Abstract
We characterized the prevalence, antibiotic susceptibility profiles, and genotypes of Staphylococcus aureus among US meat and poultry samples (n = 136). S. aureus contaminated 47% of samples, and multidrug resistance was common among isolates (52%). S. aureus genotypes and resistance profiles differed significantly among sample types, suggesting food animal–specific contamination.
Antimicrobials are used extensively in food animal production, where they are often applied subtherapeutically for growth promotion and routine disease prevention [1]. Surveys conducted by the National Antimicrobial Resistance Monitoring System (NARMS) indicate that retail meat and poultry products are frequently contaminated with multidrug-resistant Campylobacter species, Salmonella species, Enterococcus species, and Escherichia coli [2]; but little is known about the prevalence of other antibiotic-resistant pathogens in the US food supply.
Staphylococcus aureus is among the most prevalent causes of clinical infections globally and has garnered substantial public attention due to increasing mortality associated with multidrug resistance. A new multidrug-resistant S. aureus strain, ST398, has emerged that predominantly colonizes people working in food animal production. First discovered in 2003, ST398 now makes up a substantial proportion of the community-acquired methicillin-resistant S. aureus (MRSA) cases in the Netherlands [3]. Multiple studies have demonstrated the high prevalence of multidrug-resistant S. aureus, including ST398, among intensively raised swine in the European Union, Canada, and the United States [4, 5], but few studies have been conducted to measure its prevalence in US food products [6].
In the current study, we evaluated the prevalence and antibiotic susceptibility profiles of S. aureus in retail meat and poultry samples from 5 US cities. We found that S. aureus contamination was common among the samples and that distinct S. aureus populations were associated with each meat and poultry type. We further demonstrated the prevalence of multidrug resistance, including resistance to clinically important antibiotics such as ciprofloxacin, quinupristin/dalfopristin, clindamycin, erythromycin, oxacillin, and daptomycin.
MATERIALS AND METHODS
Detailed Methods.
Sample Collection.
Retail beef (ground), chicken (breasts, thighs), pork (chops, ground), and turkey (ground, cutlets) products were collected from 26 retail grocery stores in 5 US cities: Chicago; Washington, DC; Fort Lauderdale; Los Angeles; and Flagstaff.
Sample Processing.
Samples were agitated for 1 minute in 250 mL .1% peptone broth; then 30 mL of inoculated broth was mixed with 30 mL of 2X Baird Parker broth (BD) and incubated at 37°C for 18–24 h. After enrichment, 10 μL of broth was plated onto each of 6 Baird Parker agar formulations and incubated 18–24 h for growth. All agar formulations included EY tellurite enrichment (BD), and 5 plates were supplemented with one of the following antibiotics: vancomycin (8 mg/L), oxacillin (2 mg/L), ciprofloxacin (2 mg/L), tetracycline (8 mg/L), or gentamicin (8 mg/L).
Bacterial Confirmation.
Putative isolates were confirmed as S. aureus using a real-time polymerase chain reaction assay targeting the femA gene (Pathogene, LLC).
Multilocus Sequence Typing (MLST).
MLST of S. aureus was performed as described elsewhere [7].
Susceptibility Testing.
Minimum inhibitory concentrations were determined by broth microdilution (TREK Diagnostic Systems), except for linezolid and trimethoprim/sulfamethoxazole, which were also measured using Etest methods (bioMérieux). All isolates were screened for inducible clindamycin resistance following the Clinical and Laboratory Standards Institute guidelines (Appendix B of M100-S19).
Multidrug Resistance.
Multidrug resistance was reported as a single isolate resistant (intermediate or complete) to 3 or more unique antimicrobial classes.
RESULTS
We collected and tested a total of 136 meat and poultry samples from 5 US cities, encompassing 80 unique brands from 26 grocery stores. S. aureus contamination was most common among turkey samples (77%; 20/26), followed by pork (42%; 11/26), chicken (41%; 19/46), and beef (37%; 14/38). A subset of meat and poultry samples (10%; 14/136) was contaminated by multiple unique S. aureus strains as determined by MLST and susceptibility profiles, and a total of 79 unique isolates were used in subsequent analyses.
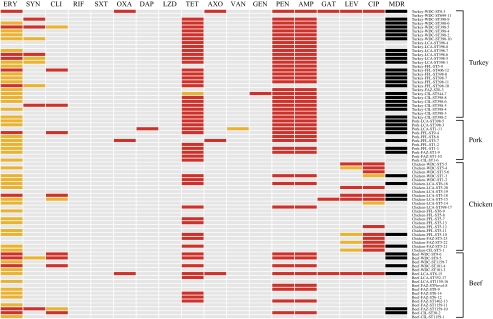
Ninety-six percent of the S. aureus isolates were resistant to at least 1 antimicrobial. Thirty-two unique susceptibility profiles were identified among the S. aureus isolates, with many resistant to multiple clinically important antimicrobial classes (Figure 1). Resistance (intermediate and complete) to tetracycline, ampicillin, penicillin, and erythromycin was highly prevalent, but resistance to other important antimicrobials was also observed, including quinupristin/dalfopristin, fluoroquinolones, oxacillin, daptomycin, and vancomycin (Figure 1). Multidrug resistance, defined as intermediate or complete resistance to 3 or more antimicrobial classes, was common among the S. aureus isolates (52%) and most prevalent among S. aureus isolates from turkey (79%; 22/28), followed by those from pork (64%; 7/11), beef (35%; 6/17), and chicken (26%; 6/23).
Figure 1.
Resistance profiles of S. aureus isolates collected in this study. Gray, susceptible; orange, intermediate resistance; red, complete resistance; black, multidrug resistant (≥3 antimicrobial classes); ERY, erythromycin; SYN, quinupristin/dalfopristin; CLI, clindamycin; RIF, rifampin; SXT, trimethoprim/sulfamethoxazole; OXA, oxacillin; DAP, daptomycin; LZD, linezolid; TET, tetracycline; AXO, ceftriaxone; VAN, vancomycin; GEN, gentamicin; PEN, penicillin; AMP, ampicillin; GAT, gatifloxacin; LEV, levofloxacin; CIP, ciprofloxacin; MDR, resistant to 3 or more antimicrobial classes.
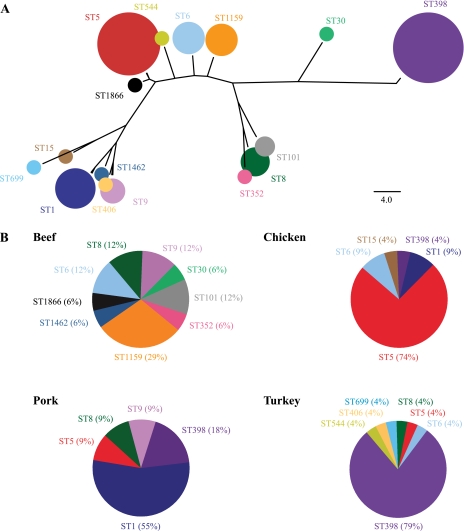
Fifteen unique MLST sequence types were identified among the S. aureus isolates, but 2 sequence types—ST5 and ST398—dominated the collection due to their high prevalence among chicken (74%) and turkey (79%) samples (Figure 2). The most common sequence types among beef and pork samples were ST1159 (29%) and ST1 (55%), respectively. The 3 MRSA isolates belonged to sequence types ST8 (beef and turkey) and ST5 (pork).
Figure 2.
Multilocus sequencing typing of food-borne S. aureus isolates. (A) Phylogenetic relationships and relative abundances of the different sequence types identified among the retail meat and poultry samples (the area of the circles is proportional to the number of isolates constituting the different sequence types). (B) Relative proportion of the isolates from each meat and poultry type made up of the different sequence types.
DISCUSSION
In the current study, we characterized the prevalence, antibiotic susceptibility profiles, and genotypes of S. aureus among US meat and poultry samples. S. aureus contaminated a substantial proportion of samples from all meat and poultry types (37–77%), with a notable 52% of isolates being multidrug resistant.
The distinct S. aureus populations on each product type suggest that food animals are the predominant source of contamination. While a portion of the S. aureus isolates may have been the result of human contamination, a uniform pattern of human-associated strains was not observed. Additional studies tracing S. aureus genotypes from farm to retail are required to definitively identify the sources of S. aureus contamination.
MRSA was isolated from one sample each of beef, turkey, and pork. Our sample size was insufficient to accurately estimate prevalence rates, but our data are consistent with a previous US-based study [6]. Higher MRSA contamination rates have been estimated among meat and poultry samples in the Netherlands, where ST398 is the dominant food-borne sequence type [8]. In contrast, the MRSA isolates from the current and previous US-based food studies were ST5 and ST8.
All isolates were screened against antibiotics that are commonly used to treat severe MRSA infections. We identified 1 vancomycin-intermediate-resistant isolate and 1 daptomycin-resistant isolate. Vancomycin, daptomycin, and their analogs were never approved for US food animal production; therefore, these findings were unexpected and may suggest origins other than US food animals.
Fluoroquinolone-resistant S. aureus isolates were uniquely prevalent among chicken products. Fluoroquinolones were used in US broiler production from 1995 to 2005, which may have selected for the fluoroquinolone-resistant S. aureus strains that exist today [9]; however, isolates collected prospectively starting prior to 1995 would be necessary to make definitive conclusions in this regard.
Our data demonstrate that retail meat and poultry are frequently contaminated with multidrug-resistant S. aureus, but the public health relevance of this finding is unclear. European and North American studies indicate that ST398 can successfully colonize and infect humans [4, 10], but few studies have investigated the risk of human colonization and infection with S. aureus from meat and poultry products [11, 12]. The European Food Safety Authority (EFSA) concluded that the risk for MRSA infection from food handling and consumption was low; however, this was based on a small number of studies [11]. Furthermore, EFSA did not evaluate the risk from methicillin-susceptible multidrug-resistant S. aureus, which is more common than MRSA among food samples.
Conventional concentrated animal feeding operations (CAFOs) provide all the necessary components for the emergence and proliferation of multidrug-resistant zoonotic pathogens. In the United States, billions of food animals are raised in densely stocked CAFOs, where antibiotics are routinely administered in feed and water for extended periods to healthy animals [1]. NARMS has shown that multidrug-resistant E. coli and Enterococcus species are prevalent among US meat and poultry products [2]. Our findings indicate that multidrug-resistant S. aureus should be added to the list of antimicrobial-resistant pathogens that routinely contaminate our food supply.
Supplementary Material
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We would like to acknowledge the scientific and logistic contributions of Jennifer Nibecker, John Gillece, Tricia O'Reilly, Michael Bork, and Laura M. Koeth.
Financial support. This work was supported with a grant from the Pew Charitable Trusts.
Potential conflicts of interest. L.B.P. and A.E.W. are consultants for the Pew Charitable Trusts. P.S.K. is a board member of PathoGene, LLC; is a consultant for Febits, Inc; has received grant support from the National Institutes of Health; has a patent with the Translational Genomics Research Institute; and holds stock options in Febits, Inc. PathoGene is partially owned by P.S.K. and D.M.E. All other authors: no conflicts.
References
- 1.Gilchrist MJ, Greko C, Wallinga DB, Beran GW, Riley DG, Thorne PS. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ Health Perspect. 2007;115:313–6. doi: 10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.[Food and Drug Administration] NARMS retail meat annual report, 2007. 2007. Available at: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm164662.htm. Accessed 29 November 2010. [Google Scholar]
- 3.van Loo I, Huijsdens X, Tiemersma E, et al. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13:1834–9. doi: 10.3201/eid1312.070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna T, Friendship R, Dewey C, Weese JS. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet Microbiol. 2008;128:298–303. doi: 10.1016/j.vetmic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Smith TC, Pearson N. The emergence of Staphylococcus aureus ST398. Vector Borne Zoonotic Dis. 2010 doi: 10.1089/vbz.2010.0072. [Epub ahead of print]. doi:10.1089/vbz.2010.007. [DOI] [PubMed] [Google Scholar]
- 6.Pu S, Han F, Ge B. Isolation and characterization of methicillin-resistant Staphylococcus aureus strains from Louisiana retail meats. Appl Environ Microbiol. 2009;75:265–7. doi: 10.1128/AEM.01110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enright MC, Spratt BG. Multilocus sequence typing. Trends Microbiol. 1999;7:482–7. doi: 10.1016/s0966-842x(99)01609-1. [DOI] [PubMed] [Google Scholar]
- 8.de Boer E, Zwartkruis-Nahuis JT, Wit B, et al. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int J Food Microbiol. 2009;134:52–6. doi: 10.1016/j.ijfoodmicro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Nelson JM, Chiller TM, Powers JH, Angulo FJ. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis. 2007;44:977–80. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- 10.van Rijen MM, Van Keulen PH, Kluytmans JA. Increase in a Dutch hospital of methicillin-resistant Staphylococcus aureus related to animal farming. Clin Infect Dis. 2008;46:261–3. doi: 10.1086/524672. [DOI] [PubMed] [Google Scholar]
- 11.[European Food Safety Authority] Assessment of the public health significance of methicillin resistant Staphylococcus aureus (MRSA) in animals and foods: Scientific opinion of the Panel on Biological Hazards. EFSA J. 2009;993:1–73. [Google Scholar]
- 12.de Jonge R, Verdier JE, Havelaar AH. Prevalence of methicillin-resistant Staphylococcus aureus amongst professional meat handlers in the Netherlands, March–July 2008. Eurosurveillance. 2010;15:1–5. doi: 10.2807/ese.15.46.19712-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.