Abstract
Purpose
The cause of most cancer deaths is incurable dissemination of cancer cells into vital organs. Current systemic therapies for disseminated cancers provide limited efficacy and are often accompanied by toxic side effects. We have recently shown that local application of EGFR-targeted PolyIC eradicates pre-established EGFR-over-expressing tumors. Here we demonstrate for the first time the high efficiency of systemic application of PolyIC/Melittin-Polyethleneimmine-Polyethyleneglycol-EGF (PolyIC/MPPE) in combination with human immune cells.
Experimental design
Cancer targeted activation of immune cells was examined in vitro and in vivo following transfection with PolyIC/MPPE. The therapeutic efficiency of the strategy was then examined on disseminated EGFR overexpressing tumors grown in SCID mice.
Results
Intravenous delivery of PolyIC/MPPE followed by intraperitoneal injection PBMC induced the complete cure of SCID mice with pre-established disseminated EGFR overexpressing tumors, with no adverse toxic effects. The immune cells and the cytokines they produce are localized to the tumor site of the treated animal and contribute decisively to the demise of the tumor cells. The immune system homes to the tumors, due to the chemokines produced by the internalized PolyIC.
Conclusion
The EGFR homing vector loaded with PolyIC can be used to treat and possibly cure patients with disseminated EGFR overexpressing tumors. The possibility of adopting this strategy to treat other tumors that express a protein capable of ligand induced internalization is discussed.
Keywords: EGFR, dsRNA, Cancer
EGFR is over-expressed in a variety of solid human tumors including non-small-cell- lung-carcinoma (NSCLC), breast cancer, glioblastoma, head and neck squamous cell carcinoma (HNSCC), colorectal cancer (CRC), adenocarcinoma, ovary cancer, bladder cancer and prostate cancer (1). The American Cancer Society's annual estimate of new cancer cases and deaths projects 1,437,180 new cancer cases in the United States in 2008 and 565,650 cancer deaths. The cause of most cancer deaths is metastasis of the cancer into internal organs, which is virtually impossible to treat by conventional methods. A significant fraction of all cancer related deaths are associated with over-expression of EGFR. Thus EGFR is one of the most important candidates for targeted cancer therapy. The two most advanced EGFR-targeted therapies are small membrane permeable EGFR kinase inhibitors and anti-EGFR antibodies, which prevent receptor activation and/or lead to receptor down-regulation. These agents induce temporary or partial remission and convey some survival benefits, but do not actually cure patients. This is most likely because EGFR is not essential for the survival of the targeted cancer cells. Recently we developed a strategy that utilizes the high level of expression of EGFR, rather than its activity per se, as the Achilles’ heel of the tumor. This was achieved by utilizing an EGFR homing chemical vector loaded with poly inosine-cytosine (PolyIC) (2). Poly IC was delivered by means of Melittin-Polyethyleneimine-Polyethyleneglycol-EGF (MPPE) (2). This is a tetra-component conjugate, consisting of 25 kDa branched polyethylenimine (brPEI), EGF as targeting ligand and polyethylene glycol (PEG) for shielding, and a synthetic derivative of the lytic peptide melittin (2). The latter was required for cytosolic delivery of poly(I:C) and therapeutic efficacy (2). The MPPE conjugates efficiently delivered poly IC, killing up to 90% of cultured cells of 3 different cancers, which over-express EGFR (2). The Intratumoral application of PolyIC/MPPE to EGFR over-expressing glioblastoma (~1× 106 receptors/cell (3)) grown intracranially and to EGFR over-expressing breast and epidermoid carcinomas grown as xenografts in nude mice, led to complete elimination of these localized tumors, curing the mice (2). Furthermore, tumors comprising a 1:1 mixture of cell over-expressing wild type EGFR and cells harboring the mutant EGFRvIII, which does not internalize the vector, were also completely eradicated (2). This “bystander effect” was due to the antiproliferative cytokines such as interferon-α (IFN-α), generated at the tumor site by the PolyIC/MPPE affected tumor cells (2).
Statement of Translational Relevance.
Metastasis of cancer into internal organs is virtually impossible to treat by conventional methods. A significant fraction of all cancer related deaths are associated with over-expression of EGFR. The current EGFR-targeted treatments induce partial therapeutic effects but do not actually cure patients. This is most likely because EGFR is not essential for the survival of the targeted cancer cells. Here we show a strategy that utilizes the high level of expression of EGFR, rather than its activity, as the Achilles’ heel of the tumor. This is achieved by utilizing an EGFR homing chemical vector loaded with poly inosine-cytosine (PolyIC). Targeted PolyIC activates cascade of antiproliferative mechanisms and induces expression of immunoactive cytokines selectively in EGFR overexpressing cells. The current study demonstrates high efficiency of the strategy in the treatment of disseminated tumor models in mice. The data presented here suggest that systemic treatment of EGFR over-expressing metastatic tumors with EGFR-targeted PolyIC may lead to a complete cure, in patients with a functional immune system.
IFN-α and other cytokines induced by PolyIC/MPPE are also potent immune activators. Activation of the immune system selectively against cancer should strongly increase efficiency of the PolyIC/MPPE therapy. Higher therapeutic efficiency should allow an effective treatment of not only local but also distant disseminated tumors, which are far more difficult to treat. Recognizing that SCID mice possess a deficient immune system we decided to examine the therapeutic efficacy of EGFR targeted PolyIC on disseminated EGFR overexpressing tumors in SCID mice and in SCID mice with a reconstituted immune system. In the current study we demonstrate that PolyIC/MPPE, together with a reconstituted immune system, can eradicate disseminated tumors in SCID mice, by systemic (IV) application. Intravenous delivery of PolyIC/MPPE, followed by intraperitoneal injection of human Peripheral Blood Mononuclear Cells (PBMCs), induced the complete cure of SCID mice with pre-established disseminated EGFR over-expressing tumors, with no obvious adverse toxic effects. We show that the internalized PolyIC attracts the immune cells to the tumor. The tumor-targeted activated immune cells increase the efficiency of the PolyIC/MPPE therapeutic effects by several fold, yet preserve its selectivity. The data presented here strongly suggest that EGFR-targeted PolyIC can be utilized to treat and possibly cure patients with metastatic tumors that over-express EGFR.
Methods
PBMC extraction
PBMCs from healthy human donors were separated on Ficoll Plaque (Pharmacia), washed twice with 50 ml RPMI 1640 medium, resuspended at a density of 4×106/ml and cultured in this medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin.
Chemotaxis
MDA-MB-468, A431 and U138MG cells grown in 96 well plates in triplicates were transfected with 2.5 mcg/ml Poly IC (pIC) or pGlu formulated with MPPE. 48 hrs after the transfection 100 microL medium of the cells were transferred to the lower chamber of Millipore chemotaxis plates with 5-micrometer pores (Millipore, Billerica, MA, USA). Five × 104 PBMC cells in 100-microL medium were added to the upper chamber, and plates were incubated for 4 hrs at 37°C. Both Lower and Upper plates were then subjected to CellTiter-Glo® Luminescent Cell Viability Assay (Promega). The assay is a homogeneous method to determine the number of viable cells in culture based on quantitation of the ATP present, which signals the presence of metabolically active cells. The procedure involves adding of single reagent (CellTiter-Glo® Reagent) directly to cells cultured in serum-supplemented medium. This results in cell lysis and generation of a luminescent signal proportional to the amount of ATP present. 100 microL of CellTiter-Glo® Reagent were added to the wells of both Lower and Upper plates. The contents were mixed for 2 minutes on an orbital shaker to induce cell lysis.10 mins later 100 microL of the mixture from each well were transferred to the new single 96 well plate (Nunc) and the luminescence was counted (arbitrary units) in luminometer.
PBMC-mediated bystander effect
100,000 MDA-MB-468 cells or A431 cells were seeded into 6 well plates and grown overnight with 2 ml medium per well (2). Cells were then transfected with PolyIC/MPPE, to a final concentration of 0.1 or 0.5 mcg/ml. 48 hrs after transfection 0.5 ml of medium from the transfected cells (“conditioned medium”) was added to 500,000 PBMCs which had been seeded 24 hrs earlier into 24 well plates and grown in 0.5 ml medium. 0.1 ml of medium from the challenged PBMCs was then exchanged for 0.1 ml medium from additional non-transfected MDA-MB-468 cells and U138MG cells (“indicator cells”) seeded on 96 well plates 24 hrs earlier. Survival of these cells was determined by methylene blue assay (2), 48 hours after challenge with the medium from the PBMCs. In parallel, to show the direct bystander effect, 0.1 ml of conditioned medium was used to replace 0.1 ml medium from non-transfected indicator cells seeded 24 hrs earlier onto 96 well plates and grown in 0.2 ml medium. Survival of these cells was determined 48 hours after addition of the conditioned medium using methylene blue.
In vitro cancer cell killing by activated PBMCs
20,000 A431, 30,000 MDA-MB-468 or 20,000 U138MG cells were seeded onto 24 well plates and grown overnight in 1 ml RPMI medium supplemented with 10% FCS and antibiotics. Cells were then transfected with PolyIC/MPPE at 0.1 mcg/ml. 24 hrs later 500,000 PBMCs/well were added to the cancer cells and co-incubated for another 24 hrs. Apoptotic cells (red fluorescence) were visualized using an Annexin-V-Biotin kit (Biosource, Inc.). To distinguish tumor cells from PBMCs, tumor cells were labeled with FITC-conjugated EGFR antibody (Biosource, Inc., green fluorescence). Cells were visualized with a fluorescent microscope and photographed using a digital camera.
Effect of PolyIC/MPPE/PBMC treatment on survival of mice with disseminated tumors
Female SCID-NOD mice (Harlan) were injected IV with 1 million A431 or MDA-MB-468 or 1×105 U138MG cells suspended in 200 μl PBS. 10 or 15 days later, the animals were randomly divided into groups (5 mice per group) and treatment was initiated with a series of intravenous injections of 20 μg PolyIC/MPPE. 24 hrs after the last PolyIC injection, the animals were injected once with four million PBMCs. Survival of the mice was analyzed afterwards. Animals were checked daily for the development of symptoms associated with the progression of implanted tumors: weight decrease, moribund state, slow movement or inability to feed or drink. Animals were sacrificed with overdose of anesthetic immediately after appearance of the above signs. Internal organs (lungs, liver and others) were examined for the presence of tumor.
Results
PolyIC/MPPE induces expression of immunoactive cytokines in A431 and MDA-MB-468
In our previous study (2) we showed that low concentration of EGFR-targeted PolyIC induced expression of IFN-α, IP-10 and Gro-α in EGFR over-expressing glioblastoma cells (U87MGwtEGFR), but not cells with low levels of EGFR (U87MG). These data support the notion that cells produce these cytokines only when a certain threshold level of dsRNA has been internalized, and that this threshold is achieved only in cells over-expressing EGFR. In this study we extended the analysis to two additional EGFR over-expressing cancer cell lines: A431 (vulval epidermoid carcinoma) and MDA-MB-468 (breast carcinoma). When these cells were transfected with PolyIC/MPPE, we detected up to 5.1 pg/ml of IFN-β; 148 pg/ml of Gro-α and 188 pg/ml of IP-10 in the growth medium (Table S1). Gro-α and IP-10 are chemokines responsible for the recruitment of T cells to the area where they are expressed (4, 5). Thus, A431 and MDA-MB-468 cells, like U87MGwtEGFR cells, secrete cytokines into the medium, following challenge with PolyIC/MPPE.
In vitro activation of human immune cells
Given the above results, we hypothesized that the cytokine-enriched medium from A431 and MDA-MB-468 cells treated with PolyIC/MPPE should attract and stimulate the immune system. We examined whether this was so, by testing the effect of medium from PolyIC-transfected cancer cells on healthy human peripheral blood mononuclear cells (PBMCs)(6). PBMCs consist of several types of immune cells (NK, T-cells, NK-T cells, macrophages). The antitumor effect of these cells has been extensively studied and many cell killing mechanisms are well established. When activated, PBMCs produce toxic cytokines, such as IFN-γ and TNF–α (6), known to be effective against various cancer cells (7, 8). Other cell killing mechanisms include Perforin/Granzyme (9, 10) and Fas Ligand/Fas (10), which efficiently destroy tumor cells (10). PBMCs also interact with each other, leading to a synergistic, highly anti-proliferative effect. For example, activated T cells and NK cells produce IFN-γ, which activates macrophages (11) and stimulates the production of TNF-α(12). Release of IL-2 into the medium correlates directly with PBMC activation (6) and can be conveniently quantified by ELISA. Thus PBMCs are a convenient system for studying the selective immune reaction against PolyIC-transfected tumor cells.
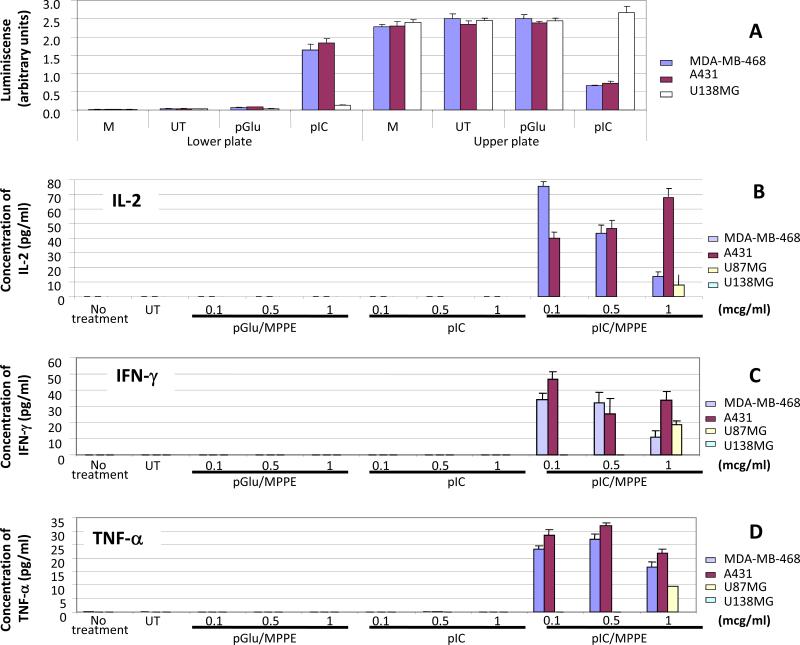
First, we examined whether the medium from PolyIC/MPPE-transfected cancer cells attracts PBMCs by chemotaxis (Fig. 1A). Using chemotactic chambers (Millipore) we show that the medium of A431 and the medium of MDA-MB-468 cells transfected with PolyIC/MPPE strongly stimulates chemotaxis of PBMCs. In contrast, medium of transfected U138MG cells virtually did not affect chemotaxis (Fig. 1A). Thus PBMCs are attracted only to the medium of EGFR overexpressing cells, which have been treated with PolyIC/MPPE.
Fig. 1. PBMCs are selectively attracted and activated by the medium of PolyIC/MPPE transfected MDA-MB-468 and A431 cells.
(A) Chemotaxis of PBMCs. MDA-MB-468, A431 and U138MG cells were grown and treated as described in Methods. 48 hrs after the transfection medium of the cells were transferred to the lower chamber of Millipore chemotaxis plates with 5-micrometer pores. PBMCs were added to the upper chamber, and plates were incubated for 4 hrs at 37°C. Both Lower and Upper plates were then subjected to CellTiter-Glo® Luminescent Cell Viability Assay (Methods) to detect both attracted PBMCs (Lower chamber) and non-attracted PBMCs (Upper chamber). The luminescence was counted (arbitrary units) in luminometer. “M”-medium only, incubated for 48 hrs with no cells. “UT”- medium of the untreated cells. Data represent means of triplicate wells ± SD. (B-D) Activation of PBMCs. 500,000 PBMCs were seeded into 24 well plates in duplicates and grown overnight in 0.5 ml medium as described in Methods. The PBMCs were then challenged with 0.5 ml of medium removed from A431, MDA-MB-468, U87MG or U138MG cells transfected with PolyIC/PEI-PEG-EGF-Mel 48 hrs after transfection. The PBMC medium was collected 48 hours after the challenge and IFN-γ, IL-2 and TNF-α were measured using ELISA assays. Transfection with naked PolyIC as well as pGlu/MPPE (poly glutamic acid/MPPE) was used as a negative control. (B) Expression of IL-2 in the medium of PBMCs. (C) Expression of IFN–γ in the medium of PBMCs. (D) Expression of TNF-α in the medium of PBMCs. “No treatment” shows expression of the cytokines in unchallenged PBMCs. “UT” shows expression of the cytokines by PBMCs challenged with the medium of untransfected cells. Data represent means of duplicate wells ± SD, and is representative of 2 experiments.
Next, we examined whether the medium from PolyIC/MPPE-transfected cancer cells activates PBMCs. Activation of PBMCs was measured using IL-2, IFN-γ and TNF-α ELISAs. PBMCs were challenged with medium from PolyIC/MPPE-transfected cancer cells. Figure 1B shows the induction of IL-2.expression by PBMCs 48 hrs after the challenge. Medium from A431 and MDA-MB-468 cells transfected with PolyIC/MPPE (0.1 mcg/ml) led the PBMCs to produce up to 165 pg/ml of IL-2. In contrast, medium from PolyIC/MPPE-treated U87MG cells (with ~12 times lower expression of EGFR than A431 and MDA-MB-468 cells) or U138MG cells (no EGFR expression (13)) did not affect PBMCs. Similar results were obtained when the expression of other cytokines was examined: Both IFN-γ (Fig. 1C) and TNF-α (Fig. 1D) were induced in PBMCs challenged with the medium from PolyIC/MPPE-transfected A431 and MDA-MB-468 cells, but not from PolyIC/MPPE-transfected U87MG and U138MG cells transfected with 0.1 mcg/ml of PolyIC/MPPE. Treatment of the cells with naked PolyIC or polyglutamic acid (pGlu) formulated with MPPE did not induce any production of the cytokines and no production of cytokines was noted in U138MG cells (that are devoid of EGFR) (13) (Fig. 1) and low levels of IL-2 and Interferon-γ in U87MG, which express about 80,000 receptors, were observed only at higher concentrations of PolyIC/MPPE (Fig. 1).
Activation of PBMCs in vivo
The selective expression of cytokines in EGFR over-expressing tumors was also confirmed in vivo (Table 1). SCID-NOD mice bearing EGFR over-expressing subcutaneous tumors on the right flank and U138MG tumors on the left flank were intravenously treated with 3 consecutive daily injections of PolyIC/MPPE followed by a single intraperitoneal injection of ten million PBMCs. Expression of cytokines was examined within the tumors as well as in blood from the animals, indicating the homing of the immune cells to the tumors. IFN-β was expressed in the EGFR over-expressing tumors only. IP-10 and Gro-α, potent T cell chemokines, were detected in the blood and, at much higher concentrations, within the EGFR over-expressing tumors (Table 1). These cytokines were expected to attract PBMCs selectively to the EGFR over-expressing tumors, where the PBMCs would be activated. In separate experiments, infiltration of the PBMCs (Fig. S1) into the EGFR over-expressing tumors of the PolyIC/+PBMCs treated animals was detected. No immune cell infiltration was detected in U138MG tumors, which do not over-express EGFR (Figures 2 and S1).
Table 1. In vivo cytokine expression pattern.
Cells were s.c. injected into right (A431 or MDA-MB-468) and left (U138MG) flanks of SCID/NOD mice. 20 days later, when the tumors reached approx 100-300 mm3, treatment was initiated as follows: 3 consecutive PolyIC/MPPE I.V. injections of 20 μg/mouse/day. 24 hrs after the last PolyIC/MPPE injection, 10 million PBMCs were injected I.P. 7 days later animals were sacrificed, tumors were extracted, homogenized with 1.5 mL extraction buffer (containing 10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100) per gram of tissue, using a homogenizer. Homogenates were centrifuged at 13,000Xg for 10 minutes at 4°C, stored at -70°C, then subjected to ELISAs. ELISAs were also performed on blood samples. ±PolyIC/MPPE/PBMC –± shows negative control with tumors extracted from untreated animals (3 animals per group), ±PolyIC/MPPE/PBMC +± shows cytokines expression from the animals treated as described above (4 animals per group). Data represent means of 3-4 animals ± SD. The numbers shows pg of cytokine per 1 gram of tissue (1ml of blood=1gr).
| MDA-MB-468 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP10 (pg/gr) | Groα (pg/gr) | IFNβ (pg/gr) | IL-2 (pg/gr) | IFNγ (pg/gr) | TNFα (pg/gr) | |||||||
| PolyIC/MPPE/PBMC | - | + | - | + | - | + | - | + | - | + | - | + |
| MDA-MB-468 | 8.6 2.2± |
89.3 ±11 |
10.6 ±7.9 |
237.6 24.7± |
0 | 7.4 ±2.6 |
0 |
56.5
±14.2 |
0 |
71
±14.4 |
0 |
1.47
±0.58 |
| U138MG | 13.4 9.5± |
5.5 ±3.8 |
14.5 ±5.9 |
8.9 ±7.2 |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood | 10.3 3± |
4.8 2.4± |
8.7 ±3.6 |
4.9 ±1.3 |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A431 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IP10 (pg/gr) | Groα (pg/gr) | IFNβ (pg/gr) | IL-2 (pg/gr) | IFNγ (pg/gr) | TNFα (pg/gr) | |||||||
| PolyIC/MPPE/PBMC | - | + | - | + | - | + | - | + | - | + | - | + |
| A431 | 1.5 ±1.6 |
108.1 26.1± |
9.4 ±6.8 |
324.8 71± |
0 | 16.3 9± |
0 |
63.3
±14.5 |
0 |
81.9
±13.02 |
0 |
2.31
±0.68 |
| U138MG | 2.9 2.8± |
7.7 9.1± |
6.6 ±4.3 |
6 ±7.6 |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood | 1.4 1.3± |
12.3 6.9± |
7.7 4.2± |
10.6 ±7.3 |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
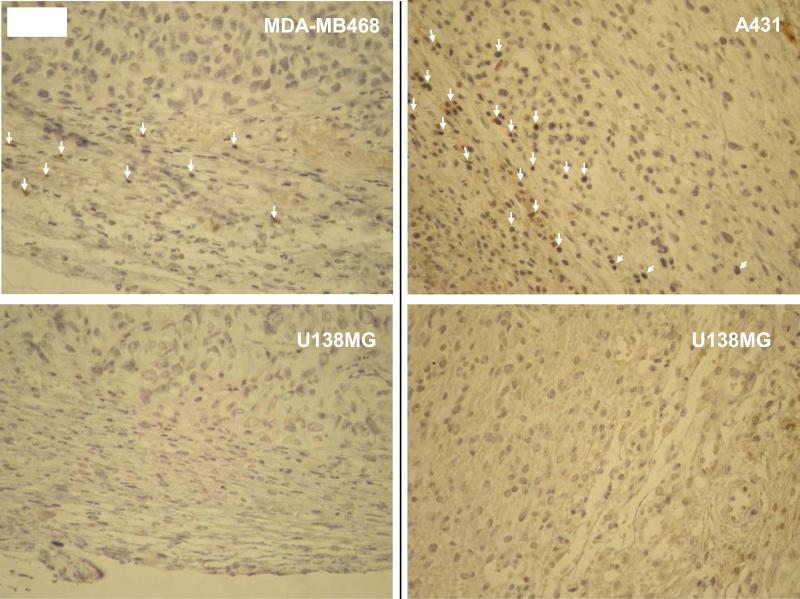
Fig. 2. Immune cells infiltrate into PolyIC/MPPE+PBMC treated EGFR over-expressing tumors.
Cells were injected s.c. into the right (A431 or MDA-MB-468) and left (U138MG) flanks of SCID-NOD mice. When the tumors reached approx 100 mm3 the treatment was initiated with 3 consecutive PolyIC/MPPE I.V. injections of 20 mcg/mouse/day. 24 hrs after the last PolyIC/MPPE injection 4 million PBMCs were injected I.P. 24 hrs later tumors were extracted, and fixed in 4% formalin. Paraffin sections were then prepared, immunostained with human antiCD3 ab and subjected to histopathological analysis. White arrows indicate CD3 positive cells.
PBMC-mediated bystander effect
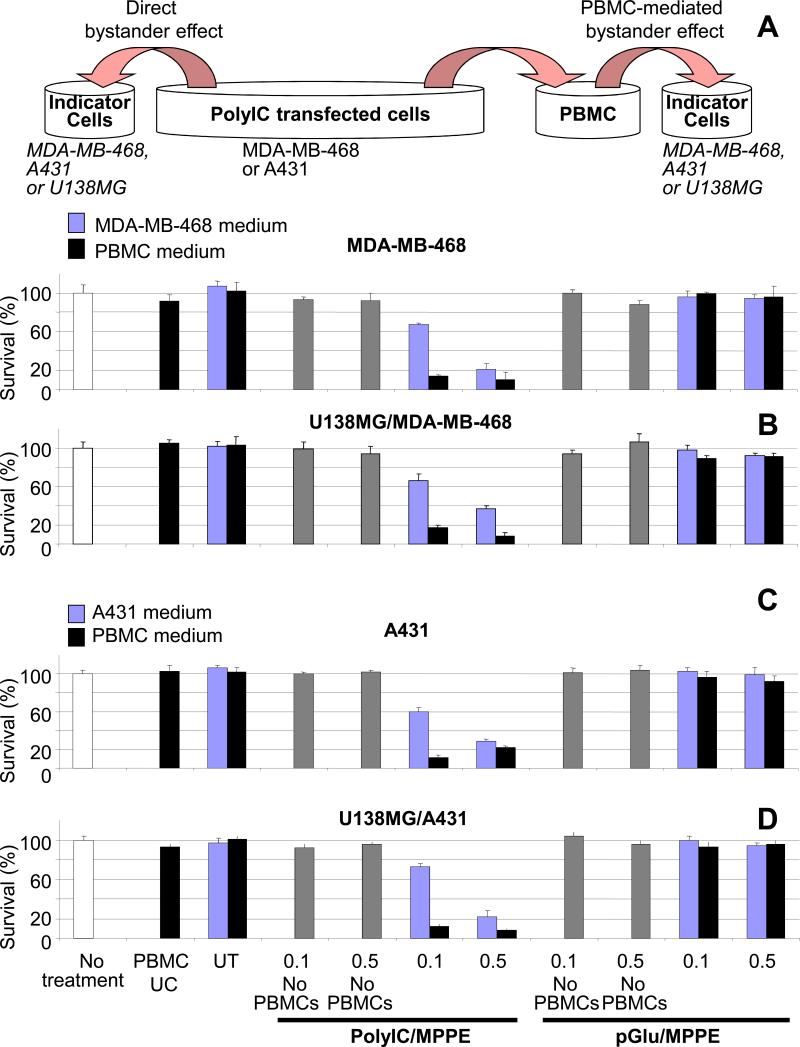
Expression of IFN–γ and TNF-α, potent antitumor cytokines, should strongly enhance bystander killing of untransfected cancer cells. In order to examine PBMC-mediated bystander effects, A431 or MDA-MB-468 cells were first transfected with PolyIC/MPPE and 48 hrs later PBMCs were challenged with the medium from the transfected cells (Methods). After another 48 hrs, medium from the challenged PBMCs was added to newly seeded, non-transfected cells (Fig. 3). The PBMC-mediated bystander effect was examined 24 and 48 hrs after medium exchange and compared with the “direct” bystander effect, mediated by medium from PolyIC/MPPE-treated A431 and MDAMB-468 cells (Fig. 3A and 3C). Both direct and PBMC-mediated bystander effects are clearly shown in Figure 3. The PBMC-mediated effect was particularly strong, killing up to 90% of the non-transfected cells. U138MG cells, which do not express EGFR at all, were also efficiently inhibited by both types of medium (Fig. 3B and 3D). No effect was observed when PolyIC was replaced by polyglutamic acid (pGlu), which similarly (to PolyIC) forms particles with MPPE, but does not induce an immune response.
Fig. 3. PBMC-mediated bystander effect.
(A) Shows experiment design (described in Methods) and the bystander effect of PolyIC transfected MDA-MB-468 cells on untransfected MDA-MB-468 cells. (B) Shows the bystander effect of poly IC transfected MDA-MB-468 cells on untransfected U138MG cells. (C) Shows the bystander effect of PolyIC transfected A431 cells on untransfected A431 cells. (D) Shows the bystander effect of PolyIC transfected A431 cells on untransfected U138MG cells.
“No treatment” shows survival of indicator cells that did not undergo any medium exchange. “PBMCs UC” shows survival of indicator cells treated with medium from unchallenged PBMCs. “UT” shows survival of indicator cells treated with medium from PBMCs challenged by the medium of untransfected cells. “No PBMCs” (gray bars) indicates survival when conditioned medium was added to PBMC growth medium but in the absence of PBMCs, and this was used 48 hours later to challenge the indicator cells. This latest control was used to detect a possible residual direct bystander effect of the conditioned medium after incubation in PBMCs medium in the absence of PBMCs. Mean values of the triplicates shown. The experiment was repeated twice.
As expected, pre-incubation of PBMC medium with neutralizing IFN-γ and TNF-α antibodies strongly inhibited PBMC-mediated bystander effects (Supplementary Fig. S2). When added together the protecting effect of the antibodies was very strong. In contrast, neutralization by single antibody, either to IFN-γ or to TNF-α, had only a slight effect. It was reported previously that Interferon-γ and TNF-α synergize in their killing effects (14, 15). It seems that at least in the presented setting each cytokine has its own strong antiproliferative effect. These results demonstrate that the combination of PolyIC/MPPE and PBMC synergize effectively killing EGFR overexpressing cancer cells.
PBMCs strongly enhance PolyIC/MPPE cancer cell killing in vitro
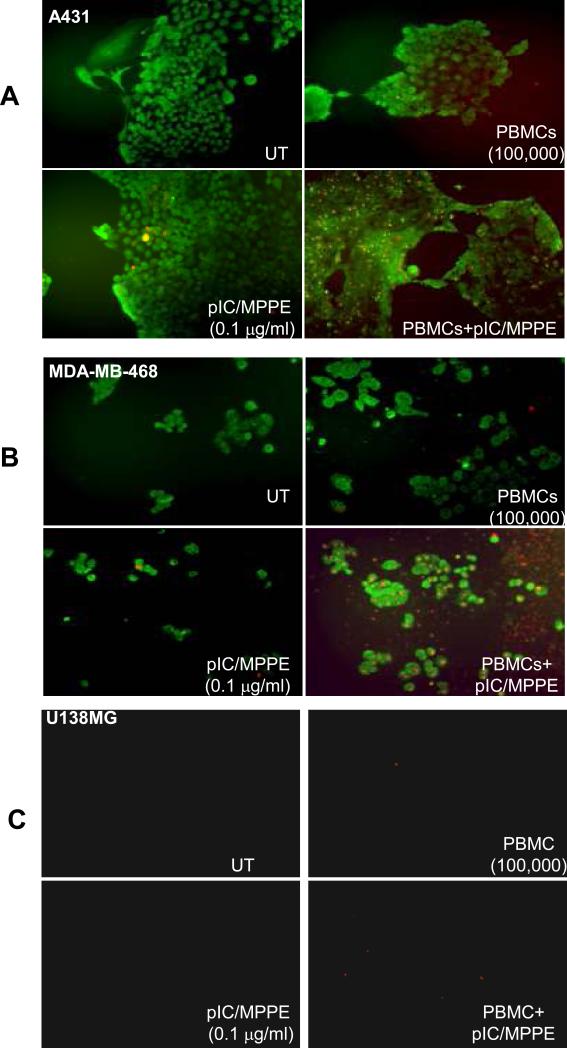
To examine the synergistic cancer killing effect, tumor cells were transfected with PolyIC/MPPE at low dose, followed by addition of PBMCs (Fig. 4). To distinguish tumor cells from PBMCs, tumor cells were labeled with FITC-conjugated EGFR antibody (Green fluorescence). Tumor cells undergoing apoptosis were detected with an Annexin-V-Biotin kit (Biosource Inc., Red fluorescence). Cells treated with either PolyIC/MPPE alone at low dose of 0.1 mcg/ml or PBMCs alone showed a very weak apoptotic signal. In contrast, a strong apoptotic signal was detected when the EGFR over-expressing cells were treated with both PolyIC/MPPE (0.1 mcg/ml) and PBMCs (Fig. 4A, B). U138MG cells did not undergo detectable apoptosis (Fig. 4C). Thus, the addition of PBMCs to PolyIC/MPPE-treated tumor cells strongly enhanced tumor cell apoptosis. Apoptotic death of EGFR over-expressing tumors was also confirmed in vivo (Supplementary Fig. S3).
Fig. 4. In vitro cancer cell killing by activated PBMCs.
Cells were grown as described in Methods. Cells were then transfected with PolyIC/MPPE at 0.1 mcg/ml. 24 hrs later 500,000 PBMCs/well were added to the cancer cells and co-incubated for another 24 hrs. Apoptotic cells (red fluorescence) were visualized using an Annexin-VBiotin kit (Biosource, Inc.). To distinguish tumor cells from PBMCs, tumor cells were labeled with FITC-conjugated EGFR antibody (Biosource, Inc., green fluorescence). Cells were visualized with a fluorescent microscope and photographed using a digital camera. (A) shows A431 cells, (B) shows MDA-MB-468 cells, (C) shows U138MG cells.
Systemic application of PolyIC/MPPE combined with PBMCs cures mice with disseminated tumors
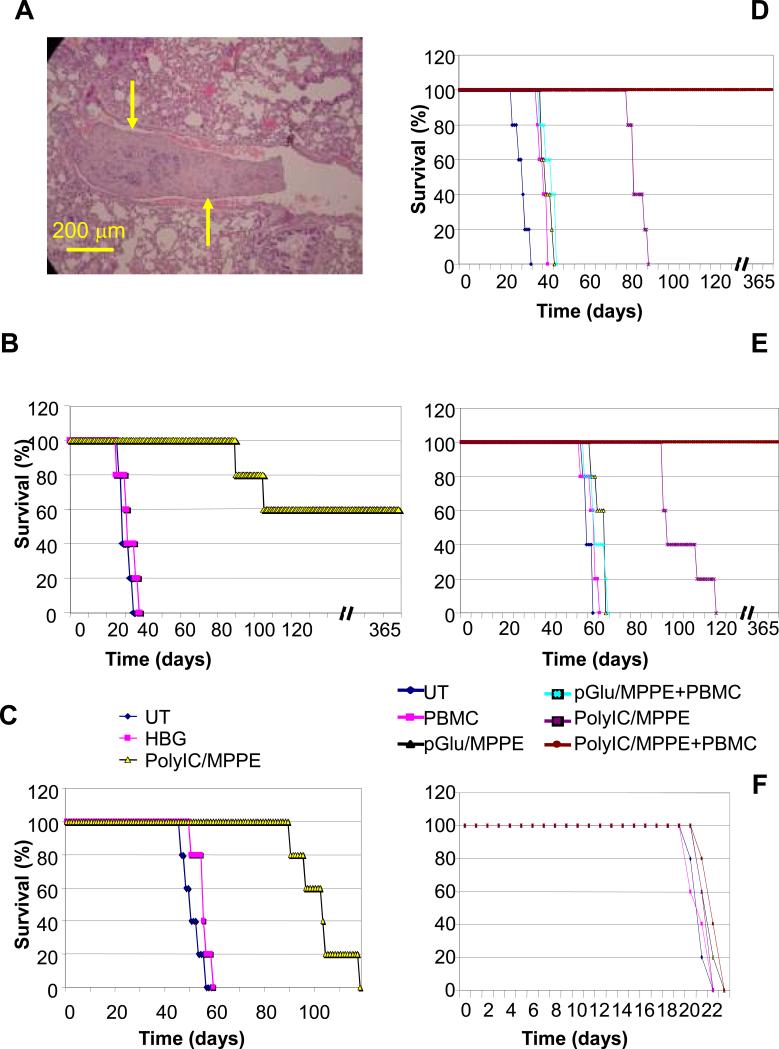
In view of our earlier finding that the PolyIC-loaded EGFR homing vector had no toxic effects on normal brain cells in tissue culture or in vivo (2), we examined whether EGFR-targeted PolyIC/MPPE could be applied systemically, for the treatment of disseminated EGFR over-expressing tumors in vivo. In the absence of a mouse model of EGFR over-expressing tumors, we injected i.v. 1 million human A431 or MDA-MB-468 cells into SCID-NOD mice. Ten days after cell injection, treatment was initiated, with two 3-day cycles and one 4-day cycle of daily injections of 20 mcg PolyIC/MPPE, with a 24 hour interval between each cycle (i.e. a total of 10 injections, spread over 12 days). Mice bearing A431 tumors that received PolyIC/MPPE survived at least 3 times longer than untreated mice, and three mice were completely cured (Fig. 5B). Mice bearing MDA-MB-468 tumors treated with PolyIC/MPPE survived up to twice as long as untreated mice (Fig. 5C)
Fig. 5. Effect of PolyIC/MPPE/PBMC treatment on survival of mice with disseminated tumors.
Disseminated tumors were established as described in Methods. (A) Histopathological analysis of mouse lungs at the time of treatment initiation (15 days after cell injection). Yellow arrows point to a tumor in a lung capillary. (B, C, F) 15 days after cell injection the animals were randomly divided into groups (5 mice per group) and the treatment was initiated with 4 consecutive intravenous injections of 20 mcg PolyIC/MPPE at 24 hr intervals. 24 hrs after the last PolyIC injection, the animals were injected once with four million PBMCs. Control groups included mice treated with pGlu/MPPE (polyglutamic acid/MPPE) to determine the effect of the conjugate without PolyIC. (B) Shows survival of animals with A431 tumors. (C) Shows survival of animals with MDA-MB-468 tumors. (F) Shows survival of animals with U138MG tumors. (D, E) 10 days after cell injection the animals were randomly divided into groups (5 mice per group) and the treatment was initiated with 3 cycles of 3 or 4 consecutive intravenous injections of 20 μg polyIC/MPPE at 24 hr intervals (total 10 injections). The interval between cycles was 48 hrs. Control groups included mice treated with pGlu/MPPE (Poly Glutamic acid/MPPE) to determine the effect of the conjugate without PolyIC and HBG buffer (Hepes Buffered Glucose)(2). (D) Shows survival of animals with A431 tumors. (E) Shows survival of animals with MDA-MB-468 tumors.
These results, combined with the finding that PBMCs strongly enhance the effect of PolyIC/MPPE in vitro, encouraged us to test whether PBMCs would similarly enhance the effect of PolyIC/MPPE in vivo. For these experiments, we waited 15 days after injection of A431 or MDA-MB-468 cells in SCID-NOD mice, at which point large tumors of up to 500 μm could be detected in the lungs (Fig 5A). Mice were then treated with 4 consecutive, daily intravenous injections of 20 mcg of PolyIC/MPPE. 24 hrs after the final PolyIC injection, the mice were injected once with four million human PBMCs (16). Reconstitution of the SCID-NOD mouse immune system using human PBMCs is a common practice (17, 18, 19). As in the earlier experiment, PolyIC/MPPE treated mice bearing A431 tumors survived longer than untreated mice. Mice that were treated with both PolyIC/MPPE and human PBMCs survived for more than a year, and did not show any signs of tumors (Fig. 5D). Similarly, mice bearing MDA-MB-468 tumors treated with PolyIC/MPPE alone survived up to twice as long as untreated mice, whereas mice treated with both PolyIC/MPPE and PBMCs survived more than a year and did not show any signs of tumors (Fig. 5E). In contrast there was virtually no change in survival rate of the mice bearing U138MG tumors, which do not express EGFR (Fig. 5F) and treated at the exactly same manner with PolyIC/MPPE and PBMCs. No visible signs of toxicity such as reduction in weight or abnormal behavior were detected, either during the treatment or afterwards. Thus, by introducing human PBMCs, we were able to significantly reduce the dosage and duration of treatment with PolyIC/MPPE and to eliminate established disseminated tumors. Hence, PBMCs play a crucial role in tumor eradication.
Discussion
Most cancer deaths are caused by metastases, and there is a great need for effective therapies. A large fraction of the most frequent and the deadliest cancers over-express the EGFR, including: lung cancer (215,020 estimated new cases and 161,840 estimated deaths in 2008 in the USA), breast cancer (184,450 estimated new cases, 40,930 estimated deaths), colon and rectal cancer (148,610 estimated new cases, 55,170 estimated deaths). EGFR over- expression is often correlated with metastasis and poor prognosis. Here we show that targeted PolyIC, delivered systemically, mobilizes immune cells to achieve complete regression of disseminated tumors.
We earlier showed that EGFR-targeted PolyIC leads to complete regression of localized xenografts in mice (2). EGFR-targeted PolyIC exerts a strong bystander effect, that is, it kills EGFR over-expressing cells as well as neighboring tumor cells, whether or not they express EGFR or its mutated version EGFRvIII (2). This is crucial, because even tumors showing strong over-expression of EGFR are commonly heterogeneous with respect to EGFR expression. At the same time, EGFR-targeted PolyIC is highly selective for tumor cells, with minimal toxic effects on the surrounding normal tissues (2) as well as on distant normal tissues. In the future more extensive toxicity studies will examine this issue, in particular attention to skin and other EGFR-rich organs, this is especially important prior to clinical development. The targeted PolyIC quickly activates multiple anti-proliferative/pro-apoptotic pathways, minimizing the likelihood of mutations leading to drug resistance. In the current study we have been able to demonstrate that the PolyIC loaded vector targeted to EGFR, can be applied systemically and therefore be utilized to treat disseminated EGFR overexpressing tumors (Fig.5). Furthermore, we demonstrate an additional advantage of the EGFR-targeted PolyIC strategy, namely, the activation of an immune reaction selectively against the tumor (Fig.5). Treatment of mice harboring tumors lacking EGFR with PolyIC/MPPE did not result in any cure, with or without PBMC (Fig.5F). In our previous study we used nude or SCID mice with significantly impaired immune system. We did not detect infiltration of the (remaining) mouse immune cells into the PolyIC/MPPE treated GBM model, despite induction of human IFN-α and other cytokines (2). Here, reconstituting of the mice immune system with human PBMCs resulted in strong yet selective immune reaction against cancer (Fig. 5 D, E). This is also demonstrated by the expression of IL-2, TNF-α and IFN-γ within the tumor, in EGFR overexpressing tumor bearing mice, treated with PolyIC/MPPE and PBMC (Table 1) but not in tumors lacking EGFR. Infiltration of immune cells, including T-cells is also demonstrated histologically (Fig. 2, Fig. S1).
It should be noted that systemic application of non-targeted PolyIC has been attempted to treat cancer (20, 21). The survival benefit was minimal while pronounced systemic toxicity was observed (20, 21). The weak effect was most likely caused by the failure to introduce a sufficient dose of PolyIC into the tumor cells. Most of the non-targeted PolyIC probably scattered through normal tissues entering into non-cancer cells and inducing toxic reactions. On the other hand EGFR-targeted PolyIC affects cancer cells only, leaving normal cells unharmed.
For a tumor to become established it must avoid elimination by the immune system. Many cancers develop mechanisms of inhibiting immune surveillance and can even grow in the presence of immune lymphocytes that recognize cancer antigens (22, 23) Furthermore, elements of the immune system can be co-opted for tumor growth (24), supporting the Virchow hypothesis (1863) that the numerous immune cells found in the vicinity of practically all malignant tumors, attest to the role of inflammation in the generation of cancer. It seems however that some of these cells possess anti-tumor activities (25), and can be “woken up” by tumor localized PolyIC, as shown here. PolyIC, a potent adjuvant, and interferon, a strong immune activator, may activate pre-existing cancer specific immune lymphocytes, in addition to attracting and activating other anti-cancer immune cells. Here we show that the cytokines induced by targeted PolyIC attract immune cells to the tumor and strongly enhance the efficiency with which cancer cells are killed. This induces a significant additive or even synergistic effect with the direct effects induced by the internalized PolyIC, leading to the complete elimination of disseminated tumors, even when treated from a distance with a low total dose of EGFR-targeted PolyIC (Figure 5D, E). Activated immune cells strongly enhance the bystander effect (Figure 3), which should facilitate the killing of heterogeneous cancers. The relatively fast immune response (Fig. 3 and Fig. 4) indicates that primarily the innate immunity is activated. It is likely though that at later stage adoptive immunity against cancer could be developed as well. Infiltration of antigen presenting cells (APC), such as macrophages, into the tumor and induction of MHC of both classes by IFNs eventually should lead to an adoptive immune response against cancer. Although our model preclude study of adoptive immune response, induction of IFNs (Tables 1, S1) and infiltration of PBMCs (Fig. 2, Fig S1) into the tumor implies possible activation of adoptive immunity that should further enhance efficiency and selectivity of the EGFR targeted PolyIC therapy.
Since PolyIC/MPPE is targeted selectively to cancer cells, we do not expect significant systemic immunotoxic reactions to occur. The fact that human PBMCs injected into mice did not induce any apparent Graft versus Host reaction, while inducing a strong antitumor reaction, supports this theory.
Intratumoral or peritumoral administration of non-targeted PolyIC has been demonstrated to be effective in anti-tumor immunotherapy (26). Such treatment is limited to localized tumors only. In contrast EGFR-targeted PolyIC is effective both by local treatment (2) and in the treatment of disseminated tumors as shown in this study.
EGFR-targeted PolyIC could potentially be combined with several cancer immunotherapies available today. These include cancer vaccines and cancer-targeted (engineered or extracted) T cells. To mediate anti-tumor effects in vivo, cancer-targeted T cells must travel to the tumor site, extravasate from the circulation, and then mediate effector functions to cause destruction of cancer cells (25). IP-10 and Gro-α strongly induced by targeted PolyIC selectively in tumor cells (Table 1, S1), should facilitate both traffic to tumor and extravasation (4, 5), while interferon should enhance T cell mediated cancer killing.
In addition, allergenic immune cell transplantation to activate graft versus tumor reactions (27) could be combined with the strategy described here. Injection of foreign PBMCs into PolyIC/MPPE treated mice in the present study (Fig. 5D, E) actually resembles such a combination. Grafted immune cells should confer a stronger antitumor effect than the patient's own immune cells.
The absence of an immunocompetent mouse model of EGFR over-expressing cancer precludes study of the activation of the mouse's own immune system by PolyIC/MPPE. However the data presented here suggest that systemic treatment of EGFR over-expressing metastatic tumors with EGFR-targeted PolyIC may lead to a complete cure, in patients with a functional immune system. In view of the success of the EGFR targeted PolyIC therapy, we propose to explore the validity of the approach for the treatment of other cancers. The chemical vector we utilize is actually built like Lego, such that the homing ligand can be modified while the other elements remain constant. Any tumor that overexpresses a surface protein that can be internalized upon ligand binding is a candidate for such therapy, once the coupling conditions have been optimized for the relevant ligand.
Supplementary Material
Acknowledgements
This study was supported by a grants from The Association for International Cancer Research (AICR), UK, and a grant from the National Cancer Institute (NIH), USA 1R01CA125500 -01A2. We would like to thank Dr. Shoshana Klein from our unit for her comments and editing. In addition, it was partially supported by the ERC advanced grant.
Abbreviations
- EGFR
Epidermal Growth Factor Receptor
- dsRNA
double stranded RNA
- MPPE
Melittin-Polyethyleneimine-Polyethyleneglycol-EGF
- PBMCs
Peripheral Blood Mononuclear Cells
References
- 1.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Shir A, Ogris M, Wagner E, Levitzki A. EGF receptor-targeted synthetic double-stranded RNA eliminates glioblastoma, breast cancer, and adenocarcinoma tumors in mice. PLoS Med. 2006;3:e6. doi: 10.1371/journal.pmed.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y, Caday CG, Nanda A, Cavenee WK, Huang HJ. Tyrphostin AG 1478 preferentially inhibits human glioma cells expressing truncated rather than wild-type epidermal growth factor receptors. Cancer Res. 1996;56:3859–61. [PubMed] [Google Scholar]
- 4.Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–80. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, Xiang J. Synergistic effect of lymphotactin and interferon gamma-inducible protein-10 transgene expression in T-cell localization and adoptive T-cell therapy of tumors. Int J Cancer. 2004;109:817–25. doi: 10.1002/ijc.20043. [DOI] [PubMed] [Google Scholar]
- 6.Kruse N, Moriabadi NF, Toyka KV, Rieckmann P. Characterization of early immunological responses in primary cultures of differentially activated human peripheral mononuclear cells. J Immunol Methods. 2001;247:131–9. doi: 10.1016/s0022-1759(00)00316-1. [DOI] [PubMed] [Google Scholar]
- 7.Alberts DS, Marth C, Alvarez RD, et al. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression-free survival. Gynecol Oncol. 2008;109:174–81. doi: 10.1016/j.ygyno.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Daniel D, Wilson NS. Tumor necrosis factor: renaissance as a cancer therapeutic? Curr Cancer Drug Targets. 2008;8:124–31. doi: 10.2174/156800908783769346. [DOI] [PubMed] [Google Scholar]
- 9.Gamero AM, Ussery D, Reintgen DS, Puleo CA, Djeu JY. Interleukin 15 induction of lymphokine-activated killer cell function against autologous tumor cells in melanoma patient lymphocytes by a CD18-dependent, perforin-related mechanism. Cancer Res. 1995;55:4988–94. [PubMed] [Google Scholar]
- 10.Mori S, Jewett A, Murakami-Mori K, Cavalcanti M, Bonavida B. The participation of the Fas-mediated cytotoxic pathway by natural killer cells is tumor-cell-dependent. Cancer Immunol Immunother. 1997;44:282–90. doi: 10.1007/s002620050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV terapy (type I/II) (2 of 2 parts). Clin Ter. 2006;157:457–68. [PubMed] [Google Scholar]
- 12.Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006;97:439–47. doi: 10.1111/j.1349-7006.2006.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu TF, Cohen KA, Ramage JG, Willingham MC, Thorburn AM, Frankel AE. A diphtheria toxin-epidermal growth factor fusion protein is cytotoxic to human glioblastoma multiforme cells. Cancer Res. 2003;63:1834–7. [PubMed] [Google Scholar]
- 14.Paludan SR. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J Leukoc Biol. 2000;67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Talmadge JE. Synergy in the toxicity of cytokines: preclinical studies. Int J Immunopharmacol. 1992;14:383–90. doi: 10.1016/0192-0561(92)90168-k. [DOI] [PubMed] [Google Scholar]
- 16.Karagiannis SN, Wang Q, East N, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–40. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 17.Lorber MI, Wilson JH, Robert ME, et al. Human allogeneic vascular rejection after arterial transplantation and peripheral lymphoid reconstitution in severe combined immunodeficient mice. Transplantation. 1999;67:897–903. doi: 10.1097/00007890-199903270-00018. [DOI] [PubMed] [Google Scholar]
- 18.King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–14. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Tai HC, Tu CF, Lee JM, et al. Long-term survival of HLA-DR15+ pig skin in SCID mice after reconstitution with human peripheral blood mononuclear cells and under short-term immunosuppression. Transplant Proc. 2008;40:570–3. doi: 10.1016/j.transproceed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Butowski N, Chang SM, Junck L, et al. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01-05). J Neurooncol. 2009;91:175–82. doi: 10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar AM, Levy HB, Ondra S, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38:1096–103. discussion 103-4. [PubMed] [Google Scholar]
- 22.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–8. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA. Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci U S A. 2008;105:12643–4. doi: 10.1073/pnas.0806877105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimura T, Nakagawa S, Ohtani T, Ito Y, Aiba S. Inhibitory effect of the polyinosinic-polycytidylic acid/cationic liposome on the progression of murine B16F10 melanoma. Eur J Immunol. 2006;36:3371–80. doi: 10.1002/eji.200636053. [DOI] [PubMed] [Google Scholar]
- 27.Ciceri F, Bonini C, Marktel S, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.