Abstract
Context
Despite the high morbidity associated with bipolar disorder (BP), few studies have prospectively studied the course of this illness in youth.
Objective
To assess the longitudinal course of BP spectrum disorders (BP-I, BP-II, and not otherwise specified [BP-NOS]) in children and adolescents.
Design
Subjects were interviewed, on average, every 9 months for an average of 2 years using the Longitudinal Interval Follow-up Evaluation.
Setting
Outpatient and inpatient units at 3 university centers.
Participants
Two hundred sixty-three children and adolescents (mean age, 13 years) with BP-I (n = 152), BP-II (n = 19), and BP-NOS (n = 92).
Main Outcome Measures
Rates of recovery and recurrence, weeks with syndromal or subsyndromal mood symptoms, changes in symptoms and polarity, and predictors of outcome.
Results
Approximately 70% of subjects with BP recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes. Analyses of weekly mood symptoms showed that 60% of the follow-up time, subjects had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of polarity, and 3% of the time, psychosis. Twenty percent of BP-II subjects converted to BP-I, and 25% of BP-NOS subjects converted to BP-I or BP-II. Early-onset BP, BP-NOS, long duration of mood symptoms, low socioeconomic status, and psychosis were associated with poorer outcomes and rapid mood changes. Secondary analyses comparing BP-I youths with BP-I adults showed that youths significantly more time symptomatic and had more mixed/cycling episodes, mood symptom changes, and polarity switches.
Conclusions
Youths with BP spectrum disorders showed a continuum of BP symptom severity from subsyndromal to full syndromal with frequent mood fluctuations. Results of this study provide preliminary validation for BP-NOS.
There has been steadily growing interest in the clinical and public health implications of bipolar disorder (BP) affecting children and adolescents. Current literature depicts the disease as devastating with substantial impairment across psychosocial domains, high risk of suicide, psychosis, significant familial aggregation, and protracted illness course in which the classically described cycles of disease followed by well periods are rarely observed.1–16
To date, few prospective studies, including clinical samples8,11–14 and a single community study,9,10 have investigated the outcome of pediatric BP. The duration of these studies varies from 1 to 5 years, with most cohorts showing 70% to 100% recovery with high rates of recurrences (≤80%), hospitalizations, psychosis, suicide attempts and completion, and poor psychosocial functioning. Retrospective studies that have followed up youth with BP for longer periods have shown similar findings.4,5,15–20
At present, it is not known if onset of BP early in life is characterized by similar or fundamentally different long-term patterns of disease progression compared with adult-onset BP.21 The pediatric samples followed up have been of small to modest sizes, and subjects have been followed up infrequently or for relatively brief periods. Other pediatric studies have been retrospective in nature or have relied on medical record reviews for depicting the follow-up course rather than direct clinical interviews. So far, no study has prospectively collected syndromal and subsyndromal course data on children and adolescents representing the full spectrum of BP phenotypes, in particular BP not otherwise specified (BP-NOS).22
This report describes the early clinical course and some relevant predictors of outcome of children and adolescents with bipolar I disorder (BP-I), BP-II, and BP-NOS recruited as part of a multisite (University of Pittsburgh, Brown University, and University of California at Los Angeles), collaborative study funded by the National Institute of Mental Health, the Course and Outcome of Bipolar Illness in Youth (COBY). The COBY study constitutes the largest pediatric BP cohort study to date, and it is the first prospective naturalistic study involving children and adolescents with BP spectrum disorders (BP-I, BP-II, and BP-NOS).
Subsequent reports will describe in more detail the clinical picture of children and adolescents with BP spectrum disorders, psychosocial course, and effects of other potential predictors of outcome such as suicidal behaviors, comorbid disorders, family psychiatric history, and treatment.
METHODS
SUBJECTS
Children and adolescents aged 7 to 17 years 11 months (mean ± SD age, 13.0 ± 3.1 years) whose primary diagnoses were DSM-IV23 BP-I or BP-II or an operationalized definition of BP-NOS were enrolled in the COBY study. Because the DSM-IV definition of BP-NOS is vague, BP-NOS was defined as the presence of clinically relevant BP symptoms that did not fulfill the DSM-IV criteria for BP-I or BP-II. In addition, subjects were required to have a minimum of elated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in the level of functioning, duration of a minimum of 4 hours within a 24-hour period, and at least 4 cumulative lifetime days meeting the criteria.
Subjects with current or lifetime diagnoses of schizophrenia, mental retardation, autism, and mood disorders secondary to substance abuse, medical conditions, or use of medications were excluded.
Subjects were recruited from consecutive admissions to outpatient clinics (65%), inpatient units (16%), advertisement (11%), and referrals from other physicians (8%) and were enrolled independent of current BP state or treatment status.
The analyses presented in this report are based on the prospective assessment of psychiatric symptoms for 263 subjects, including 151 (57%) with BP-I, 20 (8%) with BP-II, and 92 (35%) with BP-NOS who had at least 1 follow-up assessment. To date, these subjects have been prospectively interviewed every 35.5 weeks (SD, 8.5 weeks) for a mean ± SD of 94.8 ± 51.5 weeks.
As depicted in Table 1, at intake subjects with BP-I and BP-NOS were slightly younger than those with BP-II, and more BP-NOS subjects were prepubertal (all comparisons, P≤.05). Fewer BP-I subjects were living with both biological parents than BP-II and NOS subjects. Subjects with BP-II had the onset of their mood disorders significantly later and had significantly lower rates of comorbid attention-deficit/hyperactivity disorder than subjects with BP-I and BP-II (P≤.05). Subjects with BP-I had significantly more lifetime psychosis than those with BP-NOS (P = .002). There were no other differences among the 3 BP subtypes with regard to demographics or clinical characteristics, and there were no differences among groups regarding family history of mood disorders in first- and second-degree relatives.
Table 1.
Demographic and Clinical Characteristics Among Subjects With BP-I, BP-II, and BP-NOS*
| Characteristics | All Subjects (N = 263) |
BP-I (n = 152) |
BP-II (n = 19) |
BP-NOS (n = 92) |
Statistical Analysis |
P Value |
|---|---|---|---|---|---|---|
| Age, mean ± SD, y | 13.0 ± 3.1 | 13.2 ± 3.0a | 15.1 ± 2.4b | 12.1 ± 3.1c | F = 9.0 | <.001 |
| Male, % | 54.8 | 52.6 | 36.8 | 62.0 | χ2 = 4.7 | NS |
| White, % | 87.1 | 86.8 | 89.5 | 87.0 | χ2 = 0.1 | NS |
| SES, mean ± SD | 3.5 ± 1.2 | 3.6 ± 1.3 | 3.9 ± 0.9 | 3.3 ± 1.2 | K-W, 4.1 | NS |
| Living with both natural parents, % | 44.5 | 38.2a | 68.4b | 50.0ab | χ2 = 8.0 | .02 |
| Pubertal status, % at | 29/26/45 | 24/28/47 | 6/28/67 | 42/23/35 | χ2 = 13.0 | .01 |
| Tanner stage I/II–III/IV–V | ||||||
| Mood disorder, mean ± SD | ||||||
| Age of onset, y | 8.9 ± 3.9 | 9.0 ± 4.1a | 11.0 ± 3.4b | 8.4 ± 3.5a | K-W, 6.8 | .03 |
| Duration, y | 4.2 ± 2.9 | 4.4 ± 3.1 | 4.1 ± 2.4 | 3.8 ± 2.7 | K-W, 1.4 | NS |
| Lifetime comorbid diagnoses, % yes | ||||||
| Psychosis | 33.1 | 41.4a | 21.1ab | 21.7b | χ2 = 11.4 | .003 |
| ADHD | 58.6 | 61.2a | 31.6b | 59.8a | χ2 = 6.2 | .05 |
| ODD | 39.2 | 43.4 | 15.8 | 37.0 | χ2 = 5.7 | NS |
| Conduct disorder | 12.5 | 12.5 | 5.3 | 14.1 | χ2 = 1.1 | NS |
| Anxiety disorder | 38.4 | 40.8 | 42.1 | 33.7 | χ2 = 1.3 | NS |
| Substance use disorder | 9.1 | 9.9 | 5.3 | 8.7 | χ2 = 0.5 | NS |
| Family history, % | ||||||
| First-degree relative with mania | 27.6 | 29.5 | 26.3 | 24.7 | χ2 = 0.6 | NS |
| First-degree relative with depression | 69.7 | 67.1 | 84.2 | 70.8 | χ2 = 2.4 | NS |
| Second-degree relative with mania | 32.9 | 32.2 | 50.0 | 30.7 | χ2 = 2.6 | NS |
| Second-degree relative with depression | 68.3 | 68.5 | 88.9 | 63.6 | χ2 = 4.4 | NS |
Abbreviations: ADHD, attention-deficit/hyperactive disorder; BP-I, bipolar I disorder; BP-II, bipolar II disorder; BP-NOS, bipolar disorder not otherwise specified; K-W, Kruskal-Wallis; NS, not significant; ODD, oppositional defiant disorder; SES, socioeconomic status.
Values with different superscript letters were significant at P values ≤.05.
At present, the subject retention rate is 95%. Subjects who have dropped out from the study (n = 18) were significantly older (t = 3.0 [P = .003]) and non-Caucasian (χ2 = 7.9 [P = .005]), had higher rates of anxiety disorders (χ2 = 5.8 [P = .02]), and more frequently lived in a situation other than with both natural parents (χ2 = 5.3 [P = .02]) when compared with subjects who continued in the study.
PROCEDURES
Each university’s institutional review board approved the study. Assent was obtained from children and adolescents and consent from parents. We directly interviewed children and parents (about their children) for the presence of current and lifetime non-mood psychiatric disorders using the Schedule for Affective Disorders and Schizophrenia for School Age Children–Present and Lifetime Version (K-SADS-PL)24 and for the presence of mania and depression with the K-SADS–Mania Rating Scale25 and the depression section of the K-SADS-PL,26 respectively.
Longitudinal changes in psychiatric symptoms, functioning, and treatment exposure since the previous evaluation were assessed using the Longitudinal Interval Follow-up Evaluation (LIFE).27 The LIFE was administered to adolescents and parents separately. Because younger children often have problems timing their symptoms, they were interviewed with their parents. Any discrepancies between the informants’ responses were discussed, and a summary score based on all available information was determined. The LIFE9,10,27–29 evaluates the course of symptoms by identifying change points, frequently anchored by memorable dates for the subject (eg, holidays and beginning of school). The severity of ongoing symptoms, onset of new symptoms, and episode polarity for BP since the last appointment are tracked on a week-by-week basis using the LIFE Psychiatric Status Rating (PSR) scale. For DSM-IV mood disorders, the PSR scores range from 1 for no symptoms, to 2 to 4 for varying levels of subthreshold symptoms and impairment, to 5 or 6 for full criteria with different degrees of severity or impairment. Comorbid disorders and psychosis are also rated on a weekly basis on a 3-point scale of 1 to 3, where 3 indicates threshold symptoms. Family history of mood disorders was ascertained using the Family History Screen.30
The Petersen Pubertal Developmental Scale31 was used to evaluate pubertal stages, which were categorized into prepubertal (equivalent to Tanner32,33 stage I of sexual development), midpubertal (Tanner stages II and III), and postpubertal (Tanner stages IV and V). Subjects 10 years or older completed the Petersen Pubertal Developmental Scale by themselves. Subjects aged 7 to 9 years completed the Petersen Pubertal Developmental Scale with their parents’ assistance, and parents completed the form for those younger than 7 years. Socioeconomic status (SES) was ascertained using the 4-factor Hollingshead Scale.34
All assessments were completed by research staff trained to reliably administer the interviews and presented to child psychiatrists/psychologists who confirmed the diagnoses. When necessary, subjects’ medical records were reviewed. The overall K-SADS kappas for psychiatric disorders were 0.8 or more. The intraclass correlation coefficients for the K-SADS Mania Rating Scale and the depression section of the K-SADS-PL were 0.95 or more. The LIFE intraclass correlations for mood disorders were 0.80 or more.
DEFINITIONS OF COURSE OF ILLNESS
As described in the literature,28,35,36 recovery from a mood episode required 8 consecutive weeks with a PSR score of 2 or less (minimal or no symptoms). A recurrence (new episode) required a PSR score of 5 or more, with durations of 1 week for mania/hypomania and 2 weeks for depression. Rapid cycling and mixed episodes were defined as in the DSM-IV.
STATISTICAL ANALYSES
Differences between groups were analyzed using standard parametric and nonparametric univariate tests. The index episode was defined as the current or most recent episode from the date of intake. Because the index episode could have started before the subjects were recruited into the study, time to recovery was calculated from the onset of the index episode to ascertain the real duration of illness. Therefore, for some subjects the duration of episode exceeded the length of prospective follow-up. Time to recurrence was calculated from the time subjects fulfilled criteria for recovery until they met full criteria for a new mood episode. Cumulative rates of recovery and recurrence were analyzed using survival analytical methods37 stratifying subjects by BP subtype.
About 14% of the sample had their acute episode offset and recovered before intake (mean time, 44.5 ± 53.4 weeks). Analyses of the rates of recovery and recurrence with or without these subjects yielded similar results. Therefore, every subject, regardless of his or her episode status at the intake assessment, was included in this analysis.
To provide a more complete picture of the longitudinal course of BP in youth, the percentages of follow-up weeks spent asymptomatic or symptomatic in the different mood symptom status categories (eg, mania, mixed, and subsyndromal symptoms) were computed based on the PSR ratings for each subject. To compare the COBY’s BP-I data with those of a recent adult BP-I study28,29 that used comparable methods, changes in symptom status and polarity were defined and analyzed similarly to those used in the adult study. Symptom status was defined as the change in the weekly PSR ratings in levels of depressive and/or manic/hypomanic symptoms or change from/to the asymptomatic status. Change in polarity was defined as a switch between depression (PSR, ≥3) and mania/hypomania (PSR, ≥3) or vice versa with or without intervening weeks at the asymptomatic status. For these last 2 analyses, similar to those of Judd et al,28,29 weeks with mixed symptoms of depression and mania/hypomania were included together.
For all of these analyses, the effects of demographic variables, pubertal status, subtype of BP, lifetime psychosis, age of onset of BP symptoms (depression and/or mania/hypomania), and duration of illness were evaluated. Interactions among significant predictors in the models were also examined. These variables were examined univariately, and those related to the outcome of interest were included in the regression models. To assess the effects of age on the child’s outcome, the sample was dichotomized into children younger than 12 years and those 12 years or older. However, because some of the children 12 years and older had the onset of their illness when they were younger, the analyses for the effects of age and age of onset were evaluated in the following way: (1) children younger than 12 years, (2) adolescents 12 years or older with onset of BP at younger than 12 years, and (3) adolescents 12 years or older with onset of BP at 12 years or older. Models were run separately for pubertal status and the age/age of BP onset variable owing to high colinearity. For the survival analyses, the effects of these variables were evaluated using Cox proportional hazards regressions.38 For the weekly symptomatic status (ie, change in symptoms status and polarity), the same predictors were examined using linear regression models.
Unless otherwise indicated, all values are reported as mean ± SD. All P values are based on 2-tailed tests with α = .05.
RESULTS
RECOVERY AND RECURRENCE BY BP SUBTYPE
Recovery
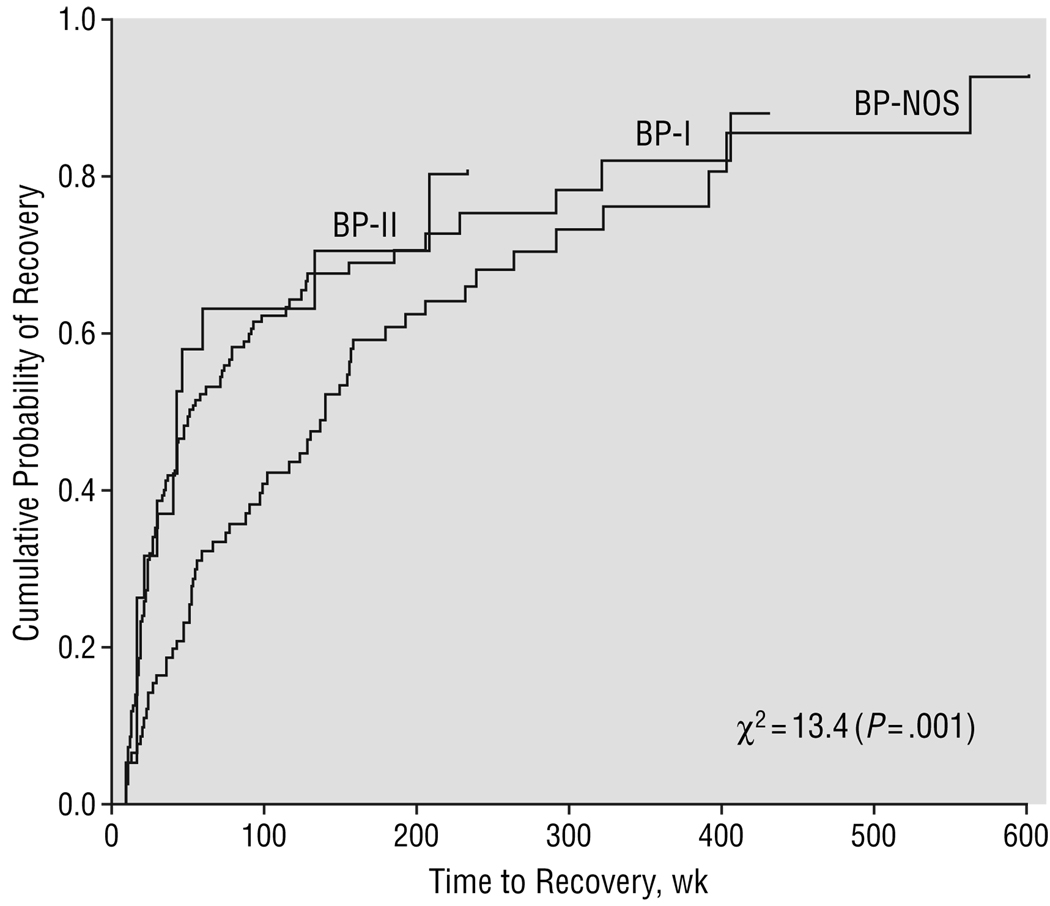
As depicted in Figure 1 and Table 2, overall 68% of subjects recovered from their index episode a median of 78 weeks after the onset of the episode. There was no significant difference in rates of recovery among the 3 BP subgroups, but subjects with BP-NOS had a significantly longer time to recovery than subjects with BP-I and BP-II (all comparisons, P≤.05).
Figure 1.
Recovery stratified by bipolar subtype. The index episode was defined as the most recent or current episode from the date of intake. To ascertain the real duration of illness, time to recovery was calculated from the onset of the index episode. Therefore, for some subjects the duration of episode exceeded the length of prospective follow-up. BP-I indicates bipolar I disorder; BP-II, bipolar II disorder; BP-NOS, bipolar disorder not otherwise specified.
Table 2.
Summary of Recovery and Recurrence by BP Subtype*
| All Subjects (N = 263) |
BP-I (n = 152) |
BP-II (n = 19) |
BP-NOS (n = 92) |
Statistical Analysis† |
P Value | |
|---|---|---|---|---|---|---|
| Rate of recovery, No./total No. (%) | 180/263 (68) | 104/152 (68) | 15/19 (79) | 61/92 (66) | NS | |
| Median time to recovery from the index episode,‡ wk | 78.3 | 52.0a | 42.1a | 140.2b | 13.4 | .001 |
| Rate of recurrence, No./total No. (%) | 101/180 (56) | 60/104 (58)ab | 13/15 (87)a | 28/61 (46)b | 8.4 | .02 |
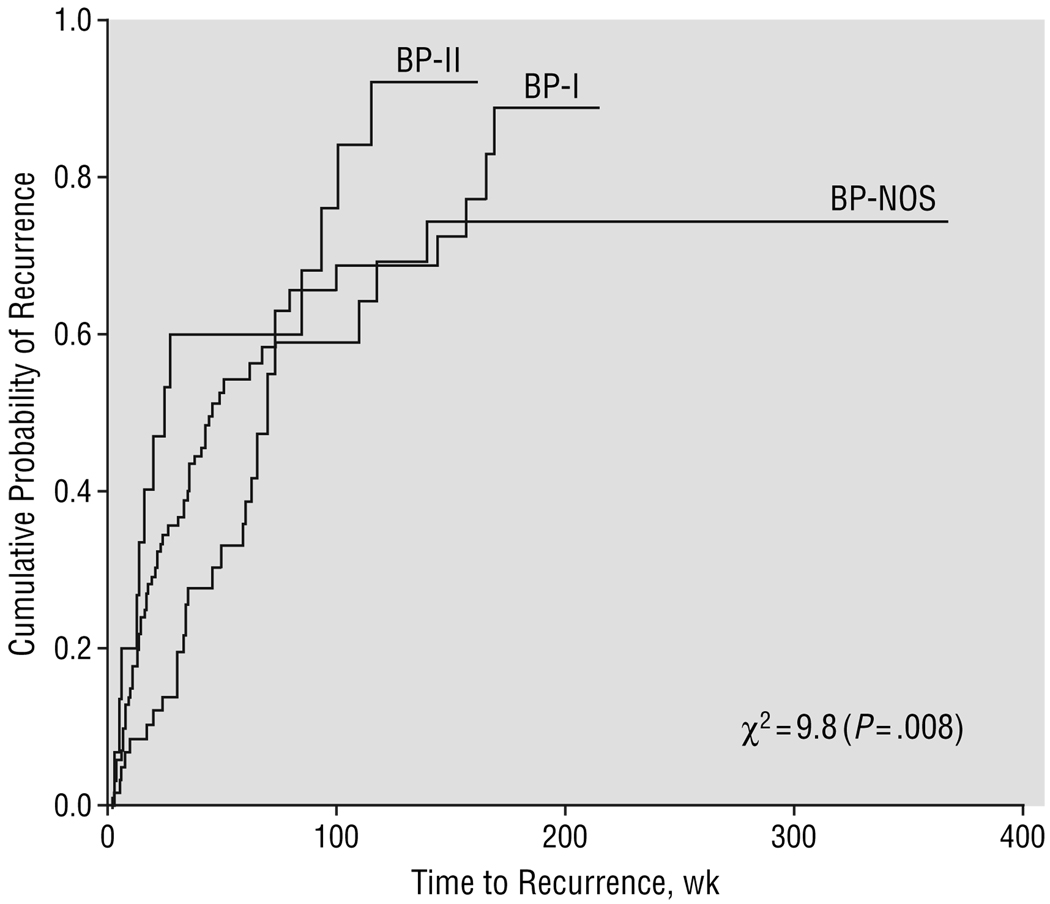
| Median time to recurrence,§ wk | 61.0 | 45.0a | 19.0a | 69.0b | 9.8 | .008 |
Abbreviations: BP-I, bipolar I disorder; BP-II, bipolar II disorder; BP-NOS: bipolar disorder not otherwise specified; NS, not significant.
Values with different superscript letters were significant at P values ≤.05.
By the χ2 test.
The index episode was defined as the most recent or current episode from the date of intake. To ascertain the real duration of illness, time to recovery was calculated from the onset of the index episode. Therefore, for some subjects the duration of episode exceeded the length of prospective follow-up.
Time to recurrence was calculated from the time subjects fulfilled criteria for recovery until they met full criteria for a new mood episode.
Cox proportional hazards regressions showed that compared with adolescents, subjects with childhood onset were 1.7 times (95% confidence interval [CI], 1.19–2.56) less likely than those with early BP onset and 1.9 times (95% CI, 1.3–2.8) less likely than those with late onset to recover. With every unit decrease in SES, subjects had a 25% lower likelihood of recovery (hazard ratio, 1.25; 95% CI, 1.10–1.43). Finally, with longer duration of BP (per year), subjects had a 10% lower likelihood of recovery (hazard ratio, 0.90; 95% CI, 0.84–0.96). Prepubertal subjects were 1.7 times (95% CI, 1.06–2.65) less likely than midpubertal subjects and 1.9 times (95% CI, 1.27–2.85) less likely than late pubertal subjects to recover. Those with BP-NOS were 2.0 times (95% CI, 1.43–2.88) less likely than BP-I subjects and 1.9 times (95% CI, 1.05–3.52) less likely than BP-II subjects to recover. No other significant predictors or interactions were found.
Recurrence
As depicted in Figure 2 and Table 2, overall 56% of subjects had at least 1 recurrence a median of 61.0 weeks after recovery of the index episode. Subjects with BP-II had higher rates of recurrence than subjects with BP-NOS and subjects with BP-NOS had significantly longer time to recurrence than those with BP-I and BP-II (all comparisons, P≤.05).
Figure 2.
Recurrence stratified by bipolar subtype. Time to recurrence was calculated from the time subjects fulfilled criteria for recovery until they met full criteria for a new mood episode. Abbreviations are explained in the legend to Figure 1.
Cox proportional hazards regressions showed that BP-I subjects were 1.7 times (95% CI, 1.06–2.67) and BP-II subjects were 2.7 times (95% CI, 1.35–5.31) more likely to have a recurrence than those with BP-NOS. Subjects with low SES had a 20% higher likelihood of recurrence (with every unit of decrease in SES) (95% CI, 0.67–0.95). No other significant predictors or interactions were found.
RECURRENCE DURING FOLLOW-UP
Subjects had a mean ± SD of 1.5 ± 0.9 syndromal recurrences. After controlling for differences in the length of the follow-up, there were no significant differences in the number of syndromal recurrences among subjects with BP-I, BP-II, and BP-NOS. Most of these recurrences were major depressive episodes (57.5%), followed by hypomania (24.2%), mania (13.7%), and mixed (4.6%). There were no significant differences in the polarity of the recurrences among subjects with BP-I, BP-II, and BP-NOS.
WEEKLY MOOD SYMPTOMATIC STATUS BY BP SUBTYPE
As depicted in Table 3, on average, subjects spent 39.7% of the follow-up time without clinically significant mood symptoms, 22.4% of the time in a DSM-IV syndromal episode, 37.9% of the time with subsyndromal symptoms, and 3.1% of the time with clinically significant symptoms of psychosis (PSR score for delusion and/or hallucinations, 3). Within the syndromal symptoms, subjects with BP-I spent significantly more weeks with syndromal mania and mixed symptoms than those with BP-NOS, and subjects with BP-II spent significantly more time with depressive symptoms than those with BP-I and BP-II (all comparisons, P≤.001). Within the subsyndromal symptoms, BP-NOS subjects spent significantly more weeks with subsyndromal mania and mixed symptoms compared with BP-I subjects, and BP-II subjects spent significantly more time with subsyndromal depression than BP-I and BP-NOS subjects (all comparisons, P≤.05).
Table 3.
Weekly Symptomatic Status by Bipolar Subtype*
| All Subjects (N = 263) |
BP-I (n = 152) |
BP-II (n = 19) |
BP-NOS (n = 92) |
Statistical Analysis |
P Value | |
|---|---|---|---|---|---|---|
| Asymptomatic, % | 39.7 ± 32.8 | 41.1 ± 34.3 | 51.8 ± 24.9 | 34.9 ± 31.0 | K-W, 4.5 | NS |
| Syndromal, % | 22.4 ± 26.3 | 27.2 ± 29.5a | 20.9 ± 19.1ab | 14.7 ± 19.3b | K-W, 12.3 | .002 |
| Pure MDD | 6.3 ± 13.5 | 6.9 ± 15.3a | 11.5 ± 11.6b | 4.2 ± 9.8a | K-W, 13.8 | .001 |
| Pure mania/hypomania | 3.9 ± 10.3 | 5.3 ± 12.7a | 2.1 ± 3.8ab | 1.9 ± 5.4b | K-W, 7.4 | .03 |
| Mixed | 2.9 ± 11.3 | 4.5 ± 14.3a | 1.8 ± 7.3ab | 0.5 ± 2.3b | K-W, 7.2 | .03 |
| Cycling | 9.3 ± 17.9 | 10.5 ± 19.7 | 5.5 ± 11.8 | 8.2 ± 15.6 | K-W, 0.1 | NS |
| Subsyndromal, % | 37.9 ± 28.2 | 31.7 ± 25.6a | 27.2 ± 20.5a | 50.4 ± 29.5b | K-W, 26.2 | <.001 |
| Mixed | 12.8 ± 21.1 | 10.4 ± 18.0a | 4.1 ± 6.1a | 18.7 ± 26.0b | K-W, 11.4 | .003 |
| Pure depression | 9.4 ± 15.1 | 8.0 ± 13.0a | 17.4 ± 22.4b | 10.1 ± 16.3a | K-W, 10.6 | .005 |
| Mania | 15.7 ± 22.9 | 13.3 ± 20.5a | 5.6 ± 7.1ab | 21.6 ± 27.1b | K-W, 6.1 | .05 |
| Psychosis (delusions and/or hallucinations), % | 3.1 ± 12.4 | 3.2 ± 11.8 | 2.3 ± 7.1 | 3.2 ± 14.3 | F = 0.05 | NS |
Abbreviations: BP-I, bipolar I disorder; BP-II, bipolar II disorder; BP-NOS: bipolar disorder not otherwise specified; K-W, Kruskal-Wallis; MDD, major depressive disorder; NS, not significant.
Indicates the percentage of follow-up weeks spent asymptomatic or symptomatic in the different mood symptom status categories. Data are presented as mean ± SD unless otherwise indicated. Values with different superscript letters were significant at P values ≤.05.
Linear regression models showed that lifetime psychosis (t = 2.96 [P = .003]) and the interaction between childhood onset of BP and low SES (t = 2.38 [P = .02]) were significant predictors of more time spent with any mood symptoms. In the same model, the main effects of low SES and childhood onset of BP (vs adolescents with late onset) remained marginally significant predictors of time spent with any mood symptoms (all comparisons, P≤.15).
Diagnosis of BP-I (vs BP-NOS, t = 2.57 [P = .01]), lower SES (t = 2.98 [P = .003]), lifetime psychosis (t = 2.78 [P = .006]), and female sex (t = 1.99 [P = .05]) were found to be significant predictors of more follow-up time spent with syndromal manic/major depressive disorder symptoms (F = 6.63 [P<.001]). There were no significant interactions.
Diagnosis of BP-NOS (vs BP-I, t = 3.56 [P<.001]; vs BP-II, t = 2.38 [P = .02]), lower SES (t = 2.74 [P = .007]), and childhood onset of BP (vs adolescents with late onset, t = 2.67 [P = .008]) were found to be significant predictors of follow-up time spent with subsyndromal mood symptoms. There were no significant interactions.
CHANGE IN SYMPTOM STATUS
Subjects experienced a mean ± SD of 34.1 ± 32.2 changes in symptom status during the entire follow-up period, or 19.8 ± 16.6 per year. Approximately 6% of the sample had 1 or fewer changes in symptom status per year. Eighty-six percent of the sample changed symptom status more than 3 times per year, 78% changed more than 5 times per year, 62% changed more than 10 times per year, and 40% changed more than 20 times per year. There was a significant difference between the BP groups in the number of total symptom changes (Kruskal-Wallis χ2 = 7.14 [P = .03]), with BP-NOS subjects showing more symptom changes than BP-I and BP-II subjects (all comparisons, P≤.05). Linear regressions show low SES (t = 2.13 [P = .03]), longer duration of mood disorder (t = 2.33 [P = .02]), lifetime psychosis (t = 3.71 [P<.001]), and BP-NOS diagnosis (vs BP-I, t = 3.15 [P = .002]) as significant predictors of greater number of changes in symptom status per year (F = 7.62 [P<.001]). There were no significant interactions.
CHANGE IN POLARITY
Shifts in polarity occurred at a mean ± SD of 26.1 ± 30.7 times during the entire follow-up period or 15.7 ± 17.0 times per year. About 19% of patients changed polarity once per year or less, 61% changed 5 or more times per year, 47% changed more than 10 times per year, and 30% changed more than 20 times per year. There were no differences in the number of changes in polarity among BP-I, BP-II, and BP-NOS subjects. Lower SES (t = 2.84 [P = .005]), lifetime psychosis (t = 3.73 [P<.001]), and BP-NOS diagnosis (vs BP-I, t = 2.88 [P = .004]) were significant predictors of greater number of changes in polarity per year (F = 8.14 [P<.001]). There were no significant interactions.
CONVERSION FROM BP-II TO BP-I AND FROM BP-NOS TO BP-I OR BP-II
During follow-up, 4 (21%) of the 19 BP-II subjects experienced conversion to BP-I; 18 (20%) of the 92 BP-NOS subjects, to BP-I; and 9 (10%) of the 92 BP-NOS subjects, to BP-II. Increased rates of conversion were associated with female sex (odds ratio, 3.2; 95% CI, 1.33–7.50) and long duration of illness (odds ratio, 0.8; 95% CI, 0.68–0.95). There were no other significant predictors of conversion.
COMMENT
To our knowledge, this is the first report to describe the psychopathological course of each of the major clinical phenotypes of BP among children and adolescents, and it is the largest cohort of pediatric subjects with BP described in the literature to date. The general findings highlight the substantial morbidity of the illness in this age group, including early age of first onset of mood disturbance, long duration, fluctuating course, high familial loading for mood and other psychiatric disorders, and high rates of comorbid disorders, particularly attention-deficit/hyperactivity disorder, disruptive behavior, and anxiety disorders. Although recovery from the index episode was observed in approximately two thirds of subjects, half of them had at least 1 full syndromal recurrence. Subjects with BP-I and BP-II recovered from their index episode and had recurrence more frequently than those with BP-NOS. In contrast, subjects with BP-NOS had a more protracted illness, but once they recovered from their index episode, they took a longer time to recur than those with BP-I and BP-II. On average, subjects had 1.5 syndromal recurrences per year, particularly depressive episodes. Complementing this information, analyses of weekly mood symptoms ascertained through the LIFE showed that subjects were symptomatic approximately 60% of the follow-up time, with about 22% of the time in full syndromal episodes and 38% of the time with subsyndromal symptoms. Subjects with BP-I had more syndromal manic/hypomanic and mixed episodes than those with BP-NOS, and subjects with BP-II had more syndromal and subsyndromal depression that those with BP-I and BP-NOS. In contrast, subjects with BP-NOS showed more subsyndromal symptoms. During the follow-up, subjects with all types of BP, and particularly those with BP-NOS, with early onset or psychosis showed numerous changes in symptoms and shifts of polarity. Approximately 20% of subjects who had an intake diagnosis of BP-II experienced conversion to BP-I, and 25% of the BP-NOS subjects experienced conversion to BP-I or BP-II. Overall, subjects whose illness started early in life or who had longer duration of illness, BP-NOS, low SES, or psychotic symptoms had worse outcome.
Several limitations of the study deserve comment. As with any prospective interview study in which subjects are asked to recall mood symptoms since the most recent assessment, the information gathered is subject to multiple sources of unreliability. Our efforts to maximize the accuracy of the information obtained included attempts to interview subjects every 6 months using well-defined anchor points to assist them with recall of critical events of importance during the interview period. This method has shown superior reliability in the COBY and other studies.9,10,27–29 Because most subjects were Caucasian and were recruited primarily from outpatient and inpatient settings, the results of this study cannot be generalized to other populations. Nevertheless, a community study using the LIFE9,10 also attested to the chronicity and high morbidity of BP spectrum disorders in youth. In addition, results pertaining to subjects with BP-II should be considered tentative because the BP-II sample was small. The definition of change in polarity used in this study and that of Judd et al28 was not meant to reflect the DSM-IV classification of rapid cycling and may have inflated the rate of shifts in polarity. However, to compare the COBY’s BP-I data with the adult data, it was necessary to use the same definition. Finally, the observed outcomes were in the context of naturalistically applied treatment. However, the COBY’s findings of recurrent and fluctuating episodes between mild and severe mood symptoms are similar to those reported by Kraepelin39 in patients with BP before pharmacological treatments were available.
Comparable to the extant pediatric BP literature,5–15 the COBY subjects first manifested emergent signs of mood disturbance early in life and had frequent comorbid psychiatric disorders, psychosis, and high familial loading for mood and other psychiatric disorders. Also in agreement with other studies,8–14 most subjects included in this study recovered from their index episode, but most had at least 1 recurrence, particularly episodes of depression. However, the rates of recovery and recurrence of syndromal episodes alone did not entirely depict the degree of morbidity reflected in the longitudinal course of BP in youth. Two thirds of the time, the COBY subjects experienced significant mood symptoms and, as reported in other pediatric studies,1,6,8 showed numerous changes in the intensity of mood symptoms and shifts in polarity. In this regard, our results are also comparable with those of recent studies of adults with BP-I and BP-II in which polyphasic episodes and interepisodic symptoms of subthreshold intensity are frequent.28,29,39–49 Nevertheless, it appears that there are developmental differences in the course of BP between children and adults.1,6,8,40,50,51 To address this issue, using the same definitions of outcome proposed by Judd et al28 and after consulting with their statisticians, we compared the COBY BP-I youth with adults with BP-I (Table 4). The COBY subjects with BP-I spent significantly more time symptomatic and had more mixed/cycling episodes, mood symptom changes, and polarity switches than adults with BP-I.28 Thus, across the age span and especially in youth, BP usually follows an ongoing changeable and sinuous course, with patients having a wide spectrum of mood symptoms ranging from mild to severe depression to mania and/or hypomania. These results substantiate what Kraepelin39 and other investigators47 and clinicians have observed and explain, at least in part, the difficulties encountered when treating subjects with BP spectrum disorders. Furthermore, it is likely that the very rapid fluctuation in mood symptoms combined with the developmental issues influencing the clinical picture of BP in youth, the difficulties children and sometimes adolescents have verbalizing their emotions, and the high rates of comorbid disorders account for the complexity and current controversies in diagnosing BP in children and adolescents.
Table 4.
Comparison of Weekly Symptom Status, Change in Symptom Status and Polarity, and Time With Psychosis Between Youth and Adults With BP-I*
| Adults With BP-I (n = 146) |
Children/Adolescents With BP-I (n = 152) |
Statistical Analysis |
P Value | |
|---|---|---|---|---|
| Weekly symptom status, %† | ||||
| Asymptomatic | 52.7 | 41.1 | t = 2.93 | .004‡ |
| Symptomatic | 47.3 | 58.9 | t = 1.01 | <.001 |
| Mania/hypomania | 6.9 | 5.3 | t = 1.36 | NS |
| MDE | 8.9 | 6.9 | t = 1.24 | NS |
| Mixed/rapid | 5.9 | 28.9 | t = 7.97 | <.001 |
| Subsyndromal | 25.3 | 21.3 | t = 1.98 | .05‡ |
| Change in symptom status, times per year§ | ||||
| Mean ± SD | 5.9 ± 7.6 | 19.6 ± 17.1 | t = 9.53 | <.001 |
| >3, % | 54 | 83.6 | χ2 = 30.3 | <.001 |
| >5, % | 34.9 | 74.3 | χ2 = 46.7 | <.001 |
| >10, % | 11.6 | 59.2 | χ2 = 73.2 | <.001 |
| >20, % | 5.5 | 38.8 | χ2 = 47.5 | <.001 |
| Change in mood polarity, times per year‖ | ||||
| Mean ± SD | 3.5 ± 7.4 | 15.7 ± 17.5 | t = 7.71 | <.001 |
| ≤1, % | 60 | 21.1 | χ2 = 47.6 | <.001 |
| >5, % | 19.2 | 59.2 | χ2 = 49.9 | <.001 |
| >10, % | 8.2 | 44.1 | χ2 = 49.2 | <.001 |
| >20, % | 4.1 | 29.6 | χ2 = 34.2 | <.001 |
| Time spent with psychotic symptoms, % | 2.3 | 3.2 | Fisher exact test | NS |
Abbreviations: BPI, bipolar I disorder; MDE, major depressive episode; NS, not significant.
Indicates the percentage of follow-up weeks spent asymptomatic or symptomatic in the different mood symptom status categories.
No longer significant after Bonferroni correction.
Indicates change in the weekly Psychiatric Status Rating in levels of depressive and/or manic/hypomanic symptoms or change from/to the asymptomatic status.
Indicates switch between depression (Psychiatric Status Rating, ≥3) and mania/hypomania (Psychiatric Status Rating, ≥3) or vice versa with or without intervening weeks at the asymptomatic status.
Although for approximately 40% of the follow-up time, the COBY subjects were free of significant mood symptoms, a substantial proportion had ongoing comorbid psychiatric disorders. Thus, unless subjects undergo prospective evaluation, accurate description of the course of BP in youth may be difficult given the phenotypic overlap between BP and certain specific symptoms of comorbid disorders such as attention-deficit/hyperactivity disorder, with which it frequently co-occurs, and may give the appearance that BP does not cycle through episodes over time.
Youth with BP showed high lifetime rates of psychosis.5,8,9 As described in the literature,5,47,52,53 the presence of psychosis was consistently associated with poor prognosis, indicating the need for intensive and prompt treatment of this symptom.
In contrast to reports in the literature of adult BP,47,49 the prevalence of substance abuse by subjects in this study was low. However, most subjects included in our study had not yet reached the age of high risk for development of substance abuse. Therefore, this finding emphasizes the importance of prompt treatment of youth with BP before they begin to use substances that could complicate the management of their mood disorder and worsen their long-term prognosis.47,49
The rate of conversion from BP-II to BP-I found in the COBY study is higher than the cumulative rate of conversion reported in the adult literature,54 possibly suggesting that BP-II is less developmentally stable in the pediatric age group. At this time, there are to our knowledge no other published longitudinal studies of children and adolescents with BP-II with which to compare the COBY’s findings. Likewise, this is the first study in the BP literature suggesting the relative instability of the BP-NOS phenotype, as approximately one third of BP-NOS subjects within the period of follow-up experienced conversion to BP-I or BP-II. In contrast, another study followed up a community sample of 54 adolescents who had subsyndromal BP symptoms and found an increased risk for major depression but not for BP.10 However, compared with the COBY’s definition of BP-NOS, this later study defined subsyndromal BP less stringently as “a distinct period of abnormally and persistently elevated, expansive, or irritable mood”10(p282) only, suggesting possibly less liability for full BP than captured by the COBY’s more restrictive definition. Furthermore, the presence of only 1 symptom, particularly irritability, does not necessarily mean that these adolescents had BP or that BP will develop.10,55
As has been reported by others,5,8,40,50,51 subjects in whom BP symptoms developed during their early childhood showed lower rates of recovery, more time in mixed/rapid cycling episodes, and more symptom and polarity changes than those whose illness started later. Also, comparable with recent adult BP studies,53,56 the results of the COBY study consistently showed that BP subjects with low SES had worse prognosis. Future studies will evaluate possible factors (eg, exposure to negative life events, lack of or poor adherence to treatment, and increased family psychopathologic characteristics) that may account for this finding.
The longitudinal course of children and adolescents with BP-NOS presented herein, together with the findings that these subjects have comparable rates of comorbidity and family history for mood disorders when compared with subjects with BP-I and BP-II, provide initial validation of the BP-NOS category as defined in the COBY. Furthermore, these results support the recommendation of the National Institute of Mental Health Consensus Roundtable22 and others1,6,48,57,58 to include children and adolescents with significant subsyndromal BP symptoms and BP-NOS in studies to further evaluate the clinical presentation of BP spectrum in youth and maximize the generalizability of the results.
In summary, children and adolescents with BP spectrum disorders, particularly those with early onset, BP-NOS, long duration, low SES, and psychosis, experienced frequent changes in symptom status and polarity in a fluctuating course showing a dimensional continuum of BP symptom severity, from subsyndromal to mood syndromes meeting full DSM-IV criteria. The enduring and rapid changeability of symptoms of this illness in children and adolescents from very early in life and at crucial stages of their lives deprives them of the opportunity for normal emotional, cognitive, and social development.1–10,58 Thus, early recognition and acute and maintenance treatment of BP spectrum disorders in children and adolescents is of utmost importance to ameliorate ongoing syndromal and subsyndromal symptoms and to reduce or prevent the serious psychosocial morbidity that usually accompanies this illness.58,59
Acknowledgment
We thank Carol Kostek for her assistance with manuscript preparation; Kristin Bruning, MD, and Jennifer Dyl, PhD, of the Course and Outcome of Bipolar Illness in Youth faculty; raters Mathew Arruda, BA, Mark Celio, BA, Jennifer Fretwell, BA, Michael Henry, BS, Risha Henry, PhD, Norman Kim, PhD, Marguerite Lee, BA, Marilyn Matzko, EdD, Heather Schwickrath, MA, Anna Van Meter, BA, and Matthew Young, BA; data personnel Amy Broz, AS, Colleen Grimm, BA, and Nicole Ryan, BA; and Editha Nottelmann, PhD, and Regina James, MD, for their continued support.
Funding/Support: This study was supported by grants MH59929 (Dr Birmaher), MH59977 (Dr Strober), and MH59691 (Dr Keller) from the National Institute of Mental Health, Rockville, Md.
Footnotes
Financial Disclosure: Dr Keller is a consultant to or has received honoraria from Bristol-Myers Squibb, Collegium Pharmaceutical, Cyberonics, Inc, Cypress Bioscience, Inc, Eli Lilly and Company, Forest Laboratories, Janssen, Merck & Co, Inc, Organon, Otsuka Pharmaceutical Co, Ltd, Pfizer, Inc, Pharmacia, PharmaStar, Sepracor Inc, Vela Pharmaceuticals Inc, and Wyeth; has received grant or research support from Eli Lilly and Company, Forest Laboratories, Merck & Co, Inc, Organon, Pfizer, Inc, and Wyeth; and is on the advisory board of Abbott Laboratories, Bristol-Myers Squibb, Cephalon, Inc, Cyberonics, Inc, Cypress Bioscience, Inc, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Janssen, Merck & Co, Inc, Mitsubishi Pharma Corporation, Novartis, Organon, Pfizer, Inc, Pharmacia, Sanofi-Synthelabo, SCIREX, Sepracor Inc, Somerset Pharmaceuticals, Inc, Vela Pharmaceuticals Inc, and Wyeth.
REFERENCES
- 1.Biederman J, Faraone SV, Wozniak J, Mick E, Kwon A, Aleardi M. Further evidence of unique developmental phenotypic correlates of pediatric bipolar disorder: findings from a large sample of clinically referred preadolescent children assessed over the last 7 years. J Affect Disord. 2004;82(suppl 1):S45–S58. doi: 10.1016/j.jad.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Weller RA, Weller EB, Tucker SG, Fristad MA. Mania in prepubertal children: has it been underdiagnosed? J Affect Disord. 1986;11:151–154. doi: 10.1016/0165-0327(86)90022-4. [DOI] [PubMed] [Google Scholar]
- 3.Brent DA, Perper JA, Moritz G, Allman C, Friend A, Roth C, Schweers J, Balach L, Baugher M. Psychiatric risk factors for adolescent suicide: a case-control study. J Am Acad Child Adolesc Psychiatry. 1993;32:521–529. doi: 10.1097/00004583-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Carlson G, Davenport Y, Jamison K. A comparison of outcome in adolescent-and late-onset bipolar manic-depressive illness. Am J Psychiatry. 1977;134:919–922. doi: 10.1176/ajp.134.8.919. [DOI] [PubMed] [Google Scholar]
- 5.Carlson GA, Bromet EJ, Driessens C, Mojtabai R, Schwartz JE. Age at onset, childhood psychopathology, and 2-year outcome in psychotic bipolar disorder. Am J Psychiatry. 2002;159:307–309. doi: 10.1176/appi.ajp.159.2.307. [DOI] [PubMed] [Google Scholar]
- 6.Findling RL, Gracious BL, McNamara NK, Youngstrom EA, Demeter CA, Calabrese JR. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3:202–210. [PubMed] [Google Scholar]
- 7.Geller B, Bolhofner K, Craney J, Williams M, DelBello M, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Geller B, Tillman R, Craney J, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- 9.Lewinsohn P, Klein D, Seeley J. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- 10.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorder during adolescence and young adulthood in a community sample. Bipolar Disord. 2000;2:281–293. doi: 10.1034/j.1399-5618.2000.20309.x. [DOI] [PubMed] [Google Scholar]
- 11.Strober M, Freeman R, Bower S, Lampert C, DeAntonio M. Recovery and relapse in adolescents with bipolar affective illness: a five-year naturalistic, prospective follow-up. J Am Acad Child Adolesc Psychiatry. 1995;34:724–731. doi: 10.1097/00004583-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Jairam R, Srinath S, Girimaji SC, Seshadri SP. A prospective 4–5 year follow-up of juvenile onset bipolar disorder. Bipolar Disord. 2004;6:386–394. doi: 10.1111/j.1399-5618.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 13.Rajeev J, Srinath S, Reddy YC, Shashikiran MG, Girimaji SC, Seshadri SP, Subbakrishna DK. The index manic episode in juvenile-onset bipolar disorder: the pattern of recovery. Can J Psychiatry. 2003;48:52–55. doi: 10.1177/070674370304800110. [DOI] [PubMed] [Google Scholar]
- 14.Srinath S, Janarolha N, Reddy YC, Girimani SR, Seshadri SR, Subbakrishna DK. A prospective study of bipolar disorder in children and adolescents from India. Acta Psychiatr Scand. 1998;98:437–442. doi: 10.1111/j.1600-0447.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- 15.Bashir M, Russell J, Johnson G. Bipolar affective disorder in adolescence: a 10-year study. Aust N Z J Psychiatry. 1987;21:36–43. doi: 10.1080/00048678709160897. [DOI] [PubMed] [Google Scholar]
- 16.Jarbin H, Ott Y, Von Knorring AL. Adult outcome of social function in adolescent-onset schizophrenia and affective psychosis. J Am Acad Child Adolesc Psychiatry. 2003;42:176–183. doi: 10.1097/00004583-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Welner A, Welner Z, Fishman R. Psychiatric adolescent inpatients: eight- to ten-year follow-up. Arch Gen Psychiatry. 1979;36:698–700. doi: 10.1001/archpsyc.1979.01780060088010. [DOI] [PubMed] [Google Scholar]
- 18.Landolt AB. Follow-up studies on circular manic-depressive reactions occurring in the young. Bull N Y Acad Med. 1957;33:65–73. [PMC free article] [PubMed] [Google Scholar]
- 19.McGlashan TH. Adolescent versus adult onset of mania. Am J Psychiatry. 1988;145:221–223. doi: 10.1176/ajp.145.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Werry JS, McClellan JM. Predicting outcome in child and adolescent (early onset) schizophrenia and bipolar disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:147–150. doi: 10.1097/00004583-199201000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Strober M, Birmaher B, Ryan N, Axelson D, Valeri S, Leonard H, Iyengar S, Gill MK, Hunt J, Keller M. Pediatric bipolar disease: current and future perspectives for study of its long-term course and treatment. Bipolar Disord. doi: 10.1111/j.1399-5618.2006.00313.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Mental Health research roundtable on prepubertal bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2001;40:871–878. doi: 10.1097/00004583-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fourth Edition. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Axelson D, Birmaher B, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the KSADS mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 26.Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semi-structured interview test-retest reliability. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 27.Keller M, Lavori P, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The longitudinal interval follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 28.Judd LL, Akiskal HS, Schettler PJ, Endicott J. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 29.Judd LL, Akiskal HS, Schettler PJ, Coryell W, Endicott J, Maser JD, Solomon DA, Leon AC, Keller MB. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 30.Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: the Family History Screen. Arch Gen Psychiatry. 2000;57:675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- 31.Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 32.Marshall W, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–295. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollingshead AB. Four-Factor Index of Social Status. New Haven, Conn: Department of Sociology, Yale University; 1975. [Google Scholar]
- 35.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 36.Birmaher B, Brent DA, Kolko D, Baugher M, Bridge J, Holder D, Iyengar S, Ulloa RE. Clinical outcome after short-term psychotherapy for adolescents with major depressive disorder. Arch Gen Psychiatry. 2000;57:29–36. doi: 10.1001/archpsyc.57.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 38.Cox DR. Regression models and life tables. J R Stat Soc [Ser A] 1972;B34:187–220. [Google Scholar]
- 39.Kraepelin E. Manic Depressive Insanity and Paranoia. Edinburgh, Scotland: E & S Livingstone; 1921. [Google Scholar]
- 40.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (SEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Turvey C, Coryell W, Amdt S, Solomon D. Polarity sequence, depression, and chronicity in bipolar I disorder. J Nerv Ment Dis. 1999;187:181–187. doi: 10.1097/00005053-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Turvey CL, Coryell WH, Solomon DA, Leon AC, Endicott J, Keller MB, Akiskal H. Long-term prognosis of bipolar I disorder. Acta Psychiatr Scand. 1999;99:110–119. doi: 10.1111/j.1600-0447.1999.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 43.Keitner GI, Solomon DA, Ryan CE, Miller IW, Mallinger A, Kupfer DJ, Frank E. Prodromal and residual symptoms in bipolar I disorder. Compr Psychiatry. 1996;37:362–367. doi: 10.1016/s0010-440x(96)90018-8. [DOI] [PubMed] [Google Scholar]
- 44.Keller M, Lavori P, Kane J, Gelenberg A, Rosenbaum JF, Walzer E, Baker M. Subsyndromal symptoms in bipolar illness: a comparison of standard and low serum levels of lithium. Arch Gen Psychiatry. 1992;49:371–376. doi: 10.1001/archpsyc.1992.01820050035005. [DOI] [PubMed] [Google Scholar]
- 45.Angst J, Gerber-Werder R, Zuberbühler HU, Gamma A. Is bipolar I disorder heterogeneous? Eur Arch Psychiatry Clin Neurosci. 2004;254:82–91. doi: 10.1007/s00406-004-0501-6. [DOI] [PubMed] [Google Scholar]
- 46.Coryell W, Turvey C, Endicott J, Leon A. Bipolar I affective disorder: predictors of outcome after 15 years. J Affect Disord. 1998;50:109–116. doi: 10.1016/s0165-0327(98)00043-3. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin F, Jamison K. Manic Depressive Illness. New York, NY: Oxford University Press Inc; 1990. [Google Scholar]
- 48.Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R. Reevaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59(suppl 1):S5–S30. doi: 10.1016/s0165-0327(00)00203-2. [DOI] [PubMed] [Google Scholar]
- 49.Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE, Jr, McElroy SL, Luckenbaugh DA, Pollio C, Kupka R, Nolen WA. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 50.Schneck CD, Miklowitz DJ, Calabrese JR, Allen MH, Thomas MR, Wisniewski SR, Miyahara S, Shelton MD, Ketter TA, Goldberg JF, Bowden CL, Sachs GS. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the systematic treatment enhancement program. Am J Psychiatry. 2004;161:1902–1908. doi: 10.1176/ajp.161.10.1902. [DOI] [PubMed] [Google Scholar]
- 51.Coryell W, Solomon D, Turvey C, Keller M, Leon AC, Endicott J, Schettler P, Judd L, Mueller T. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60:914–920. doi: 10.1001/archpsyc.60.9.914. [DOI] [PubMed] [Google Scholar]
- 52.Tohen M, Hennen J, Zarate C, Baldessarini R. Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry. 2000;157:220–228. doi: 10.1176/appi.ajp.157.2.220. [DOI] [PubMed] [Google Scholar]
- 53.Tohen M, Zarate CA, Jr, Hennen J, Khalsa H, Strakowski SM, Gebre-Medhin P, Salvatore P, Baldessarini RJ. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 54.Coryell W, Endicott J, Maser JD, Keller MB, Leon AC, Akiskal HS. Long-term stability of polarity distinctions in the affective disorders. Am J Psychiatry. 1995;152:385–390. doi: 10.1176/ajp.152.3.385. [DOI] [PubMed] [Google Scholar]
- 55.Hazell PL, Carr V, Lewin TJ, Sly K. Manic symptoms in young males with ADHD predict functioning but not diagnosis after 6 years. J Am Acad Child Adolesc Psychiatry. 2003;42:552–560. doi: 10.1097/01.CHI.0000046830.95464.33. [DOI] [PubMed] [Google Scholar]
- 56.Nolen WA, Luckenbaugh DA, Altshuler LL, Suppes T, McElroy SL, Frye MA, Kupka RW, Keck PE, Leverich GS, Post RM. Correlates of 1-year prospective outcome in bipolar disorder: results from the Stanley Foundation Bipolar Network. Am J Psychiatry. 2004;161:1447–1454. doi: 10.1176/appi.ajp.161.8.1447. [DOI] [PubMed] [Google Scholar]
- 57.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 58.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 59.Kowatch RA, Fristad MA, Birmaher B, Wagner KD, Findling RL, Hellander M. Child Psychiatric Workgroup Members. Treatment guidelines for children and adolescents with bipolar disorder: Child Psychiatric Workgroup on Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Paulus MP, Hozack NE, Zauscher BE, Frank L, Grown GG, McDowell J, Braff DL. Parietal dysfunction is associated with increased outcome-related decision-making in schizophrenia patients. Biol Psychiatry. 2002;51:995–1004. doi: 10.1016/s0006-3223(01)01358-0. [DOI] [PubMed] [Google Scholar]
- 54.Quintana J, Wong T, Ortiz-Portillo E, Kovalik E, Davidson T, Marder SR, Mazziotta JC. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry. 2003;53:12–24. doi: 10.1016/s0006-3223(02)01435-x. [DOI] [PubMed] [Google Scholar]
- 55.Danckert J, Saoud M, Maruff P. Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophr Res. 2004;70:241–261. doi: 10.1016/j.schres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PMA. PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- 57.Blakemore SJ, Oakley DA, Frith CD. Delusions of alien control in the normal brain. Neuropsychologia. 2003;41:1058–1067. doi: 10.1016/s0028-3932(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 58.Northoff G. Neuroimaging and neurophysiology. In: Caroff SN, Mann SC, Francis A, Fricchione GL, editors. Catatonia: From Psychopathology to Neurobiology. Washington, DC: American Psychiatric Publishing Inc; 2004. pp. 77–91. [Google Scholar]
- 59.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- 60.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 61.Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eyler Zorrilla LT, Jeste DV, Paulus M, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res. 2003;59:187–198. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- 63.Weiss AP, Schacter DL, Goff DC, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- 64.Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. An fMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. Am J Psychiatry. 2003;160:911–918. doi: 10.1176/appi.ajp.160.5.911. [DOI] [PubMed] [Google Scholar]
- 65.Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. doi: 10.1016/j.biopsych.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Benes FM, Berretta S. Gabaergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 67.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 68.Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11:569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- 69.Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- 70.Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull. 1984;10:430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- 71.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 72.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 73.Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol. 2002;41:331–347. doi: 10.1348/014466502760387461. [DOI] [PubMed] [Google Scholar]
- 74.Hyman SE, Fenton WS. Medicine: what are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 75.Van Elzakker M, O’Reilly RC, Rudy JW. Transitivity, flexibility, conjunctive representations, and the hippocampus, I: an empirical analysis. Hippocampus. 2003;13:334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]