Abstract
Background
Anxiety Sensitivity (AS), the tendency to fear the thoughts, symptoms, and social consequences associated with the experience of anxiety, is associated with increased risk for developing anxiety disorders. Some evidence suggests that higher scores on the Anxiety Sensitivity Index (ASI), a measure of the AS construct, are associated with activation of the anterior insular cortex during overt emotion perception. Although the ASI provides subscale scores measuring Physical, Mental Incapacitation, and Social Concerns of AS, no study has examined the relationship between these factors and regional brain activation during affect processing. We hypothesized that insular responses to fear-related stimuli would be primarily related to the Physical Concerns subscale of the ASI, particularly for a sample of subjects with specific phobias.
Methods
Adult healthy controls (HC; n=22) and individuals with specific phobia, small animal subtype (SAP; n=17), completed the ASI and underwent functional magnetic resonance imaging (fMRI) while engaged in a backward-masked affect perception task that presents emotional facial stimuli below the threshold of conscious perception.
Results
Groups did not differ in ASI, state or trait anxiety scores, or insula activation. Total ASI scores were positively correlated with activation in the right middle/anterior insula for the combined sample and for the HC and SAP groups separately. Multiple regression analysis revealed that the relationship between AS and insular activation was primarily accounted for by Physical Concerns only.
Conclusions
Findings support the hypothesized role of the right anterior insula in the visceral/interoceptive aspects of anxiety sensitivity, even in response to masked affective stimuli.
Keywords: FMRI, Neuroimaging, Anxiety Sensitivity, ASI, Insula, Specific Phobia, Healthy Controls
Anxiety can take many forms. While anxiety related to specific objects, places, or situations is fairly common, a large number of individuals find that it is not so much the external threats or circumstances they fear most—rather it is the sensations accompanying an anxious response that evoke the greatest dread. This “fear of fear,” known formally as Anxiety Sensitivity (AS, Reiss 1991), involves a propensity to fear the physical symptoms, thoughts, and social consequences that emerge during a bout of anxiety. For high AS individuals, the sensations of anxiety are perceived as potentially harmful or aversive and they become preoccupied with worries about becoming anxious (Reiss and McNally 1985). By focusing attention on the aversiveness of the physical sensations of anxiety, AS appears to amplify the effectiveness of fear conditioning, potentially serving as a predisposing and maintaining factor in many clinical anxiety conditions such as panic disorder (Barlow 2002; Clark 1986; McNally 1990).
The construct of AS has been operationalized by the 16-item Anxiety Sensitivity Index (ASI, Reiss et al. 1986), a brief assessment scale with strong psychometric properties and construct validity (Peterson and Heilbronner 1987; Rodriguez et al. 2004; Taylor et al. 1991). The ASI provides an overall index of AS, as well as three conceptually distinct subscales measuring Physical Concerns (e.g., it scares me when my heart beats rapidly), Mental Incapacitation Concerns (e.g., when I am nervous, I worry that I might be mentally ill), and Social Concerns (e.g., it is important to me not to appear nervous) (Zinbarg et al. 1997). Total ASI scores predict the severity of experimentally induced panic (Schmidt 1999; Zinbarg et al. 2001), the duration of panic disorder (Benitez et al. 2009), and the likelihood of receiving an anxiety disorder diagnosis (Schmidt and Zvolensky 2007). Some evidence suggests that the three subscale factors may be associated with specific clinical conditions (Grant et al. 2007; Rodriguez et al. 2004; Zinbarg et al. 2001).
Although it is clear that AS plays a role in the development and manifestation of clinical anxiety disorders, the neurobiological basis of this construct remains uncertain. Paulus and Stein propose a central role for the anterior insular cortex, particularly in the right hemisphere, in AS (Paulus and Stein 2006). The insula is one of the key cortical structures involved in interoceptive perception—the subjective sense of the physiological status of the body (Craig 2002; Gray et al. 2007). According to Paulus and Stein (2007), individuals who are high in AS show exaggerated interoceptive prediction signaling within the anterior insula regarding expectations for future body states, leading to a predisposition toward anxious hyperarousal and cognitive engagement (Paulus and Stein 2006). This hypothesis has received preliminary support in a functional magnetic resonance imaging (fMRI) study showing total scores on the ASI were positively correlated with activation within the anterior insula during an overt facial emotion discrimination task (Stein et al. 2007). Given the role of the insular cortex in monitoring visceral body states, it seems likely that of the three factors comprising the ASI, this relationship would be most strongly related to physical concerns. To our knowledge, no studies have examined the relationship between the three subscale factors of the ASI and insular activation within healthy or anxiety prone individuals.
Here we examined the relationship between the ASI, its subfactors, and regional brain activation using a technique known as backward stimulus masking, which bypasses “top down” cortical modulation of emotional processes by presenting emotional stimuli below the threshold of conscious visual perception (Whalen et al. 1998). This technique provides a robust method for probing the limbic circuitry involved in affective processing (Killgore and Yurgelun-Todd 2004; Morris et al. 1998; Whalen et al. 1998) and anxiety disorders (Rauch et al. 2000). Furthermore, to more fully explore the relationship between AS and task-related activation within the insula, we compared healthy control (HC) subjects with a sample of individuals meeting criteria for specific phobia, small animal subtype (SAP). The latter group was included because there is growing evidence that specific phobia is typically associated with relatively normal brain responses to most stimuli, while showing specific activation of the insula in response to fear-related (Martis et al. 2004; Wright et al. 2003) and phobia-relevant stimuli (Dilger et al. 2003; Straube et al. 2004; Wendt et al. 2008). It was hypothesized that ASI Total scores and Physical Concerns subscales would correlate positively with task-related activation of the right anterior insula during masked fearful affect perception and that these relationships would be stronger in the SAP group compared to the HC group. In contrast, Mental Incapacitation Concerns and Social Concerns were hypothesized to be unrelated to those factors. Because of the important role played by the amygdala in anxiety disorders and during masked affect perception (Rauch et al. 2000), we also evaluated the relationship between ASI and amygdala responses during masked affect perception.
Methods
Subjects
Twenty-two healthy controls (HC; Mage = 29.3; SD = 5.6 years; 9 male) and 17 individuals meeting criteria for specific animal phobia (SAP; Mage = 29.6; SD = 7.7 years; 6 male) completed a psychiatric evaluation and underwent fMRI while engaged in a masked affect perception task. Diagnoses were based on the Structured Clinical Interview for DSM-IV-TR – Patient Version (SCID-I/P) (First et al. 2002). All participants were free of psychotropic medications for at least 4 weeks prior to the time of the assessment and scan and were free of known medical or neurologic conditions that could affect brain function, additional psychiatric diagnoses, history of seizure, or significant head trauma. Written consent was obtained after a thorough explanation of the procedures. A nominal financial compensation was provided for participation. This study protocol was approved by the Partners Healthcare and McLean Hospital institutional review boards.
Anxiety Sensitivity Index
Participants were administered the 16-item version of the Anxiety Sensitivity Index (ASI) (Reiss et al. 1986). The scale provides an overall index of the fear of anxiety and its physical sensations (ASI Total), and three subscales measuring fear of somatic-physical symptoms of anxiety (Physical Concerns), fear of the loss of cognitive control (Mental Incapacitation Concerns), and fear of socially embarrassing aspects of anxiety (Social Concerns) (Zinbarg et al. 1997). Scoring was calculated as outlined in Brown et al. (Brown et al. 2003).
State-Trait Anxiety Inventory
All participants completed the State and Trait forms of the State-Trait Anxiety Inventory (STAI) (Spielberger et al. 1970). Each form consists of 20 items that either relate to current anxiety (state) or stable long term anxiety (trait). Each item is scored along a 4 point Likert scale and summed to provide an index of anxiety severity.
Masked Facial Affect Paradigm
During fMRI, participants passively viewed masked faces in two counterbalanced runs (Rauch et al. 2000; Whalen et al. 1998). Each task run lasted 5 minutes and 36 seconds and comprised 12 alternating blocks of masked neutral (2 blocks), masked happy (3 blocks), and masked fearful (3 blocks) facial expressions, and 4 blocks of a fixation baseline. Each block was 28 seconds in duration. During masked face presentations, an affective facial expression (i.e., fear, happy, neutral) from a standard set (Rauch et al. 2000) was displayed for 16 msec and masked immediately by a presentation of a neutral face from a different person for 184 msec. Within each block, 56 stimulus-mask pairs were presented at a rate of 2 per second. Stimuli were projected by LCD video onto a translucent screen that was viewed via a mirror mounted in the scanner.
Neuroimaging Methods
Scans were acquired on a 3.0 Tesla Siemens Trio whole body scanner equipped with a 12 channel head coil or a quadrature RF head coil and were scanned at one of two locations (Massachusetts General Hospital [MGH], n = 24, McLean Hospital, n = 17). For spatial normalization, high-resolution 3D MPRAGE sequences were collected for each subject (TR = 2530, TE = 3.45 msec, flip angle = 7 degrees, 1.3 mm slice thickness, 1.3 mm in-plane resolution). Functional images were acquired using a T2*-weighted sequence (TR = 2 sec, TE = 30 msec, flip angle = 90 degrees). Echoplanar images were collected over 25 axial slices (5 mm thick, 0 skip) with a 20 cm field of view and a 64×64 acquisition matrix, with an in-plane resolution of 3.125 × 3.125 × 5 mm. 168 images were collected per run.
Image Processing
Within SPM5 (Wellcome Department of Cognitive Neurology, London, UK), the images were motion corrected, co-registered to each subject's own anatomical scan, normalized to the three-dimensional space of the Montreal Neurological Institute (MNI), spatially smoothed using an isotropic Gaussian kernel (full width half maximum [FWHM] = 6 mm), and resliced to 2x2x2 mm using sinc interpolation. Time series data were convolved with the canonical hemodynamic response function.
Statistical Analysis
For each subject, a general linear model was estimated to identify activation differences between each masked affect condition relative to the other conditions (i.e., Masked Fear > Masked Neutral; Masked Happy > Masked Neutral; Masked Fear > Masked Happy). The resulting contrast images served as the input data for a series of second level random effects analyses correlating BOLD responses with ASI scores and subscales, while statistically controlling for scanner location (MGH, McLean). Because our a priori hypotheses were focused specifically on the insula and amygdala, random effects analyses were restricted to a search territory (ST) comprising those two structures bilaterally. This was accomplished by defining a region of interest mask according to the boundaries specified by the automated anatomical labeling atlas (Tzourio-Mazoyer et al. 2002) as implemented in the Wake Forest University Pickatlas Utility (Maldjian et al. 2003). To correct for multiple comparisons, all analyses were evaluated using a False Discovery Rate (FDR) correction of p < .05, k (extent) ≥ 5 voxels, within the combined amygdala-insula search territory. This mask and statistical threshold was used for all second-level random effects analyses.
These random effects analyses proceeded in several stages. First, the three contrast conditions were evaluated relative to the null hypothesis of no change via one-sample t-tests. Second, activation within the ST during the masked fear versus neutral contrast was compared directly between HC and SAP groups using an independent groups t-test. Third, the correlation between ASI Total Score and signal change within the ST during each of the three masked affect condition contrasts was evaluated for the entire sample of participants combined. This was accomplished by regressing ASI Total Score against task-related brain activation within the ST within SPM5, controlling for scanner location (MGH, McLean). Additionally, because ASI scores were correlated with measures of State and Trait Anxiety, the initial SPM5 correlation model between ASI Total and signal intensity within the ST was repeated with both State and Trait Anxiety Scores included as additional covariates in the model to rule out the potential confounding influence of anxiety. Fourth, the relationship between ASI and brain activation was examined in each group separately by extracting the eigenvariate parameter estimates from the activated voxels from the initial correlation and plotting these separately by group. The magnitude of the correlation coefficients from the two groups was compared using Fisher's r-to-z transformation. Lastly, the contribution of each ASI factor score to extracted regional activation was also examined by multiple stepwise liner regression and simple zero-order correlation analysis in SPSS 12.0.
Results
Anxiety Sensitivity Scores
Total ASI scores did not differ significantly between HC (M = 11.2, SD = 5.8) and SAP (M = 14.7, SD = 8.0) subjects, t(37) = 1.60, p = .12. Similarly, there were no significant differences between groups for Physical Concerns (HC = 5.6, SD = 5.6; SAP = 6.5, SD = 5.1; t[37] = 0.48, p = .64). The Mental Incapacitation Concerns score was lower among healthy controls (HC = 0.2, SD = 0.5; SAP = 1.4, SD = 2.2; t[17.5] = 2.46, p = .04), although this difference was not considered significant when corrected for multiple comparisons. Social Concerns did not differ significantly between groups (HC = 4.6, SD = 1.7; SAP = 5.4, SD = 2.0; t[37] = 1.30, p = .20). Additionally, groups did not differ with regard to State (HC = 27.5, SD = 5.6; SAP = 28.5, SD = 5.5; t[35] = 0.53, p = .60) or Trait (HC = 32.8, SD = 6.0; SAP = 35.1, SD = 5.1; t[35] = 1.23, p = .23) Anxiety on the STAI.
Neuroimaging Task Comparisons
Total Sample Task Activation
Separate one-sample t-tests for the Masked Fear versus Masked Neutral, Masked Happy versus Masked Neutral, and Masked Fear versus Masked Happy contrasts were not significant within the ST comprising either the amygdala or insula for the sample as a whole.
Group Comparisons
No significant differences were found between the activation patterns of the HC and SAP groups within the amygdala or insula for either the Masked Fear versus Neutral, Masked Happy versus Neutral, and Masked Fear versus Happy contrasts.
Brain Imaging Correlates of Total Anxiety Sensitivity Index Scores
Masked Fear versus Masked Neutral Contrast
As evident in Figure 1, when the sample was considered as a whole, total scores on the ASI were positively correlated with BOLD responses to masked fearful faces in a cluster of 44 voxels within the right anterior insula (MNI: x = 38, y = 2, z = 14; t = 5.08). In contrast, no significant correlation with Total ASI scores was observed for the left insula or either amygdala. Negative correlations were not observed in any of the search territories.
Figure 1.
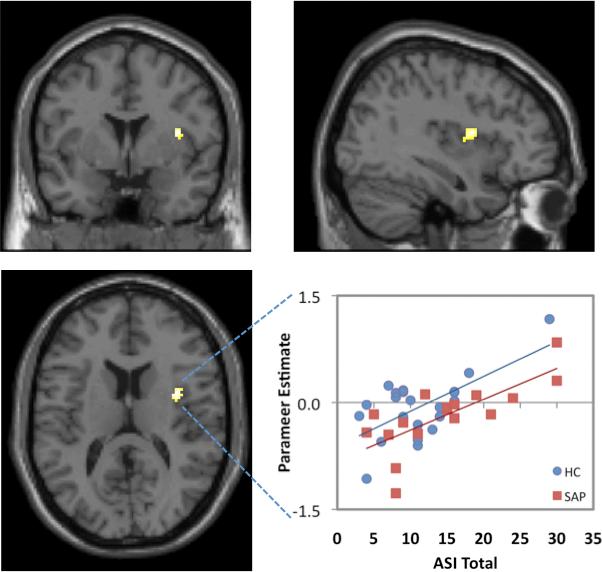
Correlations between ASI Total scores and cerebral activation during the masked fear > masked neutral contrast are shown in the coronal (top left), sagittal (top right), axial (bottom left) planes for the total combined sample (n = 39). The scatterplot (bottom right) shows that the linear relationship between ASI Total scores and extracted activation (total cluster eigenvariate) within the right insula was similar for the health control (HC; n = 22; r2 = .41), and small animal phobic (SAP; n = 17; r2 = .55) groups. Activation is significant at p < .05 (FDR corrected), k ≥ 5.
Given that Total ASI was modestly correlated with State Anxiety (r = .35, p = .04) and marginally correlated with Trait Anxiety (r = .30, p = .07) on the STAI, which were uncorrelated with each other (r = .16, p = .34), it was also important to evaluate the potential influence of State and Trait Anxiety on the observed correlations between ASI and insular activation. Therefore, we re-ran the previous regression analysis with the State and Trait scales of the STAI included as additional covariates in the model. After controlling for the effects of state and trait anxiety, this analysis continued to yield a cluster of activation (28 voxels) located within the right middle/anterior insula at essentially the same coordinates as found in the previous analysis [MNI: x = 34, y = 0, z = 12; t = 4.73). Thus, the correlation between AS and insular activation observed here does not appear to be accounted for by state or trait anxiety.
It was also of interest to evaluate whether the strength of the relationship between Total ASI scores and insula activation differed between the HC and SAP groups. Therefore, eigenvariate parameter estimates for each subject were extracted from the activated cluster in the right insula and plotted for each group separately, as shown in Figure 1. Comparison with Fisher's r-to-z transformation showed that the correlations did not differ significantly between the HC (r = .64, p = .001) and SAP (r = .74, p = .001) groups (z = 0.55, p = .59).
Masked Happy versus Masked Neutral Contrast
During this contrast, neither the amygdala nor the insula showed any significant voxelwise correlations with Total ASI scores.
Masked Fear versus Masked Happy Contrast
Similarly, neither the amygdala nor insula showed any significant voxelwise correlations with Total ASI scores.
Role of Anxiety Sensitivity Factor Scores
It was also of interest to evaluate the relative contribution of the three ASI factors (i.e., Physical Concerns, Mental Concerns, and Social Concerns) to the observed insular activation. Therefore, all three ASI factor scores were entered into a stepwise multiple linear regression analysis with the extracted parameter estimates from the right insula cluster identified in Figure 1 as the dependent variable. This analysis showed that Physical Concerns accounted for the greatest proportion of the variance in the activation of the right anterior insula (β = .60, p < .001), and that neither Mental Incapacitation Concerns nor Social concerns accounted for any additional incremental variance above and beyond that provided by Physical Concerns. Figure 2 shows the correlation scatterplots for the insula activation of each factor score separately. As evident in the figure, scores on the Physical Concerns subscale for the sample as a whole showed a significant positive correlation with BOLD signal change in the right insula (r = .60, p < .05 FDR corrected). In contrast, Mental Incapacitation Concerns (r = .38) and Social Concerns (r = .18) were not significantly linearly related to responses within the insula during the Masked Fear versus Masked Neutral contrast after FDR correction for multiple comparisons within the ST.
Figure 2.
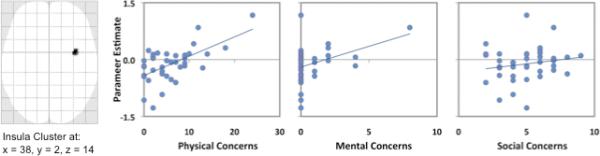
The leftmost panel shows a maximum intensity projection of the entire cluster of correlated voxels within the right insula resulting from the correlation between Total ASI scores and activation resulting from the Masked Fear > Masked Neutral contrast (p < .05 [FDR corrected], k ≥ 5). The three scatterplots show the individual associations between the extracted data from this cluster and each of the three factor indices from the ASI, including Physical Concerns (r2 = .36), Mental Concerns (r2 = .14), and Social Concerns (r2 = .03).
Discussion
As hypothesized, Total ASI scores were positively correlated with task-related activation within the right middle/anterior insular cortex during masked presentations of fearful versus neutral faces, but not for the other affect conditions. Furthermore, the relationship between AS and BOLD responses in the right insula was maintained even when the effects of state and trait anxiety were statistically controlled in the model and did not differ between HC and SAP groups. Notably, the correlation was driven predominantly by sensitivity to the Physical Concerns of anxiety, whereas Mental Incapacitation and Social Concerns were unrelated to insular activation. In contrast, activation within the other regions (i.e., left insula, right and left amygdala) was unrelated to ASI scores, suggesting that the association was specific to right insular responses rather than limbic and paralimbic activation more generally.
Interestingly, neither the insula nor the amygdala showed significant main effects of affect condition generally. In other words, without consideration of ASI scores, the task-related activation to each contrast condition was modest and did not survive correction for multiple comparisons. Instead, the present findings suggest that only the magnitude of the right insular response to the Masked Fear versus Neutral contrast is directly related to the severity of AS, particularly those aspects that involve apprehensions about the physical sensations of an anxious reaction. It is also notable that the correlation was only present for the Masked Fear versus Masked Neutral condition, suggesting that the relationship between AS and insular responsivity may also depend on the presence of implicit cues related to potential threat in the environment. The fact that the insular response derived from the Masked Fear versus Masked Happy contrast did not show a significant correlation with AS also raises the possibility that the effect may be driven partly by the level of emotional arousal communicated by the faces in addition to the negative valence of the expression (i.e., both fearful and happy expressions share high emotional arousal despite differing in valence). Further research will be necessary to clarify this issue.
For the present study, we chose to examine the relationship between ASI and insular responses in healthy individuals as well as in a psychiatric comparison group meeting diagnostic criteria for specific phobia of small animals. In contrast to panic disorder, which is characterized by severe anxiety reactions across a spectrum of situations and clearly linked to AS (Naragon-Gainey 2010), specific phobia is a relatively mild and circumscribed anxiety disorder whose relationship to AS is virtually unexplored. Contrary to our predictions, we found no group differences in ASI scores or in the responsiveness of the insula and amygdala to any of the masked affect conditions. Moreover, the linear relationship between ASI scores and insular activation was decidedly similar for both the HC and SAP groups, suggesting that greater fear of the sensations and thoughts of anxiety was associated with increased activation of the insular cortex in response to masked fearful faces, regardless of anxiety disorder status. Prior research has suggested that individuals with SAP are likely to report that their focus of apprehension is most often directed toward physical sensations elicited by the feared animal, such as disgust or revulsion (Lipsitz et al. 2002). The presently observed correlation between right insular responses and the Physical Concerns factor is consistent with the tendency for SAP patients to focus excessively on the physical responses that emerge when thinking about or confronted with the object of their phobia (e.g., feeling “creeped out” or “disgusted”). Although speculative, it seems likely that the association between insular activity and AS would be exaggerated among individuals with panic disorder, a condition involving excessive focus on the somatic/interoceptive sensations of anxiety (Barlow 2002; Clark 1986; McNally 1990), and which is associated with morphometric abnormalities within the insular cortex (Graeff and Del-Ben 2008).
The present findings are consistent with the hypothesized role of the insula in the manifestation of AS (Paulus and Stein 2006), as well as the broader involvement of the insula in a variety of neuropsychiatric disorders (Nagai et al. 2007). The insular cortex is believed to be centrally involved in monitoring interoceptive cues reflecting the physiological status of the body, integrating those sensations with expectations for future body states, and producing anticipatory signals regarding the discrepancy between the observed and expected body state to alert other cognitive and affective processing regions (Paulus and Stein 2006). Individuals with particularly elevated responsiveness of the insula to salient stimuli are hypothesized to experience an exaggerated “interoceptive prediction signal,” which increases the perceived discrepancy between the observed and expected body state (Paulus and Stein 2006). This discrepancy is believed to generate anticipatory anxiety in sensitive individuals, a factor that may contribute to the effectiveness of aversive conditioning and predispose the development of anxiety disorders (Reiss 1991). The present findings support this model, demonstrating that greater responses within the right anterior/middle insula to masked fear versus neutral faces were in fact associated with higher scores on the ASI. The present results also build upon recent findings showing that individuals scoring high on the trait of AS tend to show significantly greater insular responses during a simple facial affect matching task compared to individuals with AS scores in the average range (Stein et al. 2007) and are consistent with recent evidence suggesting that the cortical thickness of the right insula is correlated with AS among individuals with SAP but not healthy controls (Rosso et al. 2010).
The positive correlation between BOLD signal differences in the insula and ASI should also be considered in light of the nature of the masked affective stimuli used in the present study. This paradigm minimizes the effects of top down modulation of affective responses by prefrontal regions (Rauch et al. 2000), permitting an assessment of automatic affective responses with minimal influences from conscious processing. Here, variation in AS was associated with automatic insular responses during fearful affect perception, even without explicit awareness of the emotion expressed on the faces. Building on the recent work of Stein and colleagues (Paulus and Stein 2006; Stein et al. 2007), the present findings suggest that not only does AS have the potential to influence an individual's responses to affective stimuli when they are consciously and overtly perceived, but may also affect responses to stimuli that may generally go unnoticed during normal conscious processing. It may be informative for future work to examine whether individuals with high AS might be more easily primed by non-consciously perceived threat-related stimuli and whether this might account for the apparently unpredictable nature of panic symptoms.
The present study is also the first to examine the individual contributions of the three factor subscales of the ASI to the functional brain responses observed during masked affect perception. This study confirmed the hypothesis that fear of physical sensations rather than worries over mental incapacitation or social consequences arising from anxiety would drive much of the association between ASI Total scores and insular activation. Heightened concern regarding the physical sensations of anxiety was associated with greater task-related activation within regions of the brain that are critical to interoceptive monitoring of visceral sensations, bodily states, emotional reactions (Craig 2003; Wiens 2005), and the anticipation of impending physical sensations (Lovero et al. 2009). In fact, when all three ASI scales were considered together, only the Physical Concerns scale contributed significant unique variance to the prediction of insular responses during the masked fear versus neutral condition. In contrast, neither the Mental Incapacitation Concerns nor the Social Concerns subscales were correlated with any voxels within the insula or amygdala. These findings suggest that the relationship between ASI Total and insular activation is driven primarily by concerns related to the physical sensations of anxiety and that amygdala responses appear to be unrelated to the construct of AS, consistent with prior reports on conscious emotional perception (Stein et al. 2007).
In conclusion, the present findings support the critical role of the right anterior insula in AS, and further suggest that this effect is driven primarily by worries encompassed by the physical concern features of AS. Moreover, these results indicate that AS is related to insular activation in response to masked affective stimuli, complementing prior studies using overt affective stimuli. Taken together, such findings suggest that the insular responsivity related to AS may extend to visual threat-related stimuli perceived beneath the threshold of conscious awareness.
Acknowledgments
This project was supported by NIMH Grant # R01 MH070730-04 (SLR). This research was supported in part by the Intramural Research Program of the National Institutes of Health and the National Institute of Mental Health (JCB).
References
- Barlow DH. Anxiety and its disorders. Guilford Press; New York: 2002. [Google Scholar]
- Benitez CI, Shea MT, Raffa S, Rende R, Dyck IR, Ramsawh HJ, Edelen MO, Keller MB. Anxiety sensitivity as a predictor of the clinical course of panic disorder: a 1-year follow-up study. Depress Anxiety. 2009;26(4):335–42. doi: 10.1002/da.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Smits JA, Powers MB, Telch MJ. Differential sensitivity of the three ASI factors in predicting panic disorder patients’ subjective and behavioral response to hyperventilation challenge. J Anxiety Disord. 2003;17(5):583–91. doi: 10.1016/s0887-6185(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Clark DM. A cognitive approach to panic. Behav Res Ther. 1986;24(4):461–70. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Dilger S, Straube T, Mentzel HJ, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WH. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neurosci Lett. 2003;348(1):29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (11/2002 Revision) Biometrics Research Department, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Graeff FG, Del-Ben CM. Neurobiology of panic disorder: from animal models to brain neuroimaging. Neurosci Biobehav Rev. 2008;32(7):1326–35. doi: 10.1016/j.neubiorev.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Grant DM, Beck JG, Davila J. Does anxiety sensitivity predict symptoms of panic, depression, and social anxiety? Behav Res Ther. 2007;45(9):2247–55. doi: 10.1016/j.brat.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One. 2007;2(6):e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21(4):1215–23. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Lipsitz JD, Barlow DH, Mannuzza S, Hofmann SG, Fyer AJ. Clinical features of four DSM-IV-specific phobia subtypes. J Nerv Ment Dis. 2002;190(7):471–8. doi: 10.1097/00005053-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martis B, Wright CI, McMullin KG, Shin LM, Rauch SL. Functional magnetic resonance imaging evidence for a lack of striatal dysfunction during implicit sequence learning in individuals with animal phobia. Am J Psychiatry. 2004;161(1):67–71. doi: 10.1176/appi.ajp.161.1.67. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Psychological approaches to panic disorder: a review. Psychol Bull. 1990;108(3):403–19. doi: 10.1037/0033-2909.108.3.403. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry. 2007;22(6):387–94. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Naragon-Gainey K. Meta-analysis of the relations of anxiety sensitivity to the depressive and anxiety disorders. Psychol Bull. 2010;136(1):128–50. doi: 10.1037/a0018055. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Heilbronner RL. The Anxiety Sensitivity Index: construct validity and factor analytic structure. Journal of Anxiety Disorders. 1987;1:117–121. [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Reiss S. Expectancy model of fear, anxiety, and panic. Clinical Psychology Review. 1991;11:141–153. [Google Scholar]
- Reiss S, McNally RJ. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical issues in behavior therapy. Academic Press; New York: 1985. pp. 107–121. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez BF, Bruce SE, Pagano ME, Spencer MA, Keller MB. Factor structure and stability of the Anxiety Sensitivity Index in a longitudinal study of anxiety disorder patients. Behav Res Ther. 2004;42(1):79–91. doi: 10.1016/s0005-7967(03)00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Britton JC, Price LM, Gold AL, Zai D, Bruyere J, Deckersbach T, Killgore WDS, Rauch SL. Anxiety sensitivity correlates with two indices of right anterior insula structure in specific animal phobia. Depression and Anxiety. 2010;27(12):1104–1110. doi: 10.1002/da.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB. Examination of differential anxiety sensitivities in panic disorder: A test of anxiety sensitivity subdomains in predicting fearful responding in a 35% CO-sub-2 challenge. Cognitive Therapy and Research. 1999;23:3–20. [Google Scholar]
- Schmidt NB, Zvolensky MJ. Anxiety sensitivity and CO2 challenge reactivity as unique and interactive prospective predictors of anxiety pathology. Depress Anxiety. 2007;24(8):527–36. doi: 10.1002/da.20267. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004;372(3):204–8. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Taylor S, Koch WJ, Crockett DJ. Anxiety sensitivity, trait anxiety, and the anxiety disorders. Journal of Anxiety Disorders. 1991;5:293–311. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wendt J, Lotze M, Weike AI, Hosten N, Hamm AO. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45(2):205–15. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Curr Opin Neurol. 2005;18(4):442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, McMullin K, Shin LM, Rauch SL. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54(10):1067–76. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Barlow DH, Brown TA. Hierarchical structure and general factor saturation of the Anxiety Sensitivity Index: evidence and implications. Psychological Assessment. 1997;9:277–284. [Google Scholar]
- Zinbarg RE, Brown TA, Barlow DH, Rapee RM. Anxiety sensitivity, panic, and depressed mood: a reanalysis teasing apart the contributions of the two levels in the hierarchial structure of the Anxiety Sensitivity Index. J Abnorm Psychol. 2001;110(3):372–7. doi: 10.1037//0021-843x.110.3.372. [DOI] [PubMed] [Google Scholar]


