Abstract
Purpose of review
Renin cells are fundamental for the control of blood pressure, fluid electrolyte homeostasis and kidney development. This review discusses recent discoveries regarding the mechanisms that control the identity and fate of renin cells and their role in the maintenance of kidney architecture and function.
Recent findings
It is now established that cyclic AMP is a crucial factor for the regulation of the renin phenotype. Furthermore, additional factors such as microRNAs and gap junctions have recently emerged as key regulators for the maintenance and proper functioning of renin cells.
Summary
Experiments described in this review will hopefully raise new questions regarding the mechanisms that control the identity, plasticity and function of renin cells.
Keywords: cell identity, cell memory, cyclic AMP, microRNAs, plasticity
Introduction
Renin cells are specialized myoepithelioid granulated cells that synthesize, store, process and release renin, the key regulated enzyme of the renin–angiotensin system (RAS) that controls blood pressure (BP) and fluid–electrolyte homeostasis. In addition to their homeostatic/hemodynamic roles, renin cells participate in the assembling, branching and elongation of the renal arterioles [1,2]. In the adult mammalian kidney, renin cells are restricted to the wall of the afferent arteriole at the entrance to the glomerulus, thus their name juxtaglomerular cells. However, in early embryonic and fetal life, renin cells are broadly distributed along intrarenal arteries, inside the glomeruli and in a subset of tubular cells [3,4]. As development progresses, renin cells become restricted to the adult juxtaglomerular localization by differentiating into vascular smooth muscle cells, mesangial cells and a subset of tubular cells [5]. In response to challenges to homeostasis, adult animals are capable of increasing the number of renin cells, a phenomenon known as recruitment [6], by dedifferentiation of those cells derived from the renin lineage as mentioned above [5]. The ability of the cells from the renin lineage to de-differentiate and reacquire the renin phenotype suggests that the cells retain the memory to re-enact a developmental program when more renin is required to maintain BP, the constancy of the internal milieu or both. The mechanisms that govern the identity, positional information and plasticity of renin cells are discussed below.
Identity, plasticity and maintenance of renin cells
Several factors that regulate the acquisition of the identity, maintenance and developmental distribution of renin cells have recently been identified.
The cyclic AMP pathway
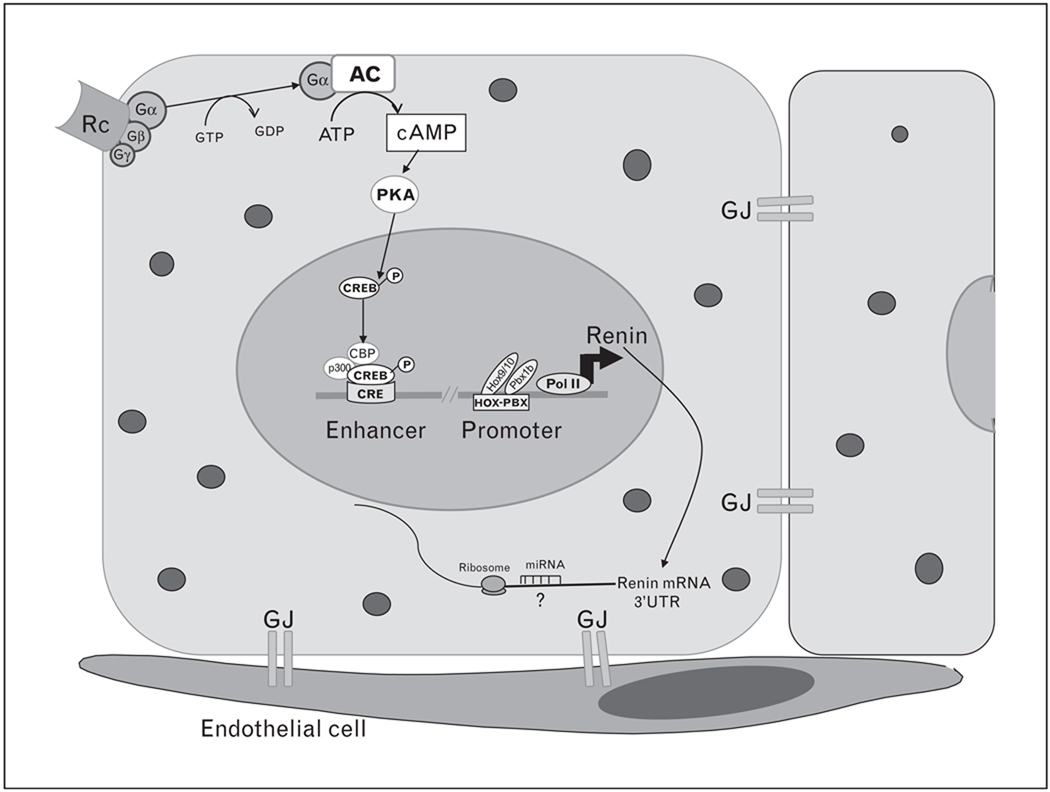
Cyclic AMP (cAMP) regulates a wide range of biological processes. Endogenous cAMP is generated from ATP after the stimulation of membrane-bound G protein-coupled receptors with the subsequent activation of adenylyl cyclase (Fig. 1). cAMP activates protein kinase A, which phosphorylates cAMP responsive element (CRE) binding (CREB) protein, which modulates renin transcription after binding to the CRE in the renin promoter. In addition to transcription and regulation of mRNA stability, cAMP also regulates renin release.
Figure 1. Major events in renin expression.
Activation of a G protein-coupled Rc by its ligand leads to conformational change transmitted to a G protein complex. The Gsα subunit is released from the complex after conversion of GTP to GDP and activates the AC, which in turn converts ATP in the second messenger cAMP. cAMP activates PKA that phosphorylates the CREB in the nucleus. Phosphorylated CREB recruits CBP and p300 and binds to the CRE in the enhancer region of the renin promoter, switching on renin mRNA transcription. At the proximal promoter, Hox and Pbx 1b paralogs bind to the HOX–PBX site and also initiate renin transcription. Posttranscriptional regulation at the 3’UTR occurs by binding proteins that increase renin mRNA stability (not depicted) and by miRNA, which repress renin translation, degrade renin mRNA or both. cAMP also stimulates renin release (not depicted). For ease of reading most of the binding sites and their interactions along the promoter are not depicted. AC, adenylyl cyclase; cAMP, cyclic AMP; CRE, cAMP responsive element; CREB, cAMP responsive element binding protein; GJ, gap junction; miRNA, microRNA; PKA, protein kinase A; Pol II, polymerase II; Rc, receptor; UTR, untranslated region.
Using renal microvessels and single renin cells subjected to the reverse hemolytic plaque assay to study renin released by individual cells, we demonstrated that stimulation of adenylyl cyclase with forskolin increased renin mRNA levels and renin release by increasing the number of cells that express and release renin [7], suggesting that the increase in circulating renin to maintain homeostasis is due mainly to an increase in the number of renin cells rather than to a substantial increase in the amount of renin per cell. Using dually labeled cells, we showed that cAMP is crucial for the reacquisition of the renin cell phenotype in vitro [8••].
Whereas β-adrenergic receptors are important for the maintenance of circulating renin basal levels [9], in the absence of β1/β2-adrenergic receptors, we found that the number of renin cells is not affected and the cells maintain their ability to respond to homeostatic challenges [9]. However, Gsα plays a fundamental role [10]. Conditional deletion of Gsα in renin cells demonstrated a marked decrease in renin cells, circulating renin with hypotension and lack of response to known stimulators of renin release [10]. The lack of Gsβ in renin cells results in an almost absence of renin expression from embryonic life with the attendant alterations of the preglomerular arterial tree [11•], emphasizing the important role of this G protein during kidney development.
In response to challenges to homeostasis, cells derived from the renin lineage maintain the plasticity to switch back to a renin phenotype and to differentiate again when the crisis passes. To identify the molecular events underlying this process, we developed a cell system that permits the study of the mechanism involved in the switching off and on of the renin gene. In this system, arteriolar smooth muscle cells derived from the renin lineage are permanently labeled with cyan fluorescent protein (CFP) and, when the cells actively transcribe renin, they are also labeled with yellow fluorescent protein (YFP) [8••]. We showed that, upon exposure to cAMP analogues, the CFP-positive cells re-expressed renin mRNA and became YFP positive. The re-expression of renin mRNA was accompanied by downregulation of β-smooth muscle actin, smoothelin and myosin heavy chain, indicating a return from a contractile to an endocrine phenotype [8••]. Activation of renin gene transcription by cAMP required chromatin remodeling with acetylation of histone 4, opening of chromatin and binding of CREB at the CRE site in the renin promoter [8••]. Access of CREB to the CRE was facilitated by action of histone acetyl transferases CREB-binding protein (CBP) and p300, known as coactivators of CREB.
The crucial role of CBP and p300 in vivo was demonstrated by simultaneous deletion of both histone acetyl transferases in renin cells that resulted not only in a remarkable decrease in the number of renin cells but also in reduced renal growth, cystic glomeruli, abnormal vessels, extensive fibrosis and renal failure [12••]. The renal abnormalities observed in these animals might be due in part to the lack of renin cells in combination with the lack of CBP and p300 in cells derived from the renin lineage, such as arteriolar smooth muscle, mesangial and tubular cells. Some of the abnormalities, including thin vessels, areas of undifferentiated tissue and abnormal Bowman’s capsule, differ from the renin knockout mice previously reported [13,14,15••], indicating that the abnormalities are not exclusively due to the lack of renin (Table 1 [10,11•,12••,13,14,15••,16–19,20••,21••,22–24]). Interestingly, mice with ablation of renin cells using diphtheria toxin [19] also showed thin vascular walls, suggesting that the renin cell per se may have additional functions for vascular growth.
Table 1.
In-vivo deletion of key regulatory molecules and their comparison with renin–angiotensin system knockouts
| Mouse strains | Circulating renin |
Kidney renin |
Renal abnormalities | Blood pressure | Ref | |
|---|---|---|---|---|---|---|
| Parenchyma | Renal arterial tree | |||||
| Ren1c knockoout | Absent | Absent | Severe hydronephrosis, medullary atrophy, interstitial fibrosis, perivascular lymphocytic infiltration | Thick wall | Decreased | [13,14] |
| Ren1c (s) knockout | NR | Absent | Severe hydronephrosis | NR | Decreased | [15••] |
| Angiotensinogen knockout | Increased | Increased | Hydronephrosis, papillary atrophy, lymphocytic infiltration | Thick wall | Decreased | [16] |
| ACE knockout | Increased | Increased | Hydronephrosis, medullary and papillary atrophy, interstitial fibrosis | Thick wall | Decreased | [17] |
| AT1aþAT1b knockout | Increased | Increased | Medullary atrophy | Thick wall | Decreased | [18] |
| Renin DTA | Reduced | Almost absent | Glomerular and tubular atrophy, areas of undifferentiated cells | Normal to thinner | Decreased | [19] |
| Dicer conditional knockout (renin cells) | Reduced | Almost absent | Characteristic striped renal fibrosis, glomerulosclerosis, glomerular and tubular dilatation, tubular atrophy | Distorted or absent vessels | Decreased | [20••] |
| Cbp/p300 conditional knockout (renin cells) | Reduced | Almost absent | Cystic glomeruli, abnormal vessels, extensive fibrosis | Thin wall | Decreased (unpublished) | [12••] |
| Gsα conditional knockout (renin cells) | Reduced | Almost absent | NR | Total number reduced and shorter | Decreased | [10,11•] |
| PPARγ conditional knockout (renin cells) | Increased | Increased | NR | Normal | Normal | [21••] |
| Connexin40 knockout | Increased | Increased | Displacement of renin cells to the extraglomerular mesangium | NR | Increased | [22–24] |
ACE, angiotensin-converting enzyme; NR, not reported; PPARγ, peroxisome proliferator-activated receptor-gamma.
Overall, those studies confirmed that the coactivators CBP and p300 are crucial for the expression and maintenance of the renin cell and validated the in-vitro data that demonstrated the essential role of the cAMP/CREB pathway for the renin cell phenotype.
Dicer and microRNAs
microRNAs (miRNAs) are a group of small noncoding RNAs, 18–22 nucleotides in length, that regulate gene expression by posttranscriptional/translational repression. These regulatory molecules are involved in many biological processes, including cell differentiation, cell proliferation, apoptosis, cancer and morphogenesis [25,26,27••]. The biogenesis of miRNAs involves the transcription in the nucleus by polymerase II and sequential enzymatic cleavage of a primary miRNA (about 100–1000 nucleotides in length) by two protein complexes involving RNase III endonucleases: the microprocessor complex of Drosha–DGCR8 in the nucleus and Dicer in the cytoplasm. As deletion of Dicer is embryonically lethal, conditional deletion studies of this enzyme have been necessary to determine whether miRNAs play a role in the development, differentiation and/or function of specific cell types, tissues and organs.
To define whether Dicer plays a role in the renin cells, we generated mice with conditional deletion of Dicer in cells of the renin lineage [20••]. These mice showed an almost absence of renin cells in the kidney with decreased expression of the renin genes, circulating renin and hypotension. In addition, they had severe renal vascular and glomerular abnormalities, some of them very different from those observed when RAS genes are deleted (Table 1 [13,14,16–18]). They had prominent stripes of renal fibrosis that replace the renal arterioles and, when present, the vessel wall was thin rather than hyperplastic/hypertrophic as observed in mice with deletion of any of the RAS genes (Table 1). As mentioned above, deletion of bothCBP/p300 in renin cells and targeting of diphtheria toxin in the renin locus also resulted in marked depletion or absence of renin cells. The fact that mice with deletion of renin per se, which is implicated in nephrovascular development [13,14], showed massive hypertrophy of themedial layer of the intrarenal arterioles suggests that the abnormalities observed inmice with conditional deletion of Dicer in renin cells are not due solely to the lack of renin but mainly to the lack of renin cells that are likely to produce additional factors (independent of renin) with hypertrophic/hyperplasic effects in smooth muscle cells of the renal arterial tree.
Overall, these results uncovered an essential role for miRNAs in the maintenance of the renin cell phenotype and the structural integrity of the kidney, and generated a new model of renal fibrosis in the absence of hypertension, activation of the RAS or both [20••].
Transcription factors and tissue specificity
Several transcription factors involved in the intricate regulation of renin gene expression at the enhancer region or proximal promoter of the renin gene have been extensively characterized [8••,28,29,30••,31,32]. Recent exciting discoveries are discussed below.
Peroxisome proliferator-activated receptor-gamma
The nuclear receptor, peroxisome proliferator-activated receptor-gamma (PPARγ), seems to control renin transcription through sites in both the enhancer and proximal promoter [33,34]. Recently, confirming previous in-vitro studies [33,34], Desch et al. [21••] using a cre-loxP system approach showed in vivo that conditional deletion of PPARγ in juxtaglomerular cells results in an increase in renin mRNA expression as well as in circulating renin. However, despite the increase in circulating renin, these mice did not show differences in BP or renal vascular resistance. Overall, those studies suggest an important role for PPARγ in the control of renin transcription in juxtaglomerular cells.
Tumor necrosis factor-alpha and reactive oxygen species
It has been shown that the cytokine tumor necrosis factor-alpha (TNFα) has a potent inhibitory effect on renin expression through the activation of nuclear factor kappa B (NFκB) by reducing the transactivating capacity of NFκB-p65 and attenuating CREB binding to CRE [35–37]. Recently, Itani et al. [38••] further explored the effects of TNFα on renin expression through the production of reactive oxygen species (ROS). Using the As4.1 cells, luciferase reporter assays and microarray analysis, this study suggested that the inhibitory effect of TNFα on renin expression is mediated not only through NFκB but also through the direct production of ROS that may in turn modulate the activity of CREB [38••].
RP-2/proximal promoter element (HOX–PBX) site
A recent study by Glenn et al. [30••] using a bacterial artificial chromosome (BAC) system expressing green fluorescent protein driven by the Ren1c promoter showed, by deletion of key elements of the renin enhancer region (including the CRE site and adjacent E-Box) and a two nucleotide mutation of the RP-2/proximal promoter element (PPE) site (HOX–PBX site, where Pbx1b and Hox Abd-B paralogs bind within the PPE), that both the renin enhancer and the PPE are important for basal expression of renin within the kidney. Whereas deletion of the renin enhancer affected renin expression in multiple tissues, the HOX–PBX site was not required for renin expression in the submandibulary gland. Using a similar approach, Tanimoto et al. [39••] generated transgenic mice with a single-nucleotide mutation at the RP-2/PPE site in a BAC system and showed that this site is essential for kidney-specific renin expression but not for extrarenal expression of renin. Thus, using highly sensitive reporting systems, those two laboratories showed in vivo that, although the enhancer is important for regulating baseline renin expression, the HOX–PBX site is critical for the tissue specificity of renin expression.
Positional information: gap junctions and connexins
Gap junctions are specialized connections between the cytoplasm of two adjacent cells that permit the free passage of ions and small molecules. They are composed of two connexons or hemichannels (contributed one per cell), which are homohexamers or heterohexamers of connexin proteins.
Juxtaglomerular cells are connected by gap junctions among themselves and with other cell types: endothelial, smooth muscle and mesangial cells. Their most abundant connexin is connexin (Cx) 40, which is expressed early in fetal life [40,41]. The adult juxtaglomerular cells also express Cx37 and Cx43 [41]. Deletion of Cx40 in mice resulted in hyperreninemic hypertension accompanied by an increase in the number of renin cells that are unusually located within the periglomerular interstitium and contain fewer and smaller secretory granules [22,23]. Cx40-mutant mice do not respond properly to the mechanisms that regulate renin release. They are less sensitive to angiotensin II and they display an inverted, paradoxical response to changes in perfusion pressure [24], raising the question of whether the renal baroreceptor mechanism that controls renin release may be, at least, partially dependent on gap junctions [42].
Recent studies [43•,44] showed that replacement of Cx40 by Cx45 (a connexin with a lower conductivity) partially rescues the defect by the re-establishment of the location of juxtaglomerular cells; however, the mice remain hypertensive. On the other hand, the study [45•] of mice with deletion of Cx37 indicated that this connexin is dispensable for the regulation of renin expression and positioning of the renin cell.
Overall, these studies suggest an important role for connexins and gap junctions in renin cell positional information and renin release. Thus, so far, Cx40 seems to be the most relevant one for the renin cell. Future experiments of conditional and time-specific deletions of single or combinations of connexins are needed to determine their specific role in the regulation of the renin expression, synthesis and release and in response to mechanical and humoral factors [42].
Intracellular versus secreted renin
Secretion of renin depends on the presence of a signal peptide in the renin protein that is encoded in the first exon (exon 1a). A renin isoform that is transcribed from an alternate exon (exon 1b) located in the first intron lacks the signal peptide and is, hence, intracellular, and is expressed in the brain and other organs [46,47]. A recent study from Xu et al. [15••] was designed to differentiate the role of secreted versus intracellular renin. The generation of a mouse with exon 1a of the Ren1c gene floxed bred to mice that express Cre recombinase in the early embryo (E2A-cre) allowed the specific deletion of the secreted renin with preservation of the intracellular renin [15••]. These mice showed a phenotype almost indistinguishable from the Ren1c null mice, indicating an essential role for secreted renin that cannot be compensated by the presence of intracellular renin.
Conclusion
As reviewed above, significant advances have recently been made in understanding the mechanisms that control renin expression, renin cell identity and renin cell plasticity.
Several questions remain to be answered: What are the signals and mechanisms that control the developmental pattern of renin distribution? Which are the participants in the network of genes and signaling systems that control the identity of the renin cell? Which are the individual, combination of miRNAs or both that control renin expression and maintenance of the juxtaglomerular cell? And, how does the renin cell contribute to renal vascular development?
Acknowledgement
The authors are thankful to the National Institutes of Health for grants DK75481 (to M.L.S.S.L.) and HL066242 (to R.A.G).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 409–410).
- 1.Reddi V, Zaglul A, Pentz ES, Gomez RA. Renin-expressing cells are associated with branching of the developing kidney vasculature. J Am Soc Nephrol. 1998;9:63–71. doi: 10.1681/ASN.V9163. [DOI] [PubMed] [Google Scholar]
- 2.Sequeira Lopez ML, Gomez RA. The role of angiotensin II in kidney embryogenesis and kidney abnormalities. Curr Opin Nephrol Hypertens. 2004;13:117–122. doi: 10.1097/00041552-200401000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Gomez RA, Chevalier RL, Sturgill BC, et al. Maturation of the intrarenal renin distribution in Wistar–Kyoto rats. J Hypertens. 1986;4:s31–s33. [Google Scholar]
- 4.Sequeira Lopez ML, Pentz ES, Robert B, et al. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol. 2001;281:F345–F356. doi: 10.1152/ajprenal.2001.281.2.F345. [DOI] [PubMed] [Google Scholar]
- 5.Sequeira Lopez ML, Pentz ES, Nomasa T, et al. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 6.Gomez RA, Chevalier RL, Everett AD, et al. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 7.Everett AD, Carey RM, Chevalier RL, et al. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest. 1990;86:169–175. doi: 10.1172/JCI114680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pentz ES, Sequeira Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol. 2008;294:H699–H707. doi: 10.1152/ajpheart.01152.2007.. This study describes the development of an in-vitro cell culture system of renin-expressing cells dually labeled with fluorescent proteins: YPF as a marker of active renin transcription and CFP as a lineage marker. Using this model, the authors show that epigenetic events such as chromatin remodeling with acetylation of histone 4 and binding of CREB at the CRE site in the renin promoter are involved in the activation of the renin gene transcription.
- 9.Kim SM, Chen L, Faulhaber-Walter R, et al. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension. 2007;50:103–109. doi: 10.1161/HYPERTENSIONAHA.107.087577. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Kim SM, Oppermann M, et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 11. Neubauer B, Machura K, Chen M, et al. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–F1012. doi: 10.1152/ajprenal.90448.2008.. This study highlights the relevance of G(s)alpha protein for renin expression and vascular development from early developmental stages.
- 12. Gomez RA, Pentz ES, Jin X, et al. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol. 2009;296:H1255–H1262. doi: 10.1152/ajpheart.01266.2008.. This study demonstrates that when the histone acetyltransferases (HATs) CBP and p300 are conditionally deleted individually in renin-expressing cells renin expression is not affected but the combined deletion of both HATs results in lack of renin cells and severe renal abnormalities.
- 13.Yanai K, Saito T, Kakinuma Y, et al. Renin-dependent cardiovascular functions and renin-independent blood-brain barrier functions revealed by renin-deficient mice. J Biol Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi N, Lopez ML, Cowhig JE, et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 15. Xu D, Borges GR, Grobe JL, et al. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension. 2009;54:1240–1247. doi: 10.1161/HYPERTENSIONAHA.109.138677.. In this study, the authors generated a secreted renin-specific knockout mouse with preservation of the intracellular renin and showed that secreted renin is crucial for maintenance of kidney morphology and function and regulation of BP.
- 16.Kim HS, Krege JH, Kluckman KD, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilgers KF, Reddi V, Krege JH, et al. Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension. 1997;29:216–221. doi: 10.1161/01.hyp.29.1.216. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchida S, Matsusaka T, Chen X, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pentz ES, Moyano MA, Thornhill BA, et al. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol. 2004;286:R474–R483. doi: 10.1152/ajpregu.00426.2003. [DOI] [PubMed] [Google Scholar]
- 20. Sequeira-Lopez ML, Weatherford ET, Borges GR, et al. The microRNA-processing enzyme Dicer maintains juxtaglomerular cells. J Am Soc Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964.. This study demonstrates a crucial role for Dicer and miRNAs in renin cells for the maintenance of the renal morphology and function.
- 21. Desch M, Schreiber A, Schweda F, et al. Increased renin production in mice with deletion of peroxisome proliferator-activated receptor-{gamma} in juxtaglomerular cells. Hypertension. 2010;55:660–666. doi: 10.1161/HYPERTENSIONAHA.109.138800.. This study shows that conditional deletion of PPARγ in renin cells results in an increased expression of renin, indicating that PPARγ is an important factor for the control of renin gene transcription.
- 22.Kurtz L, Schweda F, de Wit C, et al. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 23.Krattinger N, Capponi A, Mazzolai L, et al. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 24.Wagner C, de Wit C, Kurtz L, et al. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Tuan RS. MicroRNAs and cell differentiation in mammalian development. Birth Defects Res C Embryo Today. 2006;78:140–149. doi: 10.1002/bdrc.20070. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27. Stefani G, Slack FJ. Small noncoding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347.. This is an excellent review about the role of small noncoding RNAs in animal development.
- 28.Pan L, Black TA, Shi Q, et al. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem. 2001;276:45530–45538. doi: 10.1074/jbc.M103010200. [DOI] [PubMed] [Google Scholar]
- 29.Adams DJ, Head GA, Markus MA, et al. Renin enhancer is critical for control of renin gene expression and cardiovascular function. J Biol Chem. 2006;281:31753–31761. doi: 10.1074/jbc.M605720200. [DOI] [PubMed] [Google Scholar]
- 30. Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics. 2008;35:243–253. doi: 10.1152/physiolgenomics.00017.2008.. This study demonstrates in vivo, using a BAC transgenic technology, that the renin enhancer region is critical for the regulation of renin expression, whereas the Hox-Pbx site confers tissue specificity for renin expression.
- 31.Shi Q, Gross KW, Sigmund CD. Retinoic acid-mediated activation of the mouse renin enhancer. J Biol Chem. 2001;276:3597–3603. doi: 10.1074/jbc.M008361200. [DOI] [PubMed] [Google Scholar]
- 32.Li YC, Kong J, Wei M, et al. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todorov VT, Desch M, Schmitt-Nilson N, et al. Peroxisome proliferator-activated receptor-{gamma} is involved in the control of renin gene expression. Hypertension. 2007;50:939–944. doi: 10.1161/HYPERTENSIONAHA.107.092817. [DOI] [PubMed] [Google Scholar]
- 34.Todorov VT, Desch M, Schubert T, Kurtz A. The Pal3 promoter sequence is critical for the regulation of human renin gene transcription by peroxisome proliferator-activated receptor-gamma. Endocrinology. 2008;149:4647–4657. doi: 10.1210/en.2008-0127. [DOI] [PubMed] [Google Scholar]
- 35.Todorov VT, Volkl S, Muller M, et al. Tumor necrosis factor-alpha activates NFkappaB to inhibit renin transcription by targeting cAMP-responsive element. J Biol Chem. 2004;279:1458–1467. doi: 10.1074/jbc.M308697200. [DOI] [PubMed] [Google Scholar]
- 36.Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1046–R1051. doi: 10.1152/ajpregu.00142.2002. [DOI] [PubMed] [Google Scholar]
- 37.Todorov VT, Volkl S, Friedrich J, et al. Role of CREB1 and NF{kappa}B-p65 in the down-regulation of renin gene expression by tumor necrosis factor {alpha} J Biol Chem. 2005;280:24356–24362. doi: 10.1074/jbc.M502968200. [DOI] [PubMed] [Google Scholar]
- 38. Itani H, Liu X, Sarsour EH, et al. Regulation of renin gene expression by oxidative stress. Hypertension. 2009;53:1070–1076. doi: 10.1161/HYPERTENSIONAHA.109.130633.. This study demonstrates that TNFα inhibits renin expression not only through the activation of NFκB but also through the generation of ROS.
- 39. Tanimoto K, Sugiura A, Kanafusa S, et al. A single nucleotide mutation in the mouse renin promoter disrupts blood pressure regulation. J Clin Invest. 2008;118:1006–1016. doi: 10.1172/JCI33824.. In this study, using a BAC transgenic approach, the authors demonstrate that a single-point mutation within the RP-2/PPE site disrupts renin gene transcription, suggesting that subtle mutation in regulatory regions of the renin gene in humans could lead to BP dysregulation.
- 40.Wagner C. Function of connexins in the renal circulation. Kidney Int. 2008;73:547–555. doi: 10.1038/sj.ki.5002720. [DOI] [PubMed] [Google Scholar]
- 41.Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C. Connexin expression in renin-producing cells. J Am Soc Nephrol. 2009;20:506–512. doi: 10.1681/ASN.2008030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor? The connexin hypothesis. Kidney Int. 2009;75:460–462. doi: 10.1038/ki.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schweda F, Kurtz L, de Wit C, et al. Substitution of Cx40 with Cx45 prevents hyperreninemia and attenuates hypertension. Kidney Int. 2009;75:482–489. doi: 10.1038/ki.2008.637.. This study shows that replacement of Cx40 with one connexin with lower conductivity partially rescues the defects observed in the Cx40 knockout mouse.
- 44.Kurtz L, Gerl M, Kriz W, et al. Replacement of connexin 40 by connexin 45 causes ectopic localization of renin-producing cells in the kidney but maintains in vivo control of renin gene expression. Am J Physiol Renal Physiol. 2009;297:F403–F409. doi: 10.1152/ajprenal.00176.2009. [DOI] [PubMed] [Google Scholar]
- 45. Wagner C, Kurtz L, Schweda F, et al. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459:151–158. doi: 10.1007/s00424-009-0707-6.. This study shows that lack of Cx37 does not affect renin expression.
- 46.Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- 47.Sinn PL, Sigmund CD. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]