Abstract
The use of functional neuroimaging techniques has advanced what is known about the neural mechanisms used to support language processing in aphasia resulting from brain damage. This paper highlights recent findings derived from neuroimaging studies focused on neuroplasticity of language networks, the role of the left and right hemispheres in this process, and studies examining how treatment affects the neurobiology of recovery. We point out variability across studies as well as factors related to this variability, and we emphasize challenges that remain for research.
Introduction
The use of functional neuroimaging techniques has advanced what is known about the neural mechanisms used to support language processing in aphasia resulting from brain damage. Prior to the advent of functional neuroimaging, the network supporting language recovery was largely elucidated by lesion-deficit studies in patients. Studies are now available using positron emission tomography (PET), functional MRI (fMRI), and magnetoencephalography (MEG). PET and fMRI allow examination of processing in the spatial domain and reveal the areas of the brain that are active under certain language conditions. MEG is capable of examining the time course of language processing (as in electroencephalography) and spatial processing. However, it has been used less widely than other methods, primarily because of availability and associated costs. In this article, we discuss recent findings derived from these neuroimaging techniques in studies focused on neuroplasticity of language networks, the role of the left and right hemisphere in this process, and the effect of treatment on the neurobiology of recovery.
Neural Mechanisms of Language Recovery
It has been just over a decade since it was firmly established that neuroplasticity extends to the adult brain [1], providing neurophysiologic evidence for behavioral observations that individuals with aphasia often show substantive recovery after brain damage to left perisylvian areas, even in the chronic phase [2]. As to which brain regions and processes subserve such recovery, two primary patterns have been observed: 1) language function, premorbidly subserved by the damaged left hemisphere, is shifted to right hemisphere areas homologous to left hemisphere areas involved in affected language functions; and 2) neural tissue in the left hemisphere is recruited, extending the functional map to include perilesional regions. This tissue may be able to subsume processes typically controlled by the damaged regions, perhaps because of functional redundancy.
One issue with regard to the validity of these patterns concerns whether right and left hemisphere regions shown to be active following brain damage were engaged premorbidly in language processing. Neuroimaging studies of language processing with healthy volunteers show right hemisphere activation as well as extrasylvian recruitment across language domains [3,4]. These observations indicate that right hemisphere activation seen in studies with aphasic patients may reflect, at least in part, the large-scale neural network that subserves language under normal conditions. In a recent study, Thompson et al. [5] found right posterior perisylvian activation for verb argument structure processing in three of five patients with Broca’s aphasia who had damage to this region in the left hemisphere. However, data from 12 healthy control participants who were age-matched with the aphasic participants showed activation in the same region bilaterally. The right hemisphere activation noted for the aphasic participants may thus represent a premorbidly available network.
Not all studies, however, are clear-cut. Healthy volunteers sometimes show weak and diffuse right hemisphere activation under language processing conditions, whereas aphasic patients show relatively greater and more focal regions of activation [4]. Because of strong left hemisphere activation in healthy individuals, right brain activation may not reach threshold; however, in the case of reduced left hemisphere activity, as in aphasia, right hemisphere activation may pass the statistical thresholds imposed in neuroimaging studies. Observed increases in right hemisphere activation after stroke may therefore not reflect a true quantitative increase but, rather, the emergence of activity that was hitherto overshadowed by the language-dominant hemisphere. Further research examining language processing in healthy and aphasic participants will help to clarify these issues.
Below, we review studies showing patterns of right and left hemisphere tissue recruitment associated with recovery of language and discuss related factors. We first highlight a recent finding pertaining to blood flow changes in stroke-induced aphasia and how they may affect the activation patterns derived from neuroimaging studies of aphasia recovery [6•,7].
Stroke affects blood flow
In a recent study, Bonakdarpour et al. [6•] found that the blood oxygen level–dependent (BOLD) signal time-to-peak (TTP) is delayed in some patients with stroke-induced aphasia. Using an event-related design and a 30-second interval between stimulus presentations, allowing examination of the true hemodynamic response of the patients, three of five patients showed TTPs up to 20 seconds after stimulus, particularly in the left perisylvian region. Because many fMRI studies with stroke patients use a canonical hemodynamic response function (HRF) for data analysis, peaking at about 6 seconds following the cognitive event, it is possible and likely that activation was missed or underestimated in these studies. Thus, it is important in patient studies to examine the HRF and use it for analysis of data, particularly in event-related designs, because the shape change may alter the results.
In our own recent work [5], we measured patients’ hemodynamic responses (as reported by Bonakdarpour et al. [6•]) in 16 regions of interest (Brodmann’s areas 7, 9, 21, 22, 39, 40, 44, and 45 bilaterally) in an independent run and then used the obtained HRF patterns as weights to postevent time bins in the statistical model using a finite impulse response function. Specific contrasts were calculated for regions of interest by assigning positive and negative values to these weights.
Right versus left hemisphere recruitment
Most individuals with left hemisphere lesions resulting in aphasia, even those with large lesions encompassing the entire perisylvian region, recover language to some degree. In turn, recovered language further deteriorates following a second (right hemisphere) stroke. These observations have led researchers to believe that the right hemisphere “takes over” language function or at least plays a strong role in recovery of language [8,9]. Corroborating these behavioral observations, a number of neuroimaging studies have shown right hemisphere activation in aphasic patients during language-processing tasks. In an early PET study, Weiller et al. [10] found greater regional cerebral blood flow in the right hemisphere homologues of both Wernicke’s and Broca’s areas (ie, in the superior temporal gyrus and the inferior premotor and lateral prefrontal cortices) for Wernicke’s aphasics as compared with healthy, non–brain-damaged volunteers. Similar findings were reported by Cao et al. [11] at least 5 months after stroke. Using fMRI to examine lexical-semantic processing, they found significantly greater right hemisphere activation in the patients than in the healthy subjects. In a more recent fMRI study examining word retrieval, Perani et al. [12] also found right hemisphere activation to be associated with language processing. Their aphasic speakers with damage to the left inferior frontal area showed activation of the right hemisphere homologue of this region when performing a word-retrieval task. Notably, participants with sparing of this region in the left hemisphere activated the left rather than right inferior frontal region. Data such as these indicate that homologous right hemisphere regions can assume the function of damaged left hemisphere regions.
Several neuroimaging studies also have found activation of left hemisphere regions to be associated with language processing in aphasia [12–17], with language function extending to tissue adjacent to the damaged region. A strong interpretation of left hemisphere recruitment following brain damage is that it reflects “better” recovery. According to this view, right hemisphere recruitment may be maladaptive and reflect inefficient language processing rather than being beneficial to recovery [14,18,19]. An early study by Belin et al. [14], for example, found left anterior activation associated with improved word processing following a period of melodic intonation therapy. Supporting this position, Naeser et al. [20] reported that application of repetitive transcranial magnetic stimulation to right interior frontal cortex improved picture naming in four chronic nonfluent aphasic speakers, suggesting that this stimulation inhibited the right hemisphere and at the same time facilitated left brain processing. However, a recent finding by Winhuisen et al. [21] does not support this claim. They found that inhibiting right inferior frontal gyrus functioning using repetitive transcranial magnetic stimulation in patients with acute aphasia led to decreases in language performance on a semantic task.
Many studies have found correlations between left hemisphere activation and less severe forms of aphasia [15, 22]. However, in a study with severely impaired patients with global aphasia, Zahn et al. [23] reported no correlation between the neural recruitment site on fMRI scans and comprehension ability of the patients. Like healthy control subjects, the patients showed bilateral activation for phonetic, lexical, and semantic tasks, mainly in left extrasylvian temporal and right posterior parietal regions.
Lesion characteristics
The extent to which the brain’s hemispheres are engaged to support language recovery depends to some degree on the size and extent of the lesion. Vitali et al. [24] reported pretreatment and posttreatment fMRI data for two patients, one with a small lesion and one with a large lesion encompassing the entire perisylvian region. Results showed left hemisphere perilesional increases for the patient with the small lesion and increased activation in the right hemisphere homologue of Broca’s area in the patient with the large lesion. Grafman [25] suggested that homologous right-brain adaptation is most likely when lesions completely destroy cortical regions that serve a particular function. Transfer of function is less likely to occur when damage is incomplete because homologous sites are inhibited under normal conditions by connections from contralateral regions. When damage is incomplete, inhibitory input is retained, thereby precluding transfer of function.
The specific right hemisphere areas that show upregulation of activity are also influenced by site of lesion [26,27]. Thulborn et al. [27] reported on two patients with different lesions, one in Broca’s area and one in Wernicke’s area, in whom recovery of simple sentence (reading) comprehension was correlated with an activation shift over time to the right hemisphere homologues of their respective lesions.
An important consideration with regard to the relation between lesion characteristics and recovery concerns the precision with which the dimensions of the lesion are determined. In large groups of patients, correlations between lesion characteristics and aphasic symptoms have been found to be fairly stable [28,29]. However, recent structural fMRI studies show that lesions associated with classic types of aphasia are heterogeneous and often involve tissue regions beyond those expected [30]. In addition, perfusion weighted imaging studies show that lesion-adjacent tissue may be hypoperfused. Although not necrotic, this tissue is dysfunctional and therefore may not be recruited to support language function, in either the acute and chronic stage [31–33]. Thus, in effect, lesions often occupy more cortical tissue than structural scans reveal. Interestingly, Hillis et al. [34] have shown that, in acute stroke, reperfusion of perilesional hypoperfused areas is possible through intervention, and this reperfusion is highly correlated with improvements on specific language tasks.
Poststroke disconnections or modifications of white matter pathways also need to be considered, because they are sometimes affected by stroke, and this can influence recovery. Diffusion tensor imaging (DTI), an MRI technique that tracks the direction of water molecule movement, is useful for mapping pathways between cortical and subcortical regions. Using this method, Breier et al. [35] found a strong relation between damage to the left hemisphere superior longitudinal and arcuate fasciculi and repetition deficits (as predicted by Lichtheim and by Geschwind [36]).
More generally, lesions resulting in aphasia may not only reflect damage to cortical regions. Changes in connectivity between cortical regions also may underlie language disruption, at least in part. Using dynamic causal modeling to investigate the affected connectivity between different cortical areas in the language network in patients with primary progressive aphasia, Sonty et al. [37•] showed reduced effective connectivity between Broca’s and Wernicke’s areas in these patients, and this was correlated with semantic task performance. Research with stroke-induced aphasia using this method may show similar patterns.
Shifts from the right to the left hemisphere
Several longitudinal studies of aphasia have shown that neural tissue recruitment changes relative to time after stroke. Heiss et al. [16] showed that recovery progresses from early recruitment of right brain tissue during the spontaneous recovery period to reactivation of areas in the left hemisphere surrounding the infarction. In another study, de Boissezon et al. [38] used PET to investigate recovery in seven aphasics with subcortical lesions. Improvement after 1 year was correlated with bilateral temporal activation, with strong left hemisphere inferior temporal activation. Using fMRI 1 month after stroke and 1 year later in a patient with conduction aphasia, Fernandez et al. [39] showed a shift in activation to the perilesional (parietotemporal) cortex during phonologic task performance. Similarly, Ino et al. [40] reported on a patient recovering from letter-by-letter reading (a form of alexia) after a left basal-occipitotemporal traumatic hemorrhage who underwent fMRI 7 and 50 days after stroke. Initial activation of a bilateral network, including the right hemisphere homologue of the lesioned area (the visual word-forming area), shifted to perilesional and left superior parietal activation with successful recovery.
In an interesting study showing activation shifts over time, Saur et al. [41•] performed fMRI on 14 aphasic speakers in the acute (< 4 days after stroke), subacute (about 2 weeks after stroke), and chronic phases of recovery (4–12 months after stroke) using an auditory comprehension task. Analysis revealed little activation overall in the acute phase. A sharp increase in activation bilaterally occurred in the subacute phase, peaking in the right hemisphere homologue of Broca’s area, while in the chronic phase, activation patterns resembled those of control subjects (ie, left hemisphere perilesional activation) in the chronic phase. Both the upregulation of activity in the right hemisphere in the subacute phase and the “normalization” to left hemisphere activation peaks in the chronic phase were correlated with improved language functions, suggesting that right hemisphere structures may well have a beneficial role to play in early stages of recovery from aphasia but that ultimately it is the left hemisphere that remains best equipped to sustain effective language functions.
Effects of Treatment on the Neurobiology of Recovery
An important variable that influences the neural mechanisms of language processing is language treatment. Even studies providing short-term treatment show that activation patterns shift after treatment. During a single fMRI session, Blasi et al. [42] provided eight patients with repeated blocks of word-stem completion tasks. Results showed increased activity in the right inferior frontal region and concomitant decreased activation in the left occipital region associated with learning. These data indicate that, as animal studies have shown, the brain is an organ of plasticity and is directly affected by environmental manipulation.
Several studies using fMRI, PET, or MEG have directly charted changes in neural activation resulting from application of more long-term treatment. We reviewed 17 papers reporting the results of such studies since 2000 (Table 1 and Table 2). All showed that language improvement maps onto the brain, with activation patterns changing from pretreatment to posttreatment scans. However, the derived activation patterns differ across studies, likely because of lesion-related or language deficit–related differences in the patients studied. For example, participants across and sometimes within studies vary with regard to the type of aphasia. Other factors related to differences across studies include the type and dosage of treatment provided, the scanning tasks used to evaluate treatment effects, and the methods of data analysis employed.
Table 1.
Treatment-induced aphasia recovery studies with functional MRI
| Study | Aphasia types | LBT | Treatment | Dosage | Scan task | Activation changes* |
|---|---|---|---|---|---|---|
| Thompson et al. [56] | 2 agrammatic | Sentence production and comprehension | TUF | 20 wk; 2 1-h sess/wk | Auditory sentence–picture matching | RH STG, MTG, IFG |
| Leger et al. [49] | 1 conduction | Naming | Visually aided articulation training | 6 wk; 6 1-h sess/wk | Overt picture naming; rhyme judgment | LH IFG, SMG |
| Crosson et al. [53] | 2 nonfluent | Naming | Intention treatment | 6 wk; 5 sess/wk | Semantic category-member generation | RH pre-SMA; LH BG |
| Thompson [3] | 3 agrammatic | Sentence production and comprehension | TUF | 5 wk; 2 1-h sess/wk | Auditory sentence–picture matching | BL STG; RH prefrontal, IFG IPS |
| Cherney and Small [26] | 2 Broca | Sentence production and comprehension | Multisensory facilitation | 24 sess; 2–3 sess/wk | Oral reading; passive story comprehension | Reading: RH increase; comprehension: BL decrease |
| Davis et al. [54] | 1 Wernicke | Word comprehension | Semantic-based treatment | 4 wk; 5 1.5-h sess/wk | Lexical decision; verb generation; text listening | LH IFG; RH post-ITG |
| Fridriksson et al. [50] | 2 Broca; 1 anomic | Naming | Errorless learning | 2 wk; 4 h/d | Picture naming | BL STG, IPL |
| Wierenga et al. [55] | 2 nonfluent | Sentence production | Syntactic mapping | 32 90-min sess | Covert picture description (sentence generation) | LH IFG |
| Fridriksson et al. [51] | 2 Broca; 1 conduction | Naming | Phonologic and semantic | 2 wk; 5 2-h sess/wk | Picture naming | BL precuneus |
| Meinzer et al. [45] | 1 amnestic | Unspecified language | CIAT | 10 d; 3-h sess | Picture naming (German and French) | BL MFG, IFG, IPL |
| Vitali et al. [24] | 2 nonfluent | Naming | Phonologic cuing | 8 wk/4 wk; 5 1-h sess/wk | Picture naming | LH IFG, STG; RH homologues |
| Meinzer et al. [44] | 7 Broca; 2 Wernicke; 1 global; 1 unclassified | Unspecified language | CIAT | 10 d; 3-h sess | Picture naming | LH perilesional |
| Richter et al. [46] | 7 Broca; 7 anomic; 2 global | Unspecified language | CIAT | 2 wk; 5 3-h sess/wk | Word reading; word-stem completion | RH IFG and insula decrease |
| Thompson et al. [5] | 6 agrammatic | Sentence production and comprehension | TUF | 6 wk; 2 sess/wk | Auditory sentence verification | BL STG, IPL |
Pretreatment to posttreatment activation changes, correlated with improved language function.
BG—basal ganglia; BL—bilateral; CIAT—constraint-induced aphasia therapy; IFG—inferior frontal gyrus; IPL—inferior parietal lobule; IPS—intraparietal sulcus; ITG—inferior temporal gyrus; LBT—language behavior targeted; LH—left hemisphere; MFG—middle frontal gyrus; MTG—middle temporal gyrus; RH—right hemisphere; sess—sessions; SMA—supplementary motor area; SMG—supramarginal gyrus; STG—superior temporal gyrus; TUF—treatment of underlying forms.
Table 2.
Treatment-induced aphasia recovery studies with PET and MEG
| Study | Aphasia types | LBT | Treatment | Dosage | Scan task (study) |
Activation changes* |
|---|---|---|---|---|---|---|
| Cornelissen et al. [13] | 3 anomic | Naming | Contextual priming | 3 wk; 3 1-h sess/wk | Delayed naming (MEG) | LH inferior parietal lobule (perilesional) |
| Breier et al. [43] | 1 expressive and receptive | Unspecified; requesting items and responding to requested items | CIAT using dual card task | 3 wk; 4 3-h sess/wk | Target word comprehension (MEG) | RH language homologues: immediate increase; BL recruitment after 3 mo of treatment |
| Raboyeau et al. [52] | 2 Broca | Naming | Lexical training | 4 wk; 5 15-min sess/wk | Picture naming in English and French (PET) | RH insula and inferior frontal gyrus |
| 3 conduction | ||||||
| 4 anomic | ||||||
| 1 apraxia of speech |
Pretreatment to posttreatment activation changes, correlated with improved language function.
BL—bilateral; CIAT—constraint-induced aphasia therapy; LBT—language behavior targeted; LH—left hemisphere; MEG—magnetoencephalography; PET—positron emission tomography; RH—right hemisphere; sess—sessions.
Language and treatment variables
Table 1 and Table 2 show that the language behavior subjected to treatment as well as the type and dosage of treatment provided has varied across studies examining the neuroplastic effects of language treatment. These variables likely impact the mechanisms engaged to accommodate language-processing changes. Most of the studies have examined the effects of treatment for word-level processing, with fewer focused on treatment of sentence-level processing and on training nonspecific requesting and responding behavior (using constraint-induced aphasia therapy [43–46]). Indeed, animal studies show that auditory stimulation and motor experience strongly influence the neural organization of primary auditory and motor cortices, respectively [47,48]. Thus, it follows that treatment targeting different aspects of language processing will differentially influence the neural recruitment patterns observed following treatment.
In the domain of word-level processing, studies have focused on word retrieval, naming, and word comprehension and have used a variety of treatment approaches, including visually aided production [49], contextual priming [13], various forms of phonologic and semantic treatment [24,50–52], and intention treatment [53]. Three of the studies found left hemisphere activation to be associated with improvement [13,24,49]. Cornelissen et al. [13] found perilesional, left inferior parietal activation to be associated with improved naming. Conversely, three studies found right hemisphere recruitment [24,52,53]. Raboyeau et al. [52] showed that improved picture naming was associated with increased activation in the right inferior frontal gyrus and insula. Finally, four studies found bilateral activation to be associated with treatment improvement [45,50,51,54]. Davis et al. [54] showed activation in the left inferior frontal and the right posterior inferior temporal cortices after semantic feature treatment.
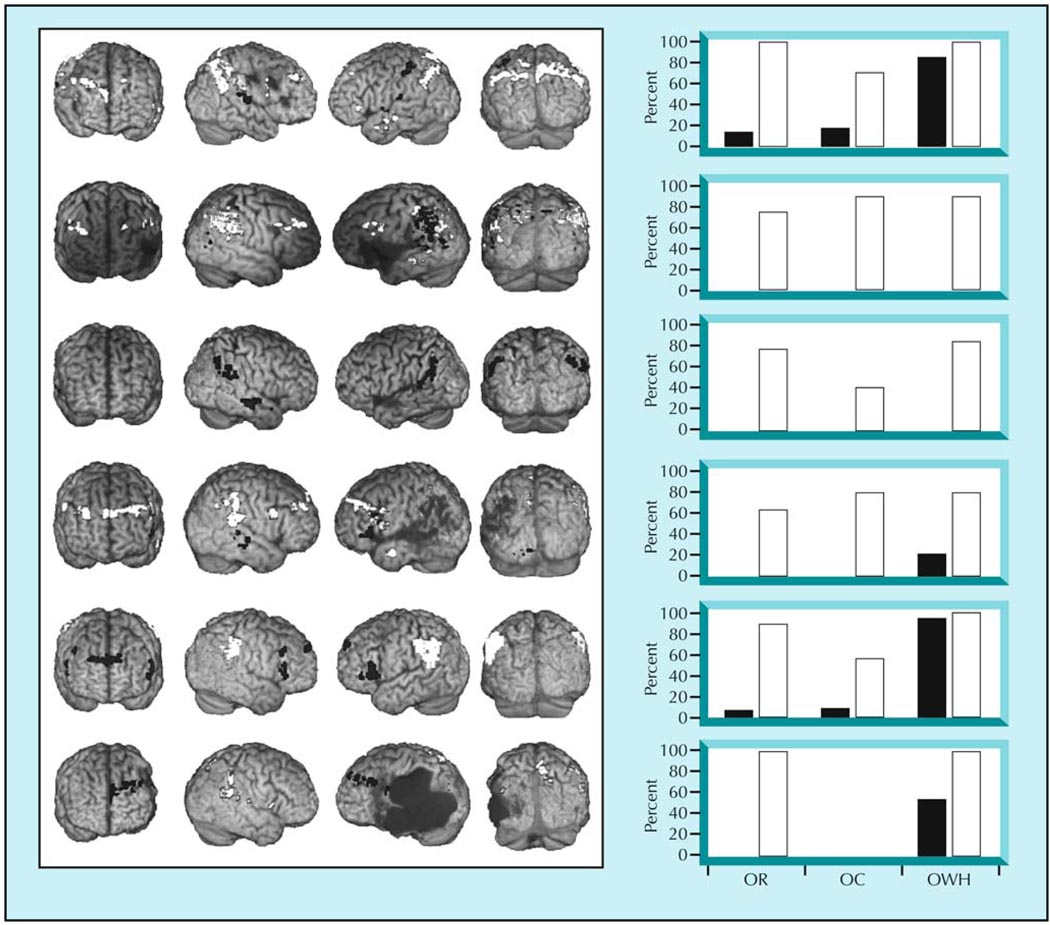
Similar variation exists for studies of sentence-level treatment. Cherney and Small [26] investigated the effects of multisensory facilitation, Wierenga et al. [55] studied syntactic mapping, and Thompson et al. [3,5,56] tested treatment of underlying forms. Notably, all studies examining the effects of sentence-level treatment have enrolled patients with nonfluent aphasia. Nevertheless, differences have been noted across studies with regard to changes in activation patterns from before to after treatment. Cherney and Small [26] found generally increased right hemisphere activation, and bilaterally decreased activation was noted for one patient. Conversely, Wierenga et al. [55] found increased left inferior frontal gyrus activation in their two patients. In our own studies [3,56], we found right inferior frontal activation to be associated with improved sentence comprehension and production. In addition, two patients showed right superior and middle temporal gyrus activation, and three showed bilateral superior temporal gyrus activation. In our recent study [5], which included six individuals with agrammatic Broca’s aphasia, we found increased right hemisphere temporoparietal activation (bilateral in patients where the lesion spared this area in the left hemisphere) to be associated with treatment effects (Fig. 1).
Figure 1.
Each row shows one individual’s activation differences for auditory verification of complex sentences between pretraining (black) and posttraining (white) functional MRI scans (false discovery rate corrected, P < 0.05; k > 3). A posttreatment temporoparietal activation increase was seen for all patients except the one represented in the third row. The graphs show each corresponding subject’s percent correct scores on behavioral production probes of object relatives (OR), object clefts (OC), and object-extracted “wh-” questions (OWH: eg, Whom did the bride carry?) before (black) and after (white) training of OR structures. (Adapted from Thompson et al. [5].)
Scanning tasks and task performance
The tasks used in the scanner to evaluate treatment-induced change have varied across studies, which likely influenced the derived activation patterns. Studies examining the effects of sentence comprehension and production treatment have used a number of tasks, including oral sentence reading and passive story comprehension [26], covert picture description [53], and auditory verification [3,5,56], which required participants to respond by pushing one of two buttons if the sentence matched a picture. Indeed, research has shown task-specific activation in patients with aphasia. Calvert et al. [57] reported on a 28-year-old woman who, after partial recovery from a left hemisphere stroke, showed a lack of activation in the inferior frontal gyrus (in either hemisphere) when performing a semantic task. However, when she performed a rhyming task, she showed prominent activation in the right hemisphere homologue of Broca’s area. Although this finding may not be surprising, it points out that activation patterns are directly tied to scanner task requirements. Neuroimaging studies of recovery from aphasia may benefit from including several tasks (tapping language performance in several domains) in order to obtain a full picture of language recovery. Researchers also should consider including nonlanguage control tasks to confirm normal, reliable activation under these conditions, in contrast to the language condition tested.
Scan task performance may also have influenced the results. This can be tricky in treatment studies, primarily because the task selected needs not only to be relevant to the language behavior(s) trained but also needs to be sufficiently challenging for the aphasic participants. If the task is too easy, it may not be sensitive to intervention effects; changes from before to after treatment may not be forthcoming if the patient can already perform the dependent task at a high level. However, if the task is too difficult, pretreatment activation may reflect effort rather than task execution. In our work, we include practice sessions in a simulated scanner prior to collecting the neuroimaging data. During practice sessions, patients are presented with tasks and stimuli that are similar but not identical to those used in the experiment. This provides data relevant to the patient’s ability to perform the task (ie, accuracy and response time), and it acclimates the patient to the scanner environment (eg, scanner noise). For patients who have highly inaccurate performances or very long reaction times even after repeated simulator sessions, the difficulty level of the task is altered or the patient is excluded from the study.
In addition, tasks that require overt responding are recommended in order to register participants’ compliance and responding ability. When covert tasks are used, it is difficult if not impossible to determine the cognitive processes engaged by the patient during task performance. Wierenga et al. [55] asked participants, while in the scanner, to silently produce sentences describing pictured events. Indeed, one cannot be certain whether the patients actually were producing sentences. Although designing a task to tap participants’ ability to produce sentences is challenging due to motion artifact, timing issues, and the like, overt tasks allow analysis of response time and accuracy, which are particularly important for detecting the effects of treatment for aphasia.
Data analysis
Another source of variability likely relates to the methods of data analysis used. In longitudinal studies, it should be taken into account that statistical comparison of activity levels in different scanning sessions can be made only through a direct comparison, as in a paired test (for fMRI, see Smith et al. [58]). Using a statistical threshold in each session separately and then counting and comparing the “surviving” voxels in a region is invalid, because noise levels may differ between sessions and there may be much activity that only just fails to meet the statistical threshold. Practically, this means that noise levels across sessions should be normalized and that general session effects should be statistically modeled. Such procedures, in turn, are not always without their own problems and may sometimes lead to underestimation of activity due to a general or session-specific “flattening” of the data.
In our work with fMRI, we combined the data of individual subjects’ scanning sessions into single models for within-subject statistical analysis, adding regressors for individual runs within each session and for the sessions themselves. Increases and decreases over time were then analyzed using direct contrasts [5].
Rather than measuring change in activation per se, some studies have used more fine-grained measures of neural activity as indices of recovery. Peck et al. [7] measured HRF changes resulting from treatment and found that their three nonfluent aphasic participants showed normalized HRFs in the presupplementary motor cortex following intention treatment for word retrieval deficits. In another interesting study, Meinzer et al. [59] used MEG to examine perilesional delta activity (slow wave forms indicative of pathological functioning due to disconnection of tissue from relevant input sources) in patients with stroke-induced aphasia, before and after constraint-induced aphasia therapy. Sixteen of 28 patients presenting with a variety of aphasia syndromes showed decreased left hemisphere delta oscillation in the vicinity of their lesions after treatment, suggesting changes in connectivity as well as an important role for perilesional tissue in aphasia recovery.
Conclusions
Perilesional left hemisphere regions and right hemisphere regions contralateral to the aphasia-inducing lesion may be recruited to support recovery of language. Several variables affect language recovery–related neuroplastic processes, including patient variables. Patients vary with regard to lesion characteristics and the language deficits that they present. Lesions may extend beyond their structural boundaries, and we therefore recommend using perfusion imaging as well as structural imaging to determine the dimensions of the lesion and, in turn, to determine candidate tissue for recovery. Because it is possible that damage to white matter connections may play a role in aphasia recovery, we recommend that future studies of language recovery use diffusion tensor imaging to evaluate the integrity of these pathways. Finally, we point out that subcortical mechanisms, particularly the basal ganglia involved in monitoring, coordinating, and adjusting cortical activation across regions and hemispheres, may play a role in the dynamic shifts of activity between the hemispheres that are crucial in different stages of recovery [53].
Patients also vary with respect to the aspects of language that are disrupted following stroke. Some show lexical-semantic impairments, some show syntax-based deficits, and some show other language problems. The severity of the aphasia may influence activation patterns, or vice versa, and should be taken into account in the design, analysis, and reporting of studies. Time after stroke also must be considered because several studies have shown a shift in activation from the right to the left hemisphere over time.
Whether or not treatment is provided, the type of treatment, and other related issues also play a role in the neurobiology of recovery. This paper highlights heterogeneity in activation patterns resulting from treatment and points out that the treatment variables applied may at least in part underlie this heterogeneity. A more programmatic approach to research in this area is needed, not only detailing the treatment provided but also examining the effects of various components of treatment. Finally, both direct and systematic replications of treatment studies are needed. With few exceptions, researchers have published single “demonstration” studies (often with only a few patients) showing pretreatment to posttreatment changes in activation patterns.
Finally, we point out experimental design and data analysis considerations that are important for research. We recommend that researchers develop a set of standards for scan task selection, patient task performance, stimulus presentation parameters, and data analysis to decrease the impact of these variables on the outcome of studies. We also suggest that researchers consider more fine-grained analysis of neurophysiologic changes associated with recovery of language. Indeed, future research that considers patient-related, treatment-related, and experiment-related variables will move us toward a better understanding of language recovery in the brain of patients with aphasia.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Merzenich M, Wright B, Jenkins W, et al. Cortical plasticity underlying perceptual, motor, and cognitive skill development: implications for neurorehabilitation. Cold Spring Harb Symp Quant Biol. 1996;61:1–8. [PubMed] [Google Scholar]
- 2.Holland A, Fromm D, DeRuyter F, Stein M. The efficacy of treatment of aphasia: a brief synopsis. J Speech Hear Res. 1996;39:525–534. doi: 10.1044/jshr.3905.s27. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CK. Plasticity of language networks. In: Baudry M, Bi X, Schrieber SS, editors. Synaptic Pasticity: Basic Mechanisms to Clinical Applications. New York: Marcel Dekker; 2005. pp. 255–270. [Google Scholar]
- 4.Crosson B, McGregor K, Gopinath KS, et al. Functional MRI of language in aphasia: a review of the literature and the methodological challenges. Neuropsychol Rev. 2007;17:157–177. doi: 10.1007/s11065-007-9024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson CK, den Ouden DB, Bonakdarpour B, et al. Neural correlates of CATE: a multiple-case study of treatment-induced recovery of sentence processing and production in agrammatism. Brain Lang. doi: 10.1016/j.neuropsychologia.2010.06.036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonakdarpour B, Parrish TB, Thompson CK. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. Neuroimage. 2007;36:322–331. doi: 10.1016/j.neuroimage.2007.02.035. This fMRI study examined the HRF in five patients with stroke-induced, agrammatic, Broca’s aphasia and four healthy volunteers using a lexical decision task and a long trial event-related design. TTP measures derived from BOLD T2*-weighted images were examined in Broca’s area, the posterior language region (Wernicke’s area and the angular and supramarginal gyri), and the occipital area bilaterally. The TTP in three of the patients was significantly longer in the anterior and posterior perisylvian regions than in the healthy volunteers and in the left primary visual cortex in the same patients
- 7.Peck KK, Moore AB, Crosson BA, et al. Functional magnetic resonance imaging before and after aphasia therapy: shifts in hemodynamic time to peak during an overt language task. Stroke. 2004;35:554–559. doi: 10.1161/01.STR.0000110983.50753.9D. [DOI] [PubMed] [Google Scholar]
- 8.Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia: two case studies. Cortex. 1989;25:555–556. doi: 10.1016/s0010-9452(89)80017-6. [DOI] [PubMed] [Google Scholar]
- 9.Willmes K, Poeck K. To what extent can aphasic syndromes be localized? Brain. 1993;116:1527–1540. doi: 10.1093/brain/116.6.1527. [DOI] [PubMed] [Google Scholar]
- 10.Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Vikingstad EM, George KP, et al. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–2340. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- 12.Perani D, Cappa SF, Tettamanti M, et al. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85:357–368. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen K, Laine M, Tarkiainen A, et al. Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci. 2003;15:444–461. doi: 10.1162/089892903321593153. [DOI] [PubMed] [Google Scholar]
- 14.Belin P, Van Eeckhout P, Zilbovicius M, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47:1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 15.Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiss WD, Kessler J, Thiel A, et al. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Kurland J, Naeser MA, Baker EH, et al. Test-retest reliability of fMRI during nonverbal semantic decisions in moderate-severe nonfluent aphasia patients. Behav Neurol. 2004;15:87–97. doi: 10.1155/2004/974094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–434. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 19.Selnes OA. Recovery from aphasia: activating the “right” hemisphere. Ann Neurol. 1999;45:419–420. [comment] [PubMed] [Google Scholar]
- 20.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Winhuisen L, Thiel A, Schumacher B, et al. The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke. 2007;38:1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
- 22.Rosen HJ, Petersen SE, Linenweber MR, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- 23.Zahn R, Drews E, Specht K, et al. Recovery of semantic word processing in global aphasia: a functional MRI study. Brain Res Cogn Brain Res. 2004;18:322–336. doi: 10.1016/j.cogbrainres.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Vitali P, Abutalebi J, Tettamanti M, et al. Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabil Neural Repair. 2007;21:152–160. doi: 10.1177/1545968306294735. [DOI] [PubMed] [Google Scholar]
- 25.Grafman J. Conceptualizing functional neuroplasticity. J Commun Disord. 2000;33:345–355. doi: 10.1016/s0021-9924(00)00030-7. quiz 355–356. [DOI] [PubMed] [Google Scholar]
- 26.Cherney LR, Small SL. Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc. 2006;12:828–842. doi: 10.1017/S1355617706061017. [DOI] [PubMed] [Google Scholar]
- 27.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 28.Kreisler A, Godefroy O, Delmaire C, et al. The anatomy of aphasia revisited. Neurology. 2000;54:1117–1123. doi: 10.1212/wnl.54.5.1117. [DOI] [PubMed] [Google Scholar]
- 29.Yang ZH, Zhao XQ, Wang CX, et al. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurol Res. 2008;30:356–360. doi: 10.1179/174313208X300332. [DOI] [PubMed] [Google Scholar]
- 30.Thompson CK, Bonakdarpour B, Fix SC, et al. Neural correlates of verb argument structure processing. J Cogn Neurosci. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridriksson J, Holland AL, Coull BM, et al. Aphasia severity: association with cerebral perfusion and diffusion. Aphasiology. 2002;16:859–871. doi: 10.1080/02687030244000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillis AE, Barker PB, Beauchamp NJ, et al. MR perfusion imaging reveals regions of hypoperfusion associated with aphasia and neglect. Neurology. 2000;55:782–788. doi: 10.1212/wnl.55.6.782. [DOI] [PubMed] [Google Scholar]
- 33.Love T, Swinney D, Wong E, Buxton R. Perfusion imaging and stroke: a more sensitive measure of the brain bases of cognitive deficits. Aphasiology. 2002;16:873–883. doi: 10.1080/02687030244000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillis AE, Kleinman JT, Newhart M, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26:8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breier JI, Hasan KM, Zhang W, et al. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 37. Sonty SP, Mesulam MM, Weintraub S, et al. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. Dynamic causal modeling and fMRI were used to study multiregional effective connectivity in eight patients with early-stage primary progressive aphasia (PPA) and eight control subjects performing semantic word matching and visual letter-matching tasks. The dynamic causal modeling analysis, which included six left hemisphere regions, showed reduced language-specific effective connectivity between Wernicke’s and Broca’s areas in the PPA group compared with the healthy control group. This decrement in connectivity was associated with semantic task accuracy
- 38.de Boissezon X, Demonet JF, Puel M, et al. Subcortical aphasia: a longitudinal PET study. Stroke. 2005;36:1467–1473. doi: 10.1161/01.STR.0000169947.08972.4f. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez B, Cardebat D, Demonet JF, et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35:2171–2176. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- 40.Ino T, Tokumoto K, Usami K, et al. Longitudinal fMRI study of reading in a patient with letter-by-letter reading. Cortex. 2008;44:773–781. doi: 10.1016/j.cortex.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 41. Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129(Pt 6):1371–1384. doi: 10.1093/brain/awl090. Fourteen patients with aphasia resulting from left middle cerebral artery infarction were scanned using fMRI as they performed an auditory comprehension task using an event-related design on three separate occasions: in the acute, subacute, and chronic stages of recovery. All patients recovered clinically. Group analysis of the fMRI data showed little early activation of left hemisphere tissue, bilateral activation peaking in the right homologue of Broca’s area and the supplementary motor area in the subacute phase, and a shift of peak activation of left hemisphere language areas in the chronic phase. This later activation matched that found in age-matched controls.
- 42.Blasi V, Young AC, Tansy AP, et al. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- 43.Breier JI, Maher LM, Schmadeke S, et al. Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: a case study. Neurocase. 2007;13:169–177. doi: 10.1080/13554790701448200. [DOI] [PubMed] [Google Scholar]
- 44.Meinzer M, Flaisch T, Breitenstein C, et al. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage. 2008;39:2038–2046. doi: 10.1016/j.neuroimage.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Meinzer M, Obleser J, Flaisch T, et al. Recovery from aphasia as a function of language therapy in an early bilingual patient demonstrated by fMRI. Neuropsychologia. 2007;45:1247–1256. doi: 10.1016/j.neuropsychologia.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Richter M, Miltner WHR, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131:1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- 47.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 48.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 49.Leger A, Demonet JF, Ruff S, et al. Neural substrates of spoken language rehabilitation in an aphasic patient: an fMRI study. Neuroimage. 2002;17:174–183. doi: 10.1006/nimg.2002.1238. [DOI] [PubMed] [Google Scholar]
- 50.Fridriksson J, Morrow-Odom L, Moser D, et al. Neural recruitment associated with anomia treatment in aphasia. Neuroimage. 2006;32:1403–1412. doi: 10.1016/j.neuroimage.2006.04.194. [DOI] [PubMed] [Google Scholar]
- 51.Fridriksson J, Moser D, Bonilha L, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. 2007;45:1812–1822. doi: 10.1016/j.neuropsychologia.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology. 2008;70:290–298. doi: 10.1212/01.wnl.0000287115.85956.87. [DOI] [PubMed] [Google Scholar]
- 53.Crosson B, Moore AB, Gopinath K, et al. Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci. 2005;17:392–406. doi: 10.1162/0898929053279487. [DOI] [PubMed] [Google Scholar]
- 54.Davis CH, Harrington G, Baynes K. Intensive semantic intervention in fluent aphasia: a pilot study with fMRI. Aphasiology. 2006;20:59–83. [Google Scholar]
- 55.Wierenga CE, Maher LM, Moore AB, et al. Neural substrates of syntactic mapping treatment: an fMRI study of two cases. J Int Neuropsychol Soc. 2006;12:132–146. doi: 10.1017/S135561770606019X. [DOI] [PubMed] [Google Scholar]
- 56.Thompson CK, Fix SC, Gitelman DG, et al. fMRI studies of agrammatic sentence comprehension before and after treatment. Brain Lang. 2000;74:387–391. [Google Scholar]
- 57.Calvert GA, Brammer MJ, Morris RG, et al. Using fMRI to study recovery from acquired dysphasia. Brain Lang. 2000;71:391–399. doi: 10.1006/brln.1999.2272. [DOI] [PubMed] [Google Scholar]
- 58.Smith SM, Beckmann CF, Ramnani N, et al. Variability in fMRI: a re-examination of inter-session differences. Hum Brain Mapp. 2005;24:248–257. doi: 10.1002/hbm.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meinzer M, Elbert T, Wienbruch C, et al. Intensive language training enhances brain plasticity in chronic aphasia. BMC Biol. 2004;2:20. doi: 10.1186/1741-7007-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]