In a longitudinal study of outcomes on atazanavir-based therapy in a large cohort of HIV-infected women, hair levels of atazanavir were the strongest independent predictor of virologic suppression. Hair antiretroviral concentrations may serve as a useful tool in HIV care.
Abstract
Background. Adequate exposure to antiretrovirals is important to maintain durable responses, but methods to assess exposure (eg, querying adherence and single plasma drug level measurements) are limited. Hair concentrations of antiretrovirals can integrate adherence and pharmacokinetics into a single assay.
Methods. Small hair samples were collected from participants in the Women's Interagency HIV Study (WIHS), a large cohort of human immunodeficiency virus (HIV)-infected (and at-risk noninfected) women. From 2003 through 2008, we analyzed atazanavir hair concentrations longitudinally for women reporting receipt of atazanavir-based therapy. Multivariate random effects logistic regression models for repeated measures were used to estimate the association of hair drug levels with the primary outcome of virologic suppression (HIV RNA level, <80 copies/mL).
Results. 424 WIHS participants (51% African-American, 31% Hispanic) contributed 1443 person-visits to the analysis. After adjusting for age, race, treatment experience, pretreatment viral load, CD4 count and AIDS status, and self-reported adherence, hair levels were the strongest predictor of suppression. Categorized hair antiretroviral levels revealed a monotonic relationship to suppression; women with atazanavir levels in the highest quintile had odds ratios (ORs) of 59.8 (95% confidence ratio, 29.0–123.2) for virologic suppression. Hair atazanavir concentrations were even more strongly associated with resuppression of viral loads in subgroups in which there had been previous lapses in adherence (OR, 210.2 [95% CI, 46.0–961.1]), low hair levels (OR, 132.8 [95% CI, 26.5–666.0]), or detectable viremia (OR, 400.7 [95% CI, 52.3–3069.7]).
Conclusions. Antiretroviral hair levels surpassed any other predictor of virologic outcomes to HIV treatment in a large cohort. Low antiretroviral exposure in hair may trigger interventions prior to failure or herald virologic failure in settings where measurement of viral loads is unavailable. Monitoring hair antiretroviral concentrations may be useful for prolonging regimen durability.
The use of combination antiretroviral therapy (cART) is the prime determinant of longevity among human immunodeficiency virus (HIV)-infected individuals, and the focus of treatment has now shifted to maintaining durable responses on existing regimens. Toward this end, robust predictive models of treatment response are needed to identify factors that may threaten virologic response and herald resistance. By the time a regimen has failed virologically, important opportunities for adherence interventions and preventing resistance have been missed. In resource-limited settings, where HIV viral load monitoring may not be routinely available, resistance mutations can accumulate by the time a regimen fails clinically or immunologically [1, 2]. A low-cost method that provides an early predictor of virologic failure would be useful in both industrialized and nonindustrialized settings to monitor responses to long-term cART [3].
Adherence to treatment, commonly assessed by self-report, is a strong contributor to cART outcomes. However, limitations of the accuracy of self-reporting and other commonly used adherence measures are well described [4]. Furthermore, interindividual variations in pharmacokinetics can lead to outcome disparities even when adherence is high, indicating the utility of an objective measure that could integrate both combined effects of adherence and individual pharmacokinetic parameters. We have developed methods for monitoring antiretroviral exposure by means of determination of antiretroviral concentrations in small-volume hair samples [5–9]. We previously reported that hair levels of protease inhibitors (PIs) were the strongest independent factor associated with short-term virologic response in individuals initiating new PI-based regimens [6]. Drug levels in hair were more closely associated with HIV RNA suppression six months after starting therapy than were self-reported adherence and other commonly applied factors, including pretreatment viral load and CD4 cell count, extent of antiretroviral or PI experience, age, or race. A better test of the ultimate value of drug levels in hair is whether these measures predict impending virologic failure. We report here on a longitudinal study that assessed how well atazanavir levels measured in hair predict treatment outcomes in a multisite observational cohort of HIV-infected women.
METHODS
The Women's Interagency HIV Study Cohort and Study Sample
The Women's Interagency HIV Study (WIHS) is the largest cohort of HIV-infected women and at-risk HIV-noninfected women studied in the United States [10]. This ongoing prospective multicenter study with sites in the San Francisco and Los Angeles, California; Chicago, Illinois; Bronx and Brooklyn, New York; and Washington, DC metropolitan areas observes participants via visits that occur at 6-month intervals. Interviewer-administered survey instruments, physical examination, and specimen collection are performed at each visit. A small sample of hair (∼10–20 strands, or 1–3 mg) is cut from the occipital region of the scalp at each study visit from every consenting HIV-seropositive woman reporting cART. All participants who reported taking an atazanavir-based reporting at any study visit during the period from April 2003 through April 2008 were included in this particular analysis. WIHS study protocols and consent materials were reviewed and approved by institutional review boards at all participating institutions.
Hair Collection, Processing, and Analysis
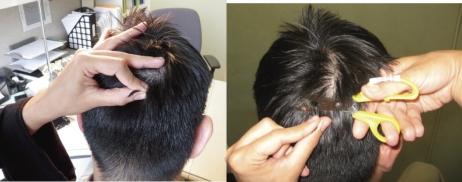
A hair specimen is collected in the WIHS if the participant reports taking antiretrovirals for at ≥1 month. Field staff at all WIHS sites have been uniformly trained on the method of collecting hair. Briefly, a small thatch of hair is cut as close as possible to the scalp from the occiput and the distal portion labeled to denote directionality (Figure 1). Methods for extraction and analyses of most of the PIs, nonnucleoside reverse transcriptase inhibitors, and tenofovir have been developed and optimized in our laboratory and reported elsewhere [6–11].
Figure 1.
Hair collection procedure for antiretroviral levels: Picture demonstrates the isolation of the 20-strand hair thatch from the occipital region of the head (left panel) and the cutting of the hair thatch right at the scalp (right panel).
Atazanavir and ritonavir levels were measured in hair samples collected at visits during which the participant reported current use. Using 2 mg of human hair, atazanavir is detected at levels as low as 0.05 ng/mg hair and ritonavir is detected at levels of as low as 0.01 ng/mg hair. The method has been validated in the range of 0.05–20 ng/mg hair for atazanavir and 0.01–4.0 ng/mg hair for ritonavir with good linearity and reproducibility. Of note, we have tested antiretrovirals in hair in this diverse WIHS cohort and found that median levels and interquartile ranges among participants with undetectable viral loads do not vary significantly by race or ethnicity.
Statistical Analysis
The primary outcome was virologic success (or “suppression”) at each study visit, defined as an HIV viral load ef <80 copies/mL. Multivariate random effects logistic regression models for repeated measures were used to estimate the association of hair drug levels with the dichotomous outcome of virologic suppression. We used the hair level at each visit to predict virologic success, and levels were analyzed both as continuous measures and as categorical variables (tertiles, quartiles, or quintiles). Also included in models were variables that could affect response, including age, race, viral load at the time of regimen initiation (continuous or dichotomized into <100,000 vs ≥100,000 copies/mL), prior antiretroviral treatment experience (dichotomized into yes vs no), and degree of PI experience (categorized into naïve to PIs, past experience with 1 PI, or treatment with ≥2 PIs), nadir and pretreatment CD4 cell counts, study year, nucleoside reverse transcriptase inhibitor (NRTI) backbone components, history of clinical AIDS, and self-reported adherence. Adherence to atazanavir was reported as the percentage of prescribed doses consumed during periods of 6 months, 30 days, and 3 days; visual analog scales aided in estimating percentages [11]. Level of adherence was analyzed either as a continuous measure or categorized into ≤74%, 75%–94%, or ≥95% over the time interval assessed. Because atazanavir is often coadministered with ritonavir, hair levels of each of these agents are substantially collinear, so separate models were run for atazanavir and ritonavir in the women receiving ritonavir-boosted atazanavir. All analyses were performed using SAS software, version 9.2 (SAS Institute).
RESULTS
Participant Demographic Characteristics
Table 1 summarizes demographic and other characteristics for the 424 WIHS participants receiving atazanavir-based cART at any time from April 2003 through April 2008. The racial and ethnic distribution of the study sample was 215 African-Americans (51%), 131 Hispanics (31%), 66 whites (15%), and 12 others (3% [Native Americans or Asian-Americans]). Each woman contributed 1–9 WIHS biannual study visits to the analysis (median, 3), for a total of 1443 person-visits. Of these 1443 person-visits, participants reported ritonavir coadministration with atazanavir in 1136 (79%). One hundred one women in the study cohort (24%) had never been treated with PIs before starting the atazanavir-based regimen, and the median pretreatment CD4 count was 281 cells/mm3 (range, 5–2046 cells/mm3). Adherence was reported as ≥95% at 1116 person-visits (77%), 75%–94% at 244 person-visits (17%), and ≤74% at 83 person-visits (6%). Viral loads were below the assay threshold (<80 copies/mL) during 918 (64%) of the 1443 person-visits. On the basis of log-likelihood statistics, assessment of atazanavir levels as quintiles fit the multivariate models better than tertile and quartile categorization schemes and better than continuous measures. Table 1 shows the hair concentration ranges represented in each quintile (first quintile, 0.05 to ≤0.658 ng/mg; second quintile, >0.658 to ≤1.78 ng/mg; third quintile, >1.78 to ≤3.13 ng/mg; fourth quintile, >3.13 to ≤5.19 ng/mg; fifth quintile, >5.19 ng/mg). A strong relationship between atazanavir levels in hair and self-reported adherence (dichotomized into <95% vs ≥95%) was observed (F statistic P < .001).
Table 1.
Characteristics of Women Participating in the Study, April 2003 Through April 2008 (N = 424)
| Characteristic | Value |
| Person-visits contributing to analysisa | 1443 |
| Age, median years (range) | 43 (21–71) |
| Race/ethnicity | |
| White (non-Hispanic) | 66 (15) |
| African-American (non-Hispanic) | 215 (51) |
| Hispanic | 131 (31) |
| Other | 12 (3) |
| Past PI treatment | |
| None | 101 (24) |
| 1 PI | 156 (37) |
| ≥2 PIs | 167 (39) |
| Pretreatment viral load, copies/mL | |
| ≥100,000 | 52 (12) |
| <100,000 | 372 (88) |
| HIV viral load, median copies/mL (range) | 5950 (80–2,500,000) |
| Pretreatment CD4 cell count, cells/mm3 | |
| <200 | 133 (31) |
| ≥200 | 291 (69) |
| CD4 cell count, median cells/mm3 (range) | 281 (5–2046) |
| Person-visits at which atazanavir is boosted with ritonavir | 1136 (79) |
| Person-visits at which viral load is undetectableb | 918 (64) |
| Adherence during past 6 months (self-reported) at person-visit | |
| 0%–74% | 83 (6) |
| 75%–94% | 244 (17) |
| ≥95% | 1116 (77) |
| Person-visits in each atazanavir hair level quintile (ng/mg), no. (%) | |
| Quintile 1 (0.05 to ≤0.658 ng/mg) | 289 (20) |
| Quintile 2 (>0.658 to ≤1.78 ng/mg) | 282 (19) |
| Quintile 3 (>1.78 to ≤3.13 ng/mg) | 297 (21) |
| Quintile 4 (>3.13 to ≤5.19 ng/mg) | 289 (20) |
| Quintile 5 (>5.19 ng/mg) | 286 (19) |
NOTE. Data are no. (%) of study participants unless otherwise indicated. PI, protease inhibitor.
Median number of visits per patient was 3 (range, 1–9); 105 women had 1 visit; 70 had 2 visits; 53 had 3 visits; 61 had 4 visits; 66 had 5 visits; 33 had 6 visits; 23 had 7 visits; 11 had 8 visits; 2 had 9 visits.
Threshold of detection, 80 copies/mL.
Association of Atazanavir Hair Levels with Virologic Suppression
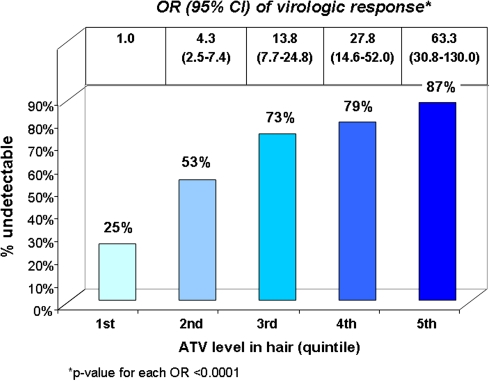
Figure 2 shows the percentage of person-visits during which viral loads were <80 copies/mL according to hair quintile. HIV RNA levels were undetectable in only 25% of visits in which hair concentrations of atazanavir were in the lowest quintile. In contrast, for person-visits in which atazanavir concentrations in hair were in the highest quintile (>5.19 ng/mg), the likelihood of maximal virologic suppression was 87%. Figure 2 also depicts the univariate relationship between hair atazanavir levels and the likelihood of virologic suppression in repeated measures analyses. The odds of viral load undetectability increased with each quintile; the odds ratio (OR) for viral suppression if the atazanavir level in hair is in the second quintile (>0.658 to ≤1.78 ng/mg) is 4.3 (95% confidence interval [CI], 2.5–7.4; P < .001), for example, and the OR for virologic response if atazanavir levels are in the top quintile is 63.3 (95% CI, 30.8–130.0; P < .001).
Figure 2.
Percent of person-visits in each hair quintile where viral load suppressed (entire study sample) and odds ratio of virologic response per hair quintile (univariate relationship): ATV, atazanavir.
Table 2 presents the multivariate analysis for virologic response in which levels of atazanavir in hair are adjusted for age, race, extent of PI experience, pretreatment viral load, and self-reported adherence. A pretreatment HIV RNA level of <100,000 copies/mL was associated with a higher odds of achieving virologic suppression over time, compared with individuals who started atazanavir-based treatment with viral loads ≥100,000 copies/mL (OR, 3.2 [95% CI, 1.5–6.9]; P = .002). Participants who were not African-American showed a trend toward a higher likelihood of virologic suppression while receiving cART than did African-Americans. Those who had been treated with ≥2 PIs prior to atazanavir showed a trend toward a lower likelihood of virologic suppression while receiving atazanavir. Self-reported adherence was associated with a higher odds of virologic suppression, with an OR of 4.0 (95% CI, 1.9–8.6; P < .001) for visits when participants reported ≥95% adherence, compared with those when participants reported <75% adherence. Adding pretreatment CD4 cell count, history of clinical AIDS, study year, or the NRTI backbone agents to the multivariate models did not significantly alter the results, so these variables were removed from the final models.
Table 2.
Multivariate Model of Hair Atazanavir Concentrations and Virologic Success (Entire Study Sample)
| Variable | OR of virologic response (95% CI) | P |
| Age, per decade | 1.05 (0.78–1.42) | .75 |
| Race | ||
| Not African-American | 1.6 (0.99–2.7) | .06 |
| African-American | Reference | … |
| Pretreatment HIV RNA level, copies/mL | ||
| <100,000 | 3.2 (1.5–6.9) | … |
| ≥100,000 | Reference | .002 |
| Past PI treatment | ||
| None | Reference | … |
| 1 PI | 0.94 (0.49–1.8) | .84 |
| ≥2 PIs | 0.56 (0.29–1.08) | .09 |
| Adherence level | ||
| 0%–74% | Reference | … |
| 75%–94% | 2.5 (1.1–5.5) | .03 |
| ≥95% | 4.0 (1.9–8.6) | <.001 |
| Hair concentration, quintile | ||
| 1 | Reference | … |
| 2 | 4.3 (2.5–7.4) | <.001 |
| 3 | 12.7 (7.1–22.8) | <.001 |
| 4 | 22.9 (12.2–43.1) | <.001 |
| 5 | 59.8 (29.0–123.2) | <.001 |
NOTE. Adjusting for pretreatment CD4 cell count, a history of clinical AIDS, study year, and/or nucleoside reverse transcriptase inhibitor backbone components used with atazanavir did not significantly alter the results of the analysis, and these variables were therefore removed from the final model. AA, African-American; CI, confidence interval; OR, odds ratio; PI, protease inhibitor.
In adjusted analyses, concentrations of atazanavir in hair were the best independent predictor of virologic suppression. An atazanavir level in the second quintile, compared with the first, yielded an OR for virologic suppression that is comparable to a self-reported adherence of ≥95%. Hair atazanavir levels in the higher quintiles were associated with progressively increasing odds of virologic suppression; women whose atazanavir levels were in the highest quintile had an OR of 59.8 (95% CI, 29.0–123.2; P <.001) for virologic suppression.
When models were repeated focusing on the 1136 person-visits during which ritonavir was coadministered with atazanavir, we found that ritonavir levels in hair were similarly the strongest predictor of subsequent virologic response. Finally, when we looked at hair level at one visit as a predictor of virologic suppression at the subsequent WIHS visit, we also saw a strong and monotonic relationship between hair level and subsequent response.
Virologic Suppression Rates in Different At-Risk Scenarios
We then investigated the key question of whether higher atazanavir exposure as indicated by hair levels could reestablish virologic suppression after a preceding lapse in adherence, exposure, or virologic suppression. We examined three separate “at risk” subgroups of the 1443 person-visits in our study: (1) those preceded by a reported adherence level of ≤95% at any previous visit (405 person-visits from 152 participants); (2) those preceded by a hair atazanavir level in the lowest quintile (0.05–0.658 ng/mg) at any previous visit (377 person-visits from 125 participants); and (3) those with an HIV viral load >1000 copies/mL at any previous visit (356 person-visits from 139 participants). Each subgroup showed lower overall rates of virologic suppression than did the entire group over time; subgroups 2 and 3 showed differences in suppression rates that were statistically significant (Table 3).
Table 3.
Rates of Virologic Suppression in Overall Cohort Compared With Rates in 3 Scenarios (Subgroups) of Failure
| Group | No. of visits | Person-visits suppressed, % | P (compared with visits not in subgroup)a,b |
| Entire cohort: all visits | 1443 | 64c | |
| Subgroup 1: self-reported adherence ≤95% at a previous visit | 405 | 58 | .11 |
| Subgroup 2: hair value in lowest quintile at a previous visit | 377 | 51 | .01 |
| Subgroup 3: viral load >1000 copies/mL at a previous visit | 356 | 38 | <.001 |
P value was obtained by comparing visits in the subgroup of interest with visits not in the subgroup (accounting for repeated measures).
Overall P value for the chance of virologic suppression for a person-visit in any of the 3 subgroups (compared with not being in any of the subgroups) is .02.
Median HIV RNA level for person-visits where viral load is not suppressed in the entire cohort, 1800 copies/mL (interquartile range, 310–17,000 copies/mL).
“Redemption” Through Improved Atazanavir Exposure
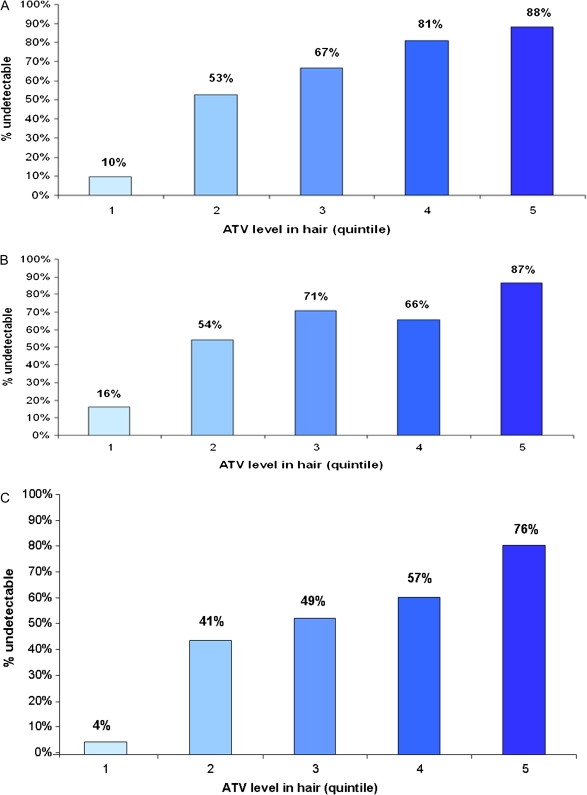
Although each at-risk subgroup had lower overall rates of virologic suppression than did the entire cohort (Table 3), each subgroup subsequently revealed a pattern of increasing rates of virologic suppression by increasing quintile of hair atazanavir level (Figures 3A, 3B, 3C). Indeed, increasing atazanavir levels in hair became even more determinative of virologic suppression (or resuppression for subgroup 3) in multivariate models for each subgroup than for the overall cohort (Table 4). The ORs for reaching a viral load of <80 copies for person-visits in which hair levels were in the highest quintile were 210.2 (95% CI, 46.0–961.1), 132.8 (95% CI, 26.5–666.0), and 400.7 (95% CI, 52.3–3069.7), respectively, for subgroups 1, 2, and 3.
Figure 3.
Percent of person-visits in each hair quintile in which viral load was suppressed for 3 subgroups in which “failure” was previously revealed (Figure 3A: subgroup 1; Figure 3B: subgroup 2; Figure 3C: subgroup 3). ATV, atazanavir.
Table 4.
Multivariate Models of Hair Atazanavir Concentrations and Virologic Success (3 Subgroups)
| Hair quintile | Subgroup 1 |
Subgroup 2 |
Subgroup 3 |
|||
| Person-visits | OR (95% CI) | Person-visits | OR (95% CI) | Person-visits | OR (95% CI) | |
| 1 | 83 | Reference | 116 | Reference | 112 | Reference |
| 2 | 91 | 14.1 (4.7–42.8) | 83 | 9.6 (3.4–27.2) | 78 | 43.8 (8.9–215.7) |
| 3 | 99 | 26.3 (8.4–82.0) | 72 | 30.0 (8.8–102.5) | 61 | 47.2 (9.3–240.2) |
| 4 | 73 | 60.5 (17.2–211.7) | 61 | 24.1 (7.0–82.4) | 60 | 82.5 (15.5–439.3) |
| 5 | 59 | 210.2 (46.0–961.1) | 45 | 132.8 (26.5–666.0) | 45 | 400.7 (52.3–3069.7) |
NOTE. All P values are <.001. All models adjusted for age, race, previous treatment with protease inhibitors, pre-atazanavir HIV viral load, and self-reported adherence. Subgroup 1: self-reported adherence ≤95% at a previous visit (n = 405); subgroup 2: hair value in lowest quintile at a previous visit (n = 377); subgroup 3: viral load >1000 copies/mL at a previous visit (n = 356). CI, confidence interval; OR, odds ratio.
DISCUSSION
In this multivariate analysis of a cohort of HIV-infected women over time, we reveal that antiretroviral concentrations in hair are the strongest independent predictor of virologic suppression. Levels of antiretroviral drugs in hair showed a monotonic relationship to the likelihood of viral suppression (OR for success, 4.3, 12.7, 22.9, and 59.8 for each increasing quintile of hair atazanavir concentration; each P < .001) in multivariate models. Because low hair antiretroviral concentrations can predict virologic failure prior to its development, this measurement may be useful in designing interventions aimed at prolonging the durability of cART.
Concentrations of antiretroviral drugs in hair samples may provide an integrated measure of behavior and biology. Levels of medications in hair reflect drug uptake from the systemic circulation over periods of weeks to months [12] and capture average, as well as individual, pharmacokinetic information. Single adherence measures or plasma antiretroviral concentrations provide “snapshots” of exposure, but a level measured in hair synthesize adherence and pharmacokinetic variability over time to provide a robust exposure measure in a single assay [13]. The value of single plasma antiretroviral levels is further limited by the so-called “white coat effect,” in which adherence transiently improves prior to clinic appointments [14], and by an inability to define meaningful therapeutic antiretroviral ranges because of substantial interindividual pharmacokinetic variability [15, 16]. Therefore, despite the importance of ensuring adequate exposure to the components of HIV regimens, no gold standard exists in current practice to assess antiretroviral exposure.
Our models show that antiretroviral exposure as measured in hair far surpasses commonly used covariates to predict HIV treatment outcomes [17]. Failed antiretroviral regimens result in substantial long-term adverse effects, including increased drug and diagnostic testing costs, as well as avoidable clinical and transmission events. Because patients who experience virologic failure on a regimen demonstrate attenuated rates of immune reconstitution on future regimens [18], substantial efforts should be made to optimize first-line cART. Preserving responses to first-line regimens are of particular import in resource-limited settings, where the average annual cost of second-line cART regimens can be up to 8 times that of first-line regimens [19]. The risk of viremia on therapy is highest in the first year after initiating cART and can be linked to increased HIV transmission rates [20]. Therefore, when initiating HIV treatment, the incorporation of an effective antiretroviral exposure measure, such as hair concentrations, during initial monitoring may avert early virologic failure, blunted responses to subsequent regimens, and the need for expensive or inaccessible second-line regimens.
Previous models of outcomes with atazanavir-based regimens have failed to define precise parameters of atazanavir exposure that increase the likelihood of virologic suppression [15, 21]. This failure could be a result of using single plasma atazanavir concentrations in these models to define exposure instead of a longer term measure. A recent report revealed that average adherence to dosage with boosted PI regimens was a better predictor of virologic suppression than was duration or frequency of missed doses [22]. Hair antiretroviral concentrations average daily exposure variability in a manner analogous to that of glycosylated hemoglobin A1C providing information on mean daily glucose levels in diabetic patients. A previous analysis by our group demonstrated that hair levels of antiretrovirals are more closely correlated with areas under the curve from intensive pharmacokinetic studies than are single plasma levels [23]. Therefore, it is not surprising that hair antiretroviral measurements predict treatment outcomes with greater accuracy than do single plasma levels. Another analysis by our group, directly comparing levels of antiretrovirals in hair with plasma levels to predict treatment responses, demonstrated the superiority of hair levels [23].
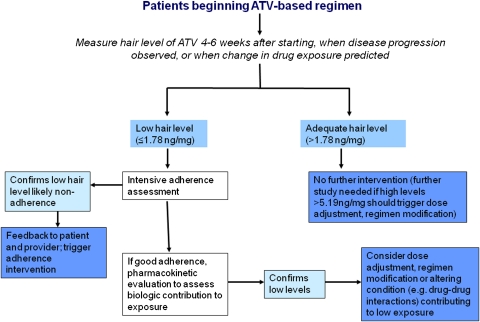
In addition to showing that atazanavir concentrations in hair predict virologic responses more strongly than self-reported adherence or other factors, we demonstrate that lapses in adherence, antiretroviral exposure, or virologic suppression are all associated with an increased likelihood of subsequent failure. Since adherence difficulties or the presence of detectable virus during therapy are well-known contributors to virologic failure, either state is likely to trigger corrective measures in the clinical setting. However, the inaccuracy of self-reported adherence, the lack of routine virologic monitoring in many resource-limited settings, and the fact that detectable viral loads when available may already indicate mutations [24] all increase the appeal of finding another tool to prospectively predict failure. Our models show that low antiretroviral hair levels portend a high risk of virologic failure; hair measures in the clinical setting could therefore trigger interventions to correct either adherence or low pharmacokinetic levels (eg, through assessing drug-drug interactions or diet) to extend regimen durability (Figure 4). Of note, only participants with hair atazanavir measurements in the higher quintiles during WIHS visits following a failure scenario had rates of virologic suppression similar to those in clinical trials. This supports the concept that the reasons for low drug levels in hair must be investigated and addressed by adherence intervention, change in regimen, or possibly dose increase (the latter requires additional study). Our group is currently planning a clinical trial assessing adherence interventions, regimen change, or dose modification of antiretrovirals based on hair measurements of anchor regimen components in a clinical setting.
Figure 4.
Possible algorithm for use of atazanavir hair levels in the clinical setting. ATV, atazanavir.
As we proposed previously [7], one possible algorithm for testing would involve measuring antiretroviral levels in hair soon after starting a new antiretroviral regimen and performing HIV viral load testing only if the hair levels fall into the lower quintiles as defined above (Figure 4). Data showing that the risk of viremia on therapy is highest during the first year after initiating cART [20] supports the use of these measures soon after regimen initiation. Data from the resource-limited setting showing that routine viral load monitoring decreases rates of virologic resistance over routine monitoring alone may argue for the use of hair measures as a surrogate for the former [25]. If the atazanavir level in hair is ≤1.78 ng/mg (first or second quintile), the rates of virologic failure approach 50% and intensive adherence interventions (vs a pharmacokinetic evaluation for low exposure if adherence is deemed adequate) should be triggered. After a patient is receiving stable HIV therapy, antiretroviral measurements using hair need not be performed routinely but only when clinical disease progression is observed (for settings where routine CD4 cell count or viral load monitoring are not available) or when an alteration in drug exposure is predicted, such as a new drug-drug interaction, pregnancy, change in dietary patterns, change in liver or renal function, and so on.
Unlike phlebotomy, hair collection is noninvasive and does not require specific skills, sterile equipment, or specialized storage conditions. The collection of hair samples for analysis merely requires a pair of scissors, and storage is at room temperature. Hair can be stored for long periods of time prior to analysis, shipped without precautions for biohazardous materials, and analyzed economically in a high-throughput hair analysis laboratory. These features may make this monitoring tool particularly advantageous in the resource-poor setting, especially when routine viral load monitoring is prohibitively expensive. We recently applied these hair measures in a nested case-control study in 2 South African public health clinics and found that low concentrations of lopinavir in hair had a high predictive value for virologic failure in that setting [25]. We are currently working on developing a lower cost, point-of-care method of analyzing antiretroviral levels in hair for resource-constrained settings to increase the feasibility of this tool. The results of the analyses presented here argue for the possibility of hair antiretroviral concentrations serving as a method of HIV therapeutic drug monitoring that may increase the durability of current antiretroviral regimens in a variety of settings.
Acknowledgments
We would like to thank the WIHS participants who contributed data to this study.
We thank the Women's Interagency HIV Study (WIHS) participants who contributed data to this study. Data were collected by the WIHS Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos, MD); Brooklyn, NY (Howard Minkoff, MD); Washington DC, Metropolitan Consortium (Mary Young, MD); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, MD); Los Angeles County/Southern California Consortium (Alexandra Levine, MD); Chicago Consortium (Mardge Cohen, MD); and Data Coordinating Center (Stephen Gange, PhD).
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authors’ contributions: M.G. developed the study protocol, provided study oversight, designed the analysis plan, interpreted the data, and wrote the paper. R.M.G. contributed to the study concept and the analysis plan and interpretation. N.A. and P.B. provided data management, contributed to the analysis plan, and performed most of the statistical analyses. R.M.G., N.A., P.B., K.A., S.J.G., H.M., M.Y., J.M., M.H.C., and G.B.S. collected WIHS participant data, helped design protocols, and critically revised the manuscript. Y.H. developed the laboratory methods for analysis of antiretroviral levels in hair.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID RO1-AI-65233) with data collected in the Women's Interagency HIV Study (WIHS). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The WIHS is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding for WIHS is also provided by the National Center for Research Resources (UCSF - CTSI Grant UL1 RR024131). M.G. was additionally supported by a Mentored Patient-Oriented Research Career Development Award (K23 A1067065) from NIAID.
Potential conflicts of interest. R.M.G. has received payment previously for manuscript preparation from HRSA and all authors have received funding from the National Institutes of Health. All other authors: no conflicts.
References
- 1.Bisson GP, Gross R, Strom JB, et al. Diagnostic accuracy of CD4 cell count increase for virologic response after initiating highly active antiretroviral therapy. AIDS. 2006;20:1613–9. doi: 10.1097/01.aids.0000238407.00874.dc. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(suppl 2):1–13. [PubMed] [Google Scholar]
- 3.Bertagnolio S, Kelley K, Hassani AS, et al. Boston, MA: 2011. Surveillance of transmitted and acquired HIV drug resistance using WHO surveys in resource-limited settings. 18th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 4.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137:696–7. doi: 10.7326/0003-4819-137-8-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:3401–9. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23:471–8. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu A, Vittinghoff E, Gandhi M, et al. 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2010. Validating measures of tenofovir drug exposure in a U.S. Pre-exposure prophylaxis trial. [Google Scholar]
- 9.Huang Y, Yang Q, Gandhi M, Greenblatt R, Gee W, Lin E. American Association of Pharmaceutical Sciences (AAPS) Annual Meeting. San Diego, CA: 2007. Sensitive analysis of ritonavir, lopinavir, atazanavir and efavirenz in human hair by LC/MS/MS for monitoring antiretroviral drug long-term exposure. [Google Scholar]
- 10.Yang Q, Liu A, Gandhi M, Greenblatt R, Gee W, Huang Y. LC/LC/MS Assay of Tenofovir in Human Hair for Pre-Exposure Prophylaxis. Association of Pharmaceutical Sciences (AAPS) Annual Meeting (New Orleans) 2010 [Google Scholar]
- 11.Huang Y, Yang Q, Koon K, Lei Y, Gee W, Lin ET, Greenblatt RM, Gandhi M. Microanalysis of the Antiretroviral Medication Nevirapine in Human Hair by LC/MS/MS for Monitoring Long-Term Drug Exposure. In press [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 13.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74–9. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 14.Beumer J, Bosman I, Maes R. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55:353–7. [PubMed] [Google Scholar]
- 15.Liu A, Gandhi M, Bacchetti P, et al. 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2011. Validating hair as a biological marker of tenofovir drug exposure in HIV pre-exposure prophylaxis (PrEP) [Google Scholar]
- 16.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9:238–46. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 17.Lescure FX, Poirier JM, Meynard JL, et al. Factors predictive of virological failure on atazanavir in 310 HIV-infected patients. AIDS. 2010;24:1593–5. doi: 10.1097/QAD.0b013e32833a2403. [DOI] [PubMed] [Google Scholar]
- 18.Ray JE, Marriott D, Bloch MT, McLachlan AJ. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005;60:291–9. doi: 10.1111/j.1365-2125.2005.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cescon A, Cooper C, Chan K, et al. Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multi-site Canadian cohort. HIV Med. 2010 doi: 10.1111/j.1468-1293.2010.00890.x. Doi: 10.1111/j.1468-1293.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 20.Trotta MP, Cozzi-Lepri A, Ammassari A, et al. Rate of CD4+ cell count increase over periods of viral load suppression: relationship with the number of previous virological failures. Clin Infect Dis. 2010;51:456–64. doi: 10.1086/655151. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, Joint United Nations Program on HIV/AIDS, United Nations Children's Fund. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report, September 2009. World Health Organization; website. http://www.who.int/hiv/pub/2009progressreport/en/index.html. Accessed 21 November 2010. [Google Scholar]
- 22.Engsig FN, Omland LH, Larsen MV, et al. Risk of high-level viraemia in HIV-infected patients on successful antiretroviral treatment for more than 6 months. HIV Med. 2010;11:457–61. doi: 10.1111/j.1468-1293.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabbiani M, Di Giambenedetto S, Ragazzoni E, et al. Mid-dosing interval concentration of atazanavir and virological outcome in patients treated for HIV-1 infection. HIV Med. 2010;11:326–33. doi: 10.1111/j.1468-1293.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 24.Parienti JJ, Ragland K, Lucht F, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis. 2010;50:1192–7. doi: 10.1086/651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M, Ameli N, Gange S, et al. 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: Concentrations of efavirenz in hair correlate strongly with 24-hour intensive PK measurements and with virologic outcomes. 16–19 February 2010. Abstract N-154. [Google Scholar]
- 26.Hawkins C, Murphy RL. Management of antiretroviral failure and resistance in developing countries. Curr Opin HIV AIDS. 2009;4:538–44. doi: 10.1097/COH.0b013e328331d2fb. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds S, Sendagire H, Newell K, et al. 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2011. Routine viral load monitoring reduces the rate of genotypic resistance to commonly used ART in Uganda. [Google Scholar]
- 28.van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31820dc0cc. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]