Abstract
Autophagy, a cellular process for organelle and protein turnover, regulates innate immune responses. We demonstrate that depletion of autophagic proteins microtubule associated protein-1 light chain 3B (LC3B) and Beclin 1 enhances caspase-1 activation and secretion of interleukin-1β and interleukin-18. Autophagic protein depletion promoted accumulation of dysfunctional mitochondria and cytosolic translocation of mitochondrial DNA (mtDNA) in response to lipopolysaccharide (LPS) and ATP in macrophages. Release of mtDNA into the cytosol depended on the NALP3 inflammasome and mitochondrial ROS. Cytosolic mtDNA contributed to IL-1β and IL-18 secretion in response to LPS and ATP. LC3B-deficient mice produced more caspase-1-dependent cytokines in two sepsis models and were susceptible to LPS-induced mortality. Our study suggests that autophagic proteins regulate NALP3-dependent inflammation by preserving mitochondrial integrity.
Autophagy, an evolutionarily-conserved cellular process, facilitates the turnover of damaged proteins and organelles such as mitochondria1. During autophagy, cytosolic constituents are engulfed into double-membrane-bound vesicles called “autophagosomes”, which are subsequently delivered to the lysosomes for degradation1. Accumulating evidence suggests that autophagic dysfunction is associated with aging and human diseases, including cancer, and neurodegenerative disorders2. Autophagy also participates in the clearance of intracellular bacteria, viruses, and protozoa from host cells2,3. In addition, autophagy affects diverse immune system functions such as antigen presentation, lymphocyte development and cytokine secretion in immune cells3. The involvement of autophagy in pro-inflammatory cytokine secretion was recently demonstrated in atg-16 deleted mice, which produce exaggerated amounts of IL-1β and IL-18 in response to LPS and other pathogen-associated molecular patterns (PAMPs)4. This observation suggested that autophagy mutations may directly or indirectly deregulate IL-1β and IL-18 secretion. However, the mechanism by which autophagy regulates cytokine secretion is poorly understood.
In macrophages, the secretion of IL-1β and IL-18 is controlled by a newly-discovered inflammatory signaling platform called the “inflammasome”5–7. The inflammasome is a multi-protein complex which mediates the cleavage and activation of caspase-1, leading to maturation and secretion of IL-1β and IL-18. The cytoplasmic receptors of the NALP (also called NLR) family are critical components of the inflammasome and interact with apoptosis-associated speck-like protein (ASC), which recruits pro caspase-1. Mice lacking NALP3, ASC or caspase-1 display a major defect in the production of mature IL-1β and IL-18 after LPS and ATP challenge, and are resistant to LPS-induced lethality8,9. Given the pivotal role of caspase-1 activation in IL-1β and IL-18 secretion and in LPS-induced inflammation in vivo, we hypothesized that autophagy-related proteins might regulate pro-inflammatory cytokine secretion via modulation of caspase-1 activation. In this study, we describe a pathway by which autophagic proteins regulate caspase-1-mediated innate immune responses through their role in preserving mitochondrial homeostasis.
RESULTS
Autophagic protein deletion enhances caspase-1 activation
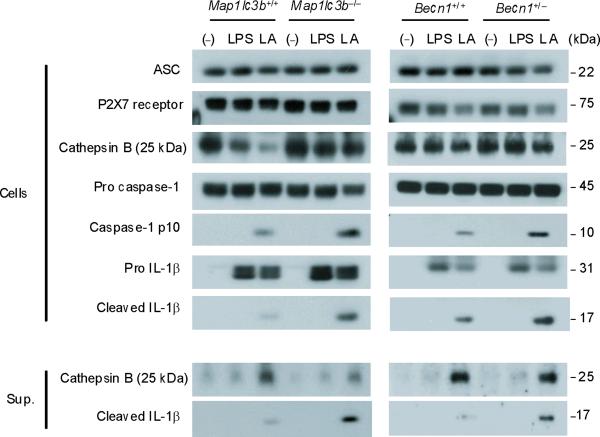
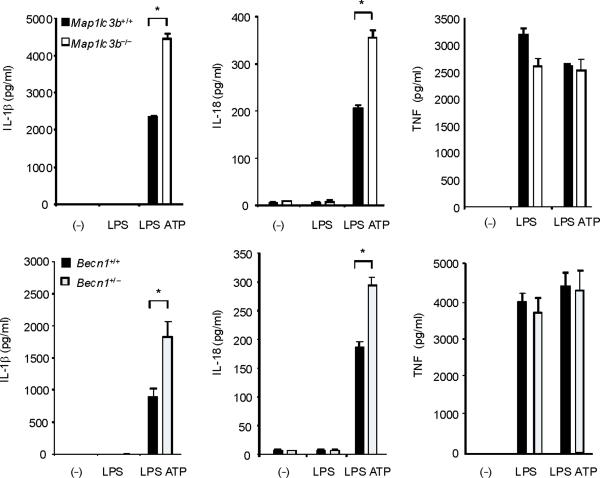
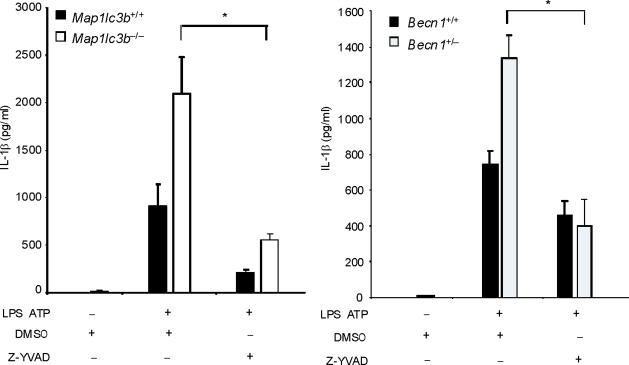
To examine the role of autophagic proteins during inflammasome activation, we pre-treated macrophages isolated from autophagy gene depleted mice with LPS and then stimulated them with ATP. ATP-driven activation of caspase-1 in LPS-primed macrophages is an established model for NALP3 inflammsome-mediated caspase-1 activation in vitro, which acts through purinergic receptor P2X7 (P2X7 receptor) and toll like receptor 4 (TLR4)-mediated signaling pathways, respectively8,9. First, we examined the effect of deficiency of LC3B on the activation of caspase-1 in thioglycollate-elicited peritoneal macrophages. LC3B is a downstream constituent of the autophagic pathway and participates in autophagosome formation and maturation1,10. Compared to wild-type Map1lc3b+/+ macrophages, Map1lc3b−/− macrophages displayed a higher level of the active, cleaved (10 kDa) form of caspase-1 in response to LPS and ATP treatment (Fig. 1a). Additionally, the cleaved form of IL-1β, produced from pro IL-1β by the action of activated caspase-1, was increased in the cell lysates and culture medium of Map1lc3b−/− macrophages (Fig. 1a). Similarly, acute siRNA-dependent knockdown of LC3B also enhanced IL-1β secretion in peritoneal macrophages (Supplementary Fig. 1). We next asked whether Beclin 1, a critical upstream regulator of autophagy, affects caspase-1 activation. Beclin 1 associates with the hVPS34-class III phosphatidylinositol-3-kinase complex, which is responsible for initiation of autophagosome formation1,2. Since homozygous deletion of Becn1 is lethal in mice, we utilized peritoneal macrophages from Becn1+/− animals11. Similar to LC3B-deficient cells, Becn1+/− macrophages displayed greater caspase-1 activation and cleaved IL-1β after LPS and ATP treatment (Fig. 1a). Moreover, IL-1β and IL-18 secretion into the medium was also increased in Map1lc3b−/− and Becn1+/− macrophages compared to corresponding wild-type macrophages (Fig. 1b). Unlike IL-1β secretion, we observed that secretion of cathepsin B, which is dependent on extracellular Ca2+ concentration12, was not increased in either Map1lc3b−/− or Becn1+/− cells (Fig. 1a). In addition, secretion of TNF in response to LPS was comparable among the genotypes (Fig. 1b). Consistent with this observation, we found that activation of NF-κB by LPS was not changed in Map1lc3b−/− macrophages relative to wild type cells (Supplementary Fig. 2). Similar increases in caspase-1 activation, IL-1β cleavage, and secretion of IL-1β and IL-18 were observed in autophagy protein deficient bone marrow-derived macrophages (BMDM) in response to LPS and ATP stimulation (Fig. 1c). Treatment of macrophages with z-YVAD-FMK, a caspase-1 inhibitor, suppressed the elevated IL-1β secretion in Map1lc3b−/− and Becn1+/− macrophages (Fig. 1d), confirming that the increased levels of IL-1β were the consequence of increased caspase-1 activity. Collectively, these results demonstrate that depletion of autophagy proteins enhance caspase-1 activation and secretion of IL-1β and IL-18 in macrophages in response to LPS and ATP.
Fig. 1. Absence of LC3B and heterozygous disruption of Beclin 1 enhance caspase-1 dependent cytokine secretion in macrophages.
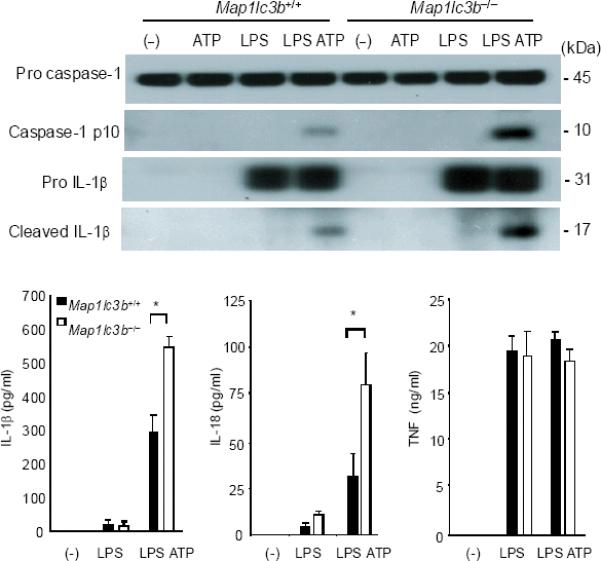
(a) Peritoneal macrophages from Map1lc3b−/− mice and Becn1+/− mice and the corresponding wild-type littermate mice were stimulated with LPS, followed by ATP treatment (L A) for 30 min. Cell lysates (Cells) and culture supernatants (Sup.) were analyzed by immunoblotting for caspase-1 and IL-1β. (b) Peritoneal macrophages were stimulated with LPS, followed by ATP treatment for 1 h. Secretion of cytokines into the media was analyzed by enzyme-linked immunosorbent assay (ELISA). (c) BMDM from Map1lc3b+/+ or Map1lc3b−/− mice were incubated with LPS, followed by ATP treatment for 30 min (immunoblotting) or 1 h (ELISA). Cell lysates were analyzed by immunoblotting for caspase-1 and IL-1β. Cell culture supernatants were analyzed by ELISA for cytokine secretion. (d) LPS-primed BMDM from Map1lc3b−/− mice and Becn1+/− mice were co-incubated with Z-YVAD-FMK (10 μM) or DMSO, followed by ATP stimulation for 1 h. IL-1β secretion into the media was analyzed by ELISA. Statistical significance was determined by the Student's t-test. *P < 0.05. Data are representative of three or four experiments (mean and s.d. in b,c,d).
Autophagic protein depletion impairs mitochondrial homeostasis
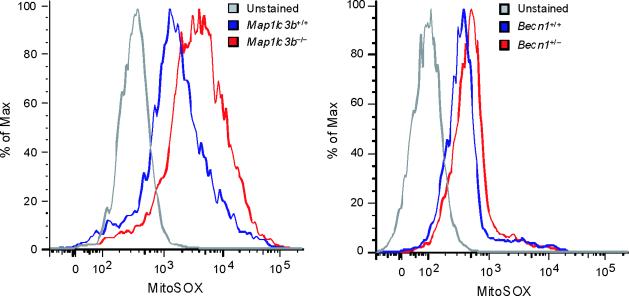
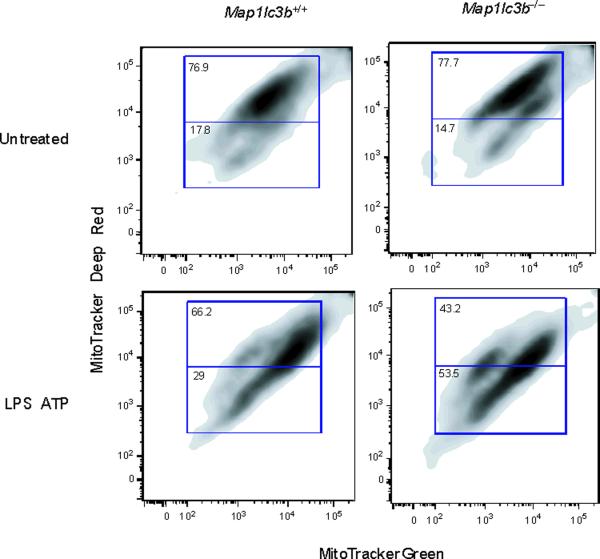
Since autophagy is critical for removing old or damaged organelles, we investigated ultrastructural phenotypes in autophagy protein deficient (Map1lc3b−/− or Becn1+/−) macrophages during LPS and ATP treatment. Under basal conditions, we did not observe morphological differences in transmission electronic micrographs (TEM) of Map1lc3b+/+ and Map1lc3b−/− macrophages (Fig. 2a). However, LPS and ATP treatment produced a greater abundance of swollen mitochondria with severely disrupted cristae in Map1lc3b−/− cells, compared to Map1lc3b+/+ macrophages (Fig. 2a). Becn1+/− cells also showed evidence of swollen or injured mitochondria in response to LPS and ATP treatment (Fig. 2b). Furthermore, we also observed that macrophages from Map1lc3b−/− and Becn1+/− mice generated higher amounts of mitochondrial superoxide anion radical (O2−) (detected by MitoSOX fluorescence) at baseline (Fig. 2c), indicative of increased mitochondrial ROS production and mitochondrial dysfunction13,14. We further assessed the functional mitochondrial pool in wild-type and autophagic-protein deleted cells using MitoTracker Deep Red, a fluorescent probe sensitive to the mitochondrial inner transmembrane potential. To measure the total mitochondrial pool, cells were counterstained with MitoTracker Green, a probe that stains mitochondrial membrane lipids independently of membrane potential13. LPS and ATP treatment decreased the percentage of intact (MitoTracker Deep Red-MitoTracker Green positive) mitochondria in Map1lc3b+/+ macrophages from 76.9% to 66.2% of the total population (Fig. 2d). In contrast, LPS and ATP treatment decreased the functional mitochondrial pool in Map1lc3b−/− macrophages, from 77.7% to 43.2% of the total mitochondria. These results reflect a ~1.6-fold increase in mitochondrial injury index in Map1lc3b−/− macrophages compared to wild-type cells (Fig. 2d). These data suggest that disruption of the autophagic protein LC3B generates a defect in mitochondrial homeostasis, which increases the basal amount of mitochondrial ROS production and renders mitochondria more susceptible to damage by LPS and ATP treatment.
Fig. 2. Depletion of autophagic proteins alters mitochondrial phenotype.
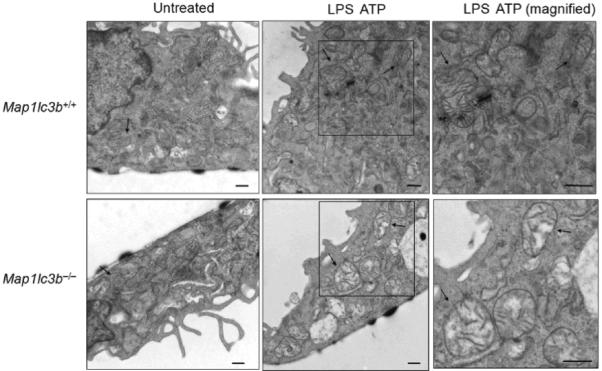
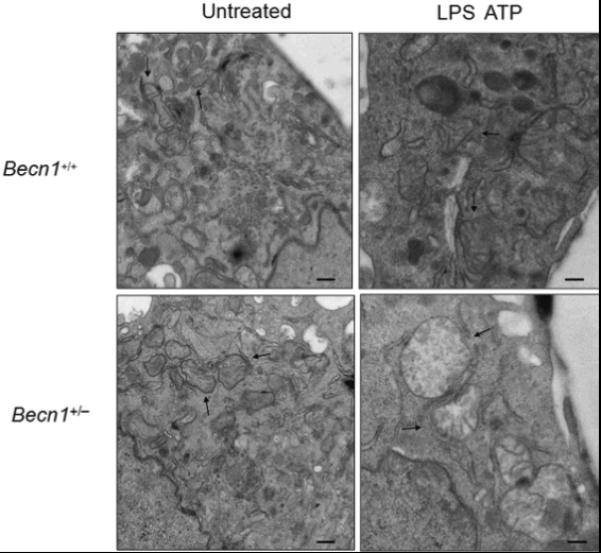
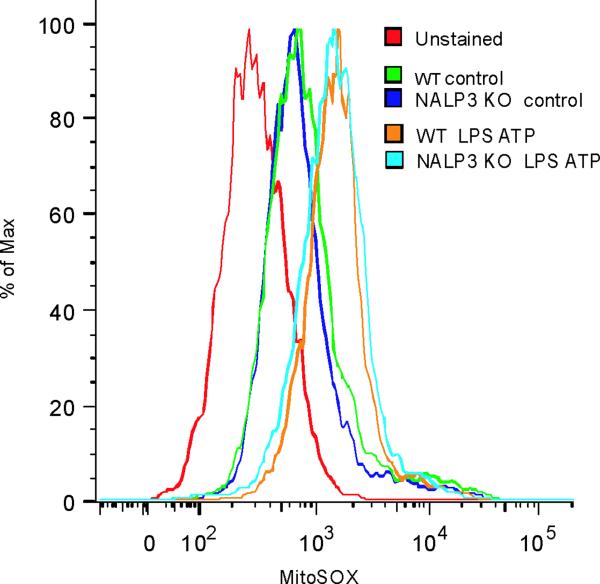
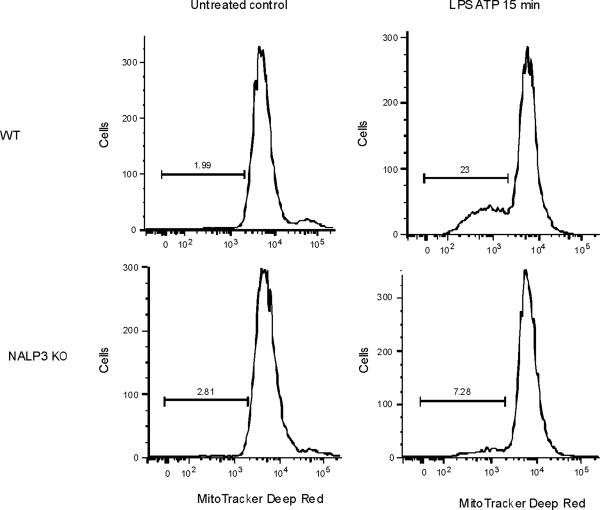
(a,b) BMDM from Map1lc3b−/− (a) or Becn1+/− (b) mice were incubated with LPS (10 ng/ml) and then stimulated with ATP (1 mM) for 30 min. Morphological changes of mitochondria were examined by TEM. Arrows indicate mitochondria. Scale bars represent 500 nm. (c) Macrophages from Map1lc3b−/− mice and Becn+/− mice were labeled with MitoSOX and analyzed by flow cytometric analyses. Representative histograms are shown. (d) Macrophages stimulated with LPS and ATP were stained with MitoTracker Deep Red and MitoTracker Green for 15 min. Representative flow cytometry plots are represented. Data are representative of three experiments.
Impaired caspase-1 activation in Rho-0 macrophages
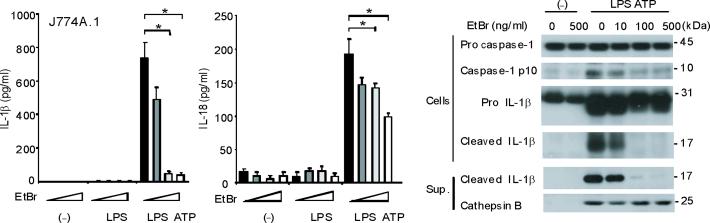
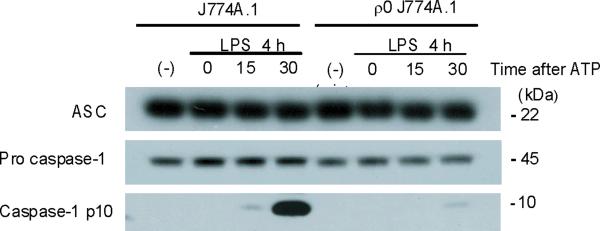
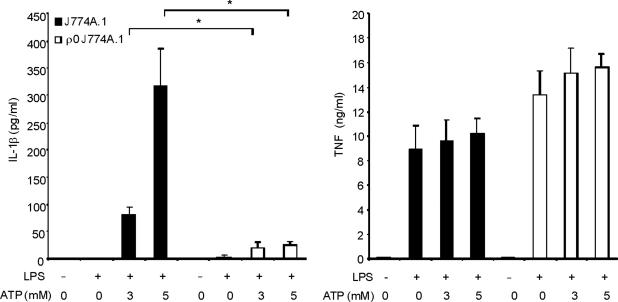
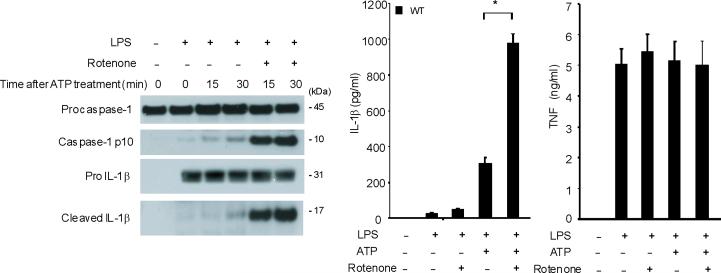
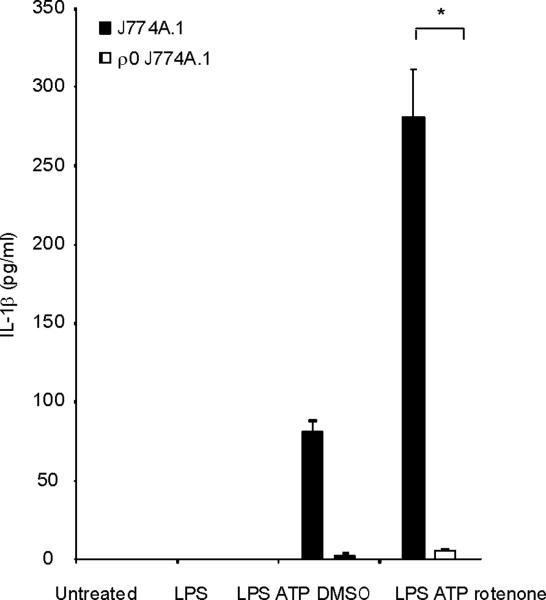
Mitochondrial abnormalities have been implicated in the pathogenesis of human diseases, such as type II diabetes, Parkinson's disease and Alzheimer's disease15–17. To assess the importance of mitochondria in caspase-1 activation, we cultured J774A.1 macrophages with ethidium bromide (EtBr) to generate macrophages with the Rho 0 (ρ0) phenotype18. In animal or human cells, chronic EtBr treatment inhibits mitochondrial DNA (mtDNA) replication, which results in the depletion of critical mitochondrial proteins encoded by the mitochondrial genome, leading to deficiency of oxidative phosphorylation as well as auxotrophy for uridine and pyruvate19,20. The ρ0 cells are widely used to investigate relationships between mtDNA, mitochondrial dysfunction and a variety of cellular processes20. We confirmed that EtBr treatment dose-dependently depleted mtDNA copy number in J774A.1 macrophages, as well as depleted the protein amounts of cytochrome c oxidase 1 (mtCOI) which is encoded by the mitochondrial genome (Supplementary Fig. 3). The protein expression of cytochrome c, a mitochondria-related protein encoded by nuclear DNA, was not affected by EtBr (Supplementary Fig. 3). The J774A.1 macrophages conditioned with 100 ng/ml EtBr, designated as ρ0 cells, displayed reduced basal and rotenone (a potent mitochondrial ROS generator) -inducible mitochondrial ROS production as measured by MitoSOX fluorescence (Supplementary Fig. 4). EtBr treatment dose-dependently inhibited caspase-1 activation IL-1β cleavage, and the secretion of IL-1β and IL-18 in response to LPS and ATP treatment, but did not decrease amounts of pro IL-1β (Fig. 3a). We also observed that EtBr treatment did not alter IL-1β mRNA level either under basal conditions or after LPS stimulation (data not shown). At the time of maximal ATP-dependent activation of caspase-1 in LPS-primed macrophages, the ρ0 macrophages in contrast displayed nearly complete attenuation of the response (Fig. 3b). In contrast to inhibition of IL-1β secretion, the ρ0 phenotype did not decrease the secretion of TNF in response to LPS and ATP (Fig. 3c). In addition, neither secretion of cathepsin B (Fig. 3a), nor protein expression of ASC were affected by EtBr treatment (Fig. 3b). These data indicate that respiring mitochondria are specifically required for caspase-1 activation in response to LPS and ATP treatment in macrophages.
Fig. 3. EtBr abolishes caspase-1 activation.
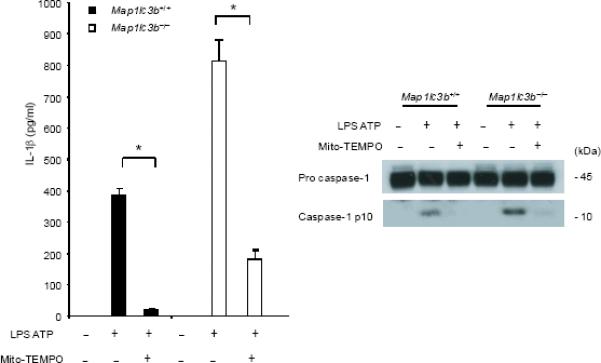
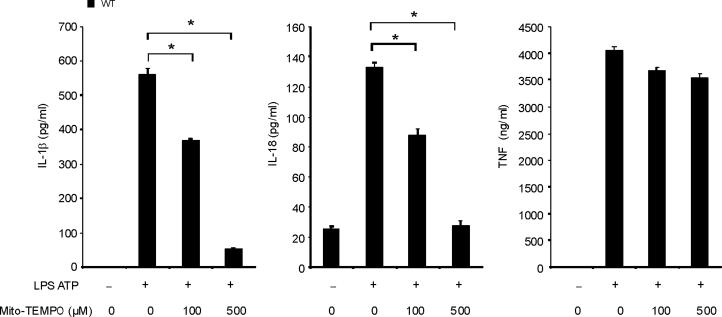
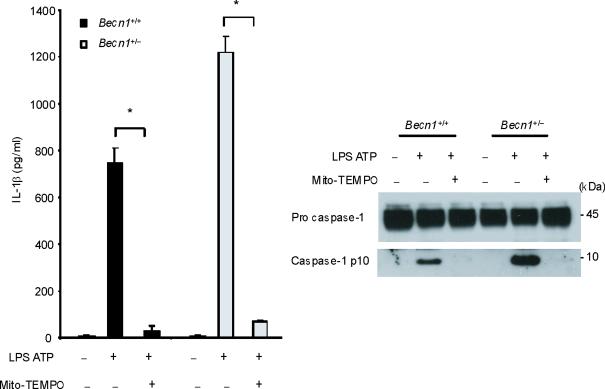
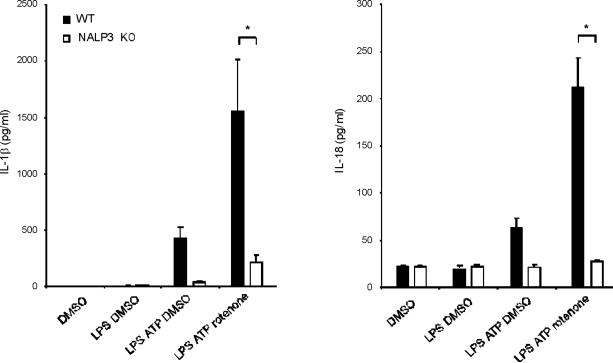
(a) J774A.1 macrophages exposed to EtBr (0, 10, 100 and 500 ng/ml) were stimulated with LPS and ATP. Cytokine secretion was analyzed by ELISA. Lysates and supernatants were immunoblotted for caspase-1 and IL-1β. (b) J774A.1 or ρ0 macrophages (EtBr; 100 ng/ml) were incubated with LPS, followed by ATP (15 min and 30 min). Lysates were immunoblotted for caspase-1 and ASC. (c) J774A.1 l or ρ0 macrophages were incubated with LPS, and ATP. Cytokine secretion was analyzed. (d) LPS-primed macrophages were incubated with rotenone (5 μM) or DMSO 1 h before ATP. Cytokine secretion was analyzed. Lysates were immunoblotted for caspase-1 and IL-1β. (e) J774A.1 or ρ0 macrophages were incubated with LPS and rotenone, followed by ATP. IL-1β secretion was analyzed by ELISA (f) Peritoneal macrophages pre-incubated with Mito-TEMPO for 1 h were stimulated with LPS and ATP. Cytokine secretion was analyzed. (g,h) Peritoneal macrophages isolated from Map1lc3b−/− (g) or Becn1+/− (h) mice were incubated with Mito-TEMPO (500 μM) for 1 h, followed by LPS and ATP. IL-1β secretion was analyzed by ELISA. Lysates were immunoblotted for caspase-1. *P < 0.05 (Student's t-test). Data represent three experiments (mean and s.d. in a,c,d,e,f,g,h).
Mitochondrial ROS and MPT regulate caspase-1
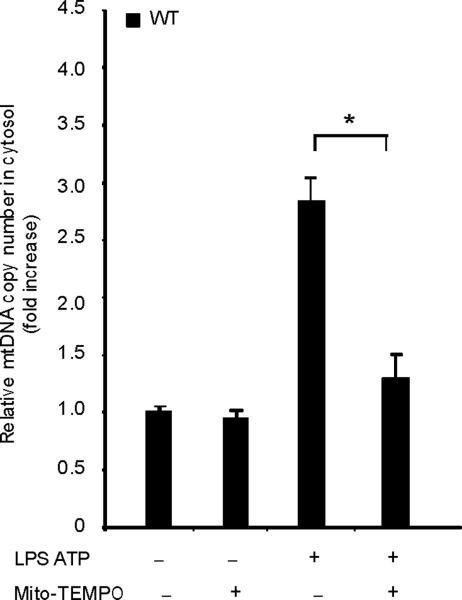
Previous studies using atg5 deleted mice have suggested a relationship between autophagic proteins and signaling through mitochondrial ROS generation in the context of viral infection13. We therefore examined the relationship between mitochondrial ROS and caspase-1 activity in macrophages stimulated with LPS and ATP. Using a mitochondrial complex I inhibitor rotenone, we observed that rotenone treatment strongly enhanced caspase-1 activation and IL-1β secretion in macrophages stimulated with LPS and ATP (Fig. 3d), whereas this compound did not effect TNF secretion. The potent enhancement of LPS and ATP-induced IL-1β secretion by rotenone was abolished in ρ0 macrophages (Fig. 3e). We next examined whether mitochondrial-specific ROS generation contributes to caspase-1 activation by LPS and ATP. Treatment of macrophages with the mitochondrial-targeted antioxidant Mito-TEMPO, a specific scavenger for mitochondrial ROS21,22, inhibited the secretion of IL-1β and IL-18 in a dose-dependent manner in BMDM, but did not affect TNF secretion in response to LPS and ATP (Fig. 3f). We also found that Mito-TEMPO did not inhibit IL-1β secretion induced by LPS and monosodium urate (MSU), which stimulates an alternate pathway to NALP3 inflammasome activation (Supplementary Fig. 5). Furthermore, the effect of Mito-TEMPO on IL-1β secretion by LPS and poly(dA:dT), representative of the absent in melanoma 2 (AIM2) inflammasome pathway23–25, was negligible compared to the effect observed in LPS and ATP model (Supplementary Fig. 5). Given the important role of mitochondrial ROS on caspase-1 activation induced by LPS and ATP in wild-type cells, we next examined the role of mitochondrial ROS on caspase-1 activation in autophagic protein-depleted macrophages. We observed that Mito-TEMPO abrogated the apparent increases in IL-1β secretion and caspase-1 activation in Map1lc3b−/− and Becn1+/− macrophages in response to LPS and ATP (Fig. 3g,h).
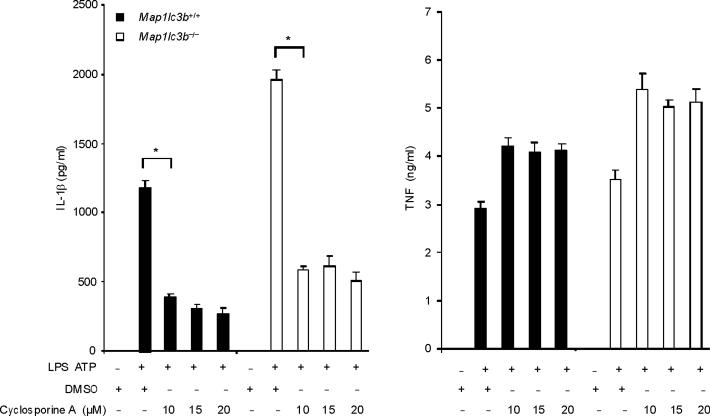
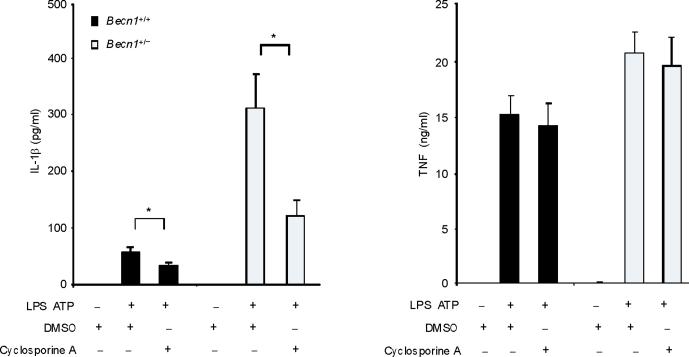
Mitochondrial ROS production is a prerequisite for mitochondrial membrane permeability transition (MPT) in several models26. Next we examined whether MPT is necessary for caspase-1 activation caused by LPS and ATP. Co-treatment with cyclosporine A, a potent inhibitor of MPT, inhibited the secretion of IL-1β, but not TNF in response to LPS and ATP in both wild-type macrophages and autophagy protein-depleted macrophages (Fig. 4a,b). Collectively, these data suggest that the stimulation of caspase-1 activation by LPS and ATP is dependent on mitochondrial ROS generation and MPT.
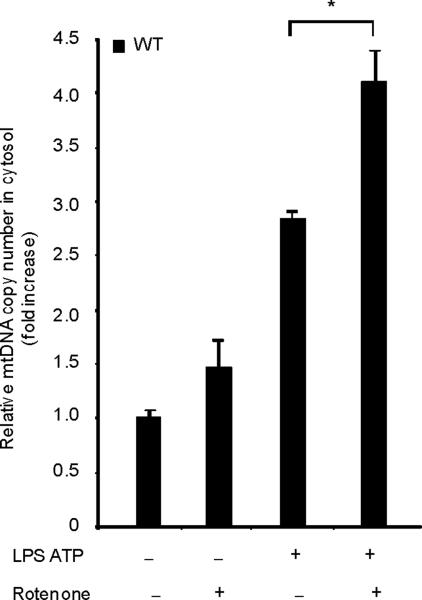
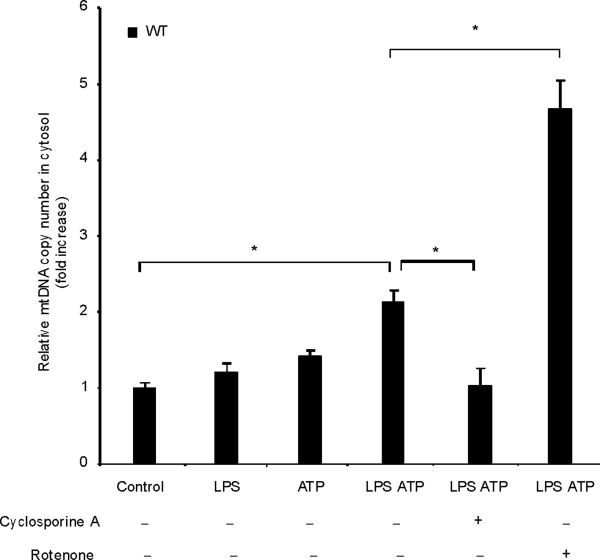
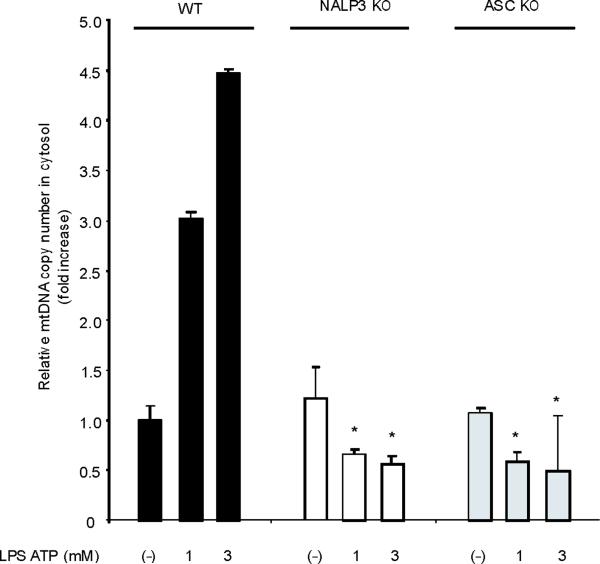
Fig. 4. Autophagic protein deficiency promotes mtDNA release into cytosol through increased MPT.
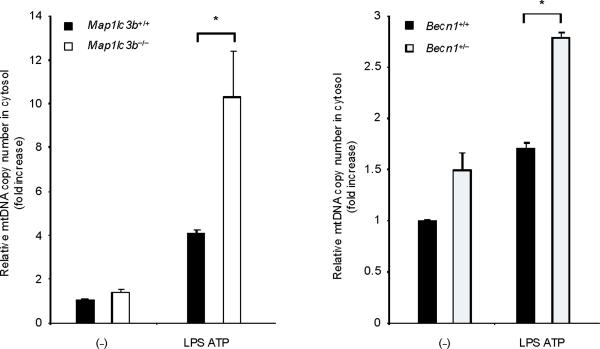
(a) LPS-primed BMDM from Map1lc3b+/+ or Map1lc3b−/− mice were co-incubated with cyclosporine A or DMSO, followed by ATP stimulation for 1 h. Cytokine secretion was analyzed by ELISA. (b) LPS-primed BMDM from Becn1+/+ or Becn1+/− mice were co-incubated with cyclosporine A (10 μM) or DMSO, followed by ATP stimulation. Cytokine secretion was analyzed by ELISA. (c) LPS-primed BMDM were incubated with rotenone (5 μM) or vehicle (DMSO) 1 h before ATP stimulation. Cytosolic mtDNA copy number was measured by quantitative PCR. (d) Macrophages pre-incubated with Mito-TEMPO (500 μM) for 1 h were incubated with LPS, followed by ATP stimulation. Cytosolic mtDNA copy number was measured by quantitative PCR. (e) LPS-primed BMDM were incubated with cyclosporine A (10 μM), rotenone (5 μM) or DMSO, followed by ATP stimulation. Cytosolic mtDNA copy number was measured by quantitative PCR. (f) LPS-primed BMDM from Map1lc3b−/− mice and Becn1+/− mice were stimulated with ATP for 30 min. Cytosolic mtDNA copy number was measured by quantitative PCR. Statistical significance was determined by the Student's t-test. *P < 0.05. Data are representative of three experiments (mean and s.d.).
Mitochondrial DNA translocates to the cytosol
MtDNA is a damage-associated molecular pattern when liberated into the extracellular space27. Since ρ0 macrophages, which are compromised for caspase-1 activation, are characterized not only by attenuation of respiratory chain activity and ROS production, but also by diminution of mtDNA content, we examined the potential role of mtDNA in caspase-1 dependent responses.
LPS and ATP induced the cytosolic accumulation of mtDNA in wild-type macrophages (Fig. 4c). The copy number of nuclear DNA (encoding 18S ribosomal RNA) in cytosol was not increased by LPS and ATP stimulation (data not shown). Rotenone treatment greatly augmented the copy number of cytosolic mtDNA in LPS and ATP treated cells (Fig. 4c). Furthermore, the mitochondria-targeted antioxidant Mito-TEMPO suppressed cytosolic mtDNA release triggered by LPS and ATP treatment (Fig. 4d). Inhibition of MPT by cyclosporine A treatment also decreased cytosolic mtDNA copy number during LPS and ATP treatment to control values (Fig. 4e). LPS and ATP treatment produced an approximately 2 fold greater degree of mtDNA translocation in Map1lc3b−/− and Becn1+/− macrophages compared to wild-type cells (Fig. 4f). These data indicate that increased activation of caspase-1 in autophagic-protein-deficient cells is accompanied by exaggerated mtDNA release into the cytosol, through increased mitochondrial membrane permeability. Furthermore, both caspase-1 activation and mtDNA release are dependent on mitochondrial ROS generation in our system.
Cytosolic mtDNA is a co-activator of caspase-1
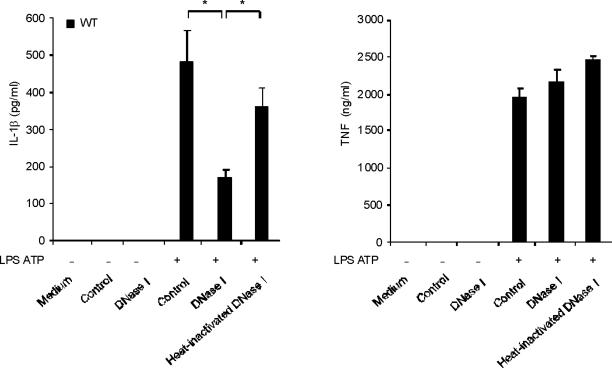
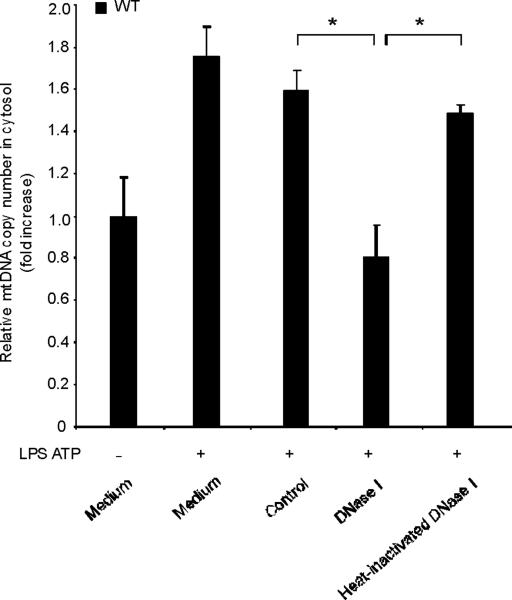
To examine the role of endogenous cytosolic mtDNA in caspase-1 activation during LPS and ATP stimulation, we transfected DNase1 into wild-type macrophages and then stimulated them with LPS and ATP. We observed that secretion of IL-1β was decreased in the cells transfected with DNase1, relative to cells transfected with control protein (LDH; lactate dehydrogenase) or with heat-inactivated DNase1 preparations (Fig. 5a). On the other hand, LPS and ATP-inducible TNF secretion was not sensitive to either DNase1 or control protein transfection (Fig. 5a). Under these conditions, DNase1 transfection effectively reversed the cytosolic accumulation of mtDNA induced by LPS and ATP, as validated by RT-PCR (Fig. 5b). Transfection of control protein or heat-inactivated DNase1 did not affect cytosolic mtDNA copy number during stimulation with LPS and ATP (Fig. 5b).
Fig. 5. Cytosolic mitochondrial DNA (mtDNA) is involved in caspase-1 activation.
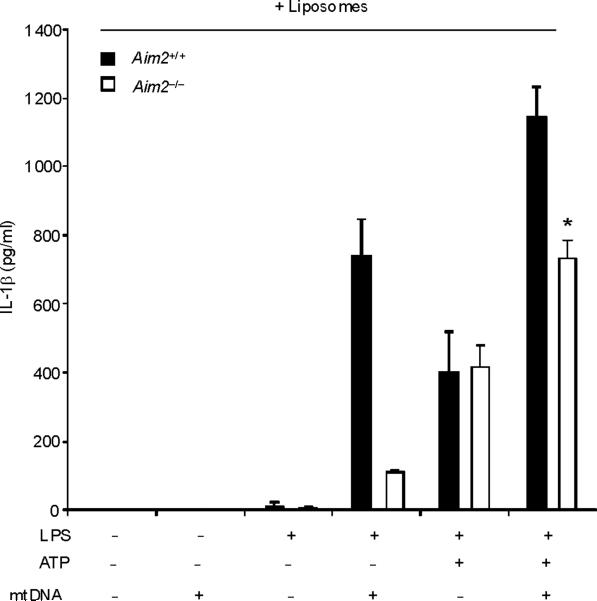
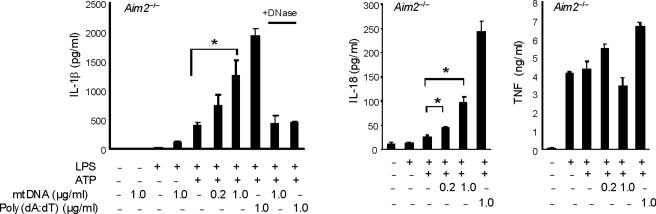
(a) BMDM were transfected with 3 μg of DNase, LDH or heat-inactivated DNase for 4 h and then stimulated with LPS, followed by ATP stimulation for 1 h. Cytokine secretion into supernatants was analyzed by ELISA. (b) BMDM were transfected with DNase, LDH or heat-inactivated DNase for 4 h and then stimulated with LPS, followed by ATP stimulation for 15 min. Cytosolic mtDNA copy number was measured by quantitative PCR. (c) BMDM from aim2+/+ or aim2−/− mice were primed with LPS (200 ng/ml) for 4 h, and then were transfected with 1 μg of mtDNA for 6 h, followed by stimulation with ATP for 1 h. IL-1β secretion was analyzed by ELISA. . *P < 0.05, versus aim2−/− cells treated with LPS and ATP (Student's t-test). (d) LPS-primed BMDM from aim2−/− mice were transfected with mtDNA, poly(dA:dT) or mtDNA pre-digested by DNase for 6 h, followed by stimulation with ATP for 1 h. Cytokine secretion was analyzed by ELISA. *P < 0.05 (Student's t-test). Data are representative of three experiments (mean and s.d.).
To directly test the bioactivity of mtDNA in isolation from other mitochondrial components, we next transfected macrophages with mtDNA in conjunction with LPS and ATP treatment. However, we recognized that transfection of exogenous DNA into macrophages was previously shown to activate the AIM2 inflammasome, a complex that is dispensable for the effects of LPS and ATP on caspase-1 activation (Fig. 5c)23–25. Therefore, we utilized aim2−/− macrophages to model the effects of mtDNA on caspase-1 activation without the effects of AIM2. In aim2+/+ macrophages, transfection of mtDNA produced enhancement of IL-1β secretion when given in addition to LPS and ATP (Figs. 5c). Approximately 50% of the enhancement on IL-1β secretion seen in wild-type macrophages after LPS, ATP and mtDNA treatment was preserved in aim2−/− cells (Fig. 5c). In the absence of ATP, as expected, IL-1β secretion by mtDNA transfection observed in aim2+/+ macrophages was impaired in aim2−/− cells (Fig. 5c), suggesting mtDNA transfection predominantly activates the AIM2 inflammasome in macrophages stimulated with LPS alone. Transfection of mtDNA also produced a dose-dependent enhancement of IL-18 secretion in aim2−/− cells stimulated with LPS and ATP, but not affect TNF production (Fig. 5d). Importantly, pretreatment with DNase1 abolished the stimulatory effects of our mtDNA preparation (Fig. 5d). Taken together, these data indicate that reducing cytosolic amounts of mtDNA coincide with an inhibition of caspase-1 activation by LPS and ATP treatment. Furthermore, we found that mtDNA can serve as an AIM2-independent co-activator of caspase-1 activity in conjunction with LPS and ATP.
NALP3 inflammasome mediates cytosolic mtDNA release
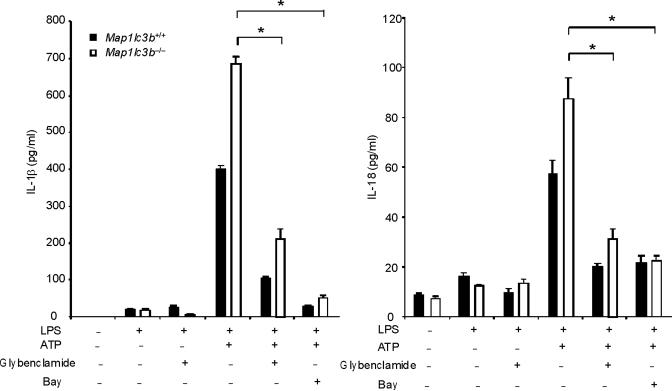
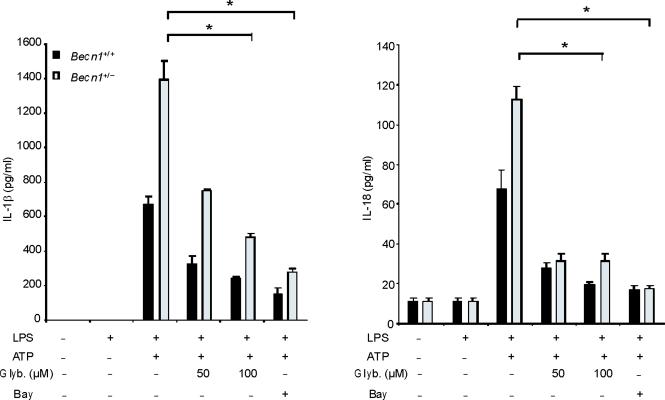
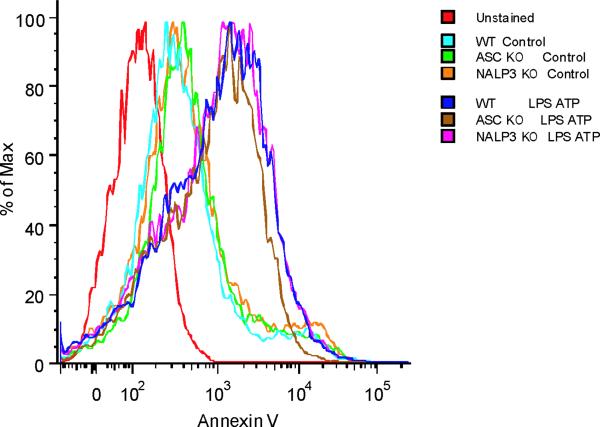
ROS generation activates the NALP3 inflammasome, a complex critical for the activation of caspase-1 by LPS and ATP28. Therefore, we examined the contribution of the NALP3 to the caspase-1 phenotype we observed in autophagic protein deficient macrophages. Glybenclamide and Bay 11-7082, inhibitors of NALP3 inflammasome activation29,30, suppressed increased secretion of IL-1β and IL-18 in Map1lc3b−/− and Becn1+/− macrophages in response to LPS and ATP (Fig. 6a,b), as well as in rotenone treated cells (Supplementary Fig. 6). Similarly, genetic deficiency of NALP3 abolished the effect of rotenone on the secretion of IL-1β and IL-18 (Fig. 6c), again suggesting that NALP3 is critically involved in mitochondrial ROS-mediated caspase-1 activation in macrophages. Since mitochondrial ROS also promotes mtDNA release, we tested whether NALP3 is involved in mtDNA release. We observed that NALP3 deficiency suppressed mtDNA release in cytosol in response to LPS and ATP treatment (Fig. 6d). Similar results were found in macrophages deficient in ASC (asc−/−) (Fig. 6e). We also observed that release of cytochrome c was mildly reduced in NALP3-deficient (nlrp3−/−) cells after the stimulation (Fig. 6d). Importantly, mitochondrial ROS generation by LPS and ATP was not affected by deficiency of NALP3 (Fig. 6f), suggesting that NALP3 is not required for mitochondrial ROS generation by LPS and ATP treatment. However, deletion of nlrp3 inhibited the loss of mitochondrial membrane potential in response to LPS and ATP treatment (Fig. 6g). These results could not be explained by a lower rate of apoptosis in nlrp3−/− macrophages compared to wild-type cells, since neither nlrp3−/− nor asc−/− macrophages displayed a difference in the overall apoptotic response to LPS and ATP stimulation, as determined by Annexin-V staining (Fig. 6h). Collectively, our data demonstrated that the increased caspase-1 activation in autophagic protein depleted cells requires the activation of the NALP3 inflammasome. Moreover, the NALP3 inflammsome is required for MPT and subsequent mtDNA translocation to the cytosol in response to LPS and ATP.
Fig. 6. NALP3 mediates mitochondrial DNA release.
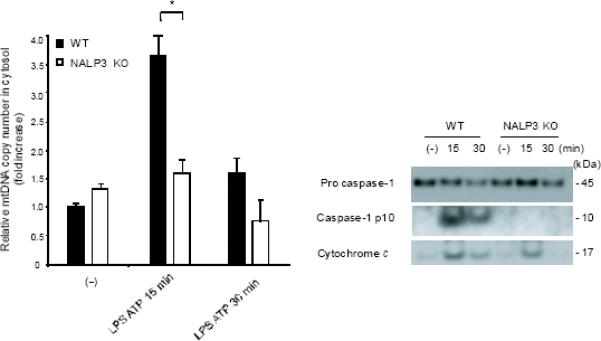
(a) LPS-primed peritoneal macrophages from Map1lc3b−/− (a) or Becn1+/− (b) mice were incubated with glybenclamide (50–100 μM), Bay 11-7082 (12 μM) or DMSO (15 min), followed by ATP. Cytokine secretion was analyzed. (c) LPS-primed nlrp3−/− macrophages were incubated with rotenone, followed by ATP (1 h). Cytokine secretion was analyzed. (d) LPS-primed nlrp3−/− BMDM were stimulated with ATP (15 or 30 min). Cytosolic mtDNA copy number was measured by qPCR. Cytosolic cytochrome c and caspase-1 activation was analyzed by immunoblotting. (e) LPS-primed nlrp3−/− or asc−/− BMDM were stimulated with ATP (15 min). Cytosolic mtDNA copy number was measured by qPCR. *P < 0.05, nlrp3−/− or asc−/− versus WT cells treated with LPS and ATP (Student's t-test). (f) LPS-primed macrophages were stained with MitoSOX prior to ATP (15 min), and analyzed by flow cytometry. Representative histograms are shown. (g) Macrophages stimulated with LPS and ATP were stained with MitoTracker Deep Red. Representative cytometry plots are shown. (h) LPS-primed peritoneal macrophages from asc−/− and nlrp3−/− mice were stimulated with ATP (30 min), and analyzed by Annexin V flow cytometry. Data represent three experiments (mean and s.d. in a,b,c,d,e).
LC3B-deficiency increases susceptibility to LPS
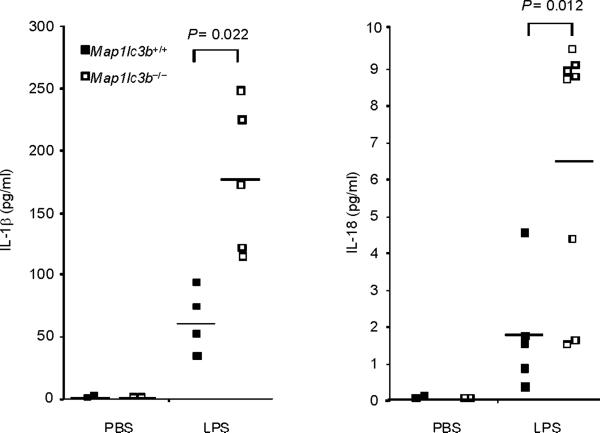
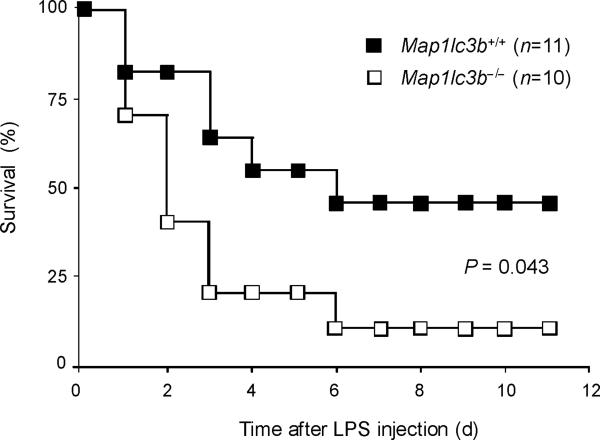
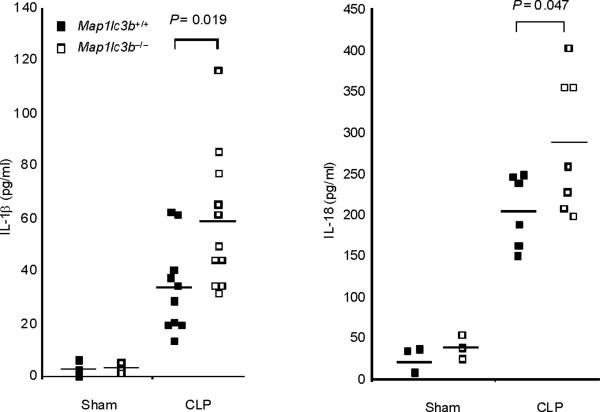
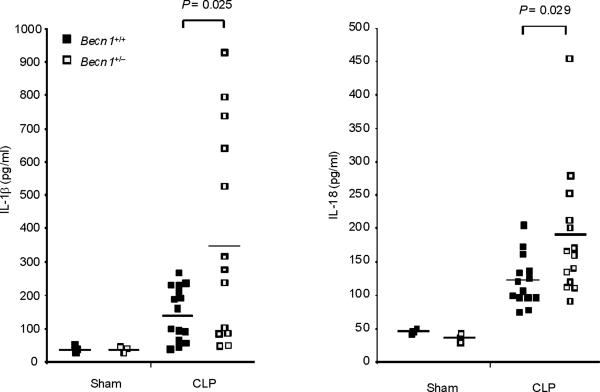
Finally, we tested the in vivo relevance of autophagic regulation of caspase-1 activity by using two different animal models of sepsis. We used endotoxemic mice which are commonly used as a septic shock model. We also examined the role of autophagic proteins on caspase-1-mediated inflammation in a cecal ligation and puncture (CLP) model which is a clinically more relevant polymicrobial sepsis model. First, we induced endotoxemia in Map1lc3b−/− mice. Consistent with observations from macrophages, serum concentrations of IL-1β and IL-18 were significantly higher in endotoxemic Map1lc3b−/− mice, compared to wild-type littermates (Fig. 7a), whereas TNF production was comparable (Supplementary Fig. 7). Importantly, Map1lc3b−/− mice were more susceptible to LPS-induced lethality than Map1lc3b+/+ mice. At 6 days post-injection, 90% of Map1lc3b−/− mice had died, whereas 50% of Map1lc3b+/+ mice survived longer than 10 days (Fig. 7b). Similar to the observations in the septic shock model, Map1lc3b−/− mice produced significantly more serum concentrations of not only IL-1β but also IL-18 in response to CLP-induced sepsis (Fig. 7c). Moreover, serum concentrations of IL-1β and IL-18 were higher in Becn1+/− mice relative to wild-type mice after CLP treatment (Fig. 7d). To correlate our findings in mice to patients with sepsis, we analyzed cytokine secretion in patients admitted to the Medical Intensive Care Unit (MICU). As the role of IL-1β in human sepsis has been investigated previously, we focused on IL-18 whose role in sepsis is not fully characterized31. We observed significantly increased IL-18 amounts in septic MICU patients compared to control patients who were admitted to the MICU for reasons unrelated to acute infection or inflammation (Supplementary Figure 8, Supplementary Table 1). IL-18 amounts were not significantly elevated in patients with Systemic Inflammatory Response Syndrome (SIRS) in the absence of sepsis. Taken together, our data demonstrate that autophagic regulation of cytokine production can occur in vivo, leading to regulation of circulating IL-1β and IL-18 levels. Moreover elevated IL-18 amounts, one of the products of caspase-1 activity, is specifically associated with severe sepsis in humans.
Fig. 7. Autophagic protein deficiency augments caspase-1-mediated inflammatory responses in vivo.
(a,b) Male map1lc3b+/+ and map1lc3b−/− mice were intraperitoneally injected with LPS (12 mg/kg). (a) Serum IL-1β and IL-18 at 24 h after the injection was analyzed by ELISA. (b) Survival of map1lc3b+/+ (n =11) and map1lc3b−/− mice (n =10) after LPS challenge. Mice were monitored over a period of 2 weeks. (c,d) Male map1lc3b−/− mice (c) and becln1+/− (d) mice were treated with cecal ligation and puncture (CLP) or with sham-laparotomy (Sham). Serum IL-1β and IL-18 at 24 h after CLP or sham-operation was analyzed by ELISA. In a,c,d, each symbol represents an individual mouse; small horizontal lines indicate the mean. P values, unpaired two-tailed Student's t-test (a,c,d) or Log-rank test; (b) Data are representative of two experiments.
DISCUSSION
In this study we found that Beclin 1 and LC3B, autophagic proteins required in the initial and late phases of autophagosome formation, act as critical regulators of caspase-1-mediated immune responses in vitro and in vivo. The mechanism by which these autophagic proteins regulate caspase-1 activation is related to their positive contributions to mitochondrial quality control. We observed that autophagic protein depleted macrophages accumulate physiologically abnormal mitochondria, which produce elevated amounts of ROS at baseline. These abnormal mitochondria are prone to undergo more severe structural derangement and dysfunction after LPS and ATP treatment. The changes to mitochondria after LPS and ATP treatment are important because we observed that the loss of mitochondrial integrity was critical for the enhanced activation of caspase-1, both in wild-type macrophages and in autophagy protein depleted (Map1lc3b−/− and Becn1+/−) macrophages. The increased mitochondrial ROS generation produced by Map1lc3b−/− and Becn1+/− macrophages was sufficient to explain their increased efficiency of caspase-1 activation in our model. As a consequence of mitochondrial ROS and subsequent MPT, we found that LPS and ATP treatment released mtDNA into the cytosolic compartment. Translocation of mtDNA into the cytosol required NALP3 inflammsome activation, and directly contributed to downstream activation of caspase-1 in response to LPS and ATP treatment. Importantly, we found that Map1lc3b−/− and Becn1+/− macrophages released mtDNA in excess quantities into the cytosol in response to LPS and ATP treatment, further explaining the increased caspase-1 activation and IL-1β and IL-18 secretion observed in these cells. While prior reports have described the important role for autophagy in mitochondrial homeostasis13,32, our results clarify the molecular link between mitochondrial quality control, as regulated by autophagy, and NALP3-dependent activation of caspase-1. We also identified endogenous cytosolic mtDNA as a novel intracellular second-messenger molecule that connects mitochondrial dysfunction to caspase-1 activation.
Mitochondrial ROS generation was a relatively upstream mechanistic step in our system, as treatment with Mito-TEMPO abolished subsequent caspase-1 activation and mtDNA translocation in response to LPS and ATP. The production of mitochondrial ROS was likely a consequence of ATP-driven activation of the P2X7 receptor33, since this was previously shown to induce mitochondrial damage34,35, and also to contribute to NALP3 inflammasome assembly9. Interestingly, we found that Mito-TEMPO did not inhibit IL-1β secretion induced by LPS and MSU. Crystalline particles such as asbestos, silica and MSU have previously shown to activate the NALP3 inflammasome, through ROS generation by phagocytic NADPH oxidase28,36. Thus, while there appears to be a general requirement for ROS in NALP3 inflammasome activation, the subcellular source ROS may differ between stimuli.
Based on previous studies6,9, we expected that NALP3 would be situated downstream of both mitochondrial ROS and cytosolic mtDNA release in the caspase-1 activation pathway. However, our results indicate that NALP3 is required at a step upstream of MPT and mtDNA release but downstream of mitochondrial ROS production in response to LPS and ATP treatment. Since deletion of the NALP3 binding partner ASC produced a similar attenuation of the response, it is likely that the NALP3 inflammasome complex is responsible for mediating MPT in our model. At present, it is unclear whether the NALP3 inflammasome is additionally responsible for more distal steps in the caspase-1 activation pathway after LPS and ATP treatment. Further research will be needed to clarify this point.
mtDNA stimulates inflammatory responses through TLR9 when released into plasma in human as a consequence of mechanical trauma27. We showed that mtDNA also mediates inflammatory responses in the intracellular space through the activation of caspase-1 in response to LPS and ATP, a NALP3 inflammsome model. Multiple groups have shown that introduction of exogenous or foreign DNA into the cytosol activates caspase-1 via the AIM2 inflammsome23,24. However, the contribution of endogenous mtDNA to caspase-1 regulation has not previously been described. Our conclusion that mtDNA is important for caspase-1 activation, in response to LPS and ATP, is based on several results. First, respiration-deficient ρ0 cells, which are also depleted for mtDNA content, produced markedly less IL-1β and IL-18 secretion when challenged with LPS and ATP. Second, transfection of DNase1 into the cytoplasm, which reduced mtDNA copy number in the cytosol, attenuated IL-1β secretion after LPS and ATP treatment in macrophages. Since nuclear DNA does not translocate to the cytoplasm after LPS and ATP treatment, the inhibitory effect of DNase1 on caspase-1 dependent cytokine secretion correlates with its ability to degrade mtDNA in the cytosol.
Third, direct transfection of mtDNA into aim2−/− macrophages, where the dominant AIM2-dependent inflammasome response to exogenous DNA in cytosol has been removed, nevertheless enhanced caspase-1 dependent cytokine secretion in response to LPS and ATP. Cytosolic mtDNA was insufficient to stimulate IL-1β and IL-18 secretion on its own but required at a minimum the presence of LPS6,7. This fact may explain why Map1lc3b−/− and Becn1+/− macrophages do not constitutively secrete elevated amounts of IL-1β and IL-18 on the basal condition, in spite of the fact that they have mildly elevated amounts of mtDNA in the cytoplasm at baseline. While the AIM2 inflammasome clearly plays a dominant role in response to transfected DNA and particular intracellular pathogens23,24, it is worth noting that other cytosolic DNA sensors potentially could serve as the dominant receptors for cytosolic mtDNA in our model37,38. Further research will be needed to clarify the downstream mechanism by which cytosolic mtDNA contributes to caspase-1 activation during LPS and ATP stimulation.
In this study, we showed the physiological relevance of autophagic proteins in regulating caspase-1-mediated inflammatory responses in two animal models of sepsis: the endotoxic shock model and the CLP model of polymicrobial sepsis. In addition, our clinical data confirmed that circulating plasma levels of IL-18 were increased in patients with sepsis. Marked autophagic vacuolization has been observed in liver biopsies obtained from patients who died of sepsis39. However, it is unclear whether this observation represented increased autophagic activity in septic patients or downstream inhibition of autophagy that led to the inappropriate accumulation of autophagosomes. Since sepsis has long been recognized to produce mitochondrial dysfunction in humans40,41, further research is needed to investigate whether autophagy is dysregulated by septic conditions and as such contributes to sepsis-induced mitochondrial dysfunction. In conclusion, the present study provides a mechanistic explanation for how autophagy, a core cellular housekeeping process, modulates caspase-1 mediated immune responses. Agents that stimulate autophagic activity may confer beneficial anti-inflammatory effects through the improvement of mitochondrial quality control and the removal of molecules such as mitochondrial ROS and cytosolic mtDNA. It might be useful to investigate the potential for therapeutic targeting of autophagic processes to mitigate inflammatory diseases.
ONLINE METHODS
Mice
Generation of Map1lc3b−/−, Becn1+/−, nlrp3−/− and aim2−/− mice were as described previously9,11,23,42. Animal care and use for all experiments was approved by the Harvard Medical Area Standing Committee on Animals, Harvard Medical School.
Reagents
The following antibodies were used: rabbit anti-mouse caspase-1 (sc-514; Santa Cruz Biotechnology), rabbit anti-mouse IL-1β (5129-100; Biovision), rabbit anti-LC3B (L7543; SIGMA), anti-mouse mitochondria COI (ab14705; abcam). Z-YVAD-FMK, Mito-TEMPO, and MSU were from ALEXIS. LPS (E. Coli) was from Invivogen. ATP, ethidium bromide (EtBr), cyclosporine A, glibenclamide, Bay 11-7082, poly(dA:dT) and rotenone were from Sigma. DNase1 was from Invitrogen.
Flow cytometric analysis
Mitochondrial ROS was measured in cells by MitoSOX (Invitrogen) staining (5 μM for 15 min at 37°C)13. To measure mitochondrial mass, cells were stained with 25 nM of MitoTracker Green FM and MitoTracker Deep Red FM (Invitrogen) (15 min at 37°C)13. Cells were washed with PBS, trypsinized and resuspended in PBS containing 1% heat inactive FBS. Apoptotic cells were detected using Annexin V (BioVision) at 10 μl/ml for 5 min. Data were acquired with a BD FACS Canto II flow cytometer (BD Biosciences) and analyzed with FlowJo analytical software (Treestar).
Cell isolation and culture
Bone marrow-derived macrophages (BMDM) were prepared as described9. Bone marrow collected from mouse femurs and tibias were plated on sterile petri dishes and incubated in DMEM containing 10% heat-inactive fetal calf serum (FCS), penicillin and streptomycin and 25% of L929-conditioned medium for 7 days. Cells were incubated with LPS (10 ng/ml) for 6 h and treated with ATP (1 mM) as described9. Peritoneal macrophages were elicited with 1 ml of 4% (vol/vol) thioglycollate solution and were collected by peritoneal lavage 4 days later43. Adherent macrophages were selected after 2 h. Cells were incubated with LPS (500 ng/ml) for 4 h, followed by treatment with ATP (5 mM)8.
Immunoblot analysis
Electrophoresis of proteins was performed as described44. Lysates were boiled 5 min in NuPAGE sample loading buffer (Invitrogen). Proteins were separated on NuPAGE 4–12% Bis-Tris Gels and transferred to nitrocellulose membranes by electroblotting.
ELISA
Mouse cytokines in culture supernatants or serum were measure by ELISA kits (R&D Systems). Human IL-18 in plasma was measured by ELISA (MBL).
Transmission electron microscopy
BMDM were fixed in 2.5% glutaraldehyde after stimulation by LPS and ATP as described before45.
Quantitative real time PCR
Total DNA was isolated from cells by using DNeasy Blood & Tissue Kit (Qiagen)27. Relative mitochondrial DNA level was measured by performing quantitative PCR using SYBR Green PCR Master Mix (Applied Biosystems). Two independent reactions were performed using established primers for mitochondrial and nuclear genes. MtDNA copy number was measured by quantitative PCR and normalized to nuclear DNA levels in a ratio of cytochrome c oxidase 1 (mtCOI) DNA over nuclear DNA (encoding 18S ribosomal RNA)13,46. The following primers were used: 18S forward, 5'-TAGAGGGACAAGTGGCGTTC-3'; 18S reverse, 5'-CGCTGAGCCAGTCAGTGT-3'; and mouse COI forward, 5'-GCCCCAGATATAGCATTCCC-3'; mouse COI reverse, 5'-GTTCATCCTGTTCCTGCTCC-3'.
For measurement of mtDNA in cytosol, 1× 107 of cells were Dounce homogenized in 100 mM Tricine-NaOH solution, pH 7.4 containing 0.25 M sucrose, 1 mM EDTA and protease inhibitor, and centrifuged at 700 g for 10 min at 4°C. The protein concentration and volume of the supernatant was normalized, followed by centrifugation at 10,000 g for 30 min at 4°C to produce a supernatant corresponding to the cytosolic fraction. DNA was isolated from 200 μl of the cytosolic fraction as described above. Copy number of mtCOI DNA was measured by quantitative real time PCR using same volume of the DNA solution.
Generation of mitochondrial DNA (mtDNA) deficient (ρ0) cells
J774A.1 macrophages were grown in DMEM supplemented with 10% FCS, pyruvate (100 μg/ml) and uridine (50 μg/ml) as described14,18. EtBr (100 ng/ml) was added to the medium for 2 weeks. Depletion of mitochondrial DNA was evaluated by quantitative real time PCR as described above. To activate caspase-1, the cells were incubated with LPS (100 ng/ml) for 4 h after changing the media with fresh complete media containing pyruvate and uridine, and then stimulated with ATP (5 mM)47.
Protein transfection
BMDM prepared at 70–80% confluent in 24 wells were washed with warm DMEM without FBS twice, and were transfected with 3 μg of DNase1 or LDH by using PULSin™ Reagent (Polyplus transfection™) for 4 h at 37°C. After removing the media, cells were incubated in complete growth media for 2 h, and then primed with LPS (4 h), followed by stimulation with ATP.
MtDNA isolation and transfection
Mouse mtDNA was isolated from thioglycollate-elicited mouse peritoneal macrophages using mitochondrial DNA isolation kit (Biovison). Peritoneal macrophages were incubated with 200 ng/ml of LPS 4 h, and then were transfected with the isolated mtDNA for 6 h using Lipofectamine 2000 (Invitrogen) as described23.
RNA interference
siRNA duplexes were from Dharmacon. Macrophages were plated on 24-well plates at 3 × 105 cells per well and transfected with 75 ng of siRNA duplex per well using HiPerFect Transfection Reagent (Qiagen).
In vivo experiments
Male mice (8–10 weeks old) were injected i.p. with 12 mg/kg of E. Coli LPS. CLP was performed as described48. The mouse cecum was exposed through a 1.5-cm incision. The cecum was ligated below the ileocecal valve with a 6-0 silk suture without causing bowel obstruction and then punctured with a 19-gauge needle (1 hole injury). Sham-operated mice underwent the same procedure without ligation and perforation of the cecum. After surgery, the mice were injected with 1 ml of physiologic saline solution subcutaneously.
Human Study
The Research Registry and Human Sample Repository for the Study of the Biology of Critical Illness (abbreviated name: Registry of Critical Ilnness (RoCI)) collects demographic, clinical information and blood specimens from patients with critical illness in the medical intensive care unit of the Brigham and Women`s Hospital (BWH). RoCI is approved by Partners Human Research Committee and operates under 2008-P-000495 protocol.
Statistical Analysis
Statistical analysis was performed using Student's t test. For the survival study, we performed the analysis by Log-rank test. Values of P < 0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to B. Levine for providing the Becn1+/− mice. We thank Emeka Ifedigbo for technical assistance. This work was supported by National Institutes of Health (NIH) grants HL079904-12, HL08554, HL060234-10 and HL097005 to A.M.K.C, by a postdoctoral fellowship from the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (NERCE; NIH/NIAID AI057159) to V.A.K.R and by NIH grants AI083713 and AI067497 to K.A.F.
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–70. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 5.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–11. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 7.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 9.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Aoki H, et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy. 2008;4:467–75. doi: 10.4161/auto.5668. [DOI] [PubMed] [Google Scholar]
- 11.Qu X, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–25. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 13.Tal MC, et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–5. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–25. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 15.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–68. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 16.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hashiguchi K, Zhang-Akiyama QM. Establishment of human cell lines lacking mitochondrial DNA. Methods Mol Biol. 2009;554:383–91. doi: 10.1007/978-1-59745-521-3_23. [DOI] [PubMed] [Google Scholar]
- 19.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–3. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 20.Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–6. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 21.Trnka J, Blaikie FH, Logan A, Smith RA, Murphy MP. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic Res. 2009;43:4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–17. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–93. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones JW, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–6. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 29.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juliana C, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahy RJ, et al. Inflammasome mRNA expression in human monocytes during early septic shock. Am J Respir Crit Care Med. 2008;177:983–8. doi: 10.1164/rccm.200703-418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen M, et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A. 2010;107:832–7. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz CM, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–9. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adinolfi E, et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–72. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Marcos M, et al. Role of sodium in mitochondrial membrane depolarization induced by P2X7 receptor activation in submandibular glands. FEBS Lett. 2005;579:5407–13. doi: 10.1016/j.febslet.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 36.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–7. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–30. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe E, et al. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab Invest. 2009;89:549–61. doi: 10.1038/labinvest.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredriksson K, et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One. 2008;3:e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.d'Avila JC, et al. Sepsis induces brain mitochondrial dysfunction. Crit Care Med. 2008;36:1925–32. doi: 10.1097/CCM.0b013e3181760c4b. [DOI] [PubMed] [Google Scholar]
- 42.Cann GM, et al. Developmental expression of LC3alpha and beta: absence of fibronectin or autophagy phenotype in LC3beta knockout mice. Dev Dyn. 2008;237:187–95. doi: 10.1002/dvdy.21392. [DOI] [PubMed] [Google Scholar]
- 43.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–18. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 44.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–72. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 45.Chen ZH, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eaton JS, Lin ZP, Sartorelli AC, Bonawitz ND, Shadel GS. Ataxiatelangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J Clin Invest. 2007;117:2723–34. doi: 10.1172/JCI31604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180:7147–57. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 48.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–47. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.