Abstract
Given that more than one-third of some cohorts of cancer survivors exhibit post-traumatic stress disorder (PTSD) symptomatology, this study examines how trauma outcomes might relate to quality of life (QOL). Eight hundred thirty survivors of adult lymphoma were assessed for PTSD, post-traumatic growth (PTG) and QOL. Structural equation modeling revealed that QOL was best explained by the model in which stressors (e.g., co-morbidities) were mediated by PTSD and PTG. Trauma outcomes mediated the relationship between specific stressors and QOL. These findings support using PTSD and PTG as a diagnostic framework in understanding symptomatology in survivors.
Keywords: Post-traumatic Stress Disorder (PTSD), post-traumatic growth (PTG), quality of life (QOL), structural equation modeling (SEM)
INTRODUCTION
The cancer experience (diagnosis, treatments, and post-treatment recovery/monitoring) is now categorized as a “traumatic event” according to the Diagnostic and Statistical Manual of Mental Disorders, (DSM-IV), in recognition that survivors are at increased risk for post-traumatic stress disorder (PTSD) symptomatology (American Psychiatric Association, 1994). Symptoms of PTSD such as re-experiencing distressing events (i.e., nightmares), avoiding cancer-related experiences such as office visits, and physiological arousal (i.e., easily startled or on edge) have been estimated in 5 to 8 percent of the adult survivors studied, and approximately 17% reported two or more symptoms (Cordova et al., 1995; Kornblith et al., 2003; Smith, Zimmerman, Williams, Preisser, & Clipp, 2008). In addition, Smith et al. (2008) found that 39% of their sample of long-term survivors of NHL exhibited symptoms (i.e., met diagnostic criteria for at least one PTSD domain). While research has largely focused on negative sequelae such as PTSD, a second focus has emerged on the initiation of positive changes resulting from the cancer experience; this is generally referred to as post-traumatic growth (PTG).
However, the effects of cancer-related outcomes including PTSD and PTG on physical, emotional, social and functional well-being and quality of life (QOL) remain largely unknown, despite the implied urgency brought about by the aging of the US population and subsequent doubling of the annual incidence from 1.3 million new cancer patients in 2000 to over 2.6 million by 2050 (Edwards et al., 2005). Furthermore, little is known about the QOL of individuals diagnosed with non-Hodgkin lymphoma (NHL), the sixth most common cancer in the US which has experienced a doubling in incidence rates since the early 1970’s (American Cancer Society, 2007). Therefore, the primary purpose of this exploratory study is to evaluate whether PTSD and PTG help to explain the role of stressors in relating to QOL in NHL survivors, thereby enhancing our understanding of the cancer experience so that processes could be targeted for intervention.
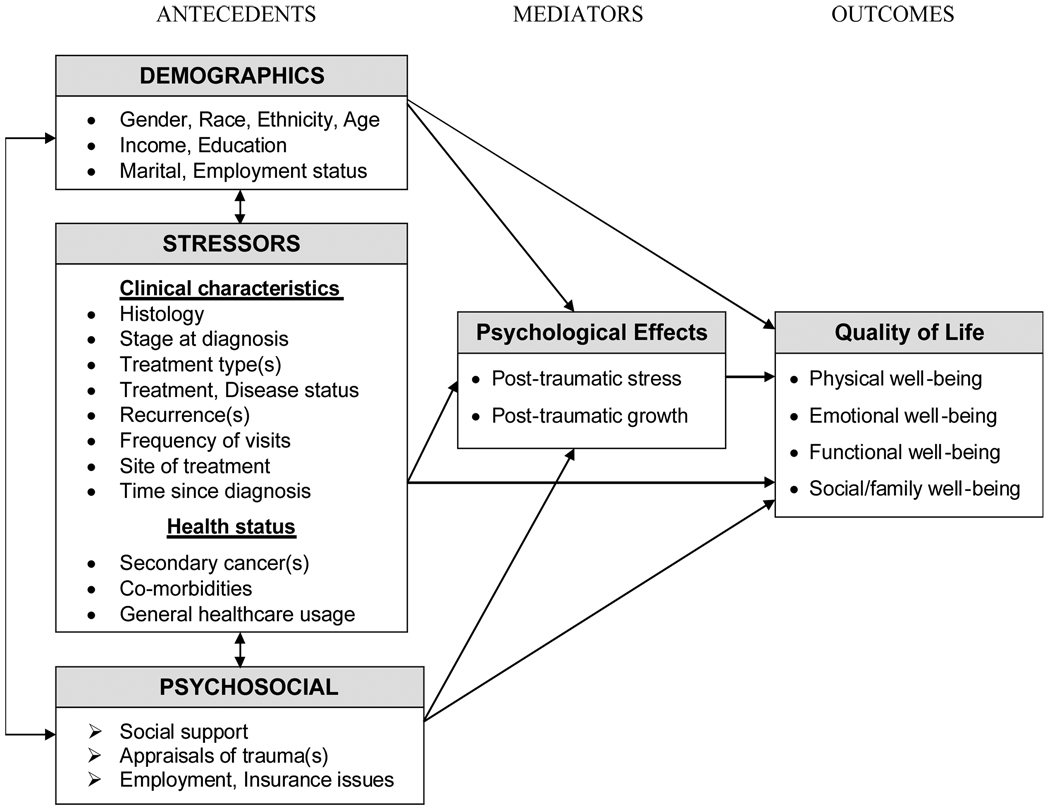
A conceptual model of QOL among cancer survivors is proposed in Figure 1, in which stress, coping, and adaptation theories (Lazarus & Folkman, 1984; Taylor & Aspinwall, 1996) emphasize the relationship between the person (demographic and psychosocial characteristics) and his or her environment (stressors). Psychological effects are a byproduct of this relationship, one of which occurs when the stress of cancer diagnosis and treatment is appraised as taxing or exceeding one’s resources and endangering one’s well-being. More specifically, the risk of developing cancer-related PTSD is directly affected by personal demographics and psychosocial characteristics and environmental stressors, which influence whether positive or negative coping strategies are employed. Alternatively, the effect can be growth inducing as in the case of PTG, which is characterized by positive changes in an individual as a result of a traumatic event. According to Techeschi and Calhoun (1996), PTG is more likely to develop when cognitive rebuilding takes into account the changed reality of one’s life after the trauma and produces schemas or views of the world as physically and psychologically safe.
FIGURE 1.
Conceptual Model of Quality of Life among Cancer Survivors
Building upon the theoretical base, antecedents identified in previous research studies that were related to QOL, PTSD, and PTG in cancer survivors were incorporated into the conceptual model (Ferrell, Dow, Leigh, Ly, & Gulasekaram, 1995; Ganz et al., 2002; Kornblith et al., 2003; Smith et al., 2008). Clinical characteristics such as cancer treatments received and health status are conceptualized as stressors, while social support, appraisals and insurance and employment-related issues represent psychosocial characteristics that can influence the outcome of these stressors. For example, the quality of social support can affect the individual’s likelihood of timely follow-up, in addition to influencing appraisals of life threat; these can either diminish or enhance the coping strategies employed by the individual, thereby leading to negative (PTSD) and/or positive (PTG) psychological effects.
More specifically, we hypothesize that PTSD and PTG directly relate to QOL and also mediate the effects of the antecedents on QOL. This hypothesis was tested using Structural Equation Modeling (SEM; Kline, 2005). While the primary aim of this exploratory study is to determine whether some or all of the antecedents have indirect and direct relationships with QOL (partial mediation), competing models are presented which examine if QOL can be better explained by the antecedents having only an indirect relationship (full mediation) or direct relationship (no mediation).
METHOD
Participants and Procedures
Potential study participants were identified through the Duke University and University of North Carolina (UNC) Lineberger Tumor Registries and contacted by mail following Institutional Review Board and physician approvals. Individuals were eligible for this study if they were diagnosed with adult NHL (≥19 years old at diagnosis, and ≥2 years post-diagnosis). Prospective participants were sent a self-administered questionnaire with other study-related materials.
Measures
Demographic, Clinical and Health Status Characteristics
Demographic information such as race, marital status and income was collected via self-report. The Tumor Registry databases were used to obtain details regarding diagnosis and treatment history. NHL histology was categorized as indolent or aggressive based on the REAL/WHO classification system (National Cancer Institute, 2007). The Self-administered Co-morbidity Questionnaire (SCQ; Sangha, Stucki, Liang, Fossel, & Katz, 2003) was used to assess other non-NHL health-related problems. In addition, selected questions were adapted for use from the Childhood Cancer Survivor Study survey (St Jude Children’s Hospital, 2008).
Psychosocial
The 20-item Medical Outcomes Study-Social Support Survey was used to measure the perceived availability of social support (Sherbourne & Stewart, 1991), with scores ranging from 20–100 (α=.97 in this study). The Appraisal of Life Threat and Treatment Intensity Questionnaire (ALTTIQ; six items, α=.80) was used to assess the extent to which cancer and its treatment are perceived to be life-threatening and intense (Stuber, Christakis, Houskamp, & Kazak, 1996). Finally, to assess employment and insurance-related situations and difficulties related to having had cancer (e.g., denials of health or life insurance applications, work promotions), 24 questions (α=.82) were derived from an instrument developed by a clinical research group (Kornblith et al., 1992).
Psychological Effects
The PTSD Checklist-Civilian Version (PCL-C) assesses symptomatology in non-combat populations by presenting a self-report symptom checklist that closely mirrors criteria set forth by the DSM-IV for a formal diagnosis of PTSD (American Psychiatric Association, 1994; Weathers, Litz, Herman, Huska, & Keane, 1993). The instructions were modified and survivors were asked to rate each PTSD symptom with respect to their diagnosis and treatment for lymphoma. In an effort to minimize scoring inflation due to treatment-related symptoms in the active disease sample, the symptom cluster scoring method was used, which employs a higher threshold for symptomatology and maps to the DSM-IV criteria for PTSD (American Psychiatric Association, 1994). The total score yielded an α=.91, and the internal consistency of the subscales were α=.88 (re-experiencing), α=.82 (avoidance), and α=.78 (arousal).
The Post-traumatic Growth Inventory (PTGI; Tedeschi & Calhoun, 1996) is a 21-item scale that was used to measure positive life changes and patients were instructed to indicate the degree to which they experienced these changes as a result of having lymphoma. The overall α=.96, with the five domains showing strong internal consistency: relating to others (α=.92); new possibilities (α=.88); personal strength (α=.86); spiritual change (α=.89); and appreciation of life (α=.80). Due to the length of the survey (28 pages) and respondent burden concerns, additional psychological variables (e.g., depression, anxiety, self-efficacy) were not included.
Quality of Life
The Functional Assessment of Cancer Therapy – General Version (FACT-G) is a 27-item self-report QOL measure with good reported internal consistency (Cella et al., 1993) and in our sample: physical well-being (α=.87); emotional well-being (α=.77); functional well-being (α=.88); social/family well-being (α=.80); and FACT-G total score (α=.93).
Statistical Methods
Pearson product moment correlations and associated statistical significance were calculated between QOL (FACT-G) and all continuous independent variables. T-tests or ANOVA compared mean FACT-G scores for those at different levels of potential categorical risk factors. Variables that were independently associated with the FACT-G score (P<.05 in the multiple linear regression model) were selected for inclusion in the SEM model, in congruence with the guidelines for testing mediation effects (Baron & Kenny, 1986). Missing data in three potential risk factors, income (10%), stage (13%), and disease status (10%) justified imputation, although the overall data set had a low level of missingness. Multiple imputation, via the Markov chain Monte Carlo (MCMC) algorithm, was used to impute values for missing data in the independent variables (excluding the mediators; Allison, 2001). Twenty datasets containing imputed values were used in the multiple linear regression and standard errors adjusted for imputation were estimated in the SAS MIANALYZE procedure (SAS Institute Inc., 2007).
The hypothesized mediation model of PTSD, PTG and QOL was tested and compared with two alternate models of no mediation and full mediation using MPLUS V4.2 (Muthen & Muthen, 2006) with the weighted least square means and variance adjusted method of estimation, an approach used when both categorical and continuous variables are included in a model. To assess the overall fit of the models, the following indices were examined: the Tucker-Lewis Index (TLI; Bentler & Bonett, 1980), the comparative fit index (CFI; Bentler, 1990), and the root mean square error of approximation (RMSEA; Steiger, 1990). Good fit is indicated by values of .90 or greater for the TLI and CFI (Hu & Bentler, 1999) and .05 or smaller for the RMSEA, while values between .05 and .08 represent adequate fit (Browne & Cudeck, 1993). Split-file analyses were conducted to confirm the absence of data-driven results. Data management and bivariate analyses were carried out with SPSS V14.0. Multiple imputation and multiple linear regression analyses were conducted using SAS V9.1.3.
RESULTS
Of the 1195 eligible survivors who were assumed to have received a mailed survey, 886 (74%) participated and provided informed consent. Participating survivors were less frequently African American (10% vs. 20%), older at study enrollment (mean age 62.8 vs. 58.8 years), and older at diagnosis (52.4 vs. 48.1 years) than non-participants (all at P<.001). The 830 survivors who completed the outcome measures were included in the analyses; their characteristics are listed in Table 1. For example, a similar number of females and males participated; 13.5% were non-Caucasian; 27.3% earned less than $30,000 annually; 40.1% had a college degree; and 40.7% were employed.
TABLE 1.
Characteristics of the Study Sample and Bivariate Associations with QOL (N=830)
| Variable | N | % | QOL a Mean or Correl. |
SD | P-value b |
|---|---|---|---|---|---|
| Demographics | |||||
| Gender | |||||
| Male | 418 | 50.3 | 85.5 | 16.9 | .917 |
| Female | 412 | 49.7 | 85.6 | 16.6 | |
| Race | |||||
| Caucasian | 718 | 86.5 | 86.3 | 16.3 | .002 |
| Non-Caucasian c | 112 | 13.5 | 80.5 | 18.7 | |
| Ethnicity | |||||
| Hispanic | 13 | 1.6 | 85.5 | 16.8 | .986 |
| Non-Hispanic | 817 | 98.4 | 85.5 | 16.8 | |
| Income level | r = .225 | <.001 | |||
| < $30,000 | 205 | 27.3 | 79.0 | 19.5 | ref<.001 |
| $30,000 – $59,999 | 229 | 30.5 | 86.1 | 16.5 | <.001 |
| $60,000 – $89,999 | 136 | 18.1 | 86.9 | 14.7 | <.001 |
| ≥ $90,000 | 181 | 24.1 | 90.0 | 13.7 | <.001 |
| Education | r = .115 | <.001 | |||
| High school or less | 228 | 27.8 | 82.3 | 18.2 | <.001 |
| Some college or trade school | 263 | 32.1 | 84.9 | 17.7 | .014 |
| College or post-grad | 328 | 40.1 | 88.3 | 14.4 | ref<.001 |
| Marital status | |||||
| Married | 618 | 74.6 | 86.5 | 16.4 | .005 |
| Not married d | 210 | 25.4 | 82.7 | 17.5 | |
| Employment status | |||||
| Employed | 334 | 40.7 | 87.6 | 15.7 | .005 |
| Not employed e | 487 | 59.3 | 84.3 | 17.3 | |
| Age at enrollment: mean (SD) | 62.8 | (13.3) | r = .099 | .054 | |
| 25–49 | 149 | 18.0 | 83.4 | 20.0 | ref.001 |
| 50–64 | 303 | 36.5 | 83.4 | 16.7 | .984 |
| 65–79 | 294 | 35.4 | 87.9 | 15.4 | .016 |
| ≥80 | 84 | 10.1 | 88.7 | 13.9 | .020 |
| Clinical Characteristics | |||||
| NHL histology | |||||
| Indolent | 414 | 52.7 | 84.8 | 17.3 | .097 |
| Aggressive | 372 | 47.3 | 86.8 | 16.1 | |
| NHL stage at diagnosis | r = −.077 | .038 | |||
| Stage I | 228 | 31.6 | 87.8 | 15.4 | ref<.202 |
| Stage II | 149 | 20.6 | 86.2 | 15.4 | .335 |
| Stage III | 145 | 20.1 | 84.9 | 18.5 | .101 |
| Stage IV | 200 | 27.7 | 84.7 | 17.3 | .049 |
| Sum of treatment types: mean (SD) | 2.1 | (1.1) | r = −.153 | <.001 | |
| Current treatment status | |||||
| Not in treatment | 711 | 86.6 | 86.7 | 16.3 | <.001 |
| Receiving treatment | 110 | 13.4 | 78.6 | 18.3 | |
| NHL disease status | |||||
| In remission or cured | 648 | 78.0 | 87.8 | 15.0 | <.001 |
| Not in remission | 102 | 12.3 | 75.2 | 20.0 | |
| Number of NHL recurrences | |||||
| 0 | 534 | 65.9 | 86.9 | 16.3 | .003 |
| ≥1 | 276 | 34.1 | 83.2 | 17.4 | |
| Frequency of NHL-related exams | r = −.234 | <.001 | |||
| Age at diagnosis: mean (SD) | 52.4 | (14.1) | r = .038 | .279 | |
| Range: | 19–87 | ||||
| Years since diagnosis: mean (SD) | 10.4 | (7.3) | r = .108 | .002 | |
| 2–4 yrs | 202 | 24.4 | 84.5 | 17.1 | ref.010 |
| 5–9 yrs | 307 | 37.0 | 84.6 | 17.4 | .909 |
| 10–14 yrs | 143 | 17.1 | 85.0 | 15.9 | .772 |
| 15–19 yrs | 85 | 10.3 | 85.8 | 17.1 | .533 |
| ≥20 yrs | 93 | 11.2 | 91.4 | 13.9 | <.001 |
| Health Status | |||||
| Secondary cancer | |||||
| Yes | 113 | 13.7 | 83.8 | 15.8 | .205 |
| No | 711 | 86.3 | 85.9 | 16.8 | |
| Co-morbidities: mean (SD) | 2.9 | (2.2) | r = −.387 | <.001 | |
| Years since last physical exam | r = −.119 | .001 | |||
| Psychosocial | |||||
| Social support: mean (SD) | 83.3 | (16.3) | r = .490 | <.001 | |
| Range: | 20–100 | ||||
| Appraisal of life threat and treatment intensity: mean (SD) | 19.4 | (5.9) | r = −.285 | <.001 | |
| Range: | 6–30 | ||||
| Employment and insurance issues related to cancer: mean (SD) | 1.1 | (2.1) | <.001 | ||
| Range: | 0–17 | ||||
| Psychological Effects | |||||
| PTSD symptom clusters: mean (SD) | 0.6 | (0.9) | r = −.621 | <.001 | |
| Range: | 0–3 | ||||
| Post-traumatic growth: mean (SD) | 60.4 | (24.6) | r = .133 | <.001 | |
| Range: | 0–105 | ||||
| FACT-G Outcomes: mean (SD) | |||||
| Physical | 22.8 | (5.6) | |||
| Emotional | 19.6 | (4.1) | |||
| Functional | 20.8 | (6.1) | |||
| Social/Family | 22.3 | (5.0) |
Note: Not all variables represent n=830 cases due to missing data; range, 722–830.
Quality of life as measured by the Functional Assessment of Cancer Therapy – General Version
P-values shown as superscripts are for the overall F-test for the categorical variable with k-1 degrees of freedom where k is the number of categories.
African-American, American Indian/Alaskan, Asian, and multiple races.
Widowed, separated, divorced, and single.
Unemployed and retired.
Relationship of QOL to Other Variables
Bivariate associations between QOL (FACT-G) and the independent variables are also given in Table 1. Among demographic and clinical variables, those who were non-Caucasian, had an annual income under $30,000, did not obtain a college degree, were not married, were not employed, were younger, were of a later stage at diagnosis, had more types of treatment, were currently receiving NHL treatment, had active disease, or had experienced at least one NHL recurrence had lower QOL (FACT-G scores; all P≤.01). Among the remaining variables, the strongest QOL relationships (all at P<.001) were for PTSD symptoms (r=−0.62), social support (r=0.49), and co-morbidity (r=−0.39).
As noted earlier, a multiple linear regression was conducted for the FACT-G total score and for those independent variables significant in the bivariate analyses (P<.05). Not having a college degree, not being employed, being a younger age at enrollment, having active NHL disease, more NHL-related visits, less time since diagnosis, more co-morbidity, less social support, more negative appraisals, and more insurance and employment issues were significantly related to lower FACT-G scores in the regression. The full model accounted for 50% of the variance.
Structural Equation Modeling
The measurement model included three latent variables (variables which are not measured directly): two exogenous variables (a variable that is not caused by another variable in the model; PTSD symptom clusters, PTG) and one fully endogenous variable (a variable that is caused by one or more variables in the model; QOL). PTSD symptom clusters had three categorical/binary indicators (re-experiencing, avoidance, arousal), PTG had five continuous indicators (relating to others, new possibilities, personal strength, spiritual change, appreciation of life), and QOL had four continuous indicators (physical, emotional, functional, social/family well-being).
The initial measurement model showed significant loadings of each observed indicator on its corresponding latent construct (all at P<.001). However, further examination of the loadings suggested that the social/family well-being indicator be dropped from QOL because its R-square (.285) was less than half of those of the remaining FACT-G domains (all at ≥ .599). Nevertheless, the social/family domain was retained due to theoretical considerations, and all four domains were included in the QOL construct for further analyses.
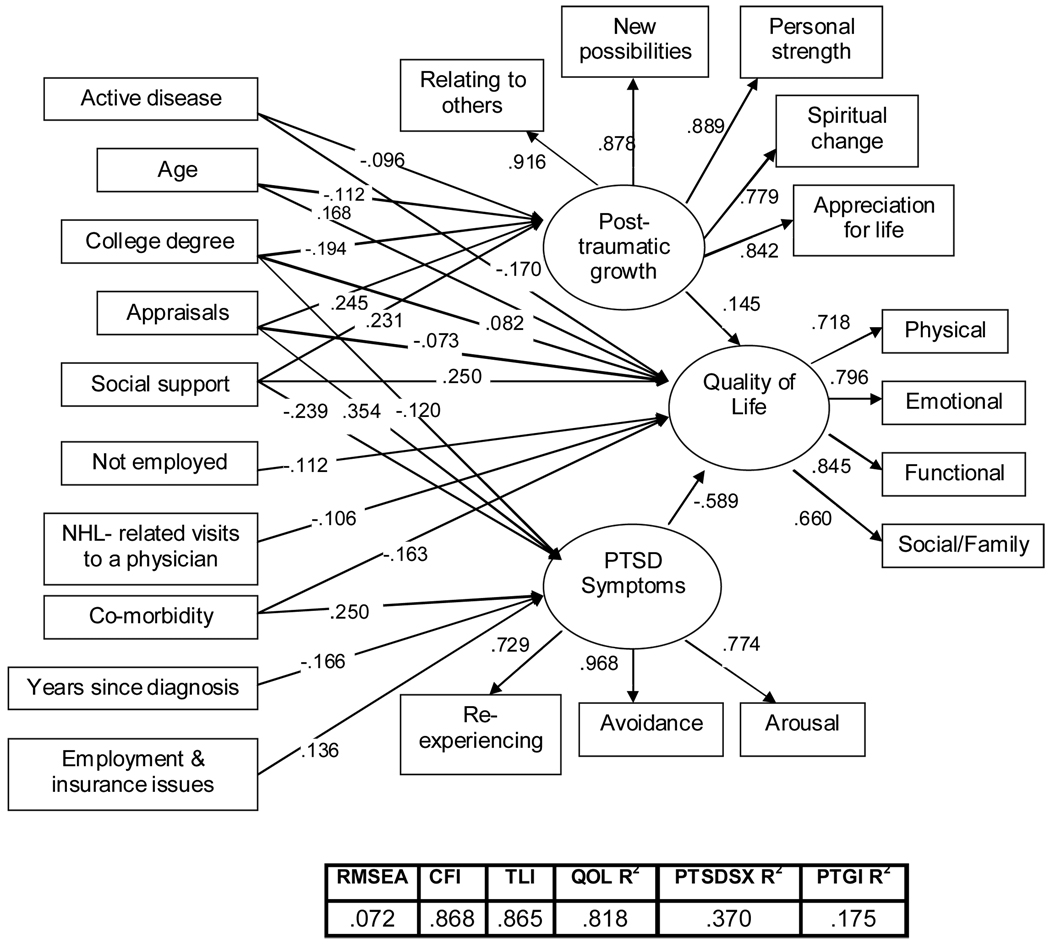
Initially, a simple, unmediated hybrid model (Model B) was tested which included only direct effects of the antecedents and mediators with QOL. As shown in Table 2, the model fit was poor, RMSEA=.104, CFI=.722, TLI=.723. However, all coefficient paths were statistically significant at P<.01, confirming the independent relationship of each variable to QOL. Next, the hypothesized partial mediation model (Model A) was tested, which included paths from the antecedents to the mediators and from the mediators to QOL. In addition, to allow for the possibility that the antecedents have relationships to QOL that are not mediated by PTSD and PTG, direct paths to QOL were included. The model fit was improved from the simple, unmediated Model B; RMSEA=.083, CFI=.827, TLI=.824. In addition, results from the split-file analysis were comparable to each other (RMSEA=.088, .090; CFI=.78, .80). The next step was to test the full mediation model (Model C), which included paths from the antecedents to the mediators and from the mediators to QOL. The model fit improved from the unmediated Model B, but slightly degraded from that of the partial mediation Model A; RMSEA=.084, CFI=.798, TLI=.818. The hypothesized Model A was trimmed to include only statistically significant paths (P<.05), which resulted in the best fitting model (Model A’), RMSEA=.072, CFI=.868, TLI=.865. As shown in Figure 2, Model A’ explained a sizable amount of the variance (81.8%) in QOL in NHL survivors.
TABLE 2.
Summary of Goodness-of-Fit Statistics for Comparative Models of QOL
| Comparison Models | Description | RMSEA | CFI | TLI |
|---|---|---|---|---|
| A | Partial mediation | .083 | .827 | .824 |
| B | No mediation | .104 | .722 | .723 |
| C | Full mediation | .084 | .798 | .818 |
| A´ | Trimmed Model A | .072 | .868 | .865 |
Note: Analyses conducted with imputed data. Model A analysis using the PTSD continuous scoring method yielded comparable results (RMSEA = .080).
FIGURE 2.
The Final Model
Note: Weighted least squares estimates for each path are significant at the P<.05 level; non-significant paths are excluded. Analyses conducted with imputed data.
In examining Figure 2 and using recommendations by Cohen (1988) and Kline (2005) about interpretations of the absolute magnitudes of path coefficients, “small” effect is indicated by standardized values <.10, “typical” or “medium” effect by values around .30, and “large” effects by values ≥ .50, we can conclude that of the mediators, PTG had a small (positive) effect while PTSD symptoms had a large (negative) effect on QOL. More specifically, PTG mediated the relationship between appraisals, social support, college education, age, and active disease on QOL while PTSD symptoms mediated some of the same variables (appraisals, social support, and education) in addition to co-morbidity, years since diagnosis and employment and insurance issues. Only two of the antecedents (not being employed and NHL-related visits to a physician) were not mediated by either PTSD symptoms or PTG; each had only a small direct (negative) relationship to QOL.
The direct and indirect relationships of demographic and clinical variables to QOL were small to medium, while health status (co-morbidity) and psychosocial (appraisals, social support) variables had greater (medium) direct and indirect relationships. For example, individuals with more social support reported more PTG, fewer PTSD symptoms and greater QOL, while those with more negative appraisals of life threat and treatment intensity reported more PTG and PTSD symptoms and lower QOL (all at P<.05).
As demonstrated by these results, PTG and PTSD symptoms were significant mediators between the antecedents and QOL. This is substantiated by the smaller direct parameter estimates of these variables in the partially mediated model (Model A) than in the unmediated model (Model B). In addition, the paths to and from the mediated variables in Model A’ were significant (all at P<.05). Furthermore, the indirect (mediated) relationships were significant (all at P<.05), according to the conservative Sobel test (Preacher & Leonardelli, 2006).
DISCUSSION
This is the first study to examine the relationship between cancer-related trauma outcomes and QOL. Our results show that PTSD symptoms and PTG help to explain the relationship between specific antecedents and QOL. Although there is an absence of comparable studies in cancer survivors, studies of war veterans have similarly found PTSD to mediate the relationship between antecedents and QOL (Magruder et al., 2004; Schnurr, Hayes, Lunney, McFall, & Uddo, 2006; Zatzick et al., 1997). Additionally, several studies conducted with cancer survivors found negative relationships between PTSD and QOL (Amir & Ramati, 2002; Meeske, Ruccione, Globe, & Stuber, 2001; Okamura, Yamawaki, Akechi, Taniguchi, & Uchitomi, 2005; Santos, Kozasa, Chauffaille Mde, Colleoni, & Leite, 2006), yet no such studies were identified in a Medline search of PTG and QOL. The dearth of literature regarding the association between PTG and QOL may be reflective of the more recent development of psychosocial models, as opposed to more traditional medical models that incorporate PTSD as a diagnostic disorder.
The Lazarus & Folkman (1984) and Taylor and Aspinwall (1996) theories appear to be a sound foundation to explain the relationships which resulted from the multiple linear regression, in which lower levels of resources (e.g., less social support, negative appraisals) were associated with lower QOL. In addition, the QOL model that we tested (Figure 2) explained a large amount of the variance in well-being (82%), indicating that it includes many key variables and is relevant to NHL survivorship. Similar to Northouse et al. (2002), we found that many of the clinical variables (e.g., histology) contributed little or no unique variance in the survivors’ QOL, while social support, appraisals, co-morbidity and PTSD symptoms were important components of the model. Future clinical and research activities need to consider the significant relationship of these variables to survivors’ QOL.
The testing of alternative SEM models describing the pathways between the antecedents, mediators and QOL led to a reduction in the number of paths, thereby enhancing parsimony. It also elucidated the relationship between variables; for example, we found that some had a direct path to QOL, others had an indirect path, and still others had both direct and indirect paths to QOL. It is through understanding and testing for mediation that we can begin to untangle the mechanisms and processes by which the cancer experience affects an individual’s QOL.
As stated by Baron and Kenny (1986), mediator variables are those that “speak to how or why such effects occur”, representing processes that could be targeted for intervention. For example, our model suggests that individuals with active disease or of an older age could be targeted for interventions aimed at enhancing PTG, while those without a college degree, more co-morbidity, more recent diagnosis or more employment and insurance issues related to cancer might benefit from treatments to reduce PTSD symptomatology as a means to improving overall QOL. In addition, individuals with less social support could benefit from interventions focused on enhancing PTG and social support, and reducing PTSD symptoms.
Several limitations warrant caution in interpreting our findings. First, since this study was cross-sectional, no definitive inferences can be made about the direction of causality between PTSD, PTG and QOL. However, our findings are consistent with the literature regarding the effects of traumatic stress on QOL and our interpretation of these findings is guided by a theoretical framework. In addition, certain variables (e.g., age and appraisals of life threat and treatment intensity at diagnosis) allow us to infer the direction of causality more than other variables (e.g., current age and level of social support). Still, longitudinal studies are needed to establish and support evidence of causality. Second, the exclusion of additional psychological variables (e.g., depression, anxiety) limits our conclusions in that these symptoms also might be important in explaining QOL. However, given the exploratory nature of this study, it was not possible to include every psychological variable of interest. Third, although several correlations reported in Table 1 were statistically significant (.115<r <.300), this was attributed more to the large sample size than the actual strength of the correlation. Fourth, although our sample was representative of NHL patients treated at two major comprehensive cancer centers in North Carolina, the findings cannot be generalized beyond this specialized population. For example, replication in other geographical areas with different types of cancer diagnoses is needed to confirm the roles of PTSD and PTG in QOL. Finally, despite our finding of adequate model fit and high R2, other theoretical frameworks may yield equivalent or better fit and could be considered in future research studies. Of note, an additional model was tested (a revision of Model A’ without the social/family FACT-G domain) which yielded a good fit, RMSEA =.050, CFI=.938, TLI=.932. This would suggest that alternative measures of QOL be considered in replication studies, as the FACT-G measurement model results were weaker than those of the remaining model components.
Implications for Clinical Practice
Despite these limitations, our findings indicate that PTSD and PTG mediated the relationship between antecedents and QOL and our conceptual model accounted for a large amount of variance in the survivors’ QOL. These findings give support to using PTSD as a diagnostic framework (and PTG, to a lesser extent) in understanding symptomatology in this population. In addition, several covariates were identified that either directly or indirectly through PTSD and/or PTG relate to QOL. Furthermore, proven therapies could be modified for use with cancer survivors to include methods that enhance PTG and address the unique features of cancer-related PTSD. For example, cognitive behavioral (CBT) and prolonged exposure techniques have been shown to be effective in reducing or eliminating PTSD symptoms in survivors of sexual assault (Foa & Meadows, 1997). Targeted treatments could be developed and implemented that minimize future-oriented intrusions (fear of recurrence, test anxiety) and enhance coping skills as survivors navigate through a range of possible aversive events such as treatments, recurrence(s), and medical surveillance.
However, given the absence of “good fit”, additional research using alternate measures and in other cancer samples is needed to improve the robustness of our findings. In addition, studies that follow patients over time are needed to help establish causality and determine if targeting PTSD symptom reduction and PTG enhancement could assist individuals in improving their QOL along the survivorship trajectory. This would help determine if efforts towards reducing PTSD symptomatology and enhancing PTG in cancer survivors as a means to improve QOL are warranted.
Acknowledgments
Funding: This research was supported by the National Cancer Institute (CA101492), Cancer Care Quality Training Grant (CA116339), American Cancer Society (DSW-0321301-SW), National Research Service Award (T32-HS000032), and the University of North Carolina Research Council. The authors wish to acknowledge the support of the non-Hodgkin lymphoma survivors who participated in our study and the project staff for their help in identifying and locating the eligible individuals for our study.
REFERENCES
- Allison PD. Missing data. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- American Cancer Society. What are the key statistics about non-Hodgkin’s lymphoma? August 2007. [Retrieved November 19, 2008];2007 from http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_non-Hodgkins_lymphoma_32.asp?sitearea=
- American Psychiatric Association. Diagnostic and statistical manual-4th edition (DSM-IV) Washington, DC: American Psychiatric Association; 1994
- Amir M, Ramati A. Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: A preliminary research. Journal of Anxiety Disorders. 2002;16(2):195–206. doi: 10.1016/s0887-6185(02)00095-6. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Academic Press; 1988. [Google Scholar]
- Cordova MJ, Andrykowski MA, Kenady DE, McGrath PC, Sloan DA, Redd WH. Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. Journal of Consulting and Clinical Psychology. 1995;63(6):981–986. doi: 10.1037//0022-006x.63.6.981. [DOI] [PubMed] [Google Scholar]
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. Journal of the National Cancer Institute. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncology Nursing Forum. 1995;22(6):915–922. [PubMed] [Google Scholar]
- Foa EB, Meadows EA. Psychosocial treatments for posttraumatic stress disorder: A critical review. Annual Review of Psychology. 1997;48:449–480. doi: 10.1146/annurev.psych.48.1.449. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. Journal of the National Cancer Institute. 2002;94(1):39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Guidelines, issues, and alternatives. Journal of Organizational Behavior. 1999;18:667–683. [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2nd ed. New York: Guilford Press; 2005. [Google Scholar]
- Kornblith AB, Anderson J, Cella DF, Tross S, Zuckerman E, Cherin E, et al. Hodgkin disease survivors at increased risk for problems in psychosocial adaptation. the cancer and leukemia group B. Cancer. 1992;70(8):2214–2224. doi: 10.1002/1097-0142(19921015)70:8<2214::aid-cncr2820700833>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Herndon JE, 2nd, Weiss RB, Zhang C, Zuckerman EL, Rosenberg S, et al. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98(4):679–689. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- Magruder KM, Frueh BC, Knapp RG, Johnson MR, Vaughan JA, 3rd, Carson TC, et al. PTSD symptoms, demographic characteristics, and functional status among veterans treated in VA primary care clinics. Journal of Traumatic Stress. 2004;17(4):293–301. doi: 10.1023/B:JOTS.0000038477.47249.c8. [DOI] [PubMed] [Google Scholar]
- Meeske KA, Ruccione K, Globe DR, Stuber ML. Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncology Nursing Forum. 2001;28(3):481–489. [PubMed] [Google Scholar]
- Muthen L, Muthen B. MPlus. Los Angeles: 2006. [Google Scholar]
- National Cancer Institute. Adult non-Hodgkin’s lymphoma (PDQ): Treatment. November 2008. [Retrieved November 19, 2008];2007 from http://www.cancer.gov/cancertopics/pdq/treatment/adult-non-hodgkins/HealthProfessional/page2#Section_17.
- Northouse LL, Mood D, Kershaw T, Schafenacker A, Mellon S, Walker J, et al. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2002;20(19):4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- Okamura M, Yamawaki S, Akechi T, Taniguchi K, Uchitomi Y. Psychiatric disorders following first breast cancer recurrence: Prevalence, associated factors and relationship to quality of life. Japanese Journal of Clinical Oncology. 2005;35(6):302–309. doi: 10.1093/jjco/hyi097. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Leonardelli GJ. Calculation for the sobel test. 2006. [Retrieved 7/20, 2007]. from http://www.psych.ku.edu/preacher/sobel/sobel.htm. [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- Santos FR, Kozasa EH, Chauffaille Mde L, Colleoni GW, Leite JR. Psychosocial adaptation and quality of life among brazilian patients with different hematological malignancies. Journal of Psychosomatic Research. 2006;60(5):505–511. doi: 10.1016/j.jpsychores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Documentation: The MIANALYZE procedure. November 2008. [Retrieved November 19, 2008];2007 from http://support.sas.com/onlinedoc/913/docMainpage.jsp.
- Schnurr PP, Hayes AF, Lunney CA, McFall M, Uddo M. Longitudinal analysis of the relationship between symptoms and quality of life in veterans treated for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2006;74(4):707–713. doi: 10.1037/0022-006X.74.4.707. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine (1982) 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Smith SK, Zimmerman S, Williams CS, Preisser JS, Clipp EC. Post-traumatic stress outcomes in non-hodgkin's lymphoma survivors. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2008;26(6):934–941. doi: 10.1200/JCO.2007.12.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jude Children’s Research Hospital. Childhood cancer survivor study, November 2008. [Retrieved November 19, 2008];2008 from http://www.stjude.org/stjude/v/index.jsp?vgnextoid=2c1325ca7e883110VgnVCM1000001e0215acRCRD.
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Stuber ML, Christakis DA, Houskamp B, Kazak AE. Posttrauma symptoms in childhood leukemia survivors and their parents. Psychosomatics. 1996;37(3):254–261. doi: 10.1016/S0033-3182(96)71564-5. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Aspinwall LG. Mediating and moderating processes in psychosocial stress: Appraisal, coping, resistance and vulnerability. In: Kaplan HB, editor. Psychosocial stress: Perspectives on structure, theory, lifecourse and methods. San Diego, CA: Academic Press; 1996. pp. 71–110. [Google Scholar]
- Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress. 1996;9(3):455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz B, Herman D, Huska JA, Keane TM. The PTSD checklist (PCL-C): Reliability, validity and diagnostic utility. Presented at the 9th Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- Zatzick DF, Marmar CR, Weiss DS, Browner WS, Metzler TJ, Golding JM, et al. Posttraumatic stress disorder and functioning and quality of life outcomes in a nationally representative sample of male vietnam veterans. The American Journal of Psychiatry. 1997;154(12):1690–1695. doi: 10.1176/ajp.154.12.1690. [DOI] [PubMed] [Google Scholar]