-induced changes in tinnitus perception. While 1-Hz rTMS is emerging as a promising, noninvasive treatment for chronic tinnitus, it is unlikely to work simply by decreasing neural activity beneath the stimulating coil. Rather, rTMS treatment probably induced generalized changes in functionally linked networks that regulate the emotional, attentional, and perceptual aspects of tinnitus which could be evaluated using more advanced methods of PET analysis than were available for this study.
Acknowledgments
This study was supported by the NIH: National Center for Research Resources Centers of Biomedical Research Excellence (COBRE) Grant Number RR20146 1UL1RR029884; National Institute of Neurological Disorders and Stroke NS39348; National Institute of Child Health and Human Development HD55677 and HD055269; and by a Tinnitus Research Consortium Grant-in-Aid. Dr Jeffery Myhill served as a study coordinator.
Footnotes
This study was performed at the University of Arkansas for Medical Sciences, Little Rock, AR
Conflict of interest: None
REFERENCES
- 1.Coles R. Epidemiology of tinnitus. Edinburgh: Churchill Livingstone; 1987. pp. 46–70. [Google Scholar]
- 2.Kadner A, Viirre E, Wester DC, et al. Lateral inhibition in the auditory cortex: an EEG index of tinnitus? Neuroreport. 2002;13:443–446. doi: 10.1097/00001756-200203250-00016. [DOI] [PubMed] [Google Scholar]
- 3.Jastreboff PJ, Gray WC, Gold SL. Neurophysiological approach to tinnitus patients. Am J Otol. 1996;17:236–240. [PubMed] [Google Scholar]
- 4.Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- 6.Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Folmer RL, Griest SE, Martin WH. Chronic tinnitus as phantom auditory pain. Otolaryngol Head Neck Surg. 2001;124:394–400. doi: 10.1067/mhn.2001.114673. [DOI] [PubMed] [Google Scholar]
- 8.Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- 9.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 10.Reyes SA, Salvi RJ, Burkard RF, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171:43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- 11.Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–199. doi: 10.1159/000276835. [DOI] [PubMed] [Google Scholar]
- 12.Kleinjung T, Eichhammer P, Langguth B, et al. Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg. 2005;132:566–569. doi: 10.1016/j.otohns.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 13.Langguth B, Eichhammer P, Wiegand R, et al. Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. Neuroreport. 2003;14:977–980. doi: 10.1097/00001756-200305230-00014. [DOI] [PubMed] [Google Scholar]
- 14.Langguth B, Kleinjung T, Landgrebe M, De Ridder D, Hajak G. rTMS for the treatment of tinnitus: the role of neuronavigation for coil positioning. Clin Neurophysiol. 2010;40:45–48. doi: 10.1016/j.neucli.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Marcondes R, Fregni F, Pascual-Leone A. Tinnitus and brain activation: insights from transcranial magnetic stimulation. Ear Nose Throat J. 2006;85:233–234. 236–238. [PubMed] [Google Scholar]
- 16.Rossi S, De Capua A, Ulivelli M, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus: a randomised, crossover, double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 2007;78:857–863. doi: 10.1136/jnnp.2006.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plewnia C, Reimold M, Najib A, Reischl G, Plontke SK, Gerloff C. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. J Neurol Neurosurg Psychiatry. 2007;78:152–156. doi: 10.1136/jnnp.2006.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richter GT, Mennemeier M, Bartel T, et al. Repetitive Transcranial Magnetic Stimulation for Tinnitus: A Case Study. Laryngoscope. 2006;116:1867–1872. doi: 10.1097/01.mlg.0000234936.82619.69. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Mennemeier M, Bartel T, et al. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope. 2007;117:529–534. doi: 10.1097/MLG.0b013e31802f4154. [DOI] [PubMed] [Google Scholar]
- 20.Dornhoffer JL, Mennemeier M. Transcranial magnetic stimulation and tinnitus: implications for theory and practice. J Neurol Neurosurg Psychiatry. 2007;78:113. doi: 10.1136/jnnp.2006.0103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langguth B, de Ridder D, Dornhoffer JL, et al. Controversy: Does repetitive transcranial magnetic stimulation/ transcranial direct current stimulation show efficacy in treating tinnitus patients? Brain Stimulation. 2008;1:192. doi: 10.1016/j.brs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Bohning DE. Introduction and overview of TMS physics. Washington, DC: American Psychiatric Press; 2000. pp. 13–44. [Google Scholar]
- 23.Cohen LG, Roth BJ, Nilsson J, et al. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- 24.Roth BJ, Momen S, Turner R. Algorithm for the design of magnetic stimulation coils. Med Biol Eng Comput. 1994;32:214–216. doi: 10.1007/BF02518921. [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 26.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wu T, Sommer M, Tergau F, Paulus W. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett. 2000;287:37–40. doi: 10.1016/s0304-3940(00)01132-0. [DOI] [PubMed] [Google Scholar]
- 28.Kimbrell TA, Little JT, Dunn RT, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 29.Fox P, Ingham R, George MS, et al. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- 30.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 31.Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990;11:434–445. doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Tai Y-C, Lin K, Hoh CK, Huang S, Hoffman E. Utilization of 3-D elastic transformation in the registration of chest x-ray CT and whole body PET. IEEE Transactions on Nuclear Science. 1997;4:1606–1612. [Google Scholar]
- 33.Mennemeier M, Triggs W, Chelette K, Woods A, Kimbrell T, Dornhoffer J. Sham Transcranial Magnetic Stimulation Using Electrical Stimulation of the Scalp. Brain Stimulat. 2009;2:168–173. doi: 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press; 1991. [Google Scholar]
- 35.Marcondes RA, Sanchez TG, Kii MA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: a double-blind controlled, clinical and neuroimaging outcome study. Eur J Neurol. 2010;17:38–44. doi: 10.1111/j.1468-1331.2009.02730.x. [DOI] [PubMed] [Google Scholar]