Abstract
The evidence suggesting a role of extensive cortical demyelization and atrophy in progressive multiple sclerosis (MS) is rapidly increasing. While conventional magnetic resonance imaging (MRI) has had a huge impact on MS by enabling an earlier diagnosis, and by providing surrogate markers for monitoring disease response to antinflammatory/immunomodulatory treatments, it is limited by the low pathological specificity and the low sensitivity to both diffuse damage in normal-appearing white matter (NAWM) and focal and diffuse damage in gray matter (GM). Advanced MRI techniques can partially overcome these limitations by providing markers more specific to the underlying pathologic substrates and more sensitive to the structural and functional “occult” brain tissue damage in patients with MS. This review describes brain and spinal cord imaging studies of MS with particular emphasis on GM imaging in both secondary- and primary-progressive MS, discusses the clinical implications of GM damage and outlines current MRI developments at high and ultra-high magnetic field strength.
Keywords: magnetic resonance imaging, secondary progressive MS, primary-progressive MS, gray matter damage
Approximately 80–85% of patients with multiple sclerosis (MS) present with a relapsing-remitting (RR) course characterized by acute relapses followed by remissions (1). The progressive course of multiple sclerosis (MS) includes the secondary progressive (SP), progressive-relapsing (PR) and the primary-progressive (PP) subtypes (1). SP-MS develops over time in patients with RR-MS and it is characterized by a gradual clinical worsening independent of relapses; patients with PP-MS present a steady progression of the disease processes since the onset in absence of exacerbations; in PR-MS, patients follow a PP-MS course and then experience relapses with continuous progression between exacerbations. Although very little is known about the mechanisms of disease progression, a recent histological study has demonstrated that both patients with SP-MS and PP-MS are characterized by extensive demyelination of the cortex and more diffuse rather than focal injury in normal-appearing white matter (NAWM). Clinical magnetic resonance imaging (MRI) is of great value in supporting MS diagnosis and in providing more sensitive outcome measures in clinical trials (2, 3); the lack of pathological specificity of routine MRI techniques, however, precludes the assessment of the neurodegenenerative features of the disease. Furthermore, while conventional MRI outcomes such as the number of Gadolinium-enhancing lesions and the number of new T2 lesions are useful in monitoring the efficacy of antinflammatory/immunomodulatory treatments, they are of limited utility in monitoring neuroprotective treatments, especially in the progressive phase of the disease (4). Advanced MRI techniques have made possible to visualize GM lesions in vivo and to quantify structural and functional damage of the cortical and sub-cortical GM (5). We will first review brain and spinal cord imaging studies that assessed GM damage in both secondary- and primary-progressive forms of MS. We shall then discuss the clinical implications of GM damage especially with regard to cognitive performance and the role of GM imaging in monitoring neuroprotective treatments. We will conclude with a discussion on high and ultra-high field MRI and its potential in elucidating the mechanisms of neuro-axonal damage in MS.
GRAY MATTER PATHOLOGY IN MULTIPLE SCLEROSIS
Cortical and sub-cortical GM pathology in MS has been neglected for long time due to the predominant interest in WM inflammatory demyelination and the difficulty in visualizing GM lesions with both conventional histochemical staining and MRI methods. In a series of brain pathology studies published in 1962, the authors found that 26% of the MS lesions were located in the cortical and subcortical GM (6). A subsequent correlative MRI-histological study confirmed the high prevalence of demyelination in GM areas (7). More recently, Kutzelnigg et al. (8) assessed NAWM and the cortex from the post-mortem tissue of 52 patients with acute, RR-MS, PP-MS, or SP-MS and from healthy controls. PP-MS and SP-MS patients were characterized by extensive demyelination of the cortex and more diffuse rather than focal injury in NAWM suggesting that progressive MS patients share a common pathology that might be different from that of RR-MS patients characterized by the prevalence of focal injury.
[Callout] In a recent post-mortem study, patients with primary progressive or secondary progressive multiple sclerosis (PP-MS or SP-MS, respectively) were characterized by extensive demyelination of the cortex and more diffuse rather than focal injury in normal appearing white matter (NAWM), suggesting that progressive MS patients share a common pathology that may differ from that of relapsing remitting multiple sclerosis (RR-MS) patients characterized by the prevalence of focal injury.
Furthermore, the lack of correlation among cortical demyelination, number of white matter lesions and the diffuse damage in normal appearing white matter (NAWM) suggested that these pathological processes occur, at least in part, independent from one another.
One of the systems proposed for the classification of cortical demyelination feature three patterns: Type I lesions are contiguous with subcortical WM lesions; type II lesions are intracortical and often perivascular; type III lesions extend from the pial surface to cortical layers 3 or 4 (9). Unlike WM lesions, GM lesions are characterized by demyelination and, to a lesser extent, microglial reaction whereas lymphocyte infiltration and blood–brain barrier (BBB) disruption are not usually found (10, 11). Axonal transection, neuronal, glial, and synaptic loss, may be found in a minority of cortical lesions. In addition to the presence of lesions, the cerebral cortex of patients with MS may also be affected by atrophy and neuronal apoptosis (10).
Sub-cortical GM is not spared by the MS pathological processes. Histophatological studies have demonstrated demyelination in the thalamus, basal ganglia, hypothalamus, hippocampus, cerebellum, and spinal cord of MS patients (8, 12, 13) Compared to cortical lesions, hypothalamic and, to a lesser extent, spinal cord GM lesions show a stronger inflammatory profile than intracortical lesions, and are characterized by the presence of activated microglia and macrophages (13). In contrast, hippocampal lesions that are very common in chronic MS autopsy cases present a pathological profile more similar to that of intra-cortical lesions (14).
CONVENTIONAL BRAIN AND SPINAL CORD MAGNETIC RESONANCE IMAGING
Clinical MRI of the brain and the spinal cord is of great value in the diagnosis of MS (2).
[Callout] Clinical magnetic resonance imagine (MRI) of the brain and the spinal cord is of great value in the diagnosis of MS.
Typically, patients with PP-MS have fewer new T2 lesions or Gadolinium-enhancing lesions than patients with other MS subtypes including those with SP-MS (15). However, a study using triple-dose enhanced MRI has demonstrated the presence of active lesions in 19 out of 45 PP-MS patients (42%) suggesting that inflammation can be substantial in subgroups of patients with early disease (16). Lesions of the spinal cord are relatively common in MS patients and occur more frequently in the cervical cord (17). In spite of the fact that locomotor disability is very common in PP-MS, the frequency of lesions found in the spinal cord of these patients is not greater than that of other MS subtypes suggesting that mechanisms other than lesions formation lead to clinical deficits (17). Conventional MRI can also demonstrate atrophy in both the brain and spinal cord of MS patients (3). Despite these advantages, however, clinical MRI is of limited utility for GM lesions detection, and lacks specificity to the pathological substrate of the disease and sensitivity to the diffuse microscopic damage in NAWM and in NAGM (3, 5).
[Callout] Despite these advantages, however, clinical MRI is of limited utility for GM lesions detection, and lacks specificity to the pathological substrate of the disease and sensitivity to the diffuse microscopic damage in NAWM and in NAGM.
MAGNETIC RESONANCE IMAGING OF CORTICAL AND SUB-CORTICAL GRAY MATTER LESIONS
Cortical lesions are poorly detectable on routine MRI sequences such as T2-weighted (T2-W) spin echo and fluid-attenuated inversion recovery (FLAIR) due to potential several reasons: 1) smaller lesions size; 2) smaller difference in T1 and T2 relaxation times between lesions and surrounding tissue in GM than in WM; 3) low inflammatory infiltration of cortical lesions and the less frequent association with blood brain barrier (BBB) breakdown; 4) partial volume effect resulting from the CSF of adjacent cortical sulci. It has been shown that an improvement in cortical lesion detection in MS can be achieved by using a double inversion recovery (DIR) (18) sequence. DIR imaging consists of two adiabatic non-selective inversion pulses applied before a Turbo Spin Echo sequence, in order to suppress the signal from two tissues with different longitudinal relaxation times simultaneously. In the brain, DIR is used to selectively image the GM by nulling the signal from WM and cerebrospinal fluid (19) (Fig. 1). DIR imaging increases cortical lesion detection rates per patient by an average of 152% when compared with FLAIR imaging and 500% when compared with conventional T2-W MRI, (20).
Fig. 1.
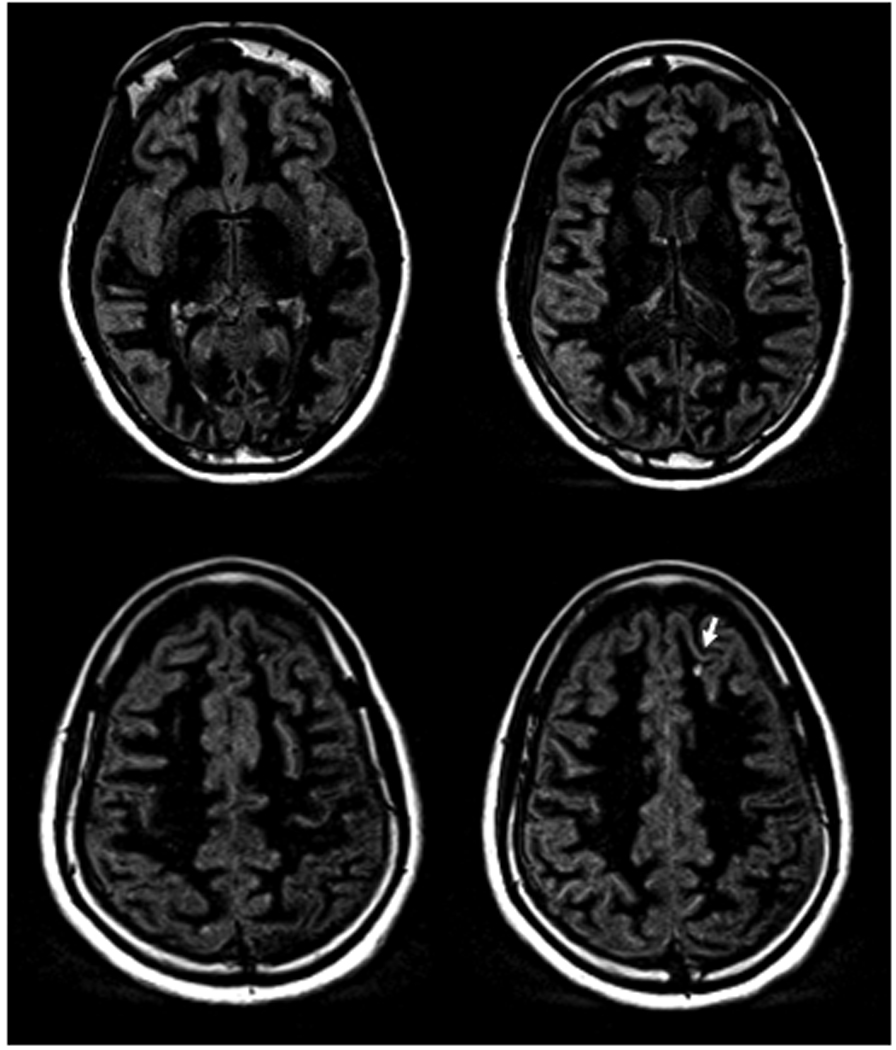
Selected axial DIR images acquired at 3 Tesla (Verio, Siemens Medical Solutions, Erlangen, Germany) from a patient with primary-progressive MS. Note the clear delineation of the gray matter resulting from the suppression of signals from the cerebral spinal fluid CSF and the white matter. The arrow indicates a cortical lesion.
In a DIR study of a large patient population, cortical lesions were detected in the majority of patients with RRMS (64%) and SPMS (70%), as well as in 37% of patients with clinically isolated syndrome (21). This finding indicates that although cortical lesions are present in all MS phenotypes, the occurrence of cortical inflammation in this disease is an early phenomenon. Importantly, patients with cortical lesions were shown to have higher expanded Disability Status Scale (EDSS) scores, a greater white matter T2 lesion load and a reduced brain volume than patients with MS who did not have cortical lesions. A 3 year longitudinal follow-up study demonstrated that the number and volume of cortical lesions increased over time at a significantly greater rate in patients with clinical progression than in patients who were clinically stable (22). Interestingly, the accumulation rate of new cortical lesions was shown to be similar in 76 patients with RR-MS and 31 patients with SP-MS (0.8 per year versus 1.0 per year), suggesting that the greater number of cortical lesions observed in patients with SP-MS probably reflects the fact that they have had the disease over a longer period of time and that the overall cortical lesion burden might affect disability progression in patients with MS. Cortical lesions have also been observed in up to 80% of patients with PP-MS and have been shown to be positively correlated with disease duration, higher EDSS scores and increased cortical atrophy and disability (23).
[Callout] Cortical lesions have also been observed in up to 80% of patients with PP-MS and have been shown to be positively correlated with disease duration, higher scores on the expanded disability status scale (EDSS) and increased cortical atrophy and disability
A large proportion of cortical lesions, however, remain undetected even when DIR imaging is used. Only 10–20% of the cortical lesions that are identified by immunohistological techniques are also detected on DIR images (24). The combination of DIR with other MRI sequences, such as a T1weighted three dimensional spoiled gradient recalled echo (25) and phase sensitive inversion recovery (26) will, hopefully, further increase the cortical lesion detection rate.
GRAY MATTER ATROPHY
Apart from visualizing cortical lesions, GM damage can be assessed by using atrophy (Fig. 2) and cortical thickness measurements (27, 28). GM volume loss has been reported in patients with different clinical phenotypes of MS and occurs since the earliest stages of the disease (29–35). Measures of GM atrophy are clinical relevant as shown by several MRI studies that have linked GM atrophy with clinical impairment, including physical disability and cognitive dysfunction (36–39). Interestingly, compared with white matter, GM atrophy is more extensive and occurs at a faster rate in the MS brain. In a longitudinal study, Fisniku et al. (40) evaluated tissue-specific atrophy in a cohort of seventy-three MS patients initially presenting with a clinically isolated syndrome (CIS) who were followed for 20 years, with clinical and MRI evaluations. The investigators found that the extent of GM atrophy in the MS patients was greater than that of WM atrophy after 20 years of disease, and that there was significantly more GM atrophy, but not WM atrophy in SP-MS versus RR-MS patients, as well as in RRMS versus CIS patients. Not only did GM atrophy correlate with disability but it also proved to be a stronger predictor of disability than focal WM lesion load and WM atrophy.
[Callout] Not only does gray matter (GM) atrophy correlate with disability but it has also proven to be a stronger predictor of disability than focal WM lesion load and WM atrophy.
In another study, Fisher et al., (41) measured the rate of GM and WM atrophy in a large group of MS patients who were followed over 4 years. They found that, although WM atrophy rate remained constant at threefold normal across all disease stages, a 3.4-fold increase of GM atrophy rate was seen in CIS patients converting to RRMS, whereas a 14-fold increase was measured in SP-MS patients. In a longitudinal MRI study of patients with PP-MS, a 1.49 % decrease in GM fraction was observed over 1 year follow-up, whereas a decrease in WM fraction was reported only in a subgroup of PP-MS patients with high Gadolinium-enhancing lesions load (16).
Fig. 2.
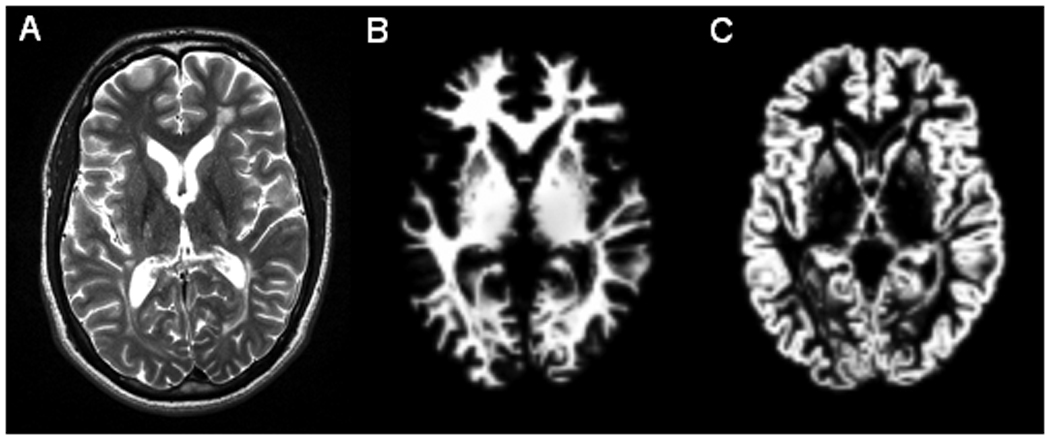
Axial T2-weighted image (A) and corresponding segmented white matter (B) and segmented gray matter (C) from a patient with secondary-progressive MS.
NON CONVENTIONAL MAGNETIC RESONANCE IMAGING AND “OCCULT” INJURY IN NORMAL-APPEARING GRAY MATTER
In addition to detection of cortical lesions and atrophy, GM pathology can be assessed in vivo using advanced quantitative MRI techniques such as magnetization transfer imaging (MTI), diffusion tensor imaging (DTI), and proton MR spectroscopy (1H-MRS). While these techniques are more sensitive to pathology in the normal-appearing GM (NAGM), they have a poorer spatial resolution than conventional MRI, which makes it difficult to distinguish between lesional and non-lesional GM damage.
MTI allows the calculation of an index, the magnetization transfer ratio (MTR), which, when reduced, indicates a diminished capacity of the protons bound to the brain macromolecules to exchange magnetization with the surrounding free water protons, thus providing an estimate of the extent of tissue disruption (42). MTR values in the brain GM were found to be reduced in patients with different MS phenotypes, although they were more pronounced in patients with PP-MS or SP-MS than in those with RR-MS. Furthermore, in patients with PP-MS, GM MTR decline reflected the rate of clinical deterioration during 3 years (43)
DTI enables the random diffusional motion of water molecules to be measured, thus providing metrics, such as mean diffusivity (MD) and fractional anisotropy (FA), which allow quantification of the size and geometry of water-filled spaces (44) DTI studies confirmed the presence of GM damage in MS and showed that the extent of such damage differs among the various disease phenotypes, being more severe in patients with SP-MS (44).Longitudinal DTI studies (44) have demonstrated a worsening of GM damage with time in patients with RR-MS, SP-MS, and PP-MS. In the latter GM diffusivity was also found to predict accumulation of disability during a 5-year period (45).
1H-MR spectroscopy allows to quantify chemical markers of the pathologic changes occurring in the brain (46) Several studies have found metabolite abnormalities, including reduced concentrations of NAA, a marker of neuroaxonal integrity and viability, and Cho, a marker of membrane turnover, and increased concentrations of myo-inositol, a marker of astrogliosis in the cortical and subcortical GM tissue (47–49) of patients with MS. While these studies could detect NAA decrease in early RR-MS and in patients with CIS suggesting that neuro-axonal injury occurs since the early stages of the disease, other studies (50, 51), found a significant decreases of Cho, creatine, and NAA concentrations in the GM of patients with the progressive forms of the disease, but not in those with RR-MS. NAA reduction has also been demonstrated in the thalamus of patients with SP-MS and RR-MS (48) and in the cerebral cortex of patients with PP-MS (52).
NON-CONVENTIONAL MAGNETIC RESONANCE IMAGING OF THE SPINAL CORD
The spinal cord is a common site of pathology in multiple sclerosis (53)and spine MRI is of great value, in addition to brain MRI, for the diagnosis of the disease (2). The majority of cord lesions are located peripherally in the cervical and thoracic segments of the cord and are limited to one to two spinal cord segments (54). Unlike in RR-MS patients who are characterized by focal abnormalities, in patients with SP-MS, cord abnormalities tend to be extensive and confluent (55) and in those with PP-MS, diffuse abnormalities are more frequent compared with the other MS phenotypes (56). Likewise, spinal cord atrophy seems to be more severe in PP-MS and SP-MS patients and to correlate with the appearance of diffuse abnormalities and locomotor disability (57). Thus, suggesting that mechanisms other than focal demyelination may be responsible for spinal cord atrophy. Conventional spine MRI is very sensitive to the presence of lesions (17), but it is unable to grade the extent of tissue injury within macroscopic lesions, as well as to detect and quantify the “occult” damage known to occur in the normal-appearing cord of patients with MS (58). Development in hardware and pulse sequences has made possible the application of quantitative MR techniques in the spinal cord for the quantification of “occult” damage in the normal-appearing cord tissue. Patients with PP-MS and SP-MS are characterized by lower cord MTR values than those with RR-MS and cervical cord MTR histogram measures are independent predictors of locomotor disability (59). A large brain and spinal cord MTI study of patients with progressive MS showed that the extent of diffuse spinal cord tissue damage measured by means of MTR in PP-MS patients matched that of SP-MS patients with similar levels of disability despite the fact they had higher MRI-visible lesion burden (60). In addition, patients with RR-MS, SP-MS and PP-MS have MD and FA histogram characteristics suggestive of diffuse cord injury (61). When patients with PP-MS are compared with those with other MS phenotypes, cord fractional anisotropy decrease is found but not differences in mean diffusivity or cord cross-sectional area (62). More recently, the feasibility of 1H-MRS in spinal cord has been shown and compared with brain and clinical findings (63, 64). A 32% decrease of NAA was detected in spinal cord, and correlated with the cerebellar subscore of the neurologic assessment. Ciccarelli and colleagues (64), have demonstrated that the combination of MR spectroscopy and diffusion MRI of the cervical cord not only provide measures that are sensitive to the tissue damage occurring in this area but enable a more comprehensive assessment of spinal cord and clinical disability.
GRAY MATTER INJURY AND PHYSICAL DISABILITY
While the correlation between WM T2 lesion volume and the Expanded Disability Status Scale (EDSS) score ranges from weak to moderate, imaging markers of GM injury show a better association with clinical disability (3). For instance, MS patients with both RR and progressive course who presented DIR detected cortical lesions were shown to have higher EDSS scores (22, 23). Furthermore, in a longitudinal study of MS patients, GM atrophy proved to be a stronger predictor of disability than focal WM lesion load and WM atrophy (40).
GRAY MATTER INJURY AND COGNITION
Cognitive impairment affects 40–65% of patients with MS, with most frequent deficits of memory, abstract reasoning, sustained attention, and information processing speed (65). Although traditionally categorized as characterized by a “sub-cortical pattern” of cognitive dysfunction, MS patients seem to be characterized by both cortical and sub-cortical pathologies as supported by the presence of injury in the cortex and deep gray matter (12). Although cognitive decline has been observed from the early stages of the disease, it is more frequent and pronounced in the progressive forms of the disease and tends to worsen over time (66, 67).
[Callout] Although cognitive decline has been observed from the early stages of the disease, it is more frequent and pronounced in the progressive forms of the disease and tends to worsen over time
Camp et al. carried out the largest survey published to date, including 63 patients with PP-MS (68) and reported cognitive dysfunction in 29% of them. In a subsequent 2-year study of PP-MS, they observed that while mean performance did not change significantly over time, in terms of individual changes, 37% of patients showed significant cognitive decline.
While earlier MRI studies focused on the impact of WM lesion burden and location, recent studies have suggested that several pathological features of MS pathology, including GM injury are likely to contribute to the presence and worsening of cognitive deficits in MS. Amato et al. (37) found that cortical atrophy is present only in cognitively impaired patients with early RR-MS and it is correlated with a poor performance on several neuropsychological tests. A 2.5-year follow-up of a subgroup of these patients showed a progressive cortical atrophy in those individuals with a deteriorating cognitive performance (69). Moreover, an increase in cortical and hippocampal lesions over time has been shown to be more frequent in patients with SP-MS and to correlate with impaired visuo-spatial memory and processing speed (70). Both cortical lesions and atrophy have been shown to be independent predictors of MS-related cognitive impairment (21).
Interestingly, brain cortical reorganization measured by means of blood oxygen level dependent MRI, could play a role in preserving cognitive performance despite the presence of disease-related structural damage (71). Conversely, the exhaustion of cortical recruitment might be among the factors responsible for a worsening of cognitive abilities (72). Recently, reduced fluctuations within the anterior regions of the default-mode network have also been shown to occur in patients with progressive MS and cognitive impairment (73).
All together these studies suggest that the combination of several MR modalities, sensitive toward different components of MS pathology, might be a valuable tool to improve the understanding of the pathophysiology of cognitive impairment in MS.
[Callout] All together these studies suggest that the combination of several MR modalities, sensitive toward different components of MS pathology, might be a valuable tool to improve the understanding of the pathophysiology of cognitive impairment in MS.
IMPLICATIONS FOR MONITORING TREATMENT
While the number of new T2 lesions and Gd-enhancing lesions is an useful outcome measures in MS trials of antinflammatory treatments, measurement of brain atrophy may be the most relevant biomarker for monitoring neurodegeneration in a clinical trial setting (4). This is particularly important for patients with primary and secondary progressive MS who are characterized by less inflammation. Furthermore, recent studies suggest that GM volume measures may represent a more sensitive tool for assessing neuroprotective therapeutic effects than whole brain and WM volumes (74). The preliminary MRI data available from patients with RR-MS (75), suggest that GM volume change in response to treatment may be a more reliable marker to distinguish disease- and treatment related brain volume changes. Although GMV may be a viable outcome measure for clinical trials investigating neuroprotection (76), further studies are needed to establish whether GM volume measurements, as well as other GM-based non-conventional MRI techniques (DIR, MTR, DTI and 1H-MRS) (77, 78) may become reliable outcome measures for MS clinical trials focusing on neuroprotection in patients with SP- and PP-MS.
HIGH AND ULTRA-HIGH FIELD STRENGTH MAGNETIC RESONANCE IMAGING
The improvement in image signal-to-noise ratio (SNR) provided by high and ultra-high field MRI systems and multi-channel radiofrequency (RF) technology can significantly improve WM lesions detection in MS patients (79). Furthermore, the higher SNR and spatial resolution, combined with the inherent greatly enhanced T2* contrast at ultra-high field strengths can improve the detection and characterization of cortical lesions (80–83). Interestingly, cortical lesions were more frequent in patients with SP-MS than in those with RR-MS (82) supporting the results of DIR studies at lower MRI field strenght. There is some evidence that high and ultra-high field MRI may be useful in imaging nuclei such as 31P, and 23Na (Fig. 3) characterized by a much lower concentration than proton nuclei (84) and in measuring metabolites such as glutamate and glutathione (GSH) present at low concentration in the brain and difficult to resolve due to overlap with other resonances (85, 86). Preliminary data from patients with RR-MS demonstrate that these techniques are sensitive not only to WM but also to GM damage suggesting their potential in elucidating the pathological mechanisms of neurodegeneration and disease progression.
Fig. 3.
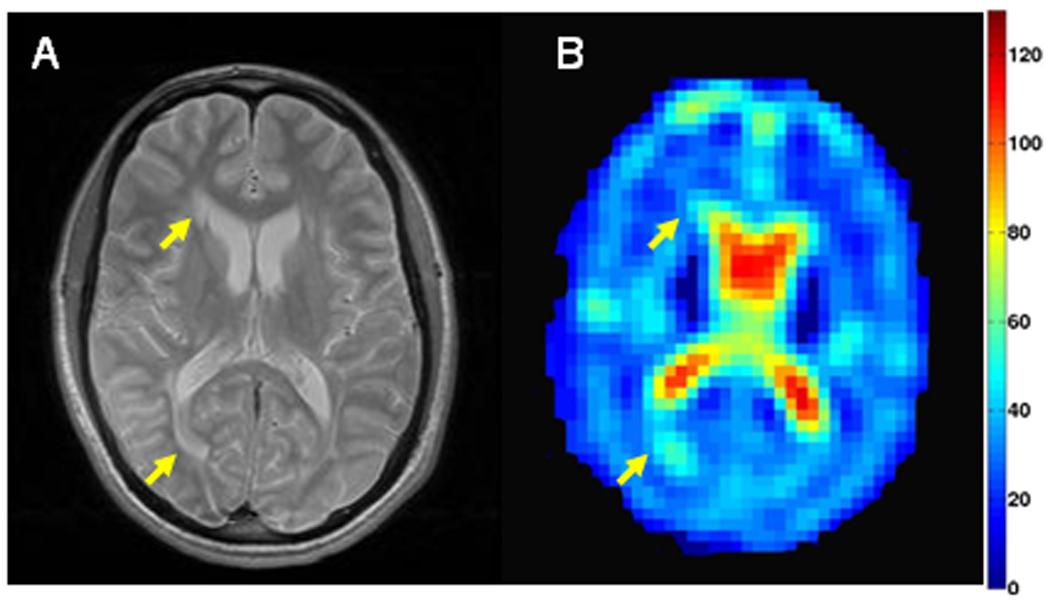
Selected axial T2-weighted image (A) and corresponding tissue sodium concentration (TSC in mM) map (B) from a patient with secondary-progressive MS. Arrows indicate lesions with increased TSC concentration.
FUTURE DIRECTIONS
While our current knowledge of cellular and molecular mechanisms of MS progression, especially in the progressive forms of the disease is limited, routine and advanced MRI techniques will undoubtedly serve as a tool for improving diagnosis, monitoring neurodegeneration and efficacy of neuroprotective treatments and identifying prognostic biomarkers. Improved detection of GM lesions in vivo by means of DIR could provide a valuable new aid for the diagnosis and follow-up of people with MS. However, additional studies will have to further validate the technique both intra- and across centers before inclusion of cortical lesions in the diagnostic MR imaging criteria could be discussed. Since the overall burden of WM MRI-visible lesions and NAWM injury do not fully account for the severity of MS cognitive impairment, future studies will have to further investigate the clinical relevance and outcome of cortical and sub-cortical GM lesions. Targets for neuroprotective drug discovery and development will emerge as we learn more about the pathophysiolgy of neuroaxonal damage. As a consequence, more tissue specific imaging tools for rapid screening of promising therapeutic agents and for monitoring their efficacy in clinical trials are needed. Measures of GM atrophy seem to be more sensitive to tissue changes over time than measures of BV and WMV and a very promising tool for distinguishing disease- and treatment related brain volume changes. However, future studies will have to establish whether GM volume measurements, as well as other GM-based non-conventional MRI techniques such as MTI, DTI and 1H-MRS may become reliable markers for monitoring neurodegeneration and neuroprotection. Finally, the advent of high and ultra-high field strength magnets and sophisticated coil technology holds great promises in terms of development and implementation of techniques with higher sensitivity and specificity to pathological mechanisms underlying disease processes.
ACKNOWLEDGMENTS
This study was supported by the US National Institute of Health [grant number R01 NS051623].
REFERENCES
- 1.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 3.Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121(Pt 1):3–24. doi: 10.1093/brain/121.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 5.Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- 6.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1962;25:315–320. doi: 10.1136/jnnp.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122(Pt 1):17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. Epub 2005 Oct 2717. [DOI] [PubMed] [Google Scholar]
- 9.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 11.van Horssen J, Brink BP, de Vries HE, van der Valk P, Bo L. The blood-brain barrier in cortical multiple sclerosis lesions. J Neuropathol Exp Neurol. 2007;66:321–328. doi: 10.1097/nen.0b013e318040b2de. [DOI] [PubMed] [Google Scholar]
- 12.Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P. Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:1101–1107. doi: 10.1097/01.jnen.0000190067.20935.42. [DOI] [PubMed] [Google Scholar]
- 13.Gilmore CP, Bo L, Owens T, Lowe J, Esiri MM, Evangelou N. Spinal cord gray matter demyelination in multiple sclerosis-a novel pattern of residual plaque morphology. Brain Pathol. 2006;16:202–208. doi: 10.1111/j.1750-3639.2006.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2007;66:819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- 15.Thompson AJ, Kermode AG, Wicks D, et al. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991;29:53–62. doi: 10.1002/ana.410290111. [DOI] [PubMed] [Google Scholar]
- 16.Sastre-Garriga J, Ingle GT, Chard DT, et al. Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain. 2005;128:1454–1460. doi: 10.1093/brain/awh498. [DOI] [PubMed] [Google Scholar]
- 17.Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology. 1993;43:2632–2637. doi: 10.1212/wnl.43.12.2632. [DOI] [PubMed] [Google Scholar]
- 18.Redpath TW, Smith FW. Technical note: use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol. 1994;67:1258–1263. doi: 10.1259/0007-1285-67-804-1258. [DOI] [PubMed] [Google Scholar]
- 19.Madelin G, Oesingmann N, Inglese M. Double Inversion Recovery MRI with fat suppression at 7 tesla: initial experience. J Neuroimaging. 2010;20:87–92. doi: 10.1111/j.1552-6569.2008.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 22.Calabrese M, Rocca MA, Atzori M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 2010;67:376–383. doi: 10.1002/ana.21906. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese M, Rocca MA, Atzori M, et al. Cortical lesions in primary progressive multiple sclerosis: a 2-year longitudinal MR study. Neurology. 2009;72:1330–1336. doi: 10.1212/WNL.0b013e3181a0fee5. [DOI] [PubMed] [Google Scholar]
- 24.Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 25.Bagnato F, Butman JA, Gupta S, et al. In vivo detection of cortical plaques by MR imaging in patients with multiple sclerosis. AJNR Am J Neuroradiol. 2006;27:2161–2167. [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson F, Poonawalla AH, Hou P, Huang F, Wolinsky JS, Narayana PA. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol. 2007;28:1645–1649. doi: 10.3174/ajnr.A0645. Epub 2007 Sep 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippi M, Rocca MA. MR imaging of gray matter involvement in multiple sclerosis: implications for understanding disease pathophysiology and monitoring treatment efficacy. AJNR Am J Neuroradiol. 2010;31:1171–1177. doi: 10.3174/ajnr.A1944. Epub 2009 Dec 1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calabrese M, Rinaldi F, Mattisi I, et al. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74:321–328. doi: 10.1212/WNL.0b013e3181cbcd03. [DOI] [PubMed] [Google Scholar]
- 29.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60:1157–1162. doi: 10.1212/01.wnl.0000055926.69643.03. [DOI] [PubMed] [Google Scholar]
- 30.Pagani E, Rocca MA, Gallo A, et al. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- 31.Prinster A, Quarantelli M, Orefice G, et al. Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. Neuroimage. 2006;29:859–867. doi: 10.1016/j.neuroimage.2005.08.034. Epub 2005 Oct 2003. [DOI] [PubMed] [Google Scholar]
- 32.Ceccarelli A, Rocca MA, Pagani E, et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage. 2008;42:315–322. doi: 10.1016/j.neuroimage.2008.04.173. Epub 2008 Apr 2020. [DOI] [PubMed] [Google Scholar]
- 33.Dalton CM, Chard DT, Davies GR, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. Epub 2004 Mar 1103. [DOI] [PubMed] [Google Scholar]
- 34.Henry RG, Shieh M, Okuda DT, Evangelista A, Gorno-Tempini ML, Pelletier D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J Neurol Neurosurg Psychiatry. 2008;79:1236–1244. doi: 10.1136/jnnp.2007.134825. Epub 2008 May 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesaros S, Rocca MA, Absinta M, et al. Evidence of thalamic gray matter loss in pediatric multiple sclerosis. Neurology. 2008;70:1107–1112. doi: 10.1212/01.wnl.0000291010.54692.85. Epub 2008 Feb 1113. [DOI] [PubMed] [Google Scholar]
- 36.Chen JT, Narayanan S, Collins DL, Smith SM, Matthews PM, Arnold DL. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. Neuroimage. 2004;23:1168–1175. doi: 10.1016/j.neuroimage.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Amato MP, Bartolozzi ML, Zipoli V, et al. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- 38.Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage. 2006;30:891–898. doi: 10.1016/j.neuroimage.2005.10.032. Epub 2005 Dec 2015. [DOI] [PubMed] [Google Scholar]
- 39.Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. Neuroimage. 2007;34:509–517. doi: 10.1016/j.neuroimage.2006.10.006. Epub 2006 Nov 2016. [DOI] [PubMed] [Google Scholar]
- 40.Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–254. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- 41.Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- 42.Filippi M, Agosta F. Magnetization transfer MRI in multiple sclerosis. J Neuroimaging. 2007;17:22S–26S. doi: 10.1111/j.1552-6569.2007.00132.x. [DOI] [PubMed] [Google Scholar]
- 43.Khaleeli Z, Altmann DR, Cercignani M, Ciccarelli O, Miller DH, Thompson AJ. Magnetization transfer ratio in gray matter: a potential surrogate marker for progression in early primary progressive multiple sclerosis. Arch Neurol. 2008;65:1454–1459. doi: 10.1001/archneur.65.11.1454. [DOI] [PubMed] [Google Scholar]
- 44.Inglese M, Bester M. Diffusion imaging in multiple sclerosis: research and clinical implications. NMR Biomed. 2010;23:865–872. doi: 10.1002/nbm.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rovaris M, Gallo A, Valsasina P, et al. Short-term accrual of gray matter pathology in patients with progressive multiple sclerosis: an in vivo study using diffusion tensor MRI. Neuroimage. 2005;24:1139–1146. doi: 10.1016/j.neuroimage.2004.10.006. Epub 2004 Nov 1126. [DOI] [PubMed] [Google Scholar]
- 46.De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology. 2007;69:1942–1952. doi: 10.1212/01.wnl.0000291557.62706.d3. [DOI] [PubMed] [Google Scholar]
- 47.Chard DT, Griffin CM, McLean MA, et al. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:2342–2352. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- 48.Inglese M, Liu S, Babb JS, Mannon LJ, Grossman RI, Gonen O. Three-dimensional proton spectroscopy of deep gray matter nuclei in relapsing-remitting MS. Neurology. 2004;63:170–172. doi: 10.1212/01.wnl.0000133133.77952.7c. [DOI] [PubMed] [Google Scholar]
- 49.Geurts JJ, Reuling IE, Vrenken H, et al. MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn Reson Med. 2006;55:478–483. doi: 10.1002/mrm.20792. [DOI] [PubMed] [Google Scholar]
- 50.Sijens PE, Mostert JP, Oudkerk M, De Keyser J. (1)H MR spectroscopy of the brain in multiple sclerosis subtypes with analysis of the metabolite concentrations in gray and white matter: initial findings. Eur Radiol. 2006;16:489–495. doi: 10.1007/s00330-005-2839-1. Epub 2005 Jul 2019. [DOI] [PubMed] [Google Scholar]
- 51.Caramanos Z, DiMaio S, Narayanan S, Lapierre Y, Arnold DL. (1)H-MRSI evidence for cortical gray matter pathology that is independent of cerebral white matter lesion load in patients with secondary progressive multiple sclerosis. J Neurol Sci. 2009;282:72–79. doi: 10.1016/j.jns.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Sastre-Garriga J, Ingle GT, Chard DT, et al. Metabolite changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch Neurol. 2005;62:569–573. doi: 10.1001/archneur.62.4.569. [DOI] [PubMed] [Google Scholar]
- 53.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976;26:26–28. doi: 10.1212/wnl.26.6_part_2.26. [DOI] [PubMed] [Google Scholar]
- 54.Bot JC, Barkhof F. Spinal-cord MRI in multiple sclerosis: conventional and nonconventional MR techniques. Neuroimaging Clin N Am. 2009;19:81–99. doi: 10.1016/j.nic.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Kidd D, Thorpe JW, Kendall BE, et al. MRI dynamics of brain and spinal cord in progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 1996;60:15–19. doi: 10.1136/jnnp.60.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nijeholt GJ, van Walderveen MA, Castelijns JA, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998;121(Pt 4):687–697. doi: 10.1093/brain/121.4.687. [DOI] [PubMed] [Google Scholar]
- 57.Lin X, Tench CR, Evangelou N, Jaspan T, Constantinescu CS. Measurement of spinal cord atrophy in multiple sclerosis. J Neuroimaging. 2004;14:20S–26S. doi: 10.1177/1051228404266265. [DOI] [PubMed] [Google Scholar]
- 58.Bergers E, Bot JC, van der Valk P, et al. Diffuse signal abnormalities in the spinal cord in multiple sclerosis: direct postmortem in situ magnetic resonance imaging correlated with in vitro high-resolution magnetic resonance imaging and histopathology. Ann Neurol. 2002;51:652–656. doi: 10.1002/ana.10170. [DOI] [PubMed] [Google Scholar]
- 59.Filippi M, Bozzali M, Horsfield MA, et al. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology. 2000;54:207–213. doi: 10.1212/wnl.54.1.207. [DOI] [PubMed] [Google Scholar]
- 60.Rovaris M, Bozzali M, Santuccio G, et al. In vivo assessment of the brain and cervical cord pathology of patients with primary progressive multiple sclerosis. Brain. 2001;124:2540–2549. doi: 10.1093/brain/124.12.2540. [DOI] [PubMed] [Google Scholar]
- 61.Valsasina P, Rocca MA, Agosta F, et al. Mean diffusivity and fractional anisotropy histogram analysis of the cervical cord in MS patients. Neuroimage. 2005;26:822–828. doi: 10.1016/j.neuroimage.2005.02.033. Epub 2005 Mar 2031. [DOI] [PubMed] [Google Scholar]
- 62.Agosta F, Benedetti B, Rocca MA, et al. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology. 2005;64:631–635. doi: 10.1212/01.WNL.0000151852.15294.CB. [DOI] [PubMed] [Google Scholar]
- 63.Blamire AM, Cader S, Lee M, Palace J, Matthews PM. Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy. Magn Reson Med. 2007;58:880–885. doi: 10.1002/mrm.21382. [DOI] [PubMed] [Google Scholar]
- 64.Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007;130:2220–2231. doi: 10.1093/brain/awm152. [DOI] [PubMed] [Google Scholar]
- 65.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 66.Minden SL, Moes EJ, Orav J, Kaplan E, Reich P. Memory impairment in multiple sclerosis. J Clin Exp Neuropsychol. 1990;12:566–586. doi: 10.1080/01688639008401002. [DOI] [PubMed] [Google Scholar]
- 67.Heaton RK, Nelson LM, Thompson DS, Burks JS, Franklin GM. Neuropsychological findings in relapsing-remitting and chronic-progressive multiple sclerosis. J Consult Clin Psychol. 1985;53:103–110. doi: 10.1037//0022-006x.53.1.103. [DOI] [PubMed] [Google Scholar]
- 68.Camp SJ, Stevenson VL, Thompson AJ, et al. Cognitive function in primary progressive and transitional progressive multiple sclerosis: a controlled study with MRI correlates. Brain. 1999;122:1341–1348. doi: 10.1093/brain/122.7.1341. [DOI] [PubMed] [Google Scholar]
- 69.Amato MP, Portaccio E, Goretti B, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol. 2007;64:1157–1161. doi: 10.1001/archneur.64.8.1157. [DOI] [PubMed] [Google Scholar]
- 70.Roosendaal SD, Moraal B, Pouwels PJ, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler. 2009;15:708–714. doi: 10.1177/1352458509102907. Epub 2009 May 2012. [DOI] [PubMed] [Google Scholar]
- 71.Filippi M, Rocca MA. Functional MR imaging in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:59–70. doi: 10.1016/j.nic.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Penner IK, Rausch M, Kappos L, Opwis K, Radu EW. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol. 2003;250:461–472. doi: 10.1007/s00415-003-1025-0. [DOI] [PubMed] [Google Scholar]
- 73.Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- 74.Zivadinov R, Reder AT, Filippi M, et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology. 2008;71:136–144. doi: 10.1212/01.wnl.0000316810.01120.05. [DOI] [PubMed] [Google Scholar]
- 75.Kelemen A, Dwyer MG, Horakova D, Vaneckova M, Havrdova E, Zivadinov R. Measurement of gray matter volume is less susceptible to pseudoatrophy effect than that of white matter or whole brain volume in patients with multiple sclerosis. Results from Avonex–Steroids–Azathioprine combination study. Mult Scler. 2008;14:S11. [Google Scholar]
- 76.Healy B, Valsasina P, Filippi M, Bakshi R. Sample size requirements for treatment effects using gray matter, white matter and whole brain volume in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:1218–1223. doi: 10.1136/jnnp.2008.154732. Epub 2009 Feb 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inglese M, van Waesberghe JH, Rovaris M, et al. The effect of interferon beta-1b on quantities derived from MT MRI in secondary progressive MS. Neurology. 2003;60:853–860. doi: 10.1212/01.wnl.0000049929.27032.29. [DOI] [PubMed] [Google Scholar]
- 78.Sajja BR, Narayana PA, Wolinsky JS, Ahn CW Group PRTM. Longitudinal magnetic resonance spectroscopic imaging of primary progressive multiple sclerosis patients treated with glatiramer acetate: multicenter study. Mult Scler. 2008;14:73–80. doi: 10.1177/1352458507079907. [DOI] [PubMed] [Google Scholar]
- 79.Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC. Comparison of multiple sclerosis lesions at 1.5 and 3.0 Tesla. Invest Radiol. 2003;38:423–427. doi: 10.1097/01.RLI.0000065426.07178.f1. [DOI] [PubMed] [Google Scholar]
- 80.Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70:2076–2078. doi: 10.1212/01.wnl.0000313377.49555.2e. [DOI] [PubMed] [Google Scholar]
- 81.Kollia K, Maderwald S, Putzki N, et al. First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. AJNR Am J Neuroradiol. 2009;30:699–702. doi: 10.3174/ajnr.A1434. Epub 2009 Jan 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;73:941–948. doi: 10.1212/WNL.0b013e3181b64bf7. Epub 2009 Jul 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 84.Inglese M, Madelin G, Oesingmann N, et al. Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 tesla. Brain. 2010;27:27. doi: 10.1093/brain/awp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. Epub 2005 Mar 1019. [DOI] [PubMed] [Google Scholar]
- 86.Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging. 2009;18:18. doi: 10.1016/j.mri.2009.06.008. [DOI] [PubMed] [Google Scholar]


