Abstract
Mitochondrial bioenergetic function is a key to cell life and death. Cells need energy not only to support their vital functions but also to die gracefully. Execution of an apoptotic program includes energy-dependent steps, including kinase signaling, formation of the apoptosome, and effector caspase activation. Under conditions of bioenergetic collapse, cells are diverted toward necrotic demise. Mitochondrial outer membrane permeabilization (MOMP) is a decisive event in the execution of apoptosis. It is also causally linked to a decline in bioenergetic function via different mechanisms, not merely due to cytochrome c dispersion. MOMP-induced bioenergetic deficiency is usually irreversible and commits cells to die, even when caspases are inactive. Here, we discuss the mechanisms by which MOMP impacts bioenergetics in different cell death paradigms.
Keywords: bioenergetics, mitochondria, apoptosis, cell death, external pathway of NADH oxidation
Introduction
Cell survival depends on critical functions of the mitochondrial membranes. The mitochondrial inner membrane (MIM) hosts the most important redox reactions converting the energy of nutrients into the pyrophosphate bond of ATP. These reactions are catalyzed by the mitochondrial electron transport chain (ETC), which transports electrons from NADH (or several other substrates) to oxygen, in the complex multistep process termed mitochondrial respiration. According to the chemiosmotic theory, mitochondrial respiration generates a transmembrane potential (Δψ) across the inner membrane, which is used by ATP synthase to phosphorylate ADP. The MIM is normally impermeable to protons and other ions. This solute barrier function of the MIM is critical for energy transduction. Permeabilization of the MIM dissipates Δψ and thereby uncouples the process of respiration from ATP synthase, halting mitochondrial ATP production.
Unlike the MIM, the mitochondrial outer membrane (MOM) is constitutively permeable to small molecules, because it contains nonselective channels formed by a family of mitochondrial porins, also known as voltage-dependent anion channel (VDAC) proteins. However, the MOM is impermeable to proteins, and this protein barrier function is essential for cell survival. It is now well established that MOM permeabilization (MOMP) is a decisive event in many forms of apoptotic cell death. MOMP allows cytochrome c and other mitochondrial apoptogenic proteins to escape into the cytoplasm, where they promote caspase activation and rapid cell death. During typical apoptosis, MOMP produces a rapid decline in mitochondrial respiration. Surprisingly, this phenomenon is not a result of the dispersal of cytochrome c throughout the cytoplasm, but rather is dependent on the activation of caspases downstream of MOMP.1 However, this loss of respiration does not mean that bioenergetic function is irrelevant to cell fate. Cells need ATP to maintain their vital functions and, somewhat paradoxically, to die by apoptosis. Execution of the apoptotic program includes energy-dependent steps, including kinase signaling upstream of MOMP and formation of the apoptosome and caspase activation downstream of MOMP. When cellular ATP levels are abnormally low, classical apoptotic triggers typically cause necrotic demise, suggesting that sustained ATP production (either via oxidative or glycolytic phosphorylation) is required for the cells to engage the apoptotic program.2–4 Decline in bioenergetic function of mitochondria that have undergone MOMP can be temporarily prevented if caspase activity is suppressed, but normally this is not sufficient to rescue the cells from progressive ATP depletion and loss of clonogenic survival5,6 (see further). In this article, we discuss the impact of MOMP on bioenergetics in different cell death scenarios as well as potential sparing mechanisms by which cells may evade rapid bioenergetic collapse after MOMP.
Mitochondrial permeability transition versus Bax/Bak-dependent MOMP
Two fundamentally different, but not mutually exclusive, mechanisms can potentially underlie MOMP. One mechanism is the mitochondrial permeability transition (MPT), which results from the opening of a nonselective pore in the MIM with an estimated diameter of 2.3 nm.7 The MPT is typically accompanied by extensive colloid osmotic swelling of the matrix, leading to mechanical rupture of the MOM. The second mechanism involves the formation of large pores in the MOM (but, remarkably, not in the MIM) and is directly promoted by two proapoptotic members of the Bcl-2 family of proteins, Bax and Bak. Although the MPT was the first mechanism suggested for MOMP,8,9 it is not currently considered a canonical apoptotic pathway. Instead, more recent evidence supports a primary role of Bax/Bak-mediated outer membrane pore formation in both physiological and pathological cell death pathways. Nevertheless, MPT-dependent cell death does occur in various models involving pathophysiological mitochondrial Ca2+ overload and oxidative stress, the conditions relevant, for example, to acute ischemia-reperfusion cell injury, several forms of neurodegeneration, and toxic stresses.10–13 In addition to Ca2+ and peroxides, the classical triggers of MPT both in vitro and in situ, a 1995 review14 listed more than 30 agents (or groups of agents) facilitating the MPT and as many as 16 classes of compounds that inhibit MPT induction by different mechanisms. Since then, the list of MPT inducers and inhibitors has further grown. However, despite this well-established phenomenology, the molecular composition of the MPT pore is ill-defined. Based on many studies, it has long been assumed that the adenine nucleotide translocator (ANT), upon binding cyclophilin D (CypD), forms the conduit of the MPT pore. Additional constituents of the pore complex were proposed to include VDAC, hexokinase II and creatine kinase (reviewed in Ref. 15). However, recent genetic knockout experiments have questioned the role of ANT and VDAC as core components of the pore.16,17
There is no solid evidence supporting a direct role of Bcl-2 family proteins in inducing or promoting MPT. On the contrary, compelling evidence obtained in systems of different levels of complexity—genetically altered mice,18,19 intact cells,20–22 digitonin-permeabilized cells,22 isolated mitochondria,23–25 and purified outer membrane vesicles26—strongly suggest that neither MPT nor even the MIMper se is involved in MOMP induction by Bax and Bak. Most persuasive are recent studies utilizing mice deficient in CypD, which is generally agreed to be a component of the MPT pore. These studies confirm the role of MPT in necrotic cell death, but not in typical apoptosis.18,19 Mitochondria lacking CypD failed to undergo classical MPT, arguing for a critical role of CypD in this event. Strikingly, CypD-deficient cells were resistant to necrosis induced in control cells by several MPT triggers, but showed typical features of apoptosis (caspase activation, Annexin V-staining) in response to staurosporine, etoposide, and other common apoptotic inducers. Apoptotic cell death in vivo showed a similar independence of CypD.18,19 In contrast, cells deficient in Bax and Bak, the MOMP effector proteins, were resistant to multiple apoptotic inducers but underwent necrotic demise in the presence of thapsigargin, an inhibitor of ER Ca2+ pumps. This type of death was associated with mitochondrial Ca2+ overload, swelling, and cytochrome c release, but not with prominent caspase activation.27
Although MPT is not required for apoptosis, it may interfere with the canonical apoptotic pathway and divert it to necrosis in the case of severe energy depletion. The concept of critical ATP level as a switch between apoptosis and necrosis3,12 has been supported by manipulating intracellular ATP concentrations using inhibitors of both mitochondrial and glycolytic ATP production (oligomycin and deoxyglucose, respectively).4 Temporary (3 h) and reversible lowering of ATP levels beyond a threshold value (by about 30%) committed cells to undergo apoptosis in the absence of additional stress stimuli. The partial loss of ATP for a longer period of time (6 h) or a short-term nearly complete ATP depletion resulted in necrosis.4 Similarly, transient glucose deprivation resulted in mostly necrotic death of cardiomyocytes treated with staurosporine, while under ATP-replenishing conditions the apoptotic phenotype was restored.28 In both scenarios, features consistent with MPT were observed.
However, a clear-cut distinction between MPT-dependent and -independent pathways is not always achievable in cellular models. As noted earlier, numerous conditions can promote MPT, and mitochondria in different cell types are known to have different sensitivity to MPT triggers. Besides, methods to assess MPT in whole cells are not “standard”. Many studies rely on sensitivity of mitochondrial depolarization, cytochrome c release and other cell death markers to cyclosporin A or less efficient MPT inhibitors, such as bongkrekic acid (BkA). This often leads to controversy concerning whether MPT is a primary cause of MOMP, a confounding factor, or simply irrelevant. For example, staurosporine-induced cell death typically displays all the hallmarks of a canonical MPT-independent apoptotic pathway, with CypD being dispensable for staurosporine-induced cytochrome c release.29 In some studies, however, cyclosporin A is capable of blocking STS-induced cell death,28 which potentially suggests an additional cyclosporin A target. An alternative explanation for this variability may be cell-type specificity: in certain cells, staurosporine causes elevation of cytosolic Ca2+ and thus may direct the cells toward the MPT-dependent pathway.30 This scenario would be similar to cytochrome c release induced by the Ca2+ mobilizing agent thapsigargin, for which CypD is reported to be indispensable.29
Although cyclosporin A targets proteins other than CypD (e.g., other cyclophilins and calcineurin), 31 it is nevertheless a more specific and potent inhibitor of MPT than another commonly used agent, BkA. The use of BkA, an inhibitor of ANT that locks the antiporter in a specific conformation, seemed justified under the assumption that ANT is a core component of the MPT pore. However, now that the role of ANT is being reconsidered, the use of BkA in cell-based models has become questionable. Furthermore, as a potent inhibitor of ATP/ADP transport, BkA is expected to have an immediate side effect on mitochondrial ATP production. Finally, even in isolated mitochondria, BkA only slightly delays the onset of MPT (typically, for several minutes29,32 in contrast to CsA, which blocks MPT completely under most conditions.
In addition to measurements of Δψ, more sophisticated methods for assessment of MPT in situ have been developed (discussed in Refs. 10,33). Also, in some paradigms, the simple observation that Δψ is sustained during cytochrome c release is sufficient to exclude the MPT as a primary cause of MOMP. An elegant single-cell imaging study employing simultaneous kinetic measurements of Δψ and cytochrome c release in apoptotic cells unequivocally supports such a scenario.21
Bioenergetics after MOMP in the presence and absence of active caspases
The concept of “harmless release of cytochrome c”22,34 has been supported by much data demonstrating functional integrity of mitochondria during MOMP. The released cytochrome c can reenter mitochondria, and its pool is initially sufficiently abundant to sustain respiration.22 However, when caspases are active, mitochondrial bioenergetics are soon impaired. Single-cell studies demonstrated that the Δψ drop lagged behind cytochrome c release only by several minutes, followed by a reduction in ATP levels.21,22 However, this loss of Δψ and ATP can be substantially delayed by pan-caspase inhibitors, such as zVAD and Q-VD. The rapid decline in bioenergetic function after MOMP was attributed to caspase cleavage of Complex I and, possibly, Complex II of the ETC.1,35 Complex IV activity was not affected, raising the possibility of limited respiration via a Complex IV-dependent bypass (as described later). A cleavage site was identified in one of the Complex I subunits (p75), and mutation at this site prevented inhibition of NADH-dependent respiration, Δψ decline and loss of ATP in apoptotic cells.1 Typically, in caspase-inhibited cells following MOMP, Δψ is not disrupted by the ATPase inhibitor oligomycin22 and therefore reflects true respiratory activity of mitochondria as opposed to Δψ generated by the hydrolysis of glycolytic ATP by ATP synthase operating in reverse.36 Δψ is sufficiently high to sustain prolonged ATP production (12–48 h, depending on the stimulus) and protein import.1,6,22,25
Although Δψ and cellular ATP pools are maintained for some time following MOMP, cells still typically exhibit a complete loss of clonogenic survival.5,6 To help clarify the mechanisms of cell death downstream of MOMP, we developed a “pure MOMP” model in which cells could be induced to undergo MOMP without the side effects of typical apoptotic cell stressors. Cells were stably transfected with a proapoptotic protein consisting of the Bax-activating protein BimS fused with a domain of estrogen receptor, resulting in the rapid and specific induction of Bax/Bak-dependent apoptosis, merely through addition of tamoxifen to the culture medium.6 This approach revealed an unexpected pattern of changes in mitochondrial function after MOMP in the presence of caspase inhibitors. Interestingly, the cells continued to divide for ~48 h after MOMP, although cellular ATP levels and, in parallel, rates of nuclear DNA replication, declined slowly over that time period, and the cells eventually succumbed to proliferation arrest. However, much earlier, at ~4–8 h after tamoxifen addition, the cells displayed impairment of specific respiratory complexes: complete loss of Complex I and ~70% decrease in Complex IV activity. Apparently, residual respiration supported by Complex II and partially inhibited Complex IV was enough to delay bioenergetic collapse. At later times, mitochondria exhibited loss of some essential proteins, a sign of progressive deterioration. Thus, MOMP itself, even if downstream caspases are inactive, precludes clonogenic cell survival, because of an early impairment of respiratory activity. An additional conclusion from this study is that oligomycin-induced inhibition of mitochondrial ATP production results in immediate cell proliferation arrest, while selective inhibition of glycolysis by 2-deoxyglucose in the presence of glutamine has no immediate effect on these cells. Thus, glycolysis alone cannot sustain cell proliferation; mitochondrial bioenergetic function is required (notwithstanding the shift toward glycolytic metabolism in some tumor cells, termed the Warburg effect). This observation is consistent with the higher efficiency of oxidative phosphorylation compared to glycolytic phosphorylation.
It was possible that loss of respiration could occur because of a gradual depletion of the cytosolic cytochrome c pool, possibly by proteasomal degradation. 37 However, the impairment of respiratory function we observed could not be corrected by the introduction of exogenous cytochrome c to permeabilized cells, although in control experiments, exogenous cytochrome c did restore respiration after MOMP was induced by treatment of permeabilized normal cells with an exogenous proapoptotic protein, tBid. Thus, degradation of cytochrome c cannot be the sole explanation for the observed reduction in respiration.6
Another possible explanation for the early loss of respiratory function is that the ETC complexes may be directly targeted by intramitochondrial proteases (e.g., by the AAA proteases38). The mechanisms by which MOMP could activate such proteases or make mitochondrial proteins accessible to them are unknown. Alternatively, specific ETC proteins could be degraded by cytoplasmic proteases that gain access to the inner membrane following MOMP. It has been reported that selective degradation of Tim23 (a key component of the mitochondrial protein import complex) by an unidentified mitochondrial protease occurs shortly after MOMP.39 However, in our “pure MOMP” model, the loss of respiratory function preceded Tim23 disappearance.6 Moreover, several mitochondrial nuclear-encoded proteins remained abundant for at least 24 h after MOMP, in agreement with the earlier study which demonstrated that the protein import function remained uncompromised in post-MOMP cells, as long as high Δψ was maintained. 25 It seems likely that mitochondrial proteins are subject to their normal degradation rates, but at later times cannot be replenished by biosynthesis and mitochondrial import. Lastly, it is conceivable that unknown proteins in the intermembrane space that are required for respiration are, along with cytochrome c, dispersed throughout the cytoplasm.
Other processes contributing to mitochondrial dysfunction may be linked to alterations in the lipids of the MIM. Several groups have reported apoptosis-associated changes in cardiolipin content, including oxidation,40 “reorganization,”41 and even relocation of cardiolipin from the MIM to other membrane compartments.42 Cardiolipin is the signature mitochondrial lipid that is obligatory required for the activity of proteins involved in energy transduction, such as ANT and Complexes III and IV of the ETC (Refs. 43 and 44 and references therein). Furthermore, oxidation of cardiolipin causes cytochrome c detachment from the MIM45 and thus compromises electron shuttling from Complex III to Complex IV. An in vitro study has reported that the proapoptotic protein tBid can selectively inhibit ANT and, therefore, ADP-stimulated respiration (but not uncoupled respiration) via binding to cardiolipin.41 However, the cell death paradigm employed in our study6 is tBid-independent and excludes such a scenario.
Spare bioenergetic mechanisms for cell survival after MOMP
In general, MOMP is a point-of-no-return in apoptosis. As mentioned earlier, MOMP leads to a loss of clonogenic survival even if effector caspase activation is blocked. Therefore, caspase inhibitors should generally be poor drugs for clinical conditions such as ischemia/reperfusion injury involving deleterious cell death. On the other hand, cancer cells often lose the ability to activate effector caspases.46–48 Also, some terminally differentiated cells can recover after MOMP.49,50 Thus, from the perspective of pharmacological intervention, the mechanisms of caspase-independent death and survival are of particular importance. How could caspase inhibition be a mechanism for long-term survival in some cells? A recent study5 showed, intriguingly, that the enforced expression of GAPDH enabled some cells that had patently undergone MOMP (as evidenced by the release of cytochrome c-GFP from mitochondria) to resume proliferating after several days and to recover their content of mitochondrial cytochrome c. Two distinct functions of GAPDH were required for this slow recovery from MOMP: glycolytic function and stimulation of autophagy, involving the upregulation of an autophagy-related gene, atg12. Exactly how the mitochondrial network might be restored in the surviving cells is still unknown. We can hypothesize, based on the results of Lartigue et al. described above, that mitochondria that have undergone MOMP deteriorate in such a progressive manner that they can never be repaired. If so, the only way a mitochondrial network can be restored is by biogenesis, which requires that at least a small pool of seed mitochondria somehow avoid undergoing MOMP. Very likely, the fragmentation of mitochondria that typically follows Bax/Bak activation51 would increase the probability that some fraction of a cell’s mitochondria remain intact. The existence of a mitochondrial quality control mechanism might help by disposing of mitochondria that eventually lose Δψ, although whether mitophagy (as opposed to autophagy in general) indeed contributes to cell rescue remains to be demonstrated. It is conceivable that damaged mitochondria, if not removed promptly, poison the cell by draining cellular ATP pools and propagating injury through increased ROS production.4
It should be noted that the ability of overexpressed GAPDH to preserve ATP production depends on whether its function represents a rate-limiting step in the glycolytic pathway. Ordinarily, another glycolytic enzyme, phosphofructokinase, serves as the rate controlling point in glycolysis, but this enzyme is upregulated in the HeLa cells used in the study. Besides, cancer cells (such as HeLa) are, in general, actively glycolytic. Remarkably, levels of GAPDH (which unwisely is often used as a gel loading control) decline by 80–90% in the post-MOMP cells.5 Therefore, GAPDH is likely to become rate-limiting. It is possible that, in other cell types, restoration of glycolytic ATP production and cell recovery may require stimulation of different enzyme(s) in the glycolytic pathway. Similarly, some cell types could employ mechanisms other than GAPDH expression to stimulate autophagy.
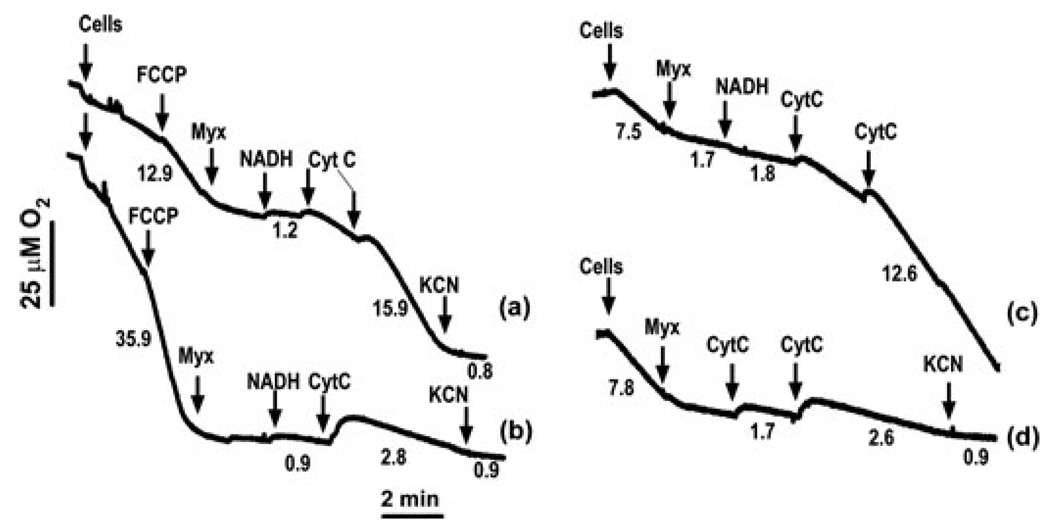
As discussed above, Complex I has emerged as a primary target for both caspase-dependent and caspase-independent injury. In caspase-inhibited cells, ATP production and Δψ are preserved (albeit temporarily) by limited respiration involving Complex II and the terminal segment of the ETC. In general, individual ETC components, in particular Complex IV, are considered to be in major functional excess,52 although it has been argued that functional redundancy of Complex IV does not exceed 20–40% in vivo.53 We hypothesize that spare respiratory capacity of Complex IV is sufficient to delay bioenergetic death and may contribute to post-MOMP cell survival. From this perspective, quantitative and mechanistic aspects of short-cut respiratory pathways warrant further investigation. One such potential mechanism is the external pathway of NADH oxidation, a phenomenon described in earlier bioenergetic studies.54 Specifically, cytochrome c can be continuously reduced by extramitochondrial NADH via the flavoprotein (Fp5)-cytochrome b5 complex residing at the MOM and then oxidized by Complex IV (cytochrome c oxidase). Thus, under conditions of inhibited ETC activity (except Complex IV), this mechanism can support respiration. Originally, it was proposed that oxidation of external NADH involves a cytochrome c shuttle between the intact MOM and cytochrome c oxidase, based on the assumption that the cytochrome c binding site of cytochrome b5 faces the intermembrane space.54 However, subsequent studies demonstrated that the rate of external NADH oxidation in intact mitochondria was extremely low and that MOM rupture was required for this process, provided that extramitochondrial cytochrome c was available.55,56 Activity of this cytochrome c redox system is sufficiently high to generate Δψ and support ATP production.55 However, this mechanism has only been studied in isolated liver mitochondria under conditions of artificial MOMP induced by digitonin and osmotic swelling. Thus, the relevance of this interesting phenomenon to mitochondrial function in normal and apoptotic cells is still hypothetical. As a step forward in this direction, we have demonstrated (Fig. 1) that the external NADH oxidation pathway can efficiently restore mitochondrial respiration in post-MOMP HeLa cells in the presence of mitochondrial inhibitors that cause complete inhibition of ETC activity upstream of Complex IV. In the experiment described in Figure 1, MOMP was induced either by in vitro action of tBid or by treatment of the cells with staurosporine. As expected, Complex I-driven respiration is significantly reduced in staurosporine-treated cells, but replenishment of mitochondria with NADH and cytochrome c is sufficient to restore respiration in these cells. In agreement with the earlier studies,55,56 external NADH oxidation was not observed in the absence of MOMP (Fig. 1, trace b). These data indicate that MOMP not only paves the way for mitochondrial damage, but at the same time permits activation of a potential spare respiratory mechanism.
Figure 1.
Mitochondrial respiration driven by oxidation of external NADH in digitonin-permeabilized HeLa cells. The cells (13 × 106 per mL) were permeabilized with 0.012% digitonin in a KCl-based incubation medium containing 10 mM succinate and 0.5 µM rotenone. Arrows indicate additions of the cells, 100 nM FCCP (an uncoupler), 2 µM myxothiazol (a Complex III inhibitor), 2 mM NADH, and cytochrome c (CytC, final concentration—80 µM). Complex IV inhibitor KCN (0.5 mM) was added at the end of the runs to terminate respiration. Curve (a) cells were treated with 0.5 µM staurosporine (in the presence of 100 µM ZVAD) for 12 h; curve (b) control cells; curves (c) and (d) digitonin-permeabilized cells were pretreated with 40 nM tBid for 5 min prior to the respiration measurements in the presence (c) or absence (d) of NADH. Numbers under the curves are respiration rates (µM O2 per min). Note that cytochrome c markedly stimulated respiration in staurosporine and tBid-treated cells (a, c) in the presence of exogenous NADH.
Acknowledgments
We thank Alexander Andreyev (UCSD) for helpful discussions. This work is supported by NIH Grants RO3DA021385 (YK) and R01GM50284 (DDN).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Ricci JE, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Ankarcrona M, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 3.Nicotera P, Melino G. Regulation of the apoptosis-necrosis switch. Oncogene. 2004;23:2757–2765. doi: 10.1038/sj.onc.1207559. [DOI] [PubMed] [Google Scholar]
- 4.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis. 2006;11:473–485. doi: 10.1007/s10495-006-5881-9. [DOI] [PubMed] [Google Scholar]
- 5.Colell A, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Lartigue L, et al. Caspase-independent mitochondrial cell death results from loss of respiration, not cytotoxic protein release. Mol. Biol. Cell. 2009;20:4871–4884. doi: 10.1091/mbc.E09-07-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crompton M, Costi A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to reperfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem. J. 1990;266:33–39. doi: 10.1042/bj2660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamzami N, et al. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skulachev VP. Membrane-linked systems preventing superoxide formation. Biosci. Rep. 1997;17:347–366. doi: 10.1023/a:1027344914565. [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 11.Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem. Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 13.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 14.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 15.Juhaszova M, et al. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann. N.Y. Acad. Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- 16.Kokoszka JE, et al. The ADP/ATP translocator is not essential for themitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baines CP, et al. Voltage-dependent anion channels are dispensable formitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baines CP, et al. Loss of cyclophilinDreveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 20.Vander Heiden MG, et al. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein JC, et al. Cytochrome c is released in a single step during apoptosis. Cell Death Differ. 2005;12:453–462. doi: 10.1038/sj.cdd.4401596. [DOI] [PubMed] [Google Scholar]
- 22.Waterhouse NJ, et al. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polster BM, Kinnally KW, Fiskum G. BH3 death domain peptide induces cell type-selective mitochondrial outer membrane permeability. J. Biol. Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- 24.Li T, et al. Dissimilar mechanisms of cytochrome c release induced by octyl glucoside-activated BAX and by BAX activated with truncated BID. Biochim. Biophys. Acta. 2010;1797:52–62. doi: 10.1016/j.bbabio.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Ahsen O, et al. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J. Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuwana T, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 27.Janssen K, et al. Inhibition of the ER Ca2+ pump forces multidrug-resistant cells deficient in Bak and Bax into necrosis. J. Cell Sci. 2009;122:4481–4491. doi: 10.1242/jcs.055772. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi J, et al. Important role of energy-dependent mitochondrial pathways in cultured rat cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1637–H1647. doi: 10.1152/ajpheart.2001.281.4.H1637. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, et al. The mitochondrial permeability transition regulates cytochrome c release for apoptosis during endoplasmic reticulum stress by remodeling the cristae junction. J. Biol. Chem. 2008;283:3476–3486. doi: 10.1074/jbc.M707528200. [DOI] [PubMed] [Google Scholar]
- 30.Norberg E, et al. An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008;15:1857–1864. doi: 10.1038/cdd.2008.123. [DOI] [PubMed] [Google Scholar]
- 31.Waldmeier PC, et al. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- 32.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. Part I. The protective mechanisms. Arch. Biochem. Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi P, et al. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 34.Von Ahsen O, et al. The ‘harmless’ release of cytochrome c. Cell Death Differ. 2000;7:1192–1199. doi: 10.1038/sj.cdd.4400782. [DOI] [PubMed] [Google Scholar]
- 35.Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 37.Cozzolino M, et al. Apoptosome inactivation rescues proneural and neural cells from neurodegeneration. Cell Death Differ. 2004;11:1179–1191. doi: 10.1038/sj.cdd.4401476. [DOI] [PubMed] [Google Scholar]
- 38.Arnold I, Langer T. Membrane protein degradation by AAA proteases in mitochondria. Biochim. Biophys. Acta. 2002;1592:89–96. doi: 10.1016/s0167-4889(02)00267-7. [DOI] [PubMed] [Google Scholar]
- 39.Goemans CG, et al. Intra-mitochondrial degradation of Tim23 curtails the survival of cells rescued from apoptosis by caspase inhibitors. Cell Death Differ. 2008;15:545–554. doi: 10.1038/sj.cdd.4402290. [DOI] [PubMed] [Google Scholar]
- 40.Jiang J, et al. Interplay between bax, reactive oxygen species production, and cardiolipin oxidation during apoptosis. Biochem. Biophys. Res. Commun. 2008;368:145–150. doi: 10.1016/j.bbrc.2008.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalvez F, et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 42.Sorice M, et al. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klingenberg M. Cardiolipin and mitochondrial carriers. Biochim. Biophys. Acta. 2009;1788:2048–2058. doi: 10.1016/j.bbamem.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 46.Devarajan E, et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- 47.Liu JR, et al. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 2002;62:924–931. [PubMed] [Google Scholar]
- 48.Wolf BB, et al. Defective cytochrome c-dependent caspase activation in ovarian cancer cell lines due to diminished or absent apoptotic protease activating factor-1 activity. J. Biol. Chem. 2001;276:34244–34251. doi: 10.1074/jbc.M011778200. [DOI] [PubMed] [Google Scholar]
- 49.Deshmukh M, Kuida K, Johnson EM., Jr Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J. Cell Biol. 2000;150:131–143. doi: 10.1083/jcb.150.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinou I, et al. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinou JC, Youle RJ. Which came first, the cytochromec release or the mitochondrial fission? Cell Death Differ. 2006;13:1291–1295. doi: 10.1038/sj.cdd.4401985. [DOI] [PubMed] [Google Scholar]
- 52.Rossignol R, et al. Mitochondrial threshold effects. Biochem. J. 2003;370:751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villani G, et al. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J. Biol. Chem. 1998;273:31829–31831. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- 54.Bernardi P, Azzone GF. Cytochrome c as an electron shuttle between the outer and inner mitochondrial membranes. J. Biol. Chem. 1981;256:7187–7192. [PubMed] [Google Scholar]
- 55.Bodrova ME, et al. Membrane potential generation coupled to oxidation of external NADH in liver mitochondria. FEBS Lett. 1998;435:269–274. doi: 10.1016/s0014-5793(98)01072-2. [DOI] [PubMed] [Google Scholar]
- 56.Lemeshko VV. Cytochrome c sorption-desorption effects on the external NADH oxidation by mitochondria: experimental and computational study. J. Biol. Chem. 2002;277:17751–17757. doi: 10.1074/jbc.M201002200. [DOI] [PubMed] [Google Scholar]