Abstract
Purpose of the review
To highlight the significance of the abnormal DNA repair mechanism in male infertility.
Recent Findings
DNA repair defects cause a variety of spermatogenic defects in mouse models. Evidence is accumulating to demonstrate the importance of DNA repair defects in human non-obstructive azoospermia. Epigenetic changes may also play a crucial role in infertility.
Summary
The DNA in the cell needs to be constantly repaired to ensure fidelity of DNA replication, to maintain genome stability and to ensure propagation of species. The DNA repair and recombination machineries are highly conserved across the species and inactivation of these pathways may lead to replication and recombination errors. This review summarizes types of DNA lesions and DNA repair pathways, particularly focusing on highly conserved meiotic regulators the DNA mismatch repair proteins. Targeted deletions of some of these proteins results in infertility and predisposes to tumor in mutant mouse models. There is evidence for loss of some of these proteins in human male infertility. Because defective DNA repair is associated with a mutator phenotype, the risk of transmission to the offspring of these otherwise infertile men conceived using an assisted reproductive technology needs further evaluation.
Key words or phareses: DNA repair, azoospermia, infertility, mismatch repair proteins, homologous recombination
Introduction
Genetic disorders like Klinefelter syndrome and Y chromosome microdeletions are recognized genetic causes of male infertility. While investigators suspect that the majority of male infertility has an underlying genetic basis; the etiology of the majority of male infertility cases remains idiopathic [1–3]. This is particularly true for men with non-obstructive azoospermia or severe oligospermia. Defects in DNA repair during spermatogenesis is thought to underlie some types of testicular failure [4,5]. DNA repair mechanisms are important to maintain the fidelity of DNA replication during mitotic, meiotic and post-meiotic development of both male and female germ cells. Three critical DNA repair pathway exist: Nucleotide excision repair (NER), Base excision repair (BER) and DNA mismatch repair (MMR). There are two additional types of DNA repair, double-stranded break repair and recombinational repair. This review focuses on our current understanding of role of these processes in the etiology of spermatogenetic defects; however, we begin with a summary of the current knowledge of types of DNA repair to set the stage for an in depth discussion of the relevance of these pathways in gametogenesis. The potential consequences of defective DNA repair during gametogenesis and the possible consequences for children conceived by ICSI who are fathered by a severely infertile male are also discussed. Errors in DNA mismatch repair is linked to a variety of cancers which may explain the link, in part, between infertility and testicular germ cell tumors.
DNA Mismatch repair
DNA repair is essential to the maintenance of genomic integrity and the basic processes are highly conserved throughout evolution. The mismatch repair (MMR) family of proteins was first characterized in bacteria. These proteins play a key role in repairing sequence mismatches that primarily arise during replication. Mismatches escape DNA polymerase proof-reading. The function of the mismatch repair proteins are not just restricted to mitosis, but also play critical roles for meiotic recombination and chromosome pairing.
The MMR system is comprised of MutS, MutL and MutH families, which are highly conserved across species. The homodimeric MutS protein recognizes DNA mismatches binds to the DNA and forms an active repair complex. Following mismatch recognition, the MutL protein serves to couple the MutS-DNA active repair complex to then induce exonuclease MutH activation. In humans and mice, there are several mismatch repair proteins homologous to the bacterial MutS and Mut L homologues, named MSH1-6, MLH1 and MLH3, and PMS1 and PMS2 (for post meiotic segregation)[6,7]. Heterodimers of MutS and MutL homologues form a tetrahetromeric complex that recognizes a single base pair mismatch, or a small loop of single strand DNA. Other potential downstream proteins with endo- and exonuclease activities (such as, exonuclease 1, EXO1), DNA polymerases and replication factors (replication protein A(RPA), proliferating cell nuclear antigen (PCNA), and replication factor C (RFC) are recruited to complete the repair process (Figure 1). In addition, the MutL heterodimers can also trigger apoptotic machinery to induce cell death when DNA repair fails. As described below, mutations or deletion of the MLH1 gene in mice and humans resulted in microsatellite instability and predisposition to the early onset of certain tumors [8,9]. Germ line mutations in MLH1 gene cause hereditary nonpolyposis colon cancer (HNPCC) [10].
Figure 1. Overview of mismatch repair in eukaryotes.
The MutSα heterodimer (MSH2-MSH6/3) binds the mismatch and recruits MutLα heterodimer (MLH1-PMS2). A conformational change upon the exchange of ADP with ATP allows sliding of the complex away from the mismatch. Latent endonuclease activity within the PMS2 subunit of MutLα introduces nicks into the discontinuous strand (indicated by pink arrows) generating 5′ entry sites for nuclease EXO1. EXO1 degrades the nicked strand and the remaining single stranded DNA is protected by RPA. POLδ and its cofactors PCNA and RFC fill in the gap with DNA ligase I completing the repair by sealing the final nick.
Mice deficient in Mlh1 gene display microsatellite instability, as well as male and female infertility (for a summary of the consequences of deletion of MLH1 and other DNA repair proteins involved in infertility in mouse models see Table 1). Failure of gametogenesis in these mice results from meiotic arrest at pachytene characterized by reduced levels of chiasmata [8,9], impaired meiotic recombination and crossing during meiosis. Similar to Mlh1 deficient mice, Pms2 or Msh2 knockout mice exhibit genomic instability; however, in contrast, these mouse mutants display male infertility due to disruption of normal chromosomal synapsis [22]. Msh2, Msh3 and Msh6 null mice exhibit mismatch repair deficiency in somatic cells but their fertility is largely unaffected, although spermatogenic defects are evident in the Msh2 deleted male mice [7,11].
Table 1.
MMR genes phenotypes in mouse knockouts
| Gene | Phenotype | Expression | Ref | |
|---|---|---|---|---|
| Fertility | Cancer | |||
| Msh2 | Fertile Some germ cell loss and SCO tubules (diameters <200 μm) | Lymphoma, GI and skin tumors | Highly expressed in mouse spermatogonia and leptotene/zygotene spermatocytes. | 11, 12, 13, 14, 15 |
| Msh3 | Fertile | Late stage GI tumors | - | 15, 16 |
| Msh4 | Infertile Failure of spermatogonial maturation beyond zygonema. | Accumulation within nuclear matrix during leptotene of prophase I. Formation of MSH4 foci on zygotene unpaired chromosomes with the number of foci decreasing until mid-pachynema. | 17 | |
| Msh5 | Infertile Incomplete and nonhomologous chromosome pairing | Lymphoma and GI tumors | Expression elevated during spermatogenesis Possible role during prophase 1 stages | 18 |
| Msh6 | Fertile Infertile | Lymphoma and GI tumors | Elevated expression observed in testis | 19, 20 |
| Mlh1 | Failure of crossing over and premature desynapsis of homologous chromosomes | Lymphoma, GI and skin tumors | Localised in discrete focis along the synaptonemal complex during pachynema in spermatocytes | 8, 9, 21 |
| Pms1 | Fertile | - | - | 21 |
| Pms2 | Male Infertile | Lymphoma | High levels in leptotene/zygotene spermatocytes | 15, 21, 22 |
| Mlh3 | Infertile | - | High levels in pachytene spermatocytes | 23 |
| Exo1 | Infertile | Lymphoma | Elevated expression observed in testis | 24 |
Two mammalian meiosis-specific MutS homologues, MSH4 and MSH5 were identified. At the time of homologous recombination and crossing over during meiosis, the chromosomes link to stabilize, through a supramolecular proteinacious structure comprised of complex aggregate of SYCP1, 2, 3 proteins known as Synaptonemal complex (SC). During recombination MSH4-MSH5 interacts with RAD51 interacts within the SC complex facilitating and stabilizing the interactions between homologs. Targeted inactivation of the meiosis-specific MutS homologues, Msh4 and Msh5 resulting in gametogenesis-specific phenotype, displaying meiotic arrest in both males and females but are not involved in DNA mismatch repair [17,25,26]. Msh4 and Msh5 null mice exhibit greatly diminished pairing of homologous chromosomes and failure of meiosis at zygotene stage. Decreased synapsis is most severe in Msh5-deficient mice. Several polymorphisms of MSH5 are reported to be associated with azoospermia or severe oligospermia [27], but the study groups thus far have been small and the functional significance of these variants remains undefined.
Less is known concerning the mismatch repair proteins and human male infertility. Maduro, et al., [4] presented evidence of genomic instability in both testicular cells and circulating white blood cells of men with non-obstructive azoospermia, predominantly those men with Sertoli cell only syndrome. Genomic instability was assessed by analyzing molecular features associated with microsatellite instability. Microsatellites are regions of repetitive sequences of nucleotides found in the genome, which may be mono-, di- or trinucleotide repeats, such as CAGCAGCAG. Deficient mismatch repair can result in expansions or contractions of the repetitive sequences and accordingly, microsatellite instability is used as a reliable marker of mismatch repair deficiency [28].
Immunohistochemistry revealed that a significant number of these non-obstructive azoospermic men displaying genomic instability also had defects in the mismatch repair proteins, MLH1 or MSH2. In some patients the protein was mislocalized in the cytoplasm, in other non-obstructive azoospermic men the protein was absent [4]. Importantly, the defect was not germ cell specific and affected the germ cells (if present), as well as the somatic cells. These types of deficient immunostaining are used to diagnose Lynch syndrome (a cancer syndrome described below). An example of mislocalization of MLH1 protein in a non-obstructive azoospermia (NOA) is shown in Figure 2. These studies are intriguing as they suggest that defects in mismatch repair proteins may underlie some forms of non-obstructive azoospermia. Furthermore, they suggest these men may be at risk of development of colorectal or other cancers associated with defective mismatch repair.
Figure 2. Subcellular localization of MLH1 protein.

Infertile control men, MLH1 displayed diffuse cytoplasmic staining with a strong perinuclear accentuation, one of the NOA patient showed aberrant localization of MLH1
Homologous Recombination Repair
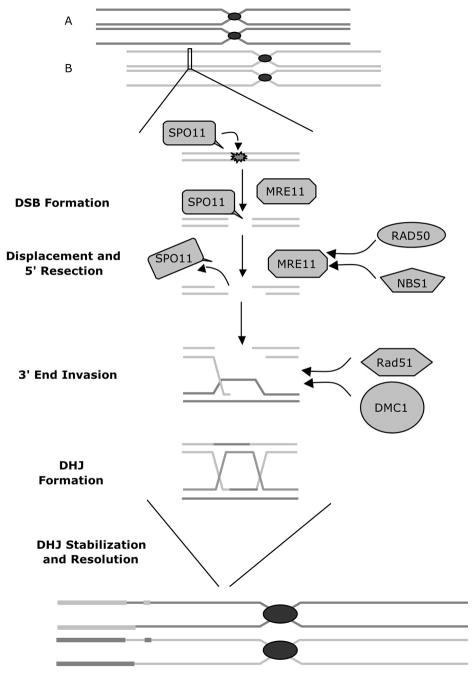
Double-strand break (DSB) is the most deleterious type of damage to somatic cellular DNA. Meiotic recombination requires endogenously induced breaking and subsequent ligation of the DNA molecules. Unrepaired or misrepaired DSBs may lead to genomic instability resulting in genetic aberrations or cell death. During the meiotic prophase, DSB are induced in a controlled manner within a DNA molecule to initiate recombination. This action is mediated by the conserved topoisomerase, SPO11 [29]. Spo11 knockout mice exhibit a failure to form DSBs into homologs; thus leading to faulty synapsis, recombination and ultimately meiotic failure [30–33]. SPO11 is then displaced from the DSB site by MRE11. MRE11 recruited at the DSB site, generates 3′ overhangs, and in concert with a multi protein complex including RAD50, NBS1 repair and process the DNA ends for homologous recombination. RAD51 and DMC1 and associated proteins are indispensable during homology and heteroduplex formation [34]. A schematic diagram of the proteins involved in homologous recombination is shown in Figure 3. Dmc1 null mice are infertile with gross defect in chromosome pairing [35,36]. Similarly, Rad51 knockout mice display embryonic lethality indicating the key role of these proteins in meiosis and development [37]. A case report of a man with spermatocyte arrest showed that he had an abnormal presence of BRCA1 with an absence of RAD51 in early and late spermatocytes associated with a failure of XY body formation suggesting a deficiency of the processing double-strand DNA breaks at the zygotene stage of meiosis [38]. Whether this is a common finding in other cases of early maturation arrest is not known.
Figure 3. Meiotic Recombination pathway.
Homologous unpaired acrocentric chromosomes are depicted above (A and B). Topoisomerase, SPO11, initiations recombination by inserting double-strand breaks (DSBs) into homologous sister chromatids. SPO11 is then displaced from the DSB site by MRE11, which in turn generates 3′ overhangs as well as recruiting RAD50 and NBS1 to repair and process the DNA ends in preparation for homologous recombination. The resulting leads to the canonical Szostak model with double Holliday junctions and crossover products.
Cell cycle checkpoint genes involved in DNA repair
If DNA damage cannot be repaired, the activity of cell cycle checkpoint genes results in apoptosis. Pachytene arrest is sometimes observed in testicular biopsies taken from infertile men. Numerous genes are involved in the regulation of DNA damage-induced cell cycle control. Mutation in one such gene ATM causes the genetic disorder Ataxia telagectesia. Ataxia telangectesia is characterized by radiosensitivity, defective cell cycle check point activation, chromosomal instability and infertility [39]. Likewise, Atm knockout mice are infertile due to meiotic arrest at zygotene-pachytene stage [40]. Aberrant telomere clustering and chromosomal fragmentation are also observed in Atm null mice [41]. ATR is an ATM-like protein, confined to the unpaired axes of meiotic chromosomes at prophase [42,43]. Mutations in Atr result in an embryonic lethal phenotype [44]. Similarly, Brca1 and Brac2 knockout mice show embryonic lethality [45], but Brac1-p53 (tumor suppressor gene implicated in elimination of germ cells in DNA damage) double-knockout mice rescued the severe phenotypes as seen in Brca1 and Brac2 but the male mice were infertile due to meiotic failure [46]. Knockout mice models of Ercc1 (excision repair cross-complementing gene 1), Msh2, Tp53 representing different DNA repair pathways (like NER, MMR and cell cycle checkpoint genes) exhibit impaired germ cell function and sperm production with overlapping phenotypic features[11]. Tp53 and Ercc1 play a critical role in DNA damage repair during spermatogenesis [11]. XRCC1, ERCC1 and ERCC2 polymorphisms are reportedly associated with a risk of idiopathic azoospermia in the Chinese [47,48], but again whether this is true for other ethnicities or races is not known nor is the functional significance of these variants.
Other DNA repair pathways
Base excision repair (BER) focuses on the base-base mismatches or lesions induced by alkylation and oxidation. The site of damage is recognized by DNA glycosylase and the mismatched bases are removed resulting in an apurine site which is cleaved by apurine endonucleases [49]. The lesion leads to a single strand break which is further repaired by the concerted action of DNA polymerases and DNA ligases [50–53]. Human genetic diseases are not yet been directly associated with BER deficiency but occurrence of polymorphisms in genes implicated in BER pathway is evident [54].
Nucleotide excision repair (NER) pathway involves repair of large loops, bulky, helix-distorting lesions [55]. NER proteins cleave the two phosphodiester bonds leaving a gap of few nucleotides which are patched and ligated by DNA polymerases [56,57]. NER are more error prone compared to BER or MMR pathways. Severe genetic diseases like Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy, are manifestation of inborn NER deficiencies [54].
Infertility and neoplasia
Infertile men have higher incidence of testicular germ cell tumors [58]. Of importance to the focus of this paper, errors in DNA mismatch repair such as some of those described above, are increasingly linked to the development of a variety of human cancers. DNA mismatch repair is associated with about 15% of colorectal cancer and with certain other tumors, namely retinoblastoma, melanoma, gastric, breast and ovarian cancers. Targeted inactivation of Exonuclease 1 protein in mice resulted in both infertility and cancer [24].
In some cases, DNA mismatch repairs are due to inherited mutations, as in Lynch Syndrome. Lynch syndrome is caused by inactivating mutations of genes that encode mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2). Generally, the Lynch syndrome patients are born with a germ line mutation in a mismatch repair gene and then develop an inactivation of the second wild-type allele in their tumors [28]. This is consistent with Knudson’s two hit hypothesis for carcinogenesis resulting from inactivation of tumor suppressor genes[28]. Apart from harboring a distinct type of colorectal cancer, these families have higher incidence of other malignancies, including those associated with multiple extracolonic cancers such as endometrial cancers, stomach, ovarian, small bowel, pancreatic, hepatobiliary tract, brain, upper uroepithelial tract and possibly prostate [59]. Upper urinary tract urothelial carcinomas have also been reported in the context of Lynch Syndrome, but incidence of testicular germ cell tumors are very rare [60]. Other possible potential mechanisms between infertility and testicular germ cell tumor includes testicular dysgenesis syndrome (if this exists), the Hiwi protein, chromosome12 aneuploidy, and Y-chromosome instability [58].
Epigenetic mechanisms of Mismatch Repair Defects in Malignancy and Infertility
Another important mechanism controlling DNA mismatch repair is methylation of the promoter regions of MMR genes. Somatic hypermethylation of MLH1-gene promoter causes inhibition of gene transcription and functionally mimics an inactivating gene mutation [28]. If both MLH1 alleles become hypermethylated, MLH1 will be deficient and microsatellite instability will occur. This route to colorectal cancer development occurs with aging in patients who do not have Lynch syndrome or mutations in the mismatch repair genes, yet develop malignancies with mismatch repair defects and microsatillite instability [61,62]. Less commonly, constitutional epimutations of MSH2 were identified in some families with early-onset colorectal and endometrial tumors [61], while the majority of those cases with constitutional MLH1 epimutation (while rare) do not have a strong family history of colorectal cancers. Although promoter hypermethylation of MMR genes in the context of infertility is yet to be explored, promoter hypermethylation and silencing of several infertility associated genes like VASA and MTHFR [63,64] was recently reported. Several other epigenetic mechanisms, like histone modification [65] and RNA interference, may also play a part. Although epimutations are reversed during the process of spermatogenesis (potentially correcting a spontaneous epigenetic error in the father), there could be transmission of the epimutation if there is a genetic factor that influences this occurrence [61].
Another potential epigenetic mechanism is the presence of SNPs (single nucleotide polymorphisms). A SNP within an important regulatory element may lead to a reduction in transcriptional output and increased propensity to hypermethylation. SNPS are more frequent in those shown to have meiotic arrest upon testicular biopsy suggesting compared to those with normal spermatogenesis who are obstructed. These mutations were confined to testicular biopsies, whereas, no polymorphisms were observed in blood from either group. Our unpublished data also show several SNPs in MLH1, MSH2 and MSH6 genes associated with non obstructive azoospermia. This observation suggests that these are de novo mutations, which arise in the germ cells during spermatogenesis.
Consequence for assisted reproductive technology
With the advent of assisted reproductive technologies, such as in vitro fertilization with intracytoplasmic sperm injection, it is now possible to bypass the natural barriers that exclude fertilization by a defective sperm. Embryos with severe DNA repair defects usually undergo spontaneous abortion. Whether incidence of congenital abnormalities following ICSI is different or not from the general population is controversial with investigators having conflicting views [66–69]. In a meta-analysis of just 8 published studies by Woldringh, et al., [66], 55 out of 1973 children born after ICSI showed abnormal karyotypes, but there was no statiscal significance or major congenital defects reported. In another study, major malformations that result in functional impairment or requiring surgical correction were observed in four children concieved by ICSI [70]. In both of the above studies, the groups were small and heterogenic. In contrast, others reported that prenatal karyotype abnormalities increase with decreasing sperm count and an increased incidence of birth defects in children conceived by ICSI [71–74]. Meta-analyses results from 25 studies suggest a statistical significance of about 30–40% increased risk of birth defects associated with ART [75]. Importantly, the incidence of birth defects represents just one measure of the safety of these procedures and it is clear that highly controlled, prospective studies of birth defects, such as those published from the Bounduelle group, and systemic defects in children conceived by ICSI are needed. Increased genetic polymorphisms seen in infertile men, raises the potential for these variants to be inherited. These polymorphisms may confer increased risk of infertility and possible increased risks of somatic defects later in life. Longer term follow up of children born through ICSI is required to understand the genetic consequences in these offspring.
Conclusion
Mismatch repair (MMR) genes are well studied in hereditary non-polyposis colorectal cancer because MMR mutation carriers are at high risk for several extracolonic malignancies. The proteins of the DNA repair pathway play a crucial role in gametogenesis. Knockout mouse models and targeted deletions of these genes involved in different repair mechanisms exhibit male and female infertility. Studies in human subjects, especially in NOA men with respect to genomic instability have progressed. Defective MMR results in a “mutator” phenotype with increased rate of spontaneous mutations and microsatellite instability. The challenges to the development of epigenetic therapies for infertility should be investigated. Further studies to elucidate the molecular mechanisms of mammalian recombination and the role of these MMR proteins in infertility are needed to provide a better insight. These characteristics may have health consequences for the men with non-obstructive azoospermia and may present a risk to their children conceived by ICSI. Thus, with the increasing knowledge of the underlying genetic cause of male infertility, patients should be encouraged for counseling before ICSI.
Acknowledgments
Supported in part by 2P01 HD 36289 and the NIH Cooperative Centers Program in Reproductive Research (U54 HD07495) from the Eunice Kennedy Shriver National Instititute of Child Health and Human Development, National Institutes of Health to DJL.
References
- 1.Gordon F, Lamb D. DNA repair genes and genomic instability in severe male factor infertility. In: Carrell DT, editor. The Genetics of Male Infertility. 2007. pp. 145–163. [Google Scholar]
- 2.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4 (Suppl):s41–49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **4.Maduro MR, Casella R, Kim E, Levy N, Niederberger C, Lipshultz LI, Lamb DJ. Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell-only syndrome. Mol Hum Reprod. 2003;9:61–68. doi: 10.1093/molehr/gag013. The review illustrates a very detailled description of the genes involved in infertility in both mouse and human and their functions and molecular role in reproduction. The genetic basis of both male and female infertility and their defects are very well summarized. The review also talks about the diagnosis and treatment in human infertility. [DOI] [PubMed] [Google Scholar]
- 5.Nudell D, Castillo M, Turek PJ, Pera RR. Increased frequency of mutations in DNA from infertile men with meiotic arrest. Hum Reprod. 2000;15:1289–1294. doi: 10.1093/humrep/15.6.1289. [DOI] [PubMed] [Google Scholar]
- 6.Baarends WM, van der Laan R, Grootegoed JA. DNA repair mechanisms and gametogenesis. Reproduction. 2001;121:31–39. doi: 10.1530/rep.0.1210031. [DOI] [PubMed] [Google Scholar]
- 7.Svetlanov A, Cohen PE. Mismatch repair proteins, meiosis, and mice: understanding the complexities of mammalian meiosis. Exp Cell Res. 2004;296:71–79. doi: 10.1016/j.yexcr.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- 9.Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- 10.Arnheim N, Shibata D. DNA mismatch repair in mammals: role in disease and meiosis. Curr Opin Genet Dev. 1997;7:364–370. doi: 10.1016/s0959-437x(97)80150-5. [DOI] [PubMed] [Google Scholar]
- 11.Paul C, Povey JE, Lawrence NJ, Selfridge J, Melton DW, Saunders PT. Deletion of genes implicated in protecting the integrity of male germ cells has differential effects on the incidence of DNA breaks and germ cell loss. PLoS One. 2007;2:e989. doi: 10.1371/journal.pone.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reitmair AH, Schmits R, Ewel A, Bapat B, Redston M, Mitri A, Waterhouse P, Mittrucker HW, Wakeham A, Liu B, et al. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet. 1995;11:64–70. doi: 10.1038/ng0995-64. [DOI] [PubMed] [Google Scholar]
- 13.de Wind N, Dekker M, van Rossum A, van der Valk M, te Riele H. Mouse models for hereditary nonpolyposis colorectal cancer. Cancer Res. 1998;58:248–255. [PubMed] [Google Scholar]
- 14.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 15.Richardson LL, Pedigo C, Ann Handel M. Expression of deoxyribonucleic acid repair enzymes during spermatogenesis in mice. Biol Reprod. 2000;62:789–796. doi: 10.1095/biolreprod62.3.789. [DOI] [PubMed] [Google Scholar]
- 16.de Wind N, Dekker M, Claij N, Jansen L, van Klink Y, Radman M, Riggins G, van der Valk M, van’t Wout K, te Riele H. HNPCC-like cancer predisposition in mice through simultaneous loss of Msh3 and Msh6 mismatch-repair protein functions. Nat Genet. 1999;23:359–362. doi: 10.1038/15544. [DOI] [PubMed] [Google Scholar]
- 17.Kneitz B, Cohen PE, Avdievich E, Zhu L, Kane MF, Hou H, Jr, Kolodner RD, Kucherlapati R, Pollard JW, Edelmann W. MutS homolog 4 localization to meiotic chromosomes is required for chromosome pairing during meiosis in male and female mice. Genes Dev. 2000;14:1085–1097. [PMC free article] [PubMed] [Google Scholar]
- 18.Her C, Wu X, Wan W, Doggett NA. Identification and characterization of the mouse MutS homolog 5: Msh5. Mamm Genome. 1999;10:1054–1061. doi: 10.1007/s003359901161. [DOI] [PubMed] [Google Scholar]
- 19.Kolodner RD, Tytell JD, Schmeits JL, Kane MF, Gupta RD, Weger J, Wahlberg S, Fox EA, Peel D, Ziogas A, et al. Germ-line msh6 mutations in colorectal cancer families. Cancer Res. 1999;59:5068–5074. [PubMed] [Google Scholar]
- 20.Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen PE, Kane MF, Lipford JR, et al. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell. 1997;91:467–477. doi: 10.1016/s0092-8674(00)80433-x. [DOI] [PubMed] [Google Scholar]
- 21.Prolla TA, Baker SM, Harris AC, Tsao JL, Yao X, Bronner CE, Zheng B, Gordon M, Reneker J, Arnheim N, et al. Tumour susceptibility and spontaneous mutation in mice deficient in Mlh1, Pms1 and Pms2 DNA mismatch repair. Nat Genet. 1998;18:276–279. doi: 10.1038/ng0398-276. [DOI] [PubMed] [Google Scholar]
- 22.Baker SM, Bronner CE, Zhang L, Plug AW, Robatzek M, Warren G, Elliott EA, Yu J, Ashley T, Arnheim N, et al. Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell. 1995;82:309–319. doi: 10.1016/0092-8674(95)90318-6. [DOI] [PubMed] [Google Scholar]
- 23.Lipkin SM, Moens PB, Wang V, Lenzi M, Shanmugarajah D, Gilgeous A, Thomas J, Cheng J, Touchman JW, Green ED, et al. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat Genet. 2002;31:385–390. doi: 10.1038/ng931. [DOI] [PubMed] [Google Scholar]
- 24.Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, Kolas NK, Russell R, Hou H, Jr, Kneitz B, et al. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries SS, Baart EB, Dekker M, Siezen A, de Rooij DG, de Boer P, te Riele H. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes Dev. 1999;13:523–531. doi: 10.1101/gad.13.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- 27.Xu K, Lu T, Zhou H, Bai L, Xiang Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of maleinfertility with azoospermia or severe oligozoospermia. Clinica Chimica Acta. 2010;411:49–52. doi: 10.1016/j.cca.2009.09.038. [DOI] [PubMed] [Google Scholar]
- **28.van Lier MG, Wagner A, van Leerdam ME, Biermann K, Kuipers EJ, Steyerberg EW, Dubbink HJ, Dinjens WN. A review on the molecular diagnostics of Lynch syndrome: a central role for the pathology laboratory. J Cell Mol Med. 2010;14:181–197. doi: 10.1111/j.1582-4934.2009.00977.x. A vivid rewiew of the molecular basis of Lynch syndrome, describing the molecular analyses, MSI analyses, hypermethylation of the promoters of the MMR genes and the limitations of molecular diognostics of lynch syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 30.Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Keeney S, Baudat F, Angeles M, Zhou ZH, Copeland NG, Jenkins NA, Manova K, Jasin M. A mouse homolog of the Saccharomyces cerevisiae meiotic recombination DNA transesterase Spo11p. Genomics. 1999;61:170–182. doi: 10.1006/geno.1999.5956. [DOI] [PubMed] [Google Scholar]
- 32.Romanienko PJ, Camerini-Otero RD. Cloning, characterization, and localization of mouse and human SPO11. Genomics. 1999;61:156–169. doi: 10.1006/geno.1999.5955. [DOI] [PubMed] [Google Scholar]
- 33.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 34.Zenvirth D, Richler C, Bardhan A, Baudat F, Barzilai A, Wahrman J, Simchen G. Mammalian meiosis involves DNA double-strand breaks with 3′ overhangs. Chromosoma. 2003;111:369–376. doi: 10.1007/s00412-002-0223-3. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida K, Kondoh G, Matsuda Y, Habu T, Nishimune Y, Morita T. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1:707–718. doi: 10.1016/s1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
- 36.Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- 37.Thacker J. A surfeit of RAD51-like genes? Trends Genet. 1999;15:166–168. doi: 10.1016/s0168-9525(99)01733-3. [DOI] [PubMed] [Google Scholar]
- 38.Sciurano RB, Rahn MI, Pigozzi MI, Olmedo SB, Solari AJ. An azoospermic man with a double-strand DNA break-processing deficiency in the spermatocyte nuclei: case report. Hum Reprod. 2006;21:1194–1203. doi: 10.1093/humrep/dei479. [DOI] [PubMed] [Google Scholar]
- 39.Meyn MS. Ataxia-telangiectasia, cancer and the pathobiology of the ATM gene. Clin Genet. 1999;55:289–304. doi: 10.1034/j.1399-0004.1999.550501.x. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 41.Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. doi: 10.1242/dev.125.20.4007. [DOI] [PubMed] [Google Scholar]
- 42.Keegan KS, Holtzman DA, Plug AW, Christenson ER, Brainerd EE, Flaggs G, Bentley NJ, Taylor EM, Meyn MS, Moss SB, et al. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 1996;10:2423–2437. doi: 10.1101/gad.10.19.2423. [DOI] [PubMed] [Google Scholar]
- 43.Moens PB, Tarsounas M, Morita T, Habu T, Rottinghaus ST, Freire R, Jackson SP, Barlow C, Wynshaw-Boris A. The association of ATR protein with mouse meiotic chromosome cores. Chromosoma. 1999;108:95–102. doi: 10.1007/s004120050356. [DOI] [PubMed] [Google Scholar]
- 44.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 45.Welcsh PL, Owens KN, King MC. Insights into the functions of BRCA1 and BRCA2. Trends Genet. 2000;16:69–74. doi: 10.1016/s0168-9525(99)01930-7. [DOI] [PubMed] [Google Scholar]
- 46.Cressman VL, Backlund DC, Avrutskaya AV, Leadon SA, Godfrey V, Koller BH. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol Cell Biol. 1999;19:7061–7075. doi: 10.1128/mcb.19.10.7061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 47.Gu AH, Liang J, Lu NX, Wu B, Xia YK, Lu CC, Song L, Wang SL, Wang XR. Association of XRCC1 gene polymorphisms with idiopathic azoospermia in a Chinese population. Asian J Androl. 2007;9:781–786. doi: 10.1111/j.1745-7262.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 48.Ji G, Gu A, Xia Y, Lu C, Liang J, Wang S, Ma J, Peng Y, Wang X. ERCC1 and ERCC2 polymorphisms and risk of idiopathic azoospermia in a Chinese population. Reprod Biomed Online. 2008;17:36–41. doi: 10.1016/s1472-6483(10)60290-8. [DOI] [PubMed] [Google Scholar]
- 49.Sousa MM, Krokan HE, Slupphaug G. DNA-uracil and human pathology. Mol Aspects Med. 2007;28:276–306. doi: 10.1016/j.mam.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Diaz M, Bebenek K, Sabariegos R, Dominguez O, Rodriguez J, Kirchhoff T, Garcia-Palomero E, Picher AJ, Juarez R, Ruiz JF, et al. DNA polymerase lambda, a novel DNA repair enzyme in human cells. J Biol Chem. 2002;277:13184–13191. doi: 10.1074/jbc.M111601200. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Beard WA, Shock DD, Prasad R, Hou EW, Wilson SH. DNA polymerase beta and flap endonuclease 1 enzymatic specificities sustain DNA synthesis for long patch base excision repair. J Biol Chem. 2005;280:3665–3674. doi: 10.1074/jbc.M412922200. [DOI] [PubMed] [Google Scholar]
- 52.Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol Cell Biol. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardoso MC, Joseph C, Rahn HP, Reusch R, Nadal-Ginard B, Leonhardt H. Mapping and use of a sequence that targets DNA ligase I to sites of DNA replication in vivo. J Cell Biol. 1997;139:579–587. doi: 10.1083/jcb.139.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *54.Chakarov S, Russev G. DNA repair and cell differentiation-does getting older means getting wiser as well? Biotecnology & Biotecnological Equipment. 2010;24:1804–1806. The review refects on nucleotide excission repair and its role on the terminal differentiation of the cell. [Google Scholar]
- 55.Huang JC, Sancar A. Determination of minimum substrate size for human excinuclease. J Biol Chem. 1994;269:19034–19040. [PubMed] [Google Scholar]
- 56.Huang JC, Svoboda DL, Reardon JT, Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5′ and the 6th phosphodiester bond 3′ to the photodimer. Proc Natl Acad Sci U S A. 1992;89:3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R, Nakazawa Y, Niimi A, Miki Y, Jaspers NG, et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell. 2010;37:714–727. doi: 10.1016/j.molcel.2010.02.009. The paper reports for the first time the NER repair mechanism and the role of three polymerases in human fibroblast. The paper reflects how the polymerase, Pol κ is recruited to the repair site by other replicaton factors. [DOI] [PubMed] [Google Scholar]
- *58.Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nat Rev Urol. 2009;6:550–556. doi: 10.1038/nrurol.2009.179. The review describes the risk of testicular cancer and testicular germ cell tumor and possible connections with male infertility. It also discusses the other potential mechanisms involved in other genetic disorders associated with male infertility. [DOI] [PubMed] [Google Scholar]
- 59.Maul JS, Warner NR, Kuwada SK, Burt RW, Cannon-Albright LA. Extracolonic cancers associated with hereditary nonpolyposis colorectal cancer in the Utah Population Database. Am J Gastroenterol. 2006;101:1591–1596. doi: 10.1111/j.1572-0241.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 60.Roupret M, Yates DR, Comperat E, Cussenot O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol. 2008;54:1226–1236. doi: 10.1016/j.eururo.2008.08.008. [DOI] [PubMed] [Google Scholar]
- *61.Hitchins MP, Ward RL. Constitutional (germline) MLH1 epimutation as an aetiological mechanism for hereditary non-polyposis colorectal cancer. J Med Genet. 2009;46:793–802. doi: 10.1136/jmg.2009.068122. A detailed description of MLH1 epimutations, characteristics and molecular mechanisms in the development of HNPCC is reviewed. [DOI] [PubMed] [Google Scholar]
- **62.Hesson LB, Hitchins MP, Ward RL. Epimutations and cancer predisposition: importance and mechanisms. Curr Opin Genet Dev. 2010;20:290–298. doi: 10.1016/j.gde.2010.02.005. Talks about the importance of epimutations in cancer and development of new therapies in cancer as well as other diseases. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto K, Koh E, Sin HS, Maeda Y, Narimoto K, Izumi K, Kobori Y, Kitamura E, Nagase H, Yoshida A, et al. Tissue-specific differentially methylated regions of the human VASA gene are potentially associated with maturation arrest phenotype in the testis. J Hum Genet. 2009;54:450–456. doi: 10.1038/jhg.2009.59. [DOI] [PubMed] [Google Scholar]
- 64.Khazamipour N, Noruzinia M, Fatehmanesh P, Keyhanee M, Pujol P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum Reprod. 2009;24:2361–2364. doi: 10.1093/humrep/dep194. [DOI] [PubMed] [Google Scholar]
- 65.Okada Y, Tateishi K, Zhang Y. Histone demethylase JHDM2A is involved in male infertility and obesity. J Androl. 2010;31:75–78. doi: 10.2164/jandrol.109.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woldringh GH, Besselink DE, Tillema AH, Hendriks JC, Kremer JA. Karyotyping, congenital anomalies and follow-up of children after intracytoplasmic sperm injection with non-ejaculated sperm: a systematic review. Hum Reprod Update. 2010;16:12–19. doi: 10.1093/humupd/dmp030. [DOI] [PubMed] [Google Scholar]
- 67.Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod. 2010;25:1594–1608. doi: 10.1093/humrep/deq103. [DOI] [PubMed] [Google Scholar]
- 68.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 69.Halliday JL, Ukoumunne OC, Baker HW, Breheny S, Jaques AM, Garrett C, Healy D, Amor D. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25:59–65. doi: 10.1093/humrep/dep364. [DOI] [PubMed] [Google Scholar]
- 70.Bonduelle M, Wilikens A, Buysse A, Van Assche E, Devroey P, Van Steirteghem AC, Liebaers I. A follow-up study of children born after intracytoplasmic sperm injection (ICSI) with epididymal and testicular spermatozoa and after replacement of cryopreserved embryos obtained after ICSI. Hum Reprod. 1998;13 (Suppl1):196–207. doi: 10.1093/humrep/13.suppl_1.196. [DOI] [PubMed] [Google Scholar]
- 71.Belva F, Henriet S, Liebaers I, Van Steirteghem A, Celestin-Westreich S, Bonduelle M. Medical outcome of 8-year-old singleton ICSI children (born >or=32 weeks’ gestation) and a spontaneously conceived comparison group. Hum Reprod. 2007;22:506–515. doi: 10.1093/humrep/del372. [DOI] [PubMed] [Google Scholar]
- 72.Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)--what are the risks? Urol Clin North Am. 2008;35:277–288. doi: 10.1016/j.ucl.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vernaeve V, Bonduelle M, Tournaye H, Camus M, Van Steirteghem A, Devroey P. Pregnancy outcome and neonatal data of children born after ICSI using testicular sperm in obstructive and non-obstructive azoospermia. Hum Reprod. 2003;18:2093–2097. doi: 10.1093/humrep/deg403. [DOI] [PubMed] [Google Scholar]
- 74.Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, Liebaers I. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17:2600–2614. doi: 10.1093/humrep/17.10.2600. [DOI] [PubMed] [Google Scholar]
- 75.Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects--a systematic review. Hum Reprod. 2005;20:328–338. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]