In a double-blind placebo-controlled trial of a Lactobacillus crispatus intravaginal suppository probiotic (LACTIN-VÜ, Osel, Inc.) for prevention of recurrent UTI (rUTI) in pre-menopausal women, LACTIN-V was safe, well tolerated, and associated with a reduction in rUTI.
Abstract
Background. Urinary tract infections (UTIs) are common among women and frequently recur. Depletion of vaginal lactobacilli is associated with UTI risk, which suggests that repletion may be beneficial. We conducted a double-blind placebo-controlled trial of a Lactobacillus crispatus intravaginal suppository probiotic (Lactin-V; Osel) for prevention of recurrent UTI in premenopausal women.
Methods. One hundred young women with a history of recurrent UTI received antimicrobials for acute UTI and then were randomized to receive either Lactin-V or placebo daily for 5 d, then once weekly for 10 weeks. Participants were followed up at 1 week and 10 weeks after intervention and for UTIs; urine samples for culture and vaginal swabs for real-time quantitative 16S ribosomal RNA gene polymerase chain reaction for L. crispatus were collected.
Results. Recurrent UTI occurred in 7/48 15% of women receiving Lactin-V compared with 13/48 27% of women receiving placebo (relative risk [RR], .5; 95% confidence interval, .2–1.2). High-level vaginal colonization with L. crispatus (≥106 16S RNA gene copies per swab) throughout follow-up was associated with a significant reduction in recurrent UTI only for Lactin-V (RR for Lactin-V, .07; RR for placebo, 1.1; P < .01).
Conclusions. Lactin-V after treatment for cystitis is associated with a reduction in recurrent UTI. Larger efficacy trials of this novel preventive method for recurrent UTI are warranted.
Clinical Trials Registration. NCT00305227.
Urinary tract infection (UTI) is an exceedingly common outpatient problem among young healthy women and results in considerable morbidity and health care cost [1–3]. A recent population survey of women in the United States found that the lifetime risk of UTI was >60% and that the estimated societal cost of UTI exceeds $25 billion over 20 years [1]. Approximately 30% of women develop frequent recurrent episodes, which often necessitate repeated antimicrobial treatment courses [4–7]. Increasing resistance to commonly used antimicrobials such as trimethoprim-sulfamethoxazole and fluoroquinolones, even among uropathogens causing community-acquired UTIs, is making treatment and prevention of these infections more problematic [8–10]. Thus, novel, safe, and effective nonantimicrobial prevention and treatment strategies are needed.
One nonantimicrobial adjunct treatment approach for which there is strong mechanistic evidence is use of a hydrogen peroxide–producing (H2O2+) lactobacillus probiotic to restore the normal vaginal microbiota in women who are prone to UTI [11–13]. We and others have demonstrated that women with recurrent UTI (rUTI) often have specific alterations in their vaginal microbiota, especially at the time of UTI, namely, increased rates of colonization with Escherichia coli and depletion of the normally predominant H2O2+ lactobacilli [14, 15]. If repletion of the normal H2O2+ lactobacilli were effective in reducing E. coli colonization and UTI, this strategy would reduce the costs and morbidity associated with UTIs, including time lost from work, health care costs, antimicrobial use, and the ultimate development of antimicrobial resistance [16]. Although there is strong in vitro evidence to support this approach, there have been few clinical studies. Randomized, double-blind, placebo-controlled studies of adequate sample size with a physiologically relevant probiotic strain are needed to ascertain whether this approach is of value.
Lactin-V (Osel) contains a carefully selected H2O2+ Lactobacillus crispatus strain CTV-05 isolated from a healthy woman's vagina and was developed as a vaginal probiotic for use in pathophysiological states characterized by detrimental alterations in vaginal flora, such as bacterial vaginosis (BV) [17, 18], rUTI, and potentially others. We recently published the results of a randomized, double-blind, phase 1 trial of the safety and tolerance of Lactin-V in women with rUTI, showing that L. crispatus CTV-05 can be given as a vaginal suppository with minimal adverse effects to healthy women with a history of rUTI [19].
Subsequently, we conducted a randomized, double-blind, placebo-controlled phase 2 trial of Lactin-V among women with rUTI. Major goals of the study were to assess the ability of the probiotic to reduce the incidence of rUTI, to evaluate whether the probiotic achieved vaginal colonization, to assess effects on the vaginal microbiota of women after treatment for UTI, and to confirm the safety of the probiotic. We found that Lactin-V reduced the risk of rUTI approximately as effectively as antimicrobial prophylaxis, achieved high-level vaginal colonization in most women, and was well tolerated. Lactin-V is a promising candidate for prevention of rUTI.
METHODS
Participants
We recruited premenopausal women aged 18–40 years with current, symptomatic, uncomplicated cystitis from the student health center at the University of Washington (Seattle, WA) from February 2006 through February 2009. Cystitis at enrollment and during follow-up was defined as 1 or more typical UTI symptoms (dysuria, frequency, or urgency) and pyuria (cell count, ≥8 white blood cells per high-power field on urinalysis) and a positive voided midstream urine culture, defined as ≥102 colony-forming units (CFUs)/mL of 1 or more uropathogens (E. coli or other Enterobacteriaciae, Enterococcus species, or group B streptococci) or ≥105 CFUs/mL of Lactobacillus species present as a single infecting organism. Eligible participants had a history of at least 1 prior symptomatic UTI treated within the past 12 months prior to the current UTI. Eligibility requirements also included the following: a normal Papanicolaou test result documented in the past year or at the screening clinic visit; not being pregnant and having regular menstrual cycles or amenorrhea for at least 6 months secondary to use of a hormonal contraceptive or hysterectomy; agreement not to use other intravaginal products, self-medication for UTI, or antimicrobial prophylaxis while using the product; agreement not to use tampons for 24 h after insertion of the product; agreement to use birth control and, if using condoms, to use nonspermicidal condoms provided by the study site personnel; and capability of understanding English and providing informed consent.
Exclusion criteria included the following: current complicated cystitis or uncomplicated pyelonephritis; a history of urologic abnormality or renal calculi; recent sexually transmitted infection (STI) or BV or a history of recurrent BV; risk factors for STI and human immunodeficiency virus (HIV) infection; pregnancy or within 2 months of pregnancy; lactation; menopause; diabetes, HIV infection, or other immunocompromied state; drug or alcohol abuse; abnormal pelvic examination results; and persistent symptoms and/or pyuria after treatment of the enrollment acute UTI.
Randomization
The study participants were randomly assigned to Lactin-V or placebo by use of a computer-generated randomized number system in blocked assignments to achieve equal sample sizes in both groups. The assigned intervention substance (Lactin-V or placebo) was packaged in identically appearing packets according to assignment and sequential study number. The list was not available for viewing by clinicians who saw the study participants or by laboratory personnel who handled their specimens.
Study Design
The participants were treated for acute UTI at visit 1 with standard therapy. At visit 2, scheduled for 7–10 d after UTI treatment, participants were randomized to receive Lactin-V (gelatin capsules with no applicator; dose, 108 CFUs/mL) or placebo vaginal suppositories for self-administration once daily for 5 d. Participants then self-administered vaginal suppositories once weekly for 10 weeks. Participants were seen in scheduled follow-up visits at 1 week (visit 3) and 10 weeks (visit 4) after beginning Lactin-V or placebo and for symptomatic UTIs. At routine follow-up or symptomatic visits, participants underwent a structured interview, including a review of adverse events, and a structured physical examination, which included the following: assessment of the appearance of external genitalia; speculum examination of the vagina and cervix; bimanual examination; vaginal wet mount and further assessment of vaginal discharge (pH test, sniff test, and microscopic examination for clue cells, yeast, and trichomonads); urine dipstick for leukocyte esterase, nitrite, and blood; and enumeration of urine white blood cells by use of a hemocytometer. Specimens were collected for urinalysis, urine, and vaginal culture. Vaginal swab specimens for real-time 16S ribosomal RNA (rRNA) gene quantitative polymerase chain reaction (qPCR) for L. crispatus to assess vaginal colonization were collected as described elsewhere [20], by use of polyurethane foam swabs (Epicenter Biotechnologies) brushed against the lateral vaginal wall, resheathed, and frozen immediately at −20°C and then held at −80°C until analysis.
If participants developed acute cystitis symptoms during the course of the study, they were instructed to contact the clinic or, if the clinic was closed, to contact the covering physicians. If the episode occurred while the clinic was open, participants came into the clinic, were evaluated for clinical parameters, and provided specimens for testing as described above. If the episode occurred while the clinic was closed, participants were instructed to collect a specimen for urine culture, refrigerate it, and bring it to clinic as soon as the clinic was open. Participants who developed symptomatic UTI during study follow-up were treated and continued on the study.
Laboratory Procedures
Laboratory procedures were performed on clinical specimens with the use of standard methods, as described elsewhere [19], and included urine dipstick testing, urinalysis, and vaginal and urine cultures for facultative bacteria and Lactobacillus species. We quantified L. crispatus in vaginal swabs collected and stored at each visit, extracting DNA and performing real-time 16S rRNA gene qPCR with an assay specific for L. crispatus, as described elsewhere [21].
L. Crispatus Colonization Levels and Patterns
On the basis of qPCR results, L. crispatus colonization was analyzed by comparing the level of L. crispatus colonization at the end of the study in the 2 groups of participants, as well as the patterns of colonization over the course of the study in each participant. High-level L. crispatus colonization was defined as having ≥106 16S RNA gene copies of L. crispatus per swab, whereas low-level colonization was defined as having <106 16S RNA gene copies of L. crispatus per swab. A high-level colonization pattern was defined within each participant as demonstrating high-level colonization of L. crispatus at the first follow-up visit (visit 3) and maintaining this level throughout follow-up (visit 4 and any symptomatic visits during follow-up). A low-level colonization pattern was defined as all other patterns not meeting these criteria.
Sample Size, Objectives, and Institutional Review Board Approval
The target sample size and final enrollment was 100 participants, 50 in each group, to provide information on the biological activity of the Lactin-V intervention and its effect on the incidence of rUTI in high-risk women, and to provide information on possible adverse events. The primary objective was to evaluate Lactin-V in healthy premenopausal women with rUTI for the ability of the probiotic to reduce the incidence of cystitis and produce high-level vaginal colonization with L. crispatus at the end of 10 weeks. Secondary objectives were to evaluate patterns of vaginal colonization with L. crispatus in the vaginal microbiota and to confirm the safety of the probiotic. The study was approved by the institutional review board of the University of Washington and registered as a clinical trial (Clinicaltrials.gov ID no. NCT00305227).
Data Analysis
All women enrolled and randomized were described using means, medians, and frequency counts. The effect of the intervention was described using relative risks (RRs) and 95% confidence intervals (CIs). Per-protocol analyses were performed and produced similar results.
RESULTS
Enrollment and Demographics
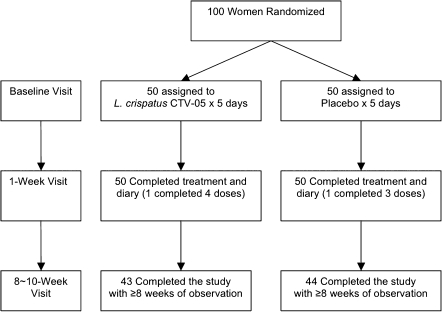
The total enrollment was 100 participants; 50 received Lactin-V and 50 received placebo (Figure 1). The median age of participants was 21 years in both groups. The median number of lifetime UTIs was 4.5. Three-quarters of the women in both groups had never been married, and 99% were sexually active with an equal median rate of sexual activity in the month prior to enrollment; <10% of women had used a spermicide-containing birth control method in the past month (Table 1).
Figure 1.
Randomization and follow-up of participants. L. crispatus CTV-05, Lactobacillus crispatus strain CTV-05.
Table 1.
Baseline Characteristics of Study Participants
| Characteristics | Participants receiving Lactin-V (n = 50) | Participants receiving placebo (n = 50) | All participants(n = 100) |
| Age in years, median (range) | 21 (18–31) | 21 (18–36) | 21 (18–36) |
| Never married | 39 (78) | 37 (74) | 76 (76) |
| Race or ethnicity | |||
| White | 33 (66) | 42 (84) | 75 (75) |
| Asian | 8 (16) | 3 (6) | 11 (11) |
| African American | 0 (0) | 1 (2) | 1 (1) |
| Native American | 0 (0) | 1 (2) | 1 (1) |
| Other | 9 (18) | 3 (6) | 12 (12) |
| Hispanic | 4 (8) | 3 (6) | 7 (7) |
| No. of UTIs in lifetime, median (range) | 4 (1–45) | 5 (1–30) | 4.5 (1–45) |
| 1–2 | 18 (36) | 12 (24) | 30 (30) |
| ≥3 | 32 (64) | 38 (76) | 70 (70) |
| Sexually active in the past month | 50 (100) | 49 (98) | 99 (99) |
| Episodes of sexual intercourse in the past month, median | 10 | 10 | 10 |
| Any spermicide usea in the past month | 5 (10) | 2 (4) | 7 (7) |
| Abnormal Papanicolaou test result | 2 (4) | 3 (6) | 5 (5) |
NOTE. Data are no. (%) of participants, unless otherwise indicated. UTI, urinary tract infection.
Diaphragm or spermicidal condom use.
Clinical Outcomes and Probiotic Effect on Vaginal Microbiota
Seven (15%) of the women receiving Lactin-V had at least 1 UTI compared with 13 (27%) of the women receiving placebo (RR, .5; 95% CI, .2–1.2) (Table 2). The prevalence of E. coli rUTIs was similar in the 2 groups (prevalence in Lactin-V group, 75%; prevalence in placebo group, 69%). On the basis of qPCR analysis, most women receiving Lactin-V achieved high-level≥ vaginal colonization with L. crispatus (≥106 16S RNA gene copies per swab). At the final visit at 10 weeks (visit 4), the prevalence of high-level L. crispatus colonization among Lactin-V recipients was 39 (93%) of 42 participants, compared with 30 (68%) of 44 placebo recipients (P = .004).
Table 2.
Urinary Tract Infection Rates by Intervention and Lactobacillus crispatus Colonization Pattern
| Intervention | No. (%) of participants developing recurrent UTI | Relative risk(95% CI) |
| Lactin-V (n = 48) | 7 (15) | .5 (.2–1.2) |
| Placebo (n = 48) | 13 (27) | … |
| Intervention, L. crispatus colonization pattern | ||
| Lactin-V, High level (n = 41) | 2 (5) | .07 (.02–.3) |
| Lactin-V, Low level (n = 7) | 5 (71) | … |
| Placebo, High level (n = 32) | 9 (28) | 1.1 (.4–3.1) |
| Placebo, Low level (n = 16) | 4 (25) | … |
NOTE. CI, confidence interval; Lactin-V, L. crispatus intravaginal suppository probiotic (Osel); UTI, urinary tract infection
Women who received Lactin-V and achieved a high-level L. crispatus vaginal colonization pattern throughout the course of the study had a significant reduction in the risk of rUTI, whereas when this high-level colonization pattern occurred in women who received placebo, it was not protective (RR for Lactin-V, .07; RR for placebo, 1.1; P< .01) (Table 2). There was no significant difference in causative organisms for rUTI events in the 2 arms of the study.
Safety and Tolerability
We confirmed the findings in the phase 1 study regarding safety and tolerance of Lactin-V [19]. Adverse effects were reported by 56% of participants who received Lactin-V and by 50% of participants who received placebo; the most common adverse effects included vaginal discharge or itching or moderate abdominal discomfort. One participant in the placebo group discontinued treatment because of adverse effects. The incidence of BV or candidal vaginitis was low in both treatment groups (0%–5%), and unlike the findings in the phase 1 study [19], there was no significant difference in rates of pyuria between the 2 groups (rate at visit 3 among women in the Lactin-V group, 13%; rate at visit 3 among women in the placebo group, 22%; rate at visit 4 among women in the Lactin-V group, 32%; rate at visit 4 amont women in the placebo group, 33%). No episodes of pyelonephritis were reported in either group.
DISCUSSION
The results of this randomized, double-blind, phase 2 trial suggest that Lactin-V, an intravaginal probiotic composed of L. crispatus CTV-05, may reduce the rate of rUTI in UTI-prone women by about one-half. Thus, among the 50 women who received Lactin-V, the rate of culture-confirmed UTI was 15%, as compared with 27% among women who received placebo (RR, .5; 95% CI, .2–1.2). Moreover, women in the Lactin-V group who achieved a high-level L. crispatus vaginal colonization pattern had a significant reduction in rUTI compared with those who did not (P < .01). Women receiving placebo had no such reduction, regardless of their pattern of colonization. The safety and tolerability of Lactin-V, previously demonstrated in a phase 1 study [19], was confirmed in this study.
Lactin-V readily achieved high-level and sustained vaginal colonization, as measured by L. crispatus qPCR. Nearly every woman who received Lactin-V had L. crispatus colonization at follow-up as shown with qPCR. In contrast, among the 14 study participants who had no L. crispatus identified using the highly sensitive qPCR method, 12 had received the placebo. Both Lactin-V recipients in whom L. crispatus colonization failed to establish during follow-up had rUTI.
We and others have shown that women with rUTI typically have depletion of the normally predominant H2O2+ lactobacilli at the time of UTI [14, 15]; however, previous studies have used standardized methods of quantifying lactobacilli cultivatable from vaginal samples on a scale of 1–4+ [22] or by using repetitive element sequence-based PCR repPCR, which also requires cultivatable lactobacilli [19]. This study is novel in its application of a quantitative molecular method to assess vaginal microbiota following UTI, and thus to more precisely define sentinel changes in vaginal microbiota following standard treatment for UTI, either with or without a specific intervention designed to repopulate the vaginal bacterial biota, namely, the Lactin-V probiotic.
We assessed L. crispatus colonization as a sentinel measure of the vaginal microbiota because surveys of healthy, premenopausal women worldwide show that this bacterium is very common, although colonization with other lactobacilli, including L. iners, L. jensenii, or L. gasseri, is also found [23–27]. Thus, using a qPCR method to assess L. crispatus colonization in women in both arms of the study allowed us to distinguish the natural recovery of the vaginal microbiota after UTI, as may have potentially occurred in the participants receiving placebo, from specific effects attributable to the intervention with the Lactin-V probiotic.
This analysis showed a most striking finding, namely, that although women who received placebo often had high concentrations of vaginal L. crispatus during follow-up, this colonization did not protect them from rUTI (RR, 1.1; 95% CI, .4–3.1), whereas those women who received Lactin-V and achieved high colonization were protected from rUTI (RR, .07; 95% CI, .02–.3; P < .01). Thus, since women in both arms of the study had reduction in lactobacillus colonization after UTI (not shown), receiving Lactin-V after treatment for acute UTI confers a significant advantage over repopulation of the vaginal microbiota with endogenous L. crispatus. We hypothesize that this advantage reflects unique properties for protection against UTI associated with repopulation by the probiotic lactobacillus isolate. This is supported by in vitro studies showing that the probiotic strain adheres to vaginal epithelial cells (VECs) in high numbers, especially cells from women with rUTI [28]. In addition, L. crispatus CTV-05 competitively inhibits uropathogenic E. coli adherence to VECs and inhibits growth of uropathogenic E. coli in vitro (Stapleton, unpublished data, 2010 unpublished data). Ongoing studies in our group are directed at understanding the mechanisms of protection in vivo and optimizing this prophylactic regimen.
In a recent review of randomized controlled trials (RCTs) of lactobacillus probiotics for BV or UTI, only 4 RCTs for prevention of UTI were identified, and only 1 of these 4 demonstrated a significant reduction in rates of recurrence [29]. Most studies did not assess vaginal colonization with lactobacilli after treatment, including the 1 positive study of probiotic prevention of UTI [29]. In our study, we found that a quantitative assessment of the vaginal microbiota was useful in determining the efficacy of this intervention to prevent rUTI.
We did not directly compare this intervention with antimicrobial prophylaxis for rUTI, but a comparison of our results with historic controls is instructive. Sen [30] reviewed multiple modalities for prevention of rUTI in 2008, noting that RCTs demonstrate efficacy of both continuous and postcoital antimicrobial prophylaxis regimens. There were more RCTs of continuous prophylaxis regimens, and the administration schemas of these regimens are more similar to our study design. Sen [30] reported that in a meta-analysis of 10 RCTs of continuous antimicrobial prophylaxis over 2–6 months of follow-up, rates of rUTI were reduced to 12% (24 of 195 participants) compared with 65% (116 of 177 participants) among placebo recipients, with a RR of rUTI that is very comparable to our findings (RR, .21; 95% CI, .13–.33).
In summary, Lactin-V treatment in women with rUTI resulted in robust and prolonged colonization with L. crispatus, with a trend of reducing the incidence of rUTI by ∼50%. The protective effects of Lactin-V were even greater in those women who achieved the most robust colonization with L. crispatus and reflect an apparent treatment advantage for Lactin-V over natural recovery of the vaginal microbiota after an episode of rUTI. This antimicrobial-sparing, well-tolerated intervention compares favorably with historical data regarding antimicrobial prophylaxis, the current standard of care for the prevention of rUTI. Our results strongly support the need for a larger, randomized, double-blind, placebo-controlled trial to confirm the efficacy of Lactin-V for prevention of rUTI in the general population of at-risk women. Studying this intervention in a population-based cohort may also supply useful information on target subsets of women who would most benefit from this type of intervention.
Supplementary Material
Acknowledgments
We are greatly indebted to D.C. Dugdale, Medical Director, and the staff at Hall Health Primary Care Center (University of Washington, Seattle, WA) for assistance with study enrollment; Ellen Cassen and Natalie DeShaw for assistance in the development of study protocols, data collection, project coordination, and data management at Hall Health Center; Cheryl Wobbe, Sheila Manuguid, and Amanda White (University of Washington Urinary Tract Infection Research Laboratory) for laboratory assistance; Peter Lee of Osel (Santa Clara, CA); and the study participants. We dedicate this manuscript to the memory of Walter Stamm.
Financial support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P01 DK053369 to W.E.S., A.E.S., M.A.-Y., T.M.H., P.L.R., C.A.C., Y.Y.-Y., and M.C.); and the Office of Research in Women's Health, National Institutes of Health (grant number SCOR P50 DK64540 to W.E.S., A.E.S., M.A.-Y., T.M.H., P.L.R., C.A.C., Y.Y.-Y., and M.C.).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Engel JD, Schaeffer AJ. Evaluation of and antimicrobial therapy for recurrent urinary tract infections in women. Urol Clin North Am. 1998;25:685–701. doi: 10.1016/s0094-0143(05)70057-4. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10:509–15. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 3.Hooton T, Stamm W. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am. 1997;11:551–81. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–9. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Mabeck CE. Treatment of uncomplicated urinary tract infection in nonpregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 7.Stamm WE, McKevitt M, Roberts PL, White NJ. Natural history of recurrent urinary tract infections in women. Rev Infect Dis. 1991;13:77–84. doi: 10.1093/clinids/13.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 9.Gupta K, Sahm DF, Mayfield D, Stamm WE. Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis. 2001;33:89–94. doi: 10.1086/320880. [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736–8. doi: 10.1001/jama.281.8.736. [DOI] [PubMed] [Google Scholar]
- 11.Redondo-Lopez V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal microflora. Rev Infect Dis. 1990;12:856–72. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 12.Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol. 2001;30:49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 13.Reid G, Bruce AW, McGroarty JA, Cheng KJ, Costerton JW. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin Microbiol Rev. 1990;1990:335–44. doi: 10.1128/cmr.3.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis. 1998;178:446–50. doi: 10.1086/515635. [DOI] [PubMed] [Google Scholar]
- 15.Pfau A, Sacks T. The bacterial flora of the vaginal vestibule, urethra and vagina in premenopausal women with recurrent urinary tract infections. J Urol. 1981;126:630–4. doi: 10.1016/s0022-5347(17)54661-3. [DOI] [PubMed] [Google Scholar]
- 16.Russo T, Johnson J. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–56. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 17.Hemmerling A, Harrison W, Schroeder A, et al. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sex Transm Dis. 2009;36:564–9. doi: 10.1097/OLQ.0b013e3181a74924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis. 2010;37:745–50. doi: 10.1097/OLQ.0b013e3181e50026. [DOI] [PubMed] [Google Scholar]
- 19.Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007;2007:35387. doi: 10.1155/2007/35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45:3270–6. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martius J, Krohn M, Hillier S, Stamm W, Holmes K, Eschenbach D. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988;71:89–95. [PubMed] [Google Scholar]
- 23.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–6. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 24.Ocana VS, Pesce de Ruiz Holgado AA, Nader-Macias ME. Selection of vaginal H2O2-generating Lactobacillus species for probiotic use. Curr Microbiol. 1999;38:279–84. doi: 10.1007/pl00006802. [DOI] [PubMed] [Google Scholar]
- 25.Pavlova SI, Kilic AO, Kilic SS, et al. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol. 2002;92:451–9. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- 26.Song YL, Kato N, Matsumiya Y, Liu CX, Kato H, Watanabe K. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37:3062–4. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallor AC, Antonio MA, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis. 2001;184:1431–6. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 28.Kwok L, Stapleton AE, Stamm WE, Hillier SL, Wobbe CL, Gupta K. Adherence of Lactobacillus crispatus to vaginal epithelial cells from women with or without a history of recurrent urinary tract infection. J Urol. 2006;176:2050–4. doi: 10.1016/j.juro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Barrons R, Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30:453–68. doi: 10.1016/j.clinthera.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Sen A. Recurrent cystitis in non-pregnant women. Clin Evid (Online). 2008 Jul 17; 2008. pii:0801. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.