Abstract
Background
Unmedicated schizophrenia patients exhibit deficits in prepulse inhibition of the acoustic startle response (PPI). Similar deficits can be induced in rodents via a variety of manipulations and these deficits can be reversed by antipsychotics. Brown Norway (BN) rats exhibit natural PPI deficits under certain parametric conditions. We treated BN rats with haloperidol or clozapine to determine if the BN rat is a useful animal model with predictive validity for the effects of antipsychotics. In addition, we also tested PD149163, a neurotensin-1 receptor agonist, which has been shown to exhibit antipsychotic-like effects in several other animal models.
Methods
BN rats received subcutaneous injections of either saline or one of two doses of haloperidol (0.5 mg/kg, 1.0 mg/kg), clozapine (7.5 mg/kg, 10 mg/kg) or PD149163 (1.0 mg/kg, 2.0 mg/kg). PPI was measured in startle chambers 30 min after injection.
Results
Systemic clozapine and PD149163 but not haloperidol facilitated PPI in BN rats (P < 0.001).
Discussion
This drug response profile suggests that the BN rat may be useful for detecting atypical antipsychotics and antipsychotics with novel mechanisms of action. The results also add to the evidence suggesting that PD149163 may have antipsychotic properties.
Keywords: schizophrenia, neurotensin, animal model, prepulse inhibition, antipsychotic
INTRODUCTION
Animal models are essential for developing new treatments for psychiatric diseases such as schizophrenia. An important characteristic required for an animal model to have utility in drug discovery is predictive validity, which for drug discovery purposes is narrowly defined as the ability to respond to established treatments for the modeled disorder with sensitivity (i.e. positive response to all drugs with established efficacy) and specificity (i.e. no response to drugs that do not have therapeutic efficacy for the disorder).
Since the core features of schizophrenia (e.g. delusions, hallucinations) do not lend themselves to direct modeling in non-human animals, animal modeling efforts have focused on biomarkers that appear to be associated with the disease. Prepulse inhibition (PPI) of the startle reflex is one such marker that has received a lot of attention and has become the basis of development of several animal models that exhibit predictive validity for the effects of antipsychotics (Geyer, Krebs-Thomson, Braff, & Swerdlow, 2001). PPI refers to the reduction in the startle response when the startle stimulus is preceded by a weak stimulus (Swerdlow & Geyer, 1998). PPI is deficient in schizophrenia patients as well as other neuropsychiatric conditions (Braff, Geyer, & Swerdlow, 2001) and converging evidence suggests that antipsychotics, particularly atypical antipsychotics, restore PPI in schizophrenia patients to normal levels (Kumari & Sharma, 2002; Leumann, Feldon, Vollenweider, & Ludewig, 2002; Oranje, Van Oel, Gispen-De Wied, Verbaten, & Kahn, 2002). In rodents psychotomimetic drugs including dopamine agonists (e.g. apomorphine and amphetamine), NMDA antagonists (e.g. dizocilpine and PCP) and serotonin agonists (e.g. DOI) can induce deficits analogous to those of schizophrenia. Typical antipsychotics reverse dopamine agonist-induced PPI deficits, while atypical antipsychotics reverse deficits produced by dopamine and serotonin agonists, and NMDA antagonists (Geyer et al., 2001).
However animal models that require pharmacological induction of PPI deficits have limitations (Feifel, Melendez, & Shilling, 2004) and thus there is an interest in models that can display predictive validity without a need for pharmacological disruption. Studies investigating the effects of antipsychotics on the normal and low baseline PPI levels in Sprague Dawley and Wistar rats have not found robust predictive validity for antipsychotic efficacy (Depoortere, Perrault, & Sanger, 1997a, 1997b). For example, clozapine consistently elevated PPI but the effects of other antipsychotics on PPI were not consistent. In contrast, we and others have found that Brattleboro rats, a rat strain that has low levels of PPI, exhibits strong predictive validity for the effects of atypical antipsychotics (Cilia et al., 2010; Feifel, Melendez, Priebe, & Shilling, 2007; Feifel et al., 2004; Feifel & Priebe, 2001; Feifel, Shilling, & Melendez, 2010). There is interest in other rodent strains that have naturally low PPI levels, analogous to schizophrenia patients that may also exhibit predictive validity for the effects of antipsychotics.
The Brown Norway (BN) rat has been shown to have naturally low PPI compared to a number of other strains when the prepulse-pulse interval is between 30 and 120 milliseconds (Conti, Costill, Flynn, & Tayler, 2005; Conti, Palmer, Vanella, & Printz, 2001; Conti & Printz, 2003; Palmer et al., 2000; Swerdlow, Breier, Mora, Ko, & Shoemaker, 2008; Swerdlow, Talledo, Sutherland, Nagy, & Shoemaker, 2006). Conti et al. (Conti et al., 2005) found that neither haloperidol, nor clozapine, characteristic members of the typical and atypical generation of antipsychotic families, respectively, attenuated PPI deficits in BN rats. However, Swerdlow et al. (2006) found that quetiapine, an atypical antipsychotic, significantly increased low PPI in BN rats.
In order to clarify the effects of antipsychotics on PPI in BN rats, we studied the effects of clozapine and haloperidol, as well as the effects of the putative antipsychotic PD149163, a neurotensin-1 receptor agonist that has been shown to facilitate PPI in several other animal models of deficient PPI (Feifel et al., 2007; Feifel et al., 2004; Feifel, Pang et al., 2010; Feifel, Reza, Wustrow, & Davis, 1999; Shilling, Melendez, Priebe, Richelson, & Feifel, 2004).
MATERIALS AND METHODS
Sixty Brown Norway rats, (250–350 grams at testing) Harlan (Harlan Laboratories, San Diego) were used in these studies. Rats were housed in groups of two or three in clear plastic chambers in a climate controlled room under a 12h/12h light/dark schedule (lights on/off - 7:00 A.M/ 7:00 P.M). They were allowed free access to food and water for the extent of the study. Behavioral testing was performed when rats were 8–10 weeks old, during the light phase of the rats’ circadian illumination schedule as startle magnitude, PPI and drug effects on PPI are stable across the circadian cycle (Weiss, Feldon, & Domeney, 1999). All experimental procedures were conducted in accordance with the University of California, San Diego guidelines for animal care and experimentation, and approved by the UCSD IACUC. A minimum of seven days after arrival drug studies began. Several studies were performed to test a range of PD149163, haloperidol and clozapine doses. Animals were injected with either saline or one of two doses of haloperidol (0.5, 1.0 mg/kg), clozapine (7.5 or 10 mg/kg) or PD149163 (1.0 or 2.0 mg/kg). Animals were tested in startle chambers 30 minutes later.
Startle Testing
Startle testing was performed in four identical startle chambers obtained from San Diego Instruments (San Diego, CA). Each chamber consisted of a clear non-restrictive Plexiglass cylinder resting on a Plexiglass platform inside a ventilated and illuminated enclosure housed in a sound-attenuated room. Continuous background white noise of 65 dB, as well as the various acoustic stimuli was produced within each chamber by a high-frequency loudspeaker (Radio Shack Supertweeter, San Diego, CA). The whole-body startle response of each animal produced vibrations of the Plexiglass cylinder, which were transduced into analog signals by a piezoelectric unit, mounted underneath the Plexiglass platform (Mansbach, Geyer, & Braff, 1988). These analog signals were then digitized and stored by an interface unit connected to a microcomputer. Startle amplitude was defined as the degree of motion detected by the piezoelectric unit.
Once in startle chambers each rat had a 5-minute acclimation period. A 65-dB white noise background was continuously present throughout the session. The acclimation was followed by a 15 minute PPI test session during which rats were presented with 40 msec broadband 120 dB burst (startle) without a prepulse, or a similar broadband burst preceded 100 msec by a 20 ms broadband burst of either 4, 8 or 12 dB above background. These four types of active stimuli were presented in addition to a neutral (no sound) stimuli condition in pseudorandom order with an average of 15 seconds between stimuli types.
A startle response was recorded for all stimuli presentations. PPI for each animal was calculated as a percentage of the pulse-alone startle magnitude using the following formula: [1− (startle magnitude after prepulse-pulse pair/startle magnitude after pulse only] × 100.
Statistical Analyses
To compare treatment groups, % PPI data for each drug were subjected to a separate two-way ANOVA with Prepulse Intensity as a within-subject factor and Drug Treatment as a between-subject factor. A significant ANOVA was followed by pair-wise comparisons using a Dunnett’s two-tailed post hoc test to compare the low and high dose groups to saline treated animals. The acoustic startle response (ASR) to the startle stimuli presented without a prepulse for each drug was subjected to ANOVA. Dunnett’s posthoc tests were used to compare the low and high dose groups to saline treated animals. Four BN rats treated with the high dose of clozapine exhibited an acoustic startle response less than 10 and were eliminated from the study. We have found that startle values below 10 result in PPI values that are unreliable, and not consistent with the rest of the data set, likely due to a floor effect.
RESULTS
PPI data are exhibited in Figure 1 (Main). PD149163: There was a main effect of Drug [F(2, 45) = 14.763, P < 0.001], a main effect of Prepulse Intensity [F(2,90) = 102.188, P < 0.001] and a significant Prepulse Intensity × Drug interaction [F(4,90) = 7.195, P = 0.001] as % PPI increased with increasing prepulses and drug-induced facilitation of PPI tended to be stronger at the higher prepulse intensities (e.g. 8 dB and 12 dB). To identify the specific prepulse intensities that exhibited significantly different PPI at each of the three prepulses, we performed ANOVAs for the prepulse (pp) 4 [F(2,47) = 1.106, NS], pp8 [F(2,47) =13.294, P < 0.001] and pp12 [F(2,47) = 27.72, P < 0.001]. Dunnett’s post hoc tests indicated that at pp8 and pp12 both doses of PD 149163 produced significantly higher % PPI compared to saline treated rats P ≤ 0.001.
Figure 1.
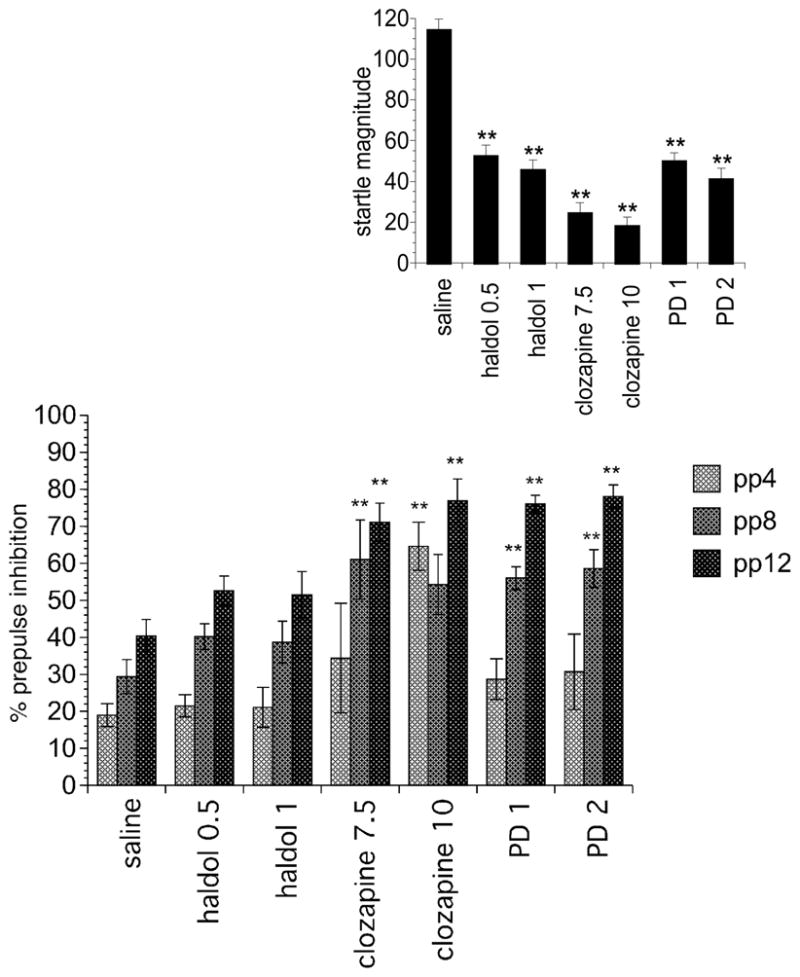
The effect of haloperidol, clozapine, and PD149163 administration on PPI (Main) and startle magnitude (Inset). Doses are mg/kg. Significantly different from saline treated represented by **P < 0.01. Data displayed as the mean±SEM.
Clozapine
There was a main effect of Drug [F(2, 31) = 10.271, P < 0.001], a main effect of Prepulse Intensity [F(2,62) = 15.504, P < 0.001] and a significant Prepulse Intensity × Drug interaction [F(4,62) = 2.536, P < 0.05] as % PPI increased with increasing prepulses and drug-induced facilitation of PPI tended to be stronger at drug-induced facilitation of PPI tended to be stronger at the higher prepulse intensities (e.g. 8 dB and 12 dB). To identify the specific prepulse intensities that exhibited significantly different PPI at each of the three prepulses, we performed ANOVAs for the prepulse (pp) 4 [F(2,33) = 9.283, P = 0.001], pp8 [F(2,33) = 5.902, P < 0.01] and pp12 [F(2,33) = 9.959, P < 0.001]. Dunnett’s post hoc tests indicated that at pp4 the high dose (P < 0.001), pp8 the low dose (P < 0.01) and at pp12 both doses (P < 0.01) of clozapine, resulted in significantly higher PPI compared to saline treated rats.
Haloperidol
The was a main effect of Prepulse Intensity [F(2,88) = 59.473, P < 0.001] as % PPI increased with increasing prepulses. There were no other significant effects.
Startle data are displayed in Figure 1 (Inset). There was a main effect of drug for PD149163 [F(2,47) = 67.038, P < 0.001], clozapine [F(2,33) = 65.192, P < 0.001], and haloperidol [F(2,46) = 53.838, P < 0.001] as all six drug groups exhibited significantly lower ASR compared to saline treated rats, P < 0.001.
DISCUSSION
Consistent with previous reports (Conti et al., 2005; Palmer et al., 2000; Swerdlow et al., 2006), saline-treated BN rats exhibited low PPI when the interstimulus interval (ISI) is set to 100 msecs (average 29.7%). In this study, we found that clozapine and PD149163 administration facilitated PPI in BN rats. In contrast, haloperidol administration had no effect on PPI in these rats. The lack of an effect of haloperidol on BN PPI in this study is consistent with the findings of Conti et al (Conti et al., 2005); whereas the ability of clozapine to increase BN PPI in this study contradicts Conti et al’s finding that clozapine had no effects. However, careful examination of their data indicates a strong trend towards clozapine attenuation of PPI deficits at 12 and 15 dB (saline vs. clozapine treated: ~22 % vs. ~30 % and ~20% vs. ~35%, respectively). Furthermore, their paper revealed that ASR was strongly suppressed by clozapine and the variability reported in ASR suggests that some rats likely exhibited extremely low ASR (<10). When ASR is extremely low, measurements of PPI may become unreliable and it is our practice not to include data from rats with extremely low ASR, as we did in this study. In the current study, PPI was not significantly increased by clozapine, when data from the four rats with extremely low PPI were included in the analysis. It is possible that the lack of PPI facilitation seen in the study reported by Conti et al. was due to strong suppression of ASR in clozapine-treated rats. Our findings are consistent with those of Swerdlow et al. (Swerdlow et al., 2006) who found that quetiapine, another atypical antipsychotic facilitated low PPI in BN rats. Conti et al’s findings suggest that BN rats are not sensitive to the effects of established antipsychotics, whereas our findings, together with Swerdlow et al.’s suggest that BN PPI deficits are selectively sensitive to atypical antipsychotics. Additional typical and atypical antipsychotics will need to be tested to confirm this notion.
PD149163 reversed BN PPI deficits at both doses administered. We have previously shown that administration of neurotensin and PD149163 produced antipsychotic-like effects on PPI (Feifel et al., 2007; Feifel et al., 2004; Feifel, Minor, Dulawa, & Swerdlow, 1997; Feifel et al., 1999). Specifically, we previously reported that PD149163 facilitated PPI in Brattleboro rats and C57 mice (Feifel et al., 2007; Feifel et al., 2004; Feifel, Pang et al., 2010), both of which have naturally low PPI like BN rats. We have also shown that PD149163 and clozapine restored deficient social memory in Brattleboro rats (Feifel et al., 2009). In addition, Azmi et al. also found that PD149163 produces pro-cognitive effects (Azmi, Norman, Spicer, & Bennett, 2006).
The mechanism implicated in the antipsychotic-like effects produced by neurotensin and neurotensin analogs such as PD149163 is unknown. However, neurotensin-1 appears to mediate this effect, since PD149163 had no effect on the PPI of C57 mice lacking these receptors (Feifel, Pang et al., 2010). Furthermore, the effects of PD149163 are more consistent with the effects of atypical antipsychotics, like clozapine, than typical antipsychotics like haloperidol (Feifel, Melendez, & Shilling, 2003; Feifel et al., 2004; Feifel et al., 1999). In this respect, PD149163’s efficacy on BN PPI is consistent with both our findings and Swerdlow et al’s finding of the efficacy of clozapine and quetiapine on BN PPI deficits, respectively.
In summary, our findings, together with that of Swerdlow et al., suggest that the BN rat is a useful genetic animal model of low PPI with predictive validity for atypical antipsychotics. Our findings also add to the growing body of evidence that PD149163 may have antipsychotic effects.
Acknowledgments
DF and PDS were partially supported by NIMH grant MH080910.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue ( PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17(4):357–362. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156(2–3):234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Cilia J, Gartlon JE, Shilliam C, Dawson LA, Moore SH, Jones DN. Further neurochemical and behavioural investigation of Brattleboro rats as a putative model of schizophrenia. J Psychopharmacol. 2010;24(3):407–419. doi: 10.1177/0269881108098787. [DOI] [PubMed] [Google Scholar]
- Conti LH, Costill JE, Flynn S, Tayler JE. Effects of a typical and an atypical antipsychotic on the disruption of prepulse inhibition caused by corticotropin-releasing factor and by rat strain. Behav Neurosci. 2005;119(4):1052–1060. doi: 10.1037/0735-7044.119.4.1052. [DOI] [PubMed] [Google Scholar]
- Conti LH, Palmer AA, Vanella JJ, Printz MP. Latent inhibition and conditioning in rat strains which show differential prepulse inhibition. Behav Genet. 2001;31(3):325–333. doi: 10.1023/a:1012287527438. [DOI] [PubMed] [Google Scholar]
- Conti LH, Printz MP. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behav Brain Res. 2003;144(1–2):11–18. doi: 10.1016/s0166-4328(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. Potentiation of prepulse inhibition of the startle reflex in rats: pharmacological evaluation of the procedure as a model for detecting antipsychotic activity. Psychopharmacology. 1997a;132(4):366–374. doi: 10.1007/s002130050357. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. Some, but not all, antipsychotic drugs potentiate a low level of prepulse inhibition shown by rats of the Wistar strain. Behavioural Pharmacology. 1997b;8(4):364–372. doi: 10.1097/00008877-199708000-00009. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;181(2):278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28(4):651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29(4):731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34(8):2011–2018. doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Research. 1997;760(1–2):80–84. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Pang Z, Shilling PD, Melendez G, Schreiber R, Button D. Sensorimotor gating in neurotensin-1 receptor null mice. Neuropharmacology. 2010;58(1):173–178. doi: 10.1016/j.neuropharm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Priebe K. Vasopressin-deficient rats exhibit sensorimotor gating deficits that are reversed by subchronic haloperidol. Biol Psychiatry. 2001;50(6):425–433. doi: 10.1016/s0006-3223(01)01100-3. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;288(2):710–713. [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Melendez G. Further characterization of the predictive validity of the Brattleboro rat model for antipsychotic efficacy. J Psychopharmacol. 2010 doi: 10.1177/0269881110388327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156(2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Kumari V, Sharma T. Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology (Berl) 2002;162(2):97–101. doi: 10.1007/s00213-002-1099-x. [DOI] [PubMed] [Google Scholar]
- Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol Psychiatry. 2002;52(7):729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94(4):507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Oranje B, Van Oel CJ, Gispen-De Wied CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. J Clin Psychopharmacol. 2002;22(4):359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse Startle Deficit in the Brown Norway Rat: A Potential Genetic Model. Behavioral Neuroscience. 2000;11(2):374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Melendez G, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT2A and an alpha1 agonist. Psychopharmacology (Berl) 2004;175(3):353–359. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Breier M, Mora AB, Ko D, Shoemaker JM. A novel rat strain with enhanced sensitivity to the effects of dopamine agonists on startle gating. Pharmacol Biochem Behav. 2008;88(3):280–290. doi: 10.1016/j.pbb.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophrenia Bulletin. 1998;24(2):285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo J, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal ‘low gating’ humans and rats. Neuropsychopharmacology. 2006;31(9):2011–2021. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J, Domeney AM. Circadian time does not modify the prepulse inhibition response or its attenuation by apomorphine. Pharmacology, Biochemistry and Behavior. 1999;64(3):501–505. doi: 10.1016/s0091-3057(99)00100-8. [DOI] [PubMed] [Google Scholar]


