Abstract
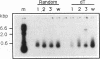
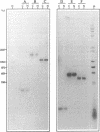
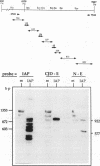
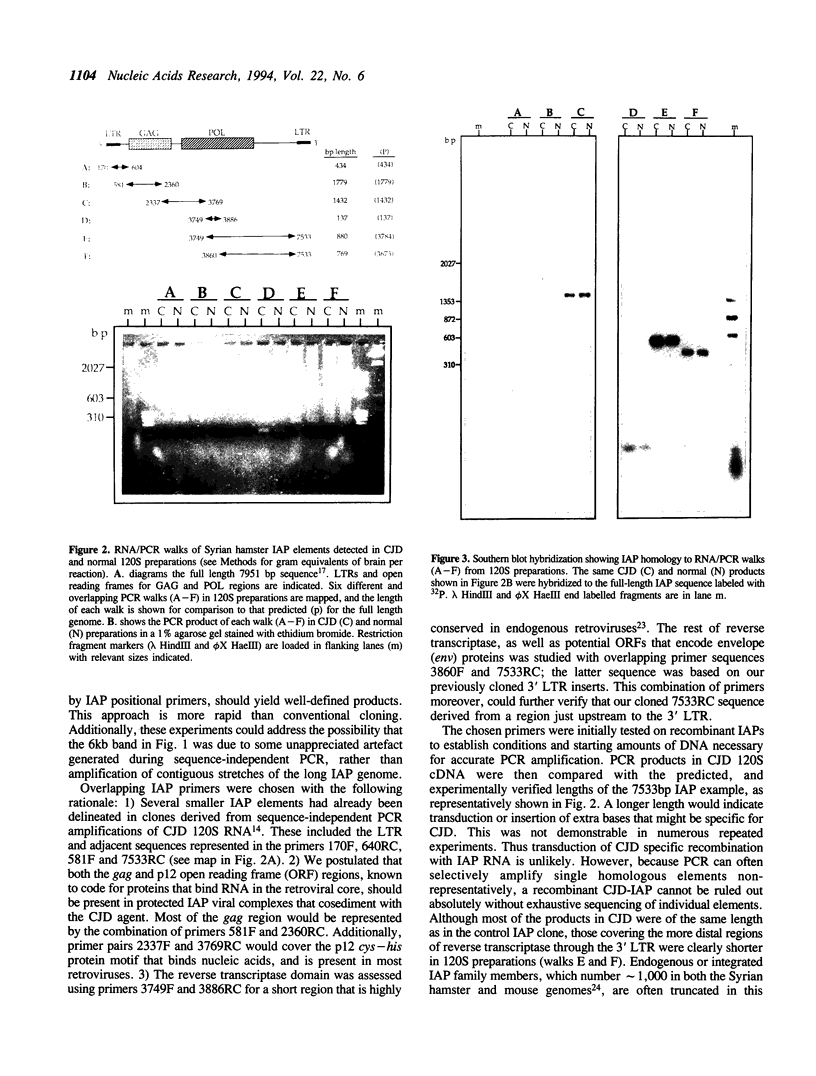
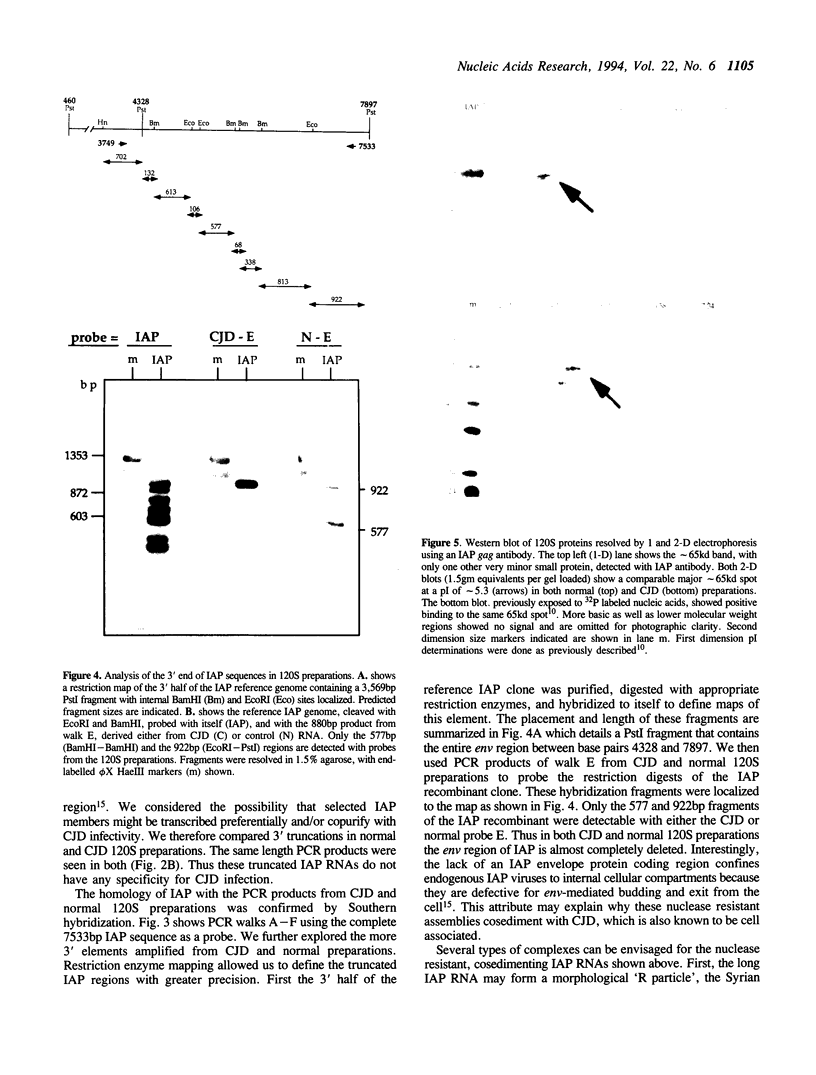
A class of viruslike agents that induces Creutzfeldt-Jakob Disease (CJD) and scrapie remains undefined at the molecular level. Several investigators believe this infectious agent is constituted by a single host protein or 'prion', and have emphasized data that would seem to exclude the presence of any viral nucleic acids. However, more rigorous evaluations in scrapie have shown reasonably abundant nucleic acids. Additionally, in highly purified 120S CJD preparations that have been treated with nucleases, RNAs as long as 6,000 bases have been detected. Few nucleic acids have been characterized in either scrapie or CJD, but previous cloning experiments delineated relatively short LTR regions of the endogenous IAP retrovirus in 120S CJD preparations. We therefore used specific primers encompassing the entire IAP genome to test for the presence of long viral RNAs, and here show approximately 5,000 contiguous bases of the IAP RNA genome can be recovered from reasonable amounts of starting brain. The 3' env region of IAP is comparably truncated in CJD and normal preparations, and we find no evidence for IAP transduction of CJD-specific sequences. Because IAP cores can coencapsidate unrelated sequences, and are unusually resistant to physical and chemical treatments, it was relevant to find if cosedimenting cognate proteins of the IAP core, such as gag, could be detected. The predicted approximately 65 kd acidic gag protein, showing appropriate antigenic and nucleic acid binding features, was apparent in both one and 2-D Western blots. This data strongly indicates specific viral complexes cofractionate with the CJD agent. Interestingly, these nuclease resistant IAPs do not appear to be in morphologically recognizable 'R' particles. This cosedimenting viral assembly therefore provides a paradigm for non-particulate CJD complexes in infectious preparations. In developing strategies to identify a CJD specific sequence, cosedimenting IAPs can be used to assess the quality, length and recovery of RNAs extracted from highly resistant viral complexes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., Williamson J. L., Borchardt L. M., Marsh R. F. Presence of mitochondrial D-loop DNA in scrapie-infected brain preparations enriched for the prion protein. J Virol. 1990 Jul;64(7):3265–3268. doi: 10.1128/jvi.64.7.3265-3268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akowitz A., Manuelidis E. E., Manuelidis L. Protected endogenous retroviral sequences copurify with infectivity in experimental Creutzfeldt-Jakob disease. Arch Virol. 1993;130(3-4):301–316. doi: 10.1007/BF01309662. [DOI] [PubMed] [Google Scholar]
- Akowitz A., Manuelidis L. A novel cDNA/PCR strategy for efficient cloning of small amounts of undefined RNA. Gene. 1989 Sep 30;81(2):295–306. doi: 10.1016/0378-1119(89)90190-x. [DOI] [PubMed] [Google Scholar]
- Akowitz A., Sklaviadis T., Manuelidis E. E., Manuelidis L. Nuclease-resistant polyadenylated RNAs of significant size are detected by PCR in highly purified Creutzfeldt-Jakob disease preparations. Microb Pathog. 1990 Jul;9(1):33–45. doi: 10.1016/0882-4010(90)90038-r. [DOI] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Forloni G., Angeretti N., Chiesa R., Monzani E., Salmona M., Bugiani O., Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993 Apr 8;362(6420):543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- Gibbons R. A., Hunter G. D. Nature of the scrapie agent. Nature. 1967 Sep 2;215(5105):1041–1043. doi: 10.1038/2151041a0. [DOI] [PubMed] [Google Scholar]
- Griffith J. S. Self-replication and scrapie. Nature. 1967 Sep 2;215(5105):1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Heidmann T. Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell. 1991 Jan 11;64(1):159–170. doi: 10.1016/0092-8674(91)90217-m. [DOI] [PubMed] [Google Scholar]
- Kellings K., Meyer N., Mirenda C., Prusiner S. B., Riesner D. Further analysis of nucleic acids in purified scrapie prion preparations by improved return refocusing gel electrophoresis. J Gen Virol. 1992 Apr;73(Pt 4):1025–1029. doi: 10.1099/0022-1317-73-4-1025. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Callahan R., Howk R. S. Immunological relationship between the structural proteins of intracisternal A-particles of Mus musculus and the M432 retrovirus of Mus cervicolor. J Virol. 1980 Mar;33(3):1211–1214. doi: 10.1128/jvi.33.3.1211-1214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983 Jul 11;11(13):4391–4408. doi: 10.1093/nar/11.13.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Taylor J. Template switching by reverse transcriptase during DNA synthesis. J Virol. 1990 Sep;64(9):4321–4328. doi: 10.1128/jvi.64.9.4321-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Fritch W. W., Kim J. H., Manuelidis L. Immortality of cell cultures derived from brains of mice and hamsters infected with Creutzfeldt-Jakob disease agent. Proc Natl Acad Sci U S A. 1987 Feb;84(3):871–875. doi: 10.1073/pnas.84.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis E. E., Gorgacz E. J., Manuelidis L. Interspecies transmission of Creutzfeldt-Jakob disease to Syrian hamsters with reference to clinical syndromes and strains of agent. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3432–3436. doi: 10.1073/pnas.75.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Manuelidis E. E. Creutzfeldt-Jakob disease and dementias. Microb Pathog. 1989 Sep;7(3):157–164. doi: 10.1016/0882-4010(89)90051-x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Murdoch G., Manuelidis E. E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Tesin D. M., Sklaviadis T., Manuelidis E. E. Astrocyte gene expression in Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5937–5941. doi: 10.1073/pnas.84.16.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Valley S., Manuelidis E. E. Specific proteins associated with Creutzfeldt-Jakob disease and scrapie share antigenic and carbohydrate determinants. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4263–4267. doi: 10.1073/pnas.82.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch G. H., Sklaviadis T., Manuelidis E. E., Manuelidis L. Potential retroviral RNAs in Creutzfeldt-Jakob disease. J Virol. 1990 Apr;64(4):1477–1486. doi: 10.1128/jvi.64.4.1477-1486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly D., Thomas C. J., Coutts R. H. Tomato aspermy virus has an evolutionary relationship with other tripartite RNA plant viruses. J Gen Virol. 1991 Jan;72(Pt 1):1–7. doi: 10.1099/0022-1317-72-1-1. [DOI] [PubMed] [Google Scholar]
- Oesch B., Groth D. F., Prusiner S. B., Weissmann C. Search for a scrapie-specific nucleic acid: a progress report. Ciba Found Symp. 1988;135:209–223. doi: 10.1002/9780470513613.ch14. [DOI] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Shih A., Misra R., Rush M. G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989 Jan;63(1):64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T. K., Manuelidis L., Manuelidis E. E. Physical properties of the Creutzfeldt-Jakob disease agent. J Virol. 1989 Mar;63(3):1212–1222. doi: 10.1128/jvi.63.3.1212-1222.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T., Akowitz A., Manuelidis E. E., Manuelidis L. Nuclease treatment results in high specific purification of Creutzfeldt-Jakob disease infectivity with a density characteristic of nucleic acid-protein complexes. Arch Virol. 1990;112(3-4):215–228. doi: 10.1007/BF01323166. [DOI] [PubMed] [Google Scholar]
- Sklaviadis T., Akowitz A., Manuelidis E. E., Manuelidis L. Nucleic acid binding proteins in highly purified Creutzfeldt-Jakob disease preparations. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5713–5717. doi: 10.1073/pnas.90.12.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklaviadis T., Dreyer R., Manuelidis L. Analysis of Creutzfeldt-Jakob disease infectious fractions by gel permeation chromatography and sedimentation field flow fractionation. Virus Res. 1992 Dec;26(3):241–254. doi: 10.1016/0168-1702(92)90016-3. [DOI] [PubMed] [Google Scholar]
- Taruscio D., Manuelidis L. Integration site preferences of endogenous retroviruses. Chromosoma. 1991 Dec;101(3):141–156. doi: 10.1007/BF00355364. [DOI] [PubMed] [Google Scholar]
- Xi Y. G., Ingrosso L., Ladogana A., Masullo C., Pocchiari M. Amphotericin B treatment dissociates in vivo replication of the scrapie agent from PrP accumulation. Nature. 1992 Apr 16;356(6370):598–601. doi: 10.1038/356598a0. [DOI] [PubMed] [Google Scholar]
- Zhou S., Yang S. Q., Standring D. N. Characterization of hepatitis B virus capsid particle assembly in Xenopus oocytes. J Virol. 1992 May;66(5):3086–3092. doi: 10.1128/jvi.66.5.3086-3092.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]