Abstract
Diets containing freeze-dried black raspberries (BRB) suppress the development of N-nitrosomethylbenzylamine (NMBA)–induced tumors in the rat esophagus. Using bioassay-directed fractionation, the anthocyanins in BRB were found to be the most active constituents for down-regulation of carcinogen-induced nuclear factor-κB and activator protein-1 expression in mouse epidermal cells in vitro. The present study was undertaken, therefore, to determine if the anthocyanins contribute to the chemopreventive activity of BRB in vivo. F344 rats consumed diets containing either (a) 5% whole BRB powder, (b) an anthocyanin-rich fraction, (c) an organic solvent-soluble extract (a–c each contained ~3.8 µmol anthocyanins/g diet), (d) an organic-insoluble (residue) fraction (containing 0.02 µmol anthocyanins/g diet), (e) a hexane extract, and (f) a sugar fraction (e and f had only trace quantities of anthocyanins), all derived from BRB. Animals were fed diets 2 weeks before treatment with NMBA and throughout the bioassay. Control rats were treated with NMBA only. Animals were killed at week 30, and esophageal tumors were enumerated. The anthocyanin treatments (diet groups a–c) were about equally effective in reducing NMBA tumorigenesis in the esophagus, indicating that the anthocyanins in BRB have chemopreventive potential. The organic-insoluble (residue) fraction (d) was also effective, suggesting that components other than berry anthocyanins may be chemopreventive. The hexane and sugar diets were inactive. Diet groups a, b, and d all inhibited cell proliferation, inflammation, and angiogenesis and induced apoptosis in both preneoplastic and papillomatous esophageal tissues, suggesting similar mechanisms of action by the different berry components.
Esophageal cancer is the third most common gastrointestinal malignancy (1) and the sixth most frequent cause of cancer death in the world (2). Squamous cell carcinoma is the predominant histologic subtype worldwide, and persons with this disease have a high rate of mortality (3). It has been estimated that more than two thirds of human cancer can be prevented through appropriate lifestyle modifications (4). Although Doll and Peto (5) reported that ~35% of human cancer mortality is caused by diet, more than 250 population-based studies, including case-control and cohort studies, indicate that persons who eat about five servings of fruit and vegetables per day have approximately half the risk of developing cancer—particularly cancers of the digestive and respiratory tracts—than do those who eat fewer than two servings per day (4). Chemoprevention can play an integral role in the overall strategy of reducing the incidence of cancer and is a potentially viable approach for reducing the risk of esophageal cancer in high-risk individuals (6).
Experimental studies provide evidence that dietary compounds prevent cancer through multiple mechanisms (4). These include the induction of cellular detoxifying and antioxidant enzymes, which protect against cellular damage caused by carcinogens and endogenously generated reactive oxygen species. Dietary compounds can also reduce cell proliferation rates, inflammation, and angiogenesis and stimulate apoptosis, normal cell differentiation, and cell-cell adhesion, among other effects (4). Because the process of cancer development involves multiple stages (i.e., initiation and promotion/progression), chemopreventive compounds have been classified broadly as blocking agents, which impede the initiation stage, or as suppressing agents, which arrest or reverse the promotion/progression stage (7).
Anthocyanins are naturally occurring polyphenolic compounds that provide pigmentation to many fruits and vegetables, such as berries, red grapes, purple sweet potato, and red cabbages (8). Berries are rich in anthocyanins, which account for ~5% to 10% of their dry weight (9). We and others have suggested that anthocyanins may be cancer preventive because they (a) inhibit cell transformation, in part, by blocking the mitogen-activated protein kinase pathway and activator protein-1 expression (10); (b) suppress inflammation by blocking the nuclear factor-κB (NF-κB) pathway and cyclooxygenase 2 (COX-2) gene expression (10); and (c) induce apoptosis in cancer cells through the activation of reactive oxygen species/c-Jun NH2-terminal kinase–mediated caspases (8).
The most commonly used preclinical animal model for esophageal squamous cell carcinoma is the F344 rat in which esophageal papillomas are induced by the nitrosamine carcinogen, N-nitrosomethylbenzylamine (NMBA; ref. 11). NMBA is administered by s.c. injection (0.25–0.5 mg/kg body weight/injection) either thrice per week for 5 weeks or once per week for 15 weeks; this results in a 100% incidence of esophageal papillomas at 25 weeks (12). In separate studies, our laboratory found that the feeding of a diet containing 5% or 10% BRB to NMBA-treated rats produced a 39% to 64% reduction in the number of esophageal papillomas when using either anti-initiation or antipromotion/progression protocols (13). We also reported that an organic solvent–soluble extract of BRB down-regulates genes associated with proliferation, inflammation, and angiogenesis in cultured mouse epidermal JB-6 Cl 41 cells (10). In a study in which 168 subfractions of this extract were tested individually for their ability to down-regulate anti–benzo(a)pyrene-7,8-diol-9,10-epoxide (BPDE)–induced NF-κB activity in JB-6 cells, the three most abundant anthocyanins [cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-(2G-xylosylrutinoside)] in BRB were found to have the highest inhibitory activity (14). The role of these anthocyanins in the prevention of esophageal tumors in vivo, however, has not been determined. The present study was conducted, therefore, to determine whether the anthocyanins in BRB play a role in the ability of the berries to prevent NMBA-induced tumors in the rat esophagus. We also determined if diets that vary in anthocyanin contents produce similar or different effects on cell proliferation, apoptosis, inflammation, and angiogenesis in NMBA-treated esophageal tissues having varying extents of neoplastic change.
Materials and Methods
Source of black raspberries and freeze drying
Black raspberries (Rubus occidentalis; BRB) of the Jewel variety were obtained from a single farm in Ohio. Ripe berries were picked mechanically, washed, and frozen at −20°C within 2 h of the time of picking. The berries were then taken to Van Drunen Farms for freeze-drying and grinding into a powder as described (13). The berry powder was then shipped frozen to the Ohio State University where it was stored at −20°C. A 100-g sample of the berry powder was shipped to Covance Laboratories for analysis of certain vitamins, minerals, carotenoids, phenolic acids, phytosterols, fungicides, pesticides, and herbicides as described (13). BRB powder was also shipped frozen to the laboratory of Dr. Stephen Hecht for preparation of BRB extracts and fractions as described below. The remaining BRB powder was stored frozen and used in an esophageal carcinogenesis bioassay conducted at the Ohio State University (also described below).
Preparation of extracts from BRB
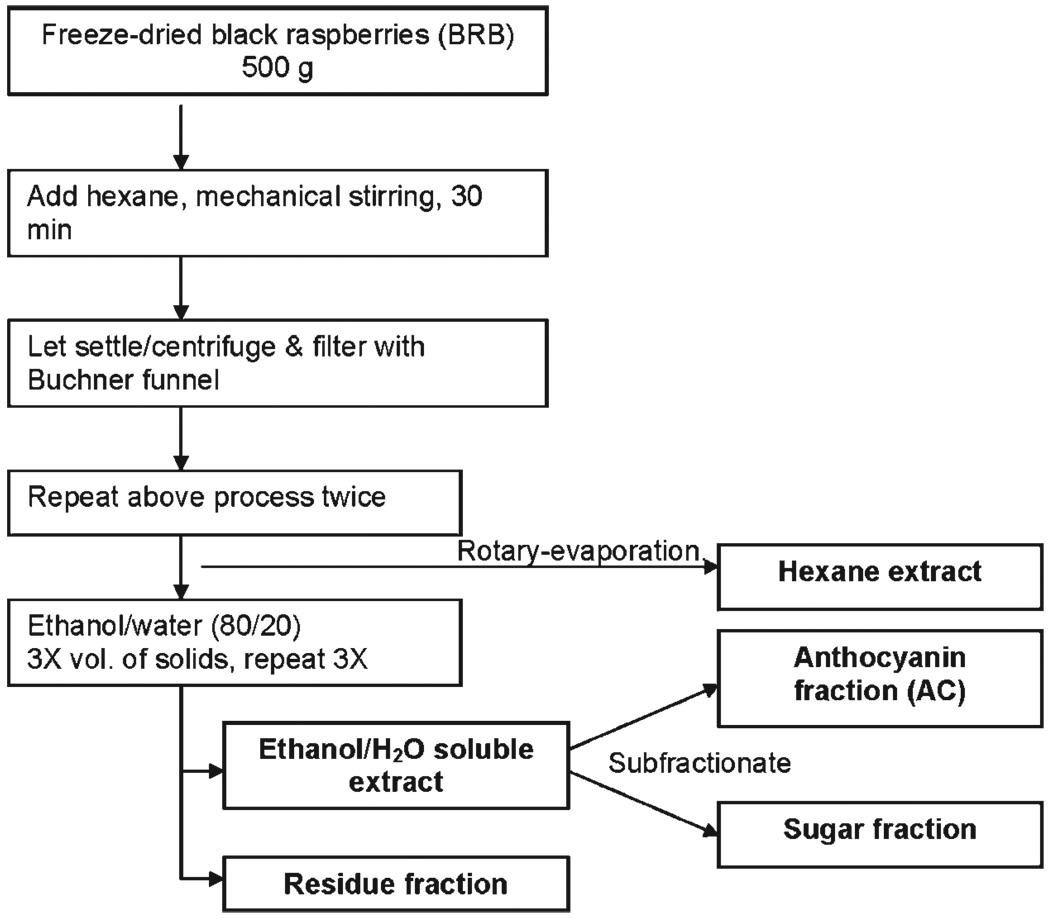
A schematic illustrating the steps used for preparation of extracts from BRB is shown in Fig. 1.
Fig. 1.
Fractionation scheme for the preparation of extracts from freeze-dried BRB.
Hexane extract
BRB (500 g) were placed in a 2,500 mL beaker with 1,500 mL hexane, and the mixture was stirred with an explosion proof mechanical stirrer and sonicated for 30 min at room temperature. The supernatant was vacuum-filtered through a Buchner funnel. This procedure was repeated twice using fresh 1,500 mL volumes of hexane, for a total extract volume of 4,500 mL. After the final extraction, the residue was dried briefly on the funnel with a stream of N2. The combined hexane extracts were concentrated to dryness by rotary evaporation at reduced pressure. The final extract was stored at −20°C.
Ethanol/H2O:80/20–soluble extract
The residue from the hexane extract was placed in a 2,500 mL beaker with 1,500 mL of ethanol/H2O:80/20, and the mixture was stirred and sonicated as above for 30 min at room temperature. The resulting mixture was filtered as above, and the procedure was repeated three more times with fresh 1,500 mL batches of ethanol/H2O:80/20. The solvents were removed to the extent possible by rotary evaporation at reduced pressure. This yielded a syrup. The remaining solvent was removed by freeze drying, as described below.
Ethanol/H2O–insoluble (residue) fraction
The berry mass obtained above after removal of the ethanol/H2O:80/20 solvent was allowed to dry for several minutes under vacuum, placed in a vacuum dessicator for 4 d, and then stored at 4°C. This fraction represents the solids in BRB and, from this point on, will be called the “residue” fraction.
Anthocyanin fraction and sugar fraction
A polystyrene-based resin, SP-700 (Mitsubishi Chemical Co.; 1.5 liters, 825 g) was activated by overnight treatment with 1.2 liters of 96% ethanol, followed by rinsing with 1.2 liters of doubly distilled H2O. The resin was stirred in the H2O for 10 min and then filtered. The filtered resin was mixed with 1.5 liters of doubly distilled H2O and introduced into a 7.5 cm (diameter) × 50 cm glass column containing a glass frit. The height of the resin in the column was about 36 cm. The packed column was washed thoroughly with 4 liters of H2O to remove any ethanol. The washed resin was poured back into a 3-liter beaker, and about 150 g of the ethanol/H2O:80/20 extract (as a concentrated syrup equivalent to about 93 g of the dried extract, or 11% of the resin weight) was then added. The mixture was stirred for 10 min to allow the anthocyanins to adsorb on the resin and then put back into the column. The column was eluted with about 2 liters of H2O at a rate of about 20 mL/min. This eluant contained the “sugar fraction.” Then, 3 liters of 95% ethanol was added to the column in steps. The first 800 mL, containing H2O that was already on the column, was collected separately and combined with the sugar fraction. The next 2 liters of the now deeply colored ethanol eluant was collected; it contained the anthocyanin fraction. The anthocyanin fraction was concentrated to a tar-like consistency using a centrifugal vacuum concentrator (Speed Vac, Thermo Savant) using a preparative rotor. The sugar fraction was concentrated by rotary evaporation under reduced pressure.
Freeze drying of the ethanol/H20:80/20–soluble extract and sugar fractions
During conventional freeze drying, the sugar fraction tended to remain as a syrup and the 80/20 extract expanded to a semisolid tar. Special techniques were necessary, therefore, to obtain these as dry fractions. The initial water content of each of these fractions was determined by freeze drying or rotary evaporating a small aliquot to dryness. The fractions were then adjusted to approximately 75% to 80% H2O content. A metal colander with numerous ~4-mm circular openings in the bottom was placed over a stainless-steel beaker, which was approximately two thirds full with 4 liters of liquid N2. Seven hundred milliliters of the sugar or the ethanol/H2O:80/20 fraction were poured into the colander over a 2-min period. The majority of the extract formed 5-mm frozen spheres at the bottom of the beaker. The liquid N2 and the spheres were poured through a wire mesh strainer. The spheres in the strainer were immediately placed in a precooled (−80°C) 10 × 6 × 1.2-in. metal tray. The tray was placed on a prechilled (−25°C) shelf in a freeze drier and vacuum was applied. The sample remained under these conditions for 4 d. The freezing shelf temperature was then allowed to equilibrate, and the sample remained under these conditions for 3 d. The result was a dry foam for the ethanol/H2O:80/20 extract and the sugar fraction. These fractions were then stored at −20°C.
Anthocyanin levels in BRB, the ethanol/H2O:80/20 extract, and the anthocyanin fraction were determined by high-performance liquid chromatography before incorporation in the diet (14). The molar percents of the three major anthocyanins in BRB were 12% cyanidin-3-O-glucoside, 34% cyanidin-3-O-(2G-xylosylrutinoside), and 53% cyanidin-3-O-rutinoside. These molar percents were very similar for the ethanol/water-soluble-soluble extract and the anthocyanin fraction.
Chemicals
NMBA, obtained from Ash Stevens, was >98% pure as determined by high-performance liquid chromatography. DMSO was purchased from Sigma.
Animals
Male F344 rats, 4 to 5 wk old, were obtained from Harlan Sprague-Dawley. The animals were housed two per cage under standard conditions (20 ± 2°C, 50 ± 10% relative humidity, 12-h light/dark cycles). Food and water were available ad libitum. Hygienic conditions were maintained by twice-weekly cage changes. The animals were fed a modified American Institute of Nutrition-76A (AIN-76A) synthetic diet (Dyets, Inc.). Body weights and food intake were recorded weekly after administration of the various diets. The animals were housed and maintained according to the recommendations of the American Association of Laboratory Animal Care.
Animal bioassay
Two weeks after arrival in the animal facility, the rats were randomly assigned into eight groups of 15 animals each, and placed on control AIN-76A diet or AIN-76A diet containing BRB powder or its fractions for the entire 30-wk bioassay. The amount of each fraction added to the diet was based on the weight percent contribution of each fraction to BRB. For the fractions containing anthocyanins (administered in groups 3–5 of Table 1), the amount of each fraction was increased to compensate for losses of anthocyanins during isolation of the fraction (as determined by high-performance liquid chromatography analysis of the anthocyanins), such that the amount of anthocyanins in each of these fractions, when added to the diet, was approximately 3.8 µmol/g diet, the same as that in BRB powder added to the diet. This concentration was confirmed by analysis of the top and bottom of the diet, before and after feeding, for anthocyanins. The groups were as follows (see Table 1): group 1, no additions to the diet or carcinogen treatment; group 2, no additions to the diet and treatment with NMBA; group 3, 5% BRB powder and NMBA; group 4, the anthocyanin fraction and NMBA; group 5, the ethanol/H2O:80/20 extract and NMBA; group 6, the residue fraction (contained 0.02 µmol anthocyanins/g diet) and NMBA; group 7, the hexane extract and NMBA; and group 8, the sugar fraction and NMBA (additions to the diet in groups 7 and 8 contained only trace quantities of anthocyanins). To maintain an isocaloric diet, the starch in the diet of animals fed 5% BRB powder was reduced by 5%. All other berry fractions were mixed with regular AIN-76A diet. After 2 wk on the diet, rats in group 1 were injected s.c. with 0.2 mL of a solution containing 20% DMSO in water (the vehicle for NMBA) once per week for 15 wk. Rats in groups 2 to 8 were injected s.c. with NMBA (0.3 mg/kg body weight) in 0.2 mL vehicle once per week for 15 wk. At 30 wk, the animals were killed by CO2 asphyxiation; the esophagus of each animal was opened longitudinally; and the surface tumors were mapped, counted, and sized. Lesions >0.5 mm in a single dimension (length, width, and height) were considered to be tumors. Tumor volume was calculated using the formula for a prolate spheroid: length × width × height × π/6, and expressed in mm3; this can also be considered as an estimation of tumor size. About one half of each esophagus and of the individual tumors was fixed in 10% neutral buffered formalin for subsequent histopathologic evaluation, and the remaining esophagus and tumors were snap frozen in liquid nitrogen for Western blot analysis.
Table 1.
Experimental design for the BRB extract bioassay
| Group | No of rats |
Treatment | Diet* | Concentration of anthocyanins in diet (µmol/g) |
Addition to diet (% by weight) |
Comments on diet preparation |
|---|---|---|---|---|---|---|
| 1 | 15 | DMSO† | AIN-76A | — | — | — |
| 2 | 15 | NMBA‡ | AIN-76A | — | — | — |
| 3 | 15 | NMBA | AIN-76A + 5% BRB | 3.8 | 5 | — |
| 4 | 15 | NMBA | AIN-76A + AC | 3.8 | 1.6 | BRB contains75.9 µmol AC per gram. The AC fraction is 12.2% by weight of BRB; therefore, this fraction should contain 75.9 µmol AC per 0.122 g. Analysis showed that 0.122 g actually contained only 38.5% of this amount due to losses in preparation of the fraction and moisture content. We, therefore, increased the amount of the fraction to compensate for losses. The calculated amount is (5% × 12.2%) / 0.385 = 1.6%. |
| 5 | 15 | NMBA | AIN-76A + ethanol/H2O soluble extract | 3.8 | 3.65 | All of the AC from BRB are in this fraction. This ethanol/H2O extract is 48.8% by weight of BRB; therefore, this fraction should contain 75.9 µmol AC per 0.488 g. Analysis showed that 0.488 g actually contained only 66.9% of this amount due to losses in preparation of the fraction. We, therefore, increased the amount of the fraction to compensate for losses. The calculated amount is (5% × 48.8%) / 0.669 = 3.65%. |
| 6 | 15 | NMBA | AIN-76A + residue fraction | 0.02 | 2.25 | The residue is 45% by weight of BRB. The calculated amount is45% × 5% = 2.25%. |
| 7 | 15 | NMBA | AIN-76A + hexane extract | Trace | 0.26 | The hexane extract is5.2% by weight of BRB. The calculated amount is 5.2% × 5% = 0.26%. |
| 8 | 15 | NMBA | AIN-76A+sugar fraction | Trace | 1.83 | The sugar fraction is 75% by weight of the ethanol/H2O soluble extract which is 48.8% by weight of BRB. the calculated amount is 75% × 48.8% × 5% = 1.83%. |
Abbreviation: AC, anthocyanin.
Berry diets were given 2 wk before, during, and after NMBA treatment throughout the entire 30-wk bioassay.
DMSO is the vehicle for NMBA.
NMBA was administered by s.c. injection (0.3 mg/kg body weight) in a volume of 0.2 mL once per week for 15 wk.
Measurement of prostaglandin E2 in serum
Four to 5 mL of whole blood were collected from each of 5 rats per group and centrifuged at 3,000 × g for 10 min. Approximately 2.5 mL of serum in the top layer of the centrifuge tube were pipetted into a cryovial and stored at −80°C. The prostaglandin E2 (PGE2) concentration was measured in 1 mL of serum per rat using an enzyme immunoassay kit (Cayman Chemical) following the manufacturer's instructions.
Immunohistochemical staining
The entire esophagus from five rats per group and 10 tumors from the same five rats per group were stained for biomarkers of cell proliferation (Ki-67), apoptosis (TUNEL), inflammation (COX-2, NF-κB p50, and CD45), and angiogenesis (CD34). Paraffin-embedded (for Ki-67, TUNEL, COX-2, NF-κB p50, and CD34) and frozen (for CD45) tissues were cut at 4 µm and placed on positively charged slides. Slides with specimens were then placed in a 60°C oven for 1 h, cooled, deparaffinized, and rehydrated through xylenes and graded ethanol solutions to water. All slides were treated for 5 min with a 3% H2O2 solution in water to block endogenous peroxidase. Antigens were retrieved by placing the slides in a vegetable steamer in Dako Target Retrieval Solution for 25 min, after which they were cooled for 15 min. The slides were then placed on a Dako Autostainer for automated staining. Primary antibodies, antibody dilutions, and incubation times and temperatures used were as follows: Ki-67, dilution 1:10, incubation for 1 h at room temperature; COX-2 (Lab Vision Corp.), dilution 1:10, incubation for 1 h at room temperature; NF-kB p50 (Santa Cruz Biotechnology), dilution 1:600, incubation for 1 h at room temperature; CD45 (BD Biosciences), dilution 1:25, incubation for 1 h at room temperature; and CD34 (R&D Systems), dilution 1:800, incubation for 30 min at room temperature. The slides were then stained with their respective secondary antibody. The slides were counterstained with hematoxylin, dehydrated through a graded series of ethanol, and coverslipped. The ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon) was used for TUNEL staining following the manufacturer's instructions.
Computer-assisted image analysis
Tissues stained for Ki-67, COX-2, NF-κB p50, CD45, CD34, and for apoptotic cells by TUNEL were viewed and photographed at ×200 with a bright-field microscope mounted with a high-resolution spot camera (see Supplementary Fig. S1). The camera was interfaced with a computer containing a matrix frame grabber board and image analysis software (Simple PCI Imaging Systems, Compix, Inc.). For all antigens, 20 fields per the entire esophagus from 5 animals per group and 30 fields from the 10 tumors in each group were analyzed and the staining intensities were quantified by image analysis software as described (13).
Western blot analysis
Esophagi from five animals per group, and 10 tumors from the same five animals per group, were pooled separately and homogenized in lysis buffer. The homogenates were centrifuged at 14,000 × g for 10 min at 4°C. Protein concentrations were measured using a BCA protein assay kit (Pierce Chemical). A total of 50 µg protein was resolved on precasted SDS-PAGE gels (Bio-Rad), and Western blotting was done as described earlier (15). p42/44 (Cell Signaling Technology), Bcl-2 (Cell Signaling Technology), Bax (Cell Signaling Technology), COX-2 (Thermo Fisher Scientific), vascular endothelial growth factor (VEGF, Santa Cruz Biotechnology), hypoxia-inducible factor 1α (HIF-1α, Santa Cruz Biotechnology), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Santa Cruz Biotechnology) antibodies were used for identification of their respective proteins.
Statistical analysis
Body weight, food consumption, tumor multiplicity and volume, immunohistochemical staining data, and serum PGE2 levels were compared using ANOVA and an unpaired t test StatView (SAS Institute). A P value < 0.05 was considered to be statistically significant.
Results
General observations
No significant differences in animal body weights were found in any of the groups during the first 25 weeks of the bioassay. During the last 5 weeks, however, NMBA-treated animals consuming the 5% BRB diet (group 3) had significantly lower body weights than did all other groups (P < 0.05; data not shown). The animals in group 3 also consumed less food than did all other groups during weeks 24 to 30 of the study (P < 0.05; data not shown). We examined each tumor by light microscopy for histopathologic features of squamous cell carcinoma (e.g., loss of cell polarity; nuclear atypia; keratin “pearl” formation; cellular invasion through the basement membrane into the underlying stroma, blood vessels, and lymphatics) and saw no tumors with these features; all tumors were papillomas. Typically, in this model system, and at the dose of NMBA used, the animals succumb to the occlusive effects of papillomas in the esophagus before carcinomas develop.
Effects of the various diets on NMBA-induced rat esophageal tumorigenesis
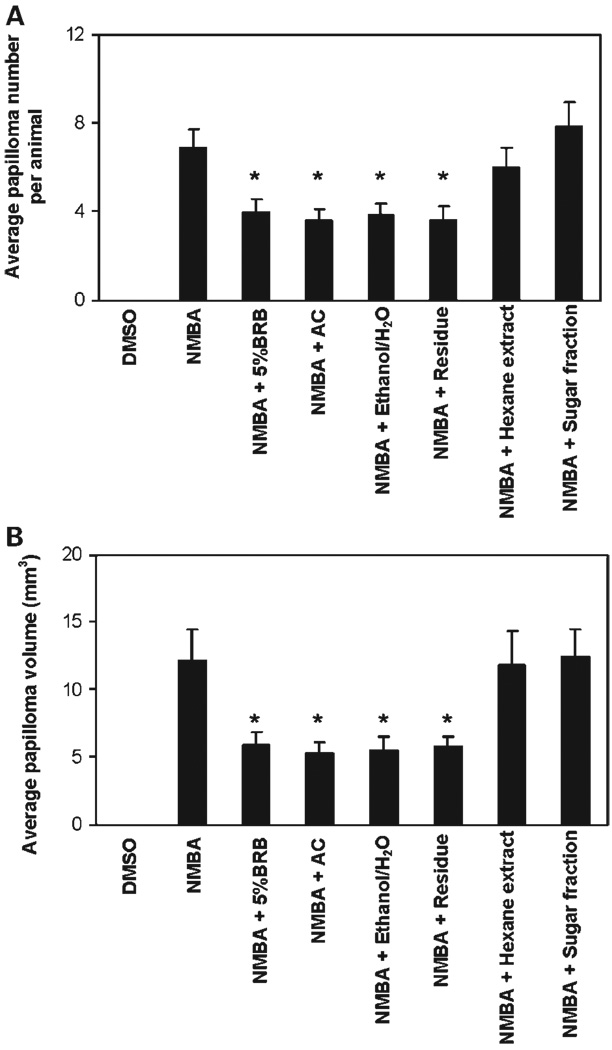
The effects of the different diets on the number and volume of NMBA-induced esophageal papillomas at 30 weeks are shown in Fig. 2A and B, respectively. As indicated, diets containing either 5% whole BRB powder, the anthocyanin fraction, or the ethanol/H2O–soluble extract (each containing ~3.8 µmol anthocyanins/g diet) were about equally effective in reducing both the number and volume of NMBA-induced papillomas. Intriguingly, the diet containing the residue fraction was approximately equally as effective as the three anthocyanin-containing diets in reducing both the number and volume of NMBA-induced papillomas. The hexane extract (mainly nonpolar compounds) and the sugar fraction (mainly glucose and fructose) were ineffective in reducing the number and volume of esophageal papillomas in NMBA-treated rats.
Fig. 2.
Effect of diets containing BRB or BRB residues/fractions on papilloma development in NMBA-treated rat esophagus. Rats treated with NMBA + 5% BRB, NMBA + anthocyanin (AC) fraction, NMBA + ethanol/H2O–soluble extract (Ethanol/H2O), and NMBA + residue fraction (Residue) had fewer papillomas (A) and smaller papilloma volume (B) than rats treated with NMBA only. Columns, mean (n = 15); bars, SD. *, significantly lower (P < 0.05) than rats treated with NMBA only.
Effects of the various diets on cell proliferation, apoptosis, inflammation, and angiogenesis
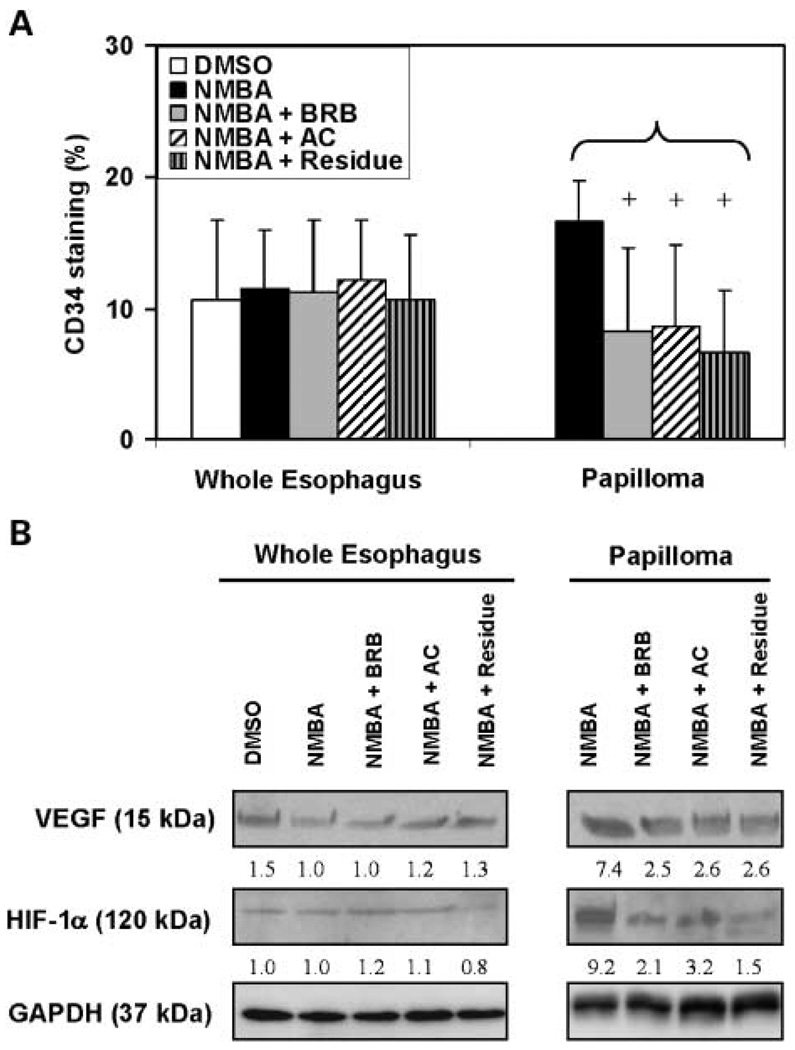
The above results indicated that the three diets (i.e., 5% BRB, anthocyanin fraction, and ethanol/H2O extract) containing approximately the same quantity of anthocyanins were about equally effective in preventing NMBA-induced esophageal carcinogenesis. These results suggested that the chemopreventive efficacy of BRB resides predominantly in their content of anthocyanins. However, the diet containing the residue fraction, which had only low amounts of anthocyanins, was nearly as effective in preventing esophageal carcinogenesis as the anthocyanin-rich diets (Fig. 2A and B). In view of this, we elected to conduct some initial studies to determine whether diets containing different amounts of anthocyanins might vary in their effects on cellular and molecular variables associated with carcinogenesis. To determine this, we selected esophagi, papillomas, and sera from animals fed diets containing either 5% BRB, the anthocyanin fraction, or the residue fraction and evaluated the effects of these diets on cell proliferation, apoptosis, inflammation, and angiogenesis. Whole esophagi (containing a mixture of normal, hyperplastic, and dysplastic tissue) and papillomas from the three diet groups, and from NMBA-treated and vehicle control rats, were stained for Ki-67 as a measure of cell proliferation, TUNEL for apoptosis, COX-2, NF-κB p50 and CD45 for inflammation, and CD34 for angiogenesis.
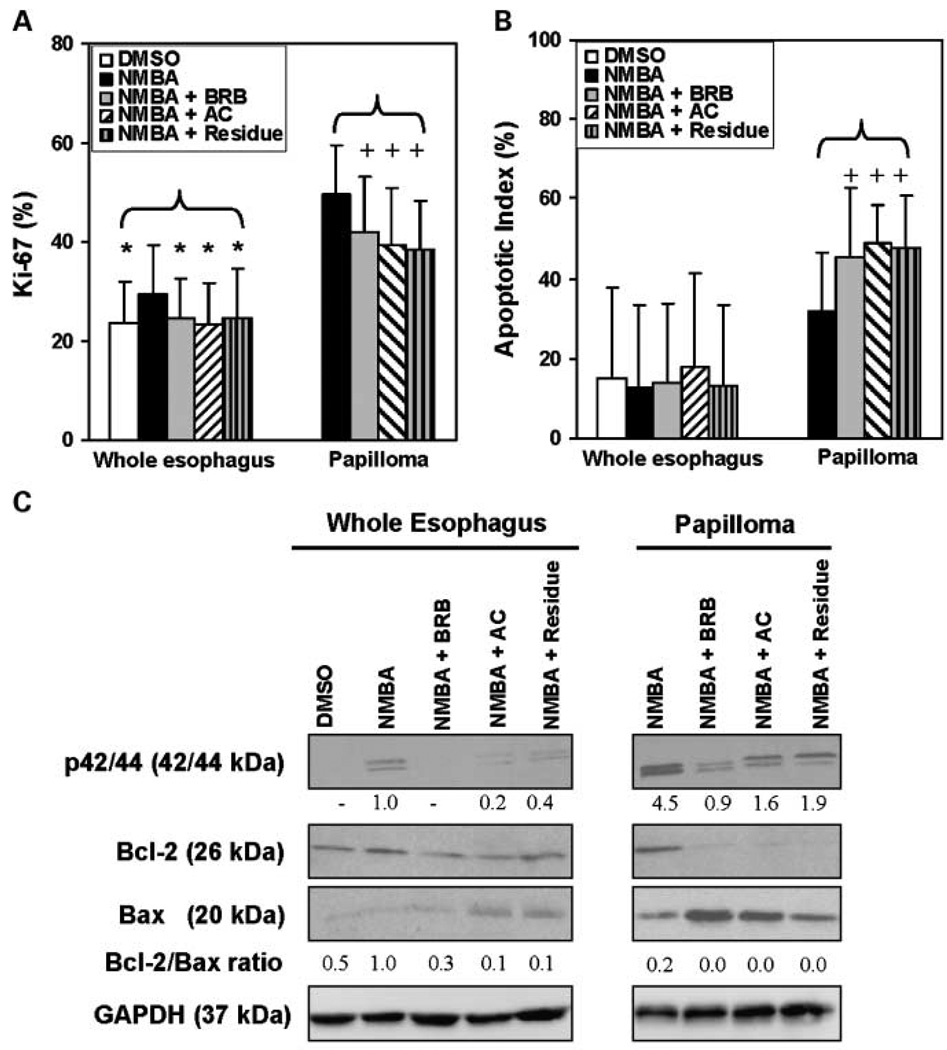
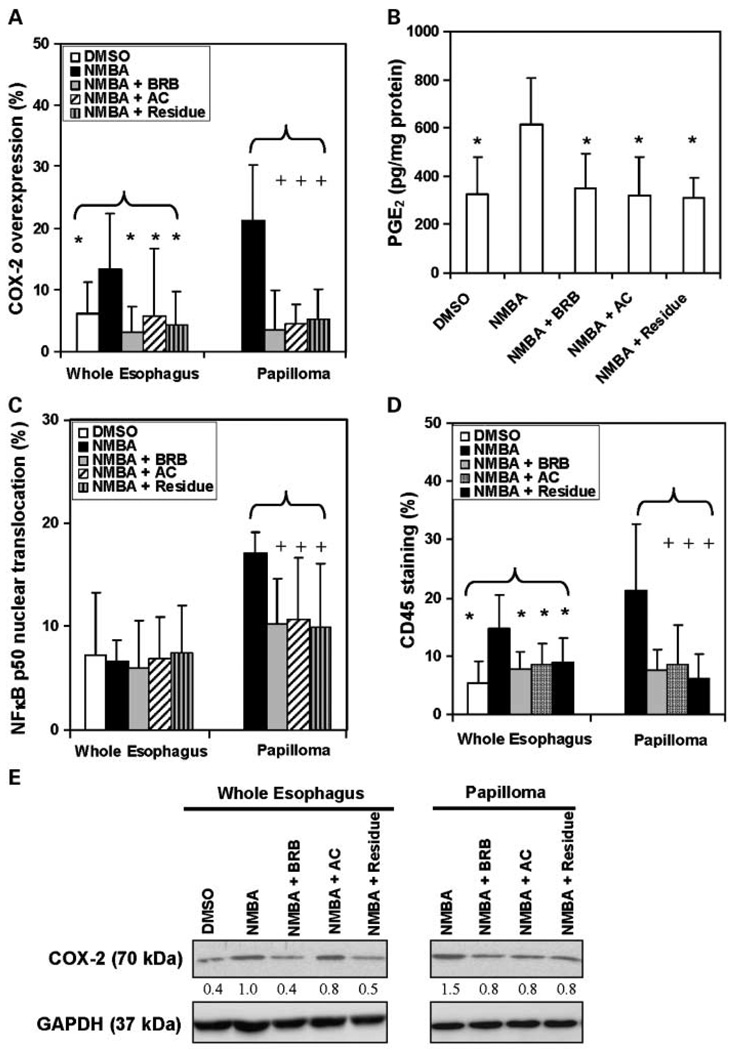
The immunohistochemical staining of Ki-67 (Fig. 3A), COX-2 (Fig. 4A), and CD45 (Fig. 4D) in the whole esophagus of rats treated with NMBA only was significantly (P < 0.05) higher than in the esophagus of rats treated with the vehicle control. In contrast, the TUNEL (Fig. 3B), NF-κB p50 (Fig. 4C), and CD34 (Fig. 5A) expressions in whole esophagi of NMBA-treated animals were similar to those in the vehicle controls. The up-regulation of Ki-67, COX-2, and CD45 in NMBA-treated whole esophagus was reduced significantly (P < 0.05) by all three diets (Figs. 3A and 4A and D). In rats treated with NMBA only, Ki-67, TUNEL, COX-2, NF-κB p50, CD45, and CD34 staining was higher in papillomas than in whole esophagus. All three diets significantly (P < 0.05) reduced the staining of Ki-67, COX-2, NF-κB p50, CD45, and CD34 (see Figs. 3A; 4A, C, D; and 5A, respectively), and increased TUNEL staining (Fig. 3B), in the papillomas. These results suggest that the anthocyanins and unknown compounds in the residue fraction reduce cell proliferation, inflammation, and angiogenesis, and induce apoptosis in NMBA-treated rat esophagus.
Fig. 3.
Effects of dietary administration of BRB and two BRB fractions on cell proliferation and apoptosis in the whole esophagus and in papillomas. Quantification of immunohistochemical staining of Ki-67 (A) and apoptotic index (TUNEL; B). Columns, mean (n = 5); bars, SD. *, P < 0.05 for all groups versus NMBA-treated esophagus. +, P < 0.05 for all groups versus NMBA-induced papillomas. C, p44/42 mitogen-activated protein kinase (Erk1/2), Bcl-2, and Bax protein expression in pooled esophagi from 5 animals and 10 pooled tumors from the same 5 animals per group was determined by Western blot analysis. GAPDH was used as loading control. The densitometry analysis was done and fold changes for p44/42 (Erk1/2) and Bcl-2-to-Bax ratios are depicted in the figure, with that of whole esophagi from the NMBA-only group arbitrarily set at 1.0. There were no papillomas in DMSO control animals.
Fig. 4.
Effects of dietary administration of BRB and two BRB fractions on inflammatory markers in the whole esophagus and in papillomas. Quantification of immunohistochemical staining of (A) COX-2 intensity, (C) NF-κB p50 nuclear translocation, and (D) CD45 intensity in whole rat esophagus and papillomas. Columns, mean (n = 5); bars, SD. *, P < 0.05 for all groups versus NMBA-treated esophagus. +, P < 0.05 for all groups versus NMBA-induced papillomas. B, PGE2 serum level. Columns, mean (n = 5); bars, SD. *, P < 0.05 versus NMBA control. E, COX-2 protein expression in pooled esophagi from 5 animals and 10 pooled tumors from the same 5 animals per group was determined by Western blot analysis. GAPDH was used as loading control. The densitometry analysis was done and fold changes for COX-2 protein expression are depicted in the figure, with that of whole esophagi from NMBA-only group arbitrarily set at 1.0. There were no papillomas in DMSO control animals.
Fig. 5.
Effects of dietary administration of BRB and two BRB fractions on angiogenesis in the whole esophagus and in papillomas. A, quantification of immunohistochemical staining of CD34 staining intensity on whole esophagus and papillomas. Columns, mean (n = 5); bars, SD. +, P < 0.05 for all groups versus NMBA-induced papillomas. B, VEGF and HIF-1α protein expression in pooled esophagi from 5 animals and 10 pooled tumors from the same 5 animals per group was determined by Western blot analysis. VEGF antibody detected the 188-, 164-, and 120-amino-acid splice variants of VEGF. The densitometry analysis was done and fold changes for VEGF and HIF-1α protein expression are depicted in the figure, with that of whole esophagi from NMBA-only group arbitrarily set at 1.0. GAPDH was used as loading control. There were no papillomas in DMSO control animals.
In an effort to confirm the immunohistochemistry results, we examined the expression of proteins involved in cell proliferation, inflammation, angiogenesis, and apoptosis by Western blot analysis. These proteins included p42/44 as a measure of cell proliferation, Bcl-2, and Bax for apoptosis; COX-2 for inflammation; and both VEGF and HIF-1α for angiogenesis. The results of these analyses are presented in Figs. 3C, 4E, and 5B. As indicated, all three diets reduced the expression levels of proteins involved in cell proliferation (Fig. 3C), inflammation (Fig. 4E), and angiogenesis (Fig. 5B), and increased those associated with apoptosis (Fig. 3C) in NMBA-treated rat esophagus. In addition, the levels of PGE2 in the serum of NMBA-treated rats fed diets containing 5% BRB, the anthocyanin fraction, or the residue fraction were significantly (P < 0.05) lower than in NMBA-treated rats fed control diet (Fig. 4B).
Discussion
We reported previously that three of the four anthocyanins [cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, and cyanidin-3-O-(2G-xylosylrutinoside)] in BRB were the most active berry components in down-regulating BPDE-induced NF-κB expression in cultured mouse epidermal cells (14). Another anthocyanin, cyanidin-3-O-sambubioside, was not identified as active probably due to its very low concentration in the berries (14). The present study extends these observations to an in vivo model system in which the anthocyanins were found to be important berry components in inhibiting esophageal carcinogenesis in the rat. It is likely that the inhibitory effects of the anthocyanins are due to their localized absorption into esophageal tissue for the following reasons: (a) Pharmacokinetic studies have shown that anthocyanin absorption into the bloodstream of both rodents and humans fed anthocyanin-containing diets is minimal (i.e., ~1% of the amount administered in the diet). Thus, systemic delivery of anthocyanins to the esophagus of rats fed either the anthocyanin-rich diet or 5% BRB is probably insufficient to prevent cancer (8). (b) Rats in the present study were fed berry diets ad libitum, thus permitting localized absorption of the anthocyanins and other berry compounds into the esophagus at all hours of the day and night. In contrast, in a human clinical trial of subjects with Barrett's esophagus, the daily administration of BRB in a slurry of water over a period of 30 minutes for 6 months failed to affect segment length of Barrett's lesions (16). This may have been due to the fact that the transit time of the berry slurry across the human esophagus was very rapid and did not permit sufficient time for anthocyanins and other berry compounds to be absorbed into the lesions. (c) Treatment of strain A mice with a diet containing 5% and 10% freeze-dried strawberries, which contain two anthocyanins (17), failed to prevent carcinogen-induced lung tumors even when the carcinogens were administered in fractionated doses rather than in a single bolus dose (18). Presumably, the active compounds in the berries did not reach the lung in sufficient quantities to be protective. In contrast, dietary 5% and 10% strawberries were effective in preventing NMBA-induced esophageal cancer in rats (19). These observations suggest that if anthocyanins are to be effective in the prevention of esophageal cancer in humans, they will need to be administered in formulations that enhance their absorption into esophageal tissues.
An interesting observation in this study was that the ethanol/H2O–insoluble (residue) fraction of BRB was found to be as effective as both whole 5% BRB, the anthocyanin fraction and the ethanol/H2O–soluble extract (each containing ~3.8 µmol anthocyanins/g diet) in suppressing NMBA-induced rat esophageal tumorigenesis (Fig. 2). The residue fraction represents about 45% of whole BRB powder and likely contains cellulose, hemicelluloses, pectin, lignin, protein, and ellagitannins (20). Ellagitannins, in particular, may be involved in the chemopreventive activity of the residue observed here (21, 22). We have previously shown that dietary ellagic acid inhibits the metabolic activation of NMBA and inhibits NMBA tumorigenesis in the rat esophagus (23, 24). Because the residue fraction was as effective as the anthocyanin fraction and the ethanol/H2O–soluble extract, and all of them were as active as whole 5% BRB, it is possible that compounds in the residue may interfere with the inhibitory activity of the anthocyanins, or reverse. Currently, we are attempting to identify the active constituents in the residue fraction after which we will evaluate these constituents in combination with the anthocyanins for prevention of esophageal tumorigenesis in rats.
Our results showed that biomarkers associated with cell proliferation, apoptosis (except Bax), inflammation, and angiogenesis were increased significantly in NMBA-induced papillomas relative to the whole esophagus (Figs. 3–5). Although whole BRB, the anthocyanin fraction, and the residue fraction have different chemical compositions, they all modulated the selected biomarkers of cell proliferation, apoptosis, inflammation, and angiogenesis in whole esophagus and in papillomas. Thus, and perhaps not surprisingly, these biomarkers did not prove useful for distinguishing the three diets on a mechanistic basis. When the active constituents in the residue fraction are identified, it may be more informative to compare the cellular and molecular effects of these constituents with those of the anthocyanins to identify potential differences in mechanisms of action.
Our results suggest that all three diets, and the diet containing the ethanol/H2O extract, might have therapeutic use in the treatment of esophageal tumors because the papilloma volumes in these groups were smaller than those in the NMBA-only group (Fig. 2B). In addition, histopathologic studies showed that all four berry diets reduced the occurrence of NMBA-induced preneoplastic lesions (data not shown). Finally, our data suggest that the absorption of berry compounds into papillomas is sufficient to influence the expression of the biomarkers measured in this study.
The Ki-67 and COX-2 results in the present study are similar to those in previous studies in our laboratory in which both biomarkers were found to be increased in preneoplastic esophageal tissues (relative to normal esophagus) at 15, 25, and 35 weeks of the bioassay and reduced in expression at all time points by 5% BRB (13, 25). The effect of the 5% BRB diet on Ki-67 and COX-2 expression in papillomas however was not determined in the previous study. Therefore, results from the present study extend the previous observations by showing that all three berry diets decreased Ki-67 and COX-2 expression in both preneoplastic esophagus and in papillomas. Moreover, this is the first report of the ability of BRB and its components to induce apoptosis in NMBA-treated rat esophagus by decreasing Bcl-2 and increasing Bax protein expression (Fig. 3C).
NMBA is by far the most potent inducer of tumors in the rat esophagus. As for other nitrosamines, the first step in the metabolic activation of NMBA involves hydroxylation of the methylene carbon by esophageal cytochrome P450 enzymes (11). This reaction produces an α-hydroxy derivative that spontaneously decomposes to methyldiazohydroxide and benzaldehyde. Methyldiazohydroxide leads to the formation of a methylcarbonium ion, the ultimate electrophilic species that methylates guanine residues at the N7 and O6 positions. The O6-methylguanine adduct is particularly important for carcinogenesis because it is poorly repaired and leads to single base mispairing in DNA (26–28). Our laboratory has shown the ability of dietary BRB to reduce the formation of O6-methylguanine adducts in esophageal DNA (13). Inhibition of DNA adduct formation might be expected to inhibit tumor initiation, in part, because one of the principal initiation events in rat esophageal carcinogenesis is mutational activation of the H-ras oncogene (29, 30). Activation of this gene by NMBA is associated with GC→AT transition mutations in the second base of codon 12; these mutations are consistent with the formation of O6-methylguanine adducts in DNA. In the current study, BRB and BRB fractions were administered to the animals 2 weeks before NMBA treatment to account for the possibility that the different berry fractions might influence NMBA metabolism and DNA adduct formation. Although we did not measure O6-methylguanine adducts in DNA, the fact that the different fractions were about equally as effective as whole berries in preventing cancer suggests that they all influenced NMBA metabolism and DNA adduct formation.
In summary, results from the present study indicate that the anthocyanins in BRB are important for their chemopreventive potential. In addition, the residue of BRB seems to be equally as effective as the anthocyanin fraction in preventing esophageal cancer in rats, and studies are ongoing to identify the active constituents in the residue. Finally, our data suggest that berry fractions may have therapeutic value for the treatment of esophageal tumorigenesis and, perhaps, consideration should be given to the use of these in conjunction with radiotherapy and/or chemotherapy.
Supplementary Material
Acknowledgments
We thank Ron Nines for his assistance in the animal bioassays and Mark von Keitz of the Bio Technology Institute, University of Minnesota, for expert advice on the use of the freeze drier.
Grant support: National Cancer Institute grant CA103180 and U.S. Department of Agriculture grant 38903-03560.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2. [PubMed] [Google Scholar]
- 2.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Souza RF. Molecular and biologic basis of upper gastrointestinal malignancy-esophageal carcinoma. Surg Oncol Clin N Am. 2002;11:257–272. doi: 10.1016/s1055-3207(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 4.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 5.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 6.Stoner GD, Wang LS, Zikri N, et al. Cancer prevention with freeze-dried berries and berry components. Semin Cancer Biol. 2007;17:403–410. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–1674. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H, Li J, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr Cancer. 2006;54:69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- 11.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 12.Siglin JC, Barch DH, Stoner GD. Effects of dietary phenethyl isothiocyanate, ellagic acid, sulindac and calcium on the induction and progression of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Carcinogenesis. 1995;16:1101–1106. doi: 10.1093/carcin/16.5.1101. [DOI] [PubMed] [Google Scholar]
- 13.Kresty LA, Morse MA, Morgan C, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 14.Hecht SS, Huang C, Stoner GD, et al. Identification of cyanidin glycosidesas constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFκB and AP-1 activity. Carcinogenesis. 2006;27:1617–1626. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LS, Huang YW, Liu S, Yan P, Lin YC. Conjugated linoleic acid induces apoptosis through estrogen receptor α in human breast tissue. BMC Cancer. 2008;8:208. doi: 10.1186/1471-2407-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kresty LA, Frankel WL, Hammond CD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54:148–156. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 17.Andersen ØM, Fossen T, Torskangerpoll K, Fossen A, Hauge U. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin. Phytochemistry. 2004;65:405–410. doi: 10.1016/j.phytochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Carlton PS, Kresty LA, Stoner GD. Failure of dietary lyophilized strawberries to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-and benzo[a] pyrene-induced lung tumorigenesis in strain A/J mice. Cancer Lett. 2000;159:113–117. doi: 10.1016/s0304-3835(00)00464-x. [DOI] [PubMed] [Google Scholar]
- 19.Stoner GD, Kresty LA, Carlton PS, Siglin JC, Morse MA. Isothiocyanates and freeze-dried strawberries as inhibitors of esophageal cancer. Toxicol Sci. 1999;52:95–100. doi: 10.1093/toxsci/52.2.95. [DOI] [PubMed] [Google Scholar]
- 20.Voragen FGJ, Timmers JPJ, Linssen JPH, Schols HA, Pilnik W. Methods of analysis for cell-wall polysaccharides of fruit and vegetables. Z Lebensm Unters Forsch. 1983;177:251–256. [Google Scholar]
- 21.Ross HA, McDougall GJ, Stewart D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry. 2007;68:218–228. doi: 10.1016/j.phytochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Mullen W, Yokota T, Lean MEJ, Crozier A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MS. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 23.Mandal S, Shivapurkar NM, Galati AJ, Stoner GD. Inhibition of N-nitrosobenzylmethylamine metabolism and DNA binding in cultured rat esophagusby ellagic acid. Carcinogenesis. 1988;9:1313–1316. doi: 10.1093/carcin/9.7.1313. [DOI] [PubMed] [Google Scholar]
- 24.Mandal S, Stoner GD. Inhibition of N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlton PS, Kresty LA, Siglin JC, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–446. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 27.Craddock VM, Henderson AR. Effect of N-nitrosamines carcinogenic for oesophagus on O6-alkyl-guanine-DNA-methyl transferase in rat oesophagus and liver. J Cancer Res Clin Oncol. 1986;111:229–236. doi: 10.1007/BF00389238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogdson M, Wiessler M, Kleihues P. Preferential methylation of target organ DNA by the esophageal carcinogen N-nitrosomethylbenzylamine. Carcinogenesis. 1980;1:861–866. doi: 10.1093/carcin/1.10.861. [DOI] [PubMed] [Google Scholar]
- 29.Lozano JC, Nakazawa H, Cross MP, Cabral R, Yamasaki H. G to A mutations in p53 and Ha-ras genes in esophageal papillomas induced by N-nitrosomethylbenzylamine in two strains of rats. Mol Carcinog. 1994;9:33–39. doi: 10.1002/mc.2940090107. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, You M, Reynolds SH, Stoner GD, Anderson MW. Mutational activation of the cellular Harvey rasoncogene in rat esophageal papillomas induced by methylbenzylnitrosamine. Cancer Res. 1990;50:1591–1595. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.