Abstract
Reduction of immunosuppression (RI) is commonly used to treat post-transplant lymphoproliferative disorder (PTLD) in solid organ transplant recipients. We investigated the efficacy, safety and predictors of response to RI in adult patients with PTLD. 67 patients were managed with RI alone and 30 patients were treated with surgical excision followed by adjuvant RI. The response rate to RI alone was 45% (complete response - 37%, partial response - 8%). The relapse rate in complete responders was 17%. Adjuvant RI resulted in a 27% relapse rate. The acute rejection rate following RI-containing strategies was 32% and a second transplant was feasible without relapse of PTLD. The median survival was 44 months in patients treated with RI alone and 9.5 months in patients who remained on full immunosuppression (p=0.07). Bulky disease, advanced stage and older age predicted lack of response to RI. Survival analysis demonstrated predictors of poor outcome - age, dyspnea, B symptoms, LDH level, hepatitis C, bone marrow and liver involvement. Patients with none or 1 of these factors had a 3-year overall survival of 100% and 79% respectively. These findings support the use of RI alone in low-risk PTLD and suggest factors that predict response and survival.
Introduction
Post-transplantation lymphoproliferative disorder (PTLD) is a heterogeneous group of lymphoid proliferations that arise in patients following solid organ or hematopoietic stem-cell transplantation(1, 2). These neoplasms are associated with activation of Epstein-Barr virus (EBV) in 70–90% of cases(3) and are the result of pharmacologic immunosuppression, which permits B-cell proliferation in the absence of appropriate T-cell regulation(4). Therefore, reduction of immunosuppression (RI) is often the first line of therapy for this disorder. RI is a powerful therapy for PTLD, as it allows recovery of the physiologic immune surveillance of EBV-transformed cells(5). In some patients, it is curative and abrogates the need for specific anti-neoplastic therapies (6–11).
Previous attempts to define the efficacy and safety of RI for PTLD remain of limited value due to small numbers of patients(12–18). Complete response rates following RI are reported between 0–74%; this wide range likely reflects heterogeneity of patient populations and the non-standardized management of RI. Retrospective studies reveal that in many centers the proportion of patients who are managed with RI alone is low and often chemotherapy and/or rituximab are administered up front(12, 15, 19–21). In this report, we describe the response rates to RI when used alone as initial therapy for PTLD and determine predictors of response and survival.
Methods
Patients
Between August 1988 and June 2008, 162 adult solid organ recipients were diagnosed with PTLD at the University of Pennsylvania. Staging procedures included CT scans of the chest, abdomen and pelvis; additional modalities (MRI, endoscopies) were used to investigate specific symptoms or imaging findings. Imaging of the brain and cytological examination of CSF were performed when neurologic signs or symptoms were present. A bone marrow biopsy was reserved for patients with abnormal blood counts or disseminated disease. RI included discontinuation of mycophenolate-mofetil or azathioprine in most cases and reduction of the dose of calcineurin inhibitors (CNI) and steroids (when applicable), usually by targeting a lower blood level. Lung and heart recipients on tacrolimus were targeted to reach a blood level of 4–8 ng/ml (normally 8–12) if diagnosed within the first year or 4–6 ng/ml if diagnosed later (normally 6–8). In liver and kidney recipients tacrolimus target levels were 2–3 and 3–5 respectively. Similar rules were used for patients on cyclosporine. Close monitoring was used to adjust doses according to blood levels, PTLD response, organ function and signs of rejection. We categorized patients into “complete withdrawal” when all immunosuppressive drugs were withdrawn as opposed to “partial withdrawal”. Patients who remained on low dose steroids were still considered “complete withdrawal”.
We obtained data on clinical and pathologic characteristics, anti-neoplastic therapies and tumor responses. The WHO classification of hematopoietic tumors was used for histologic classification of PTLD(2). EBV positivity was defined as either a positive in-situ hybridization for EBV-encoded RNA (EBER) or a positive immunohistochemical stain for the Latent Membrane Protein (LMP). A negative LMP stain without an accompanying EBER stain was considered non-diagnostic(2). Tumor responses were graded according to the Response Evaluation Criteria In Solid Tumors (RECIST). Failure of RI was defined as either PTLD-related death, progression of disease, or initiation of second-line therapy. Finally, we collected information on outcome of the allograft, second transplantation following graft loss, and overall survival. We focused on first-line therapy in order to isolate the response to RI alone, which was defined as the intent-to-treat using modulation of the immunosuppressive regimen with no further intervention until progression or lack of response. Data were analyzed separately for patients who were managed with RI alone (n=67), treated with complete surgical excision of their tumor followed by RI (n=30) or managed with other 1st-line modalities with or without RI (n=51). The Institutional Review Board of the University of Pennsylvania approved the study.
Statistical Analysis
Patient characteristics were compared with the t or χ2 test as appropriate. Survival curves were estimated by the Kaplan-Meier method and comparisons were determined by the logrank test. The Cox proportional hazards model was used for univariate analysis of survival and logistic regression modeling was used to analyze predictors of response. Following the identification of significant predictors of response by univariate analysis, a step-wise multivariable logistic regression model was used to select variables with independent predictive significance. Hazard ratios (HRs) and odds ratios (ORs) were calculated with 95% confidence intervals (95% CI). All statistical tests were two-sided, and P values less than 0.05 were considered significant. The analysis was conducted in SAS Release 9.1.3 (SAS Institute, Cary, NC).
Results
Patients
Of 162 adult patients diagnosed with PTLD, patients who were diagnosed at autopsy (n=9) were excluded and patients with missing response data (n=5) were only included in the survival analysis. The median number of annual PTLD diagnoses was 7 (range 2–12). Of 148 evaluable patients, 67 patients were treated with RI alone as initial therapy (“RI alone” group). We did not exclude patients who died quickly after initiation of therapy or patients who were deemed ineligible for any other line of therapy, in order to capture the variability in patient characteristics within this group and prevent a bias towards healthier patients.
Thirty patients were treated with surgical excision of a localized PTLD lesion, followed by RI (“adjuvant RI” group). These patients had an isolated skin lesion (n=15), gastrointestinal lesion (n=3) or graft PTLD (n=12). These patients were in remission following surgical excision and RI was instituted as adjuvant therapy.
Patient characteristics and clinical presentation
Table 1 displays the characteristics of patients categorized according to their first line therapy – RI alone, adjuvant RI and other modalities. RI alone was used across organ types, disease stages and in early (<1 year from transplant) and late-occurring PTLD. Complete surgical excision followed by adjuvant RI was more commonly done in kidney transplant recipients and less often in liver recipients.
Table 1.
Patient characteristics and clinical presentation
| RI alone as initial therapy | Surgery followed by adjuvant RI | Other first-line therapies* with or without RI | |
|---|---|---|---|
| Number of patients | 67 | 30 | 51 |
| Gender: | |||
| Male | 48 (72%) | 18 (60%) | 33 (65%) |
| Female | 19 (28%) | 12 (40%) | 18 (35%) |
| Allograft type: | ★ | ||
| Heart | 9 (13%) | 5 (17%) | 10 (20%) |
| Lung | 13 (19%) | 5 (17%) | 9 (17%) |
| Kidney | 27 (40%) | 15 (50%) | 17 (33%) |
| Kidney+Pancreas | 2 (3%) | 3 (10%) | 4 (8%) |
| Liver | 16 (24%) | 1 (3%) | 10 (20%) |
| Pancreas | 0 | 1 (3%) | 0 |
| Liver+Kidney | 0 | 0 | 1 (2%) |
| Mean age at transplant (years) | 45.6 | 41 | 45.6 |
| Mean age at PTLD diagnosis (years) | 50.7 | 46.2 | 51.5 |
| Median time from transplant to PTLD (range) | 871 days (56–8402) | 825 days (9–6166) | 963 days (6–6771) |
| Immunosuppressive regimen at diagnosis | |||
| CSA+MMF | 7 (11%) | 0 | 5 (10%) |
| TAC+MMF | 12 (19%) | 5 (19%) | 9 (18%) |
| CSA+AZA | 22 (35%) | 16 (59%) | 16 (33%) |
| TAC+AZA | 5 (8%) | 1 (4%) | 5 (10%) |
| CSA alone | 6 (10%) | 1 (4%) | 8 (16%) |
| TAC alone | 9 (14%) | 1 (4%) | 5 (10%) |
| AZA alone | 2 (3%) | 3 (11%) | 1 (2%) |
| Unknown | 4 | 3 | 2 |
| Steroids at diagnosis | |||
| Yes | 56 (88%) | 26 (93%) | 42 (86%) |
| No | 8 (12%) | 2 (7%) | 7 (14%) |
| Unknown | 3 | 2 | 2 |
| Hep. B – Yes | 5 (11%) | 0 | 2 (7%) |
| No | 40 (89%) | 14 (100%) | 26 (93%) |
| Unknown | 22 | 16 | 23 |
| Hep. C – Yes | 4 (11%) | 1 (8%) | 4 (20%) |
| No | 33 (89%) | 11 (92%) | 16 (80%) |
| Unknown | 30 | 18 | 31 |
| Stage | ★ | ||
| I/II | 34 (52%) | 30 (100%) | 25 (52%) |
| III/IV | 32 (48%) | 0 (0%) | 23 (48%) |
| Unknown | 1 | 0 | 3 |
| ★ | |||
| Single site | 18 (27%) | 30 (100%) | 19 (40%) |
| Multiple sites | 48 (73%) | 0 (0%) | 29 (60%) |
| Unknown | 1 | 0 | 3 |
| Previous grafts: | |||
| 1 | 60 (90%) | 28 (93%) | 47 (92%) |
| >1 | 7 (10%) | 2 (7%) | 4 (8%) |
| Previous rejection episodes – Yes | 31 (52%) | 11 (41%) | 24 (57%) |
| No | 29 (48%) | 16 (59%) | 18 (43%) |
| Unknown | 7 | 3 | 9 |
| B symptoms ¶ – Yes | 40 (61%) | 16 (57%) | 31 (65%) |
| No | 26 (39%) | 12 (43%) | 17 (35%) |
| Unknown | 1 | 2 | 3 |
| Weight loss – | ★ | ||
| Yes | 29 (43%) | 6 (21%) | 17 (35%) |
| No | 38 (57%) | 22 (79%) | 31 (65%) |
| Unknown | 0 | 2 | 3 |
| Fever – Yes | 23 (34%) | 14 (50%) | 20 (42%) |
| No | 44 (66%) | 14 (50%) | 28 (58%) |
| Unknown | 2 | 3 | |
| Night sweats- Yes | 14 (21%) | 5 (18%) | 8 (17%) |
| No | 53 (79%) | 23 (82%) | 40 (83%) |
| Unknown | 0 | 2 | 3 |
| Bulky disease ¶ - | ★ | ||
| Yes | 10 (15%) | 0 (0%) | 7 (15%) |
| No | 57 (85%) | 30 (100%) | 40 (85%) |
| Unknown | 0 | 0 | 4 |
| Extranodal disease – Yes | |||
| No | 48 (73%) | 27 (90%) | 39 (83%) |
| Unknown | 18 (27%) | 3 (10%) | 8 (17%) |
| 1 | 0 | 4 | |
| Involvement of the graft – | |||
| Yes | 16 (24%) | 12 (40%) | 18 (38%) |
| No | 51 (76%) | 18 (60%) | 29 (62%) |
| Unknown | 0 | 0 | 4 |
| CNS involvement – | ★ | ||
| Primary | 0 | 0 | 3 (6%) |
| Secondary | 1 (2%) | 0 | 3 (6%) |
| None | 65 (98%) | 30 (100%) | 44 (88%) |
| unknown | 1 | 0 | 1 |
| BM involvement – | |||
| Yes | 5 (15%) | 0 | 2 (9%) |
| No | 28 (85%) | 9 (100%) | 21 (91%) |
| Unknown | 34 | 21 | 28 |
| Elevated Creatinine – | ★ | ||
| Yes | 41 (63%) | 26 (93%) | 34 (74%) |
| No | 24 (37%) | 2 (7%) | 12 (26%) |
| Unknown | 2 | 2 | 5 |
| Elevated LDH – Yes | 40 (65%) | 15 (75%) | 29 (74%) |
| No | 22 (35%) | 5 (25%) | 10 (26%) |
| Unknown | 5 | 10 | 12 |
| HistologyΔ: | ★ | ||
| Polymorphic | 25 (37%) | 13 (43%) | 31 (61%) |
| Monomorphic | 42 (63%) | 17 (57%) | 20 (39%) |
| Mono. Subtypes: | |||
| DLBCL | 34 (51%) | 12 (40%) | 16 (31%) |
| Plasmacytoma-like | 4 (6%) | 2 (7%) | 2 (4%) |
| Hodgkin-like | 2 (3%) | 0 | 0 |
| Burkitt-like | 2 (3%) | 0 | 1 (2%) |
| T-cell | 0 | 0 | 1 (2%) |
| NOS | 0 | 3 (10%) | 0 |
| EBV positivity~ - Yes | 42 (70%) | 16 (80%) | 28 (67%) |
| No | 18 (30%) | 4 (20%) | 14 (33%) |
| Unknown | 7 | 10 | 10 |
| CD20 expression – Yes | |||
| No | 46 (79%) | 10 (67%) | 24 (71%) |
| Unknown | 12 (21%) | 5 (33%) | 10 (29%) |
| 9 | 15 | 17 | |
P<0.05 compared to RI alone.
Other therapies include surgery, radiotherapy, rituximab and cytotoxic chemotherapy.
B symptoms include weight loss, night sweats and fever. Bulky disease defined as a greater than 7cm mass or lymph node.
The WHO classification of hematopoietic tumors was used for classification of PTLD(2).
EBV positivity was defined as either a positive EBER in-situ hybridization or positive LMP stain. A negative LMP stain without an accompanying EBER stain was considered non-diagnostic (2).
There were no significant differences in organ involvement, most clinical symptoms and lab abnormalities (Table 1 and Supplementary Table 1). Patients presenting with abnormal renal function were more prevalent in the adjuvant RI group, reflecting the high proportion of kidney recipients and patients with graft PTLD in that group.
The RI groups were compared with 51 patients that were treated up front with other modalities, (Table 1). Significant baseline imbalance was found in PTLD histologic subtypes; monomorphic PTLD was diagnosed in 42/67 (63%) patients treated with RI alone (diffuse large B-cell lymphoma-like, n=34; Hodgkin lymphoma-like, n=2; plasmacytoma-like, n=4; Burkitt-like, n=2) compared to only 20/51 (39%) patients treated with other first-line therapies (DLBCL-like, n=16; plasmacytoma-like, n=2; Burkitt-like, n=1; T-cell PTLD, n=1), implying a selection of patients with monomorphic PTLD for treatment with RI alone (p=0.011). Only one patient with CNS involvement was treated with RI alone while the majority of these patients were treated with rituximab, chemotherapy and/or intrathecal chemotherapy (p=0.041). We found no significant differences in organ type, EBV positivity or CD20 expression between the groups.
In patients treated with RI alone, 17/62 patients (27%) underwent full withdrawal of immunosuppression as initial therapy: 9 kidney, 7 liver and 1 kidney/pancreas recipients. 43/62 (69%) underwent partial withdrawal: 14 kidney, 7 liver, 9 heart, 12 lung, 1 kidney/pancreas (p=0.001 for organ distribution). Two liver recipients (3%) were switched from their regimen to sirolimus. Data was not available for 5 patients. In patients treated with surgery and adjuvant RI, full withdrawal was done prior to graft removal and all other patients underwent partial withdrawal shortly after surgery.
Response to reduction of immunosuppression
Sufficient data were available to grade the responses of 62/67 patients treated with RI alone (Table 2). The overall response rate (CR + PR) was 45% and an additional 18% experienced stable disease. Among 67 patients treated with RI alone, 40 patients (60%) required second-line therapy, which included rituximab (40%), cytotoxic chemotherapy (39%) & radiotherapy (15%), either alone or in combination. Only 4 relapsed/refractory patients (6%) never received 2nd-line therapy. The median time to failure (TTF) of RI in the RI alone group was 45 days (range 1–2573) in non-responders. Among patients who had a complete response, only 4 patients (17%) relapsed and required additional therapy.
Table 2.
Response rates in patients treated with RI alone
| RI Alone as Initial Therapy | |
|---|---|
| Overall Response | 28/62 (45%) |
| Complete Response | 23/62 (37%) |
| Partial Response | 5/62 (8%) |
| Stable Disease | 11/62 (18%) |
| Progressive Disease | 23/62 (37%) |
| Unknown | 5 |
Grading of response was performed using the Response Evaluation Criteria in Solid Tumors (RECIST).
The outcome of patients treated with surgery and adjuvant RI was favorable and only 8/30 patients (27%) relapsed at a median of 5 months (range 1–86).
Allograft outcome
Table 3 displays the proportion of patients who experienced an acute rejection episode following RI, and Figure S1 displays the organ-specific outcome in the RI alone group. The acute rejection rate was 40% in patients treated with RI alone, 25% in patients treated with adjuvant RI (excluding graft removals) and 45% in patients who received rituximab with RI. Concurrent cytotoxic chemotherapy and RI resulted in a low (17%) rate of acute rejection. Five kidney recipients and one lung recipient underwent a second transplantation more than one year after resolution of their PTLD and did not experience a relapse. The degree of RI (complete vs. partial) did not predict rejection (OR 1.51; 95% CI [0.43, 5.31], P=0.52).
Table 3.
Acute allograft rejection in patients treated with reduction of immunosuppression (RI) alone and in combination
| Regimen | Incidence of Acute Graft Rejection |
|---|---|
| RI alone | 20/50 (40%) |
| Surgery followed by adjuvant RI | 4/16 (25%)* |
| RI + rituximab | 5/11 (45%) |
| RI + cytotoxic chemotherapy | 3/24 (17%) |
| Any RI containing 1st-line Regimen | 32/101 (32%) |
Out of patients who did not undergo surgical removal of the graft.
Survival
Kaplan-Meier estimates of survival for RI alone and adjuvant RI are presented in Figure 1. The median follow-up was 64 months. The median survival for patients treated with RI alone was 44 months (3-year OS, 55%) and the median survival for patients treated with adjuvant RI was not reached (3-year OS, 65%). For comparison, we plotted the survival of patients that were maintained on full immunosuppression throughout the treatment (“No RI” group, n=23). These patients were treated with rituximab, chemotherapy or both and did not undergo RI due to concurrent rejection (n=12), previous rejection episodes (n=4) or complex presentation with infections and/or multi-organ failure (n=7). The characteristics of these patients placed them at high risk, partially explaining their poor outcome in comparison with the RI alone group (median survival 9.5 months; 3-year OS, 34%; p=0.07).
Figure 1.
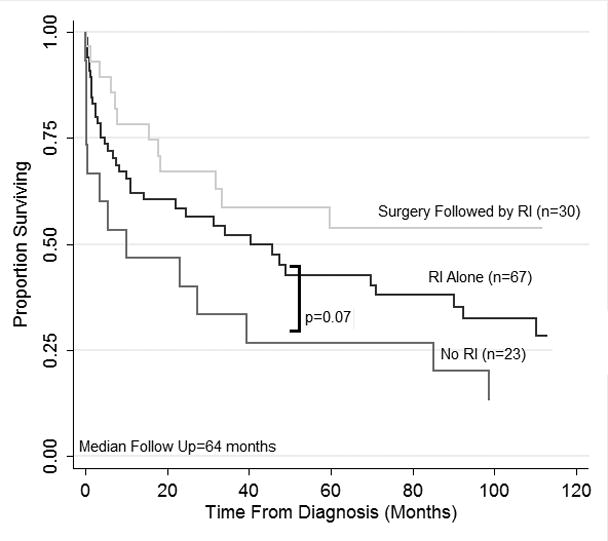
Overall survival of patients with PTLD who were treated with reduction of immunosuppression (RI) alone as initial therapy (n=67), complete surgical excision of a PTLD lesion, followed by adjuvant RI (n=30), and no RI throughout treatment (n=23). Survival estimates are plotted using the Kaplan-Meier method. P=0.07 for RI alone versus No RI represents a logrank test.
Predictors of survival and response to RI
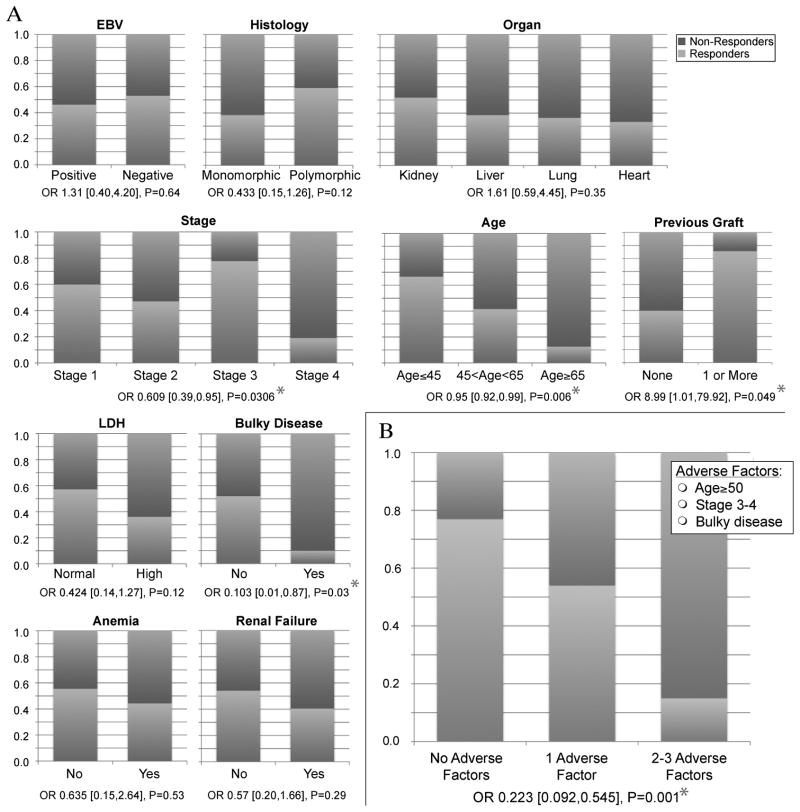
We evaluated multiple variables for prediction of survival and response to RI using the Cox proportional hazards model and logistic regression, respectively (Tables 4–5 and Figure 2). Early disease stage at presentation predicted favorable response to RI alone. Patients with isolated graft PTLD were considered stage I. Early stage did not only predict response to RI but to any 1st-line therapy, when analyzed on 153 patients who had sufficient data (OR 0.32; 95% CI [0.15, 0.68], p=0.003). An alternative classification of tumor spread (single vs. multiple sites), suggested previously (22), did not predict response or survival better than the Ann Arbor classification. Older age was a significant predictor of poor response to RI alone (OR 0.95 per year; 95% CI [0.92, 0.99], p=0.006), and responses were rare in patients older than 65 years (Figure 2A). Anorexia at diagnosis and bulky disease (greater than 7 cm at diagnosis), were significant predictors of poor response (ORs 0.27 and 0.103 respectively). A history of multiple allografts was associated with a better response to RI. Although most of the patients who had previous allografts represented kidney recipients, response to RI was not limited to one organ type. Responses were slightly more common among kidney recipients but recipients of other organs demonstrated good responses as well (p=0.35 for kidney vs. non-kidney; Figure 2A). Non-significant differences in response to RI were demonstrated between major histologic subtypes, EBV status and late vs. early PTLD. The response rates among patients with uncommon histologic subtypes were 3/4 for plasmacytoma (all 3 achieved CR), 0/2 for Hodgkin-like PTLD and 1/2 for Burkitt-like lesions (PR in one responder).
Table 4.
Predictors of response to RI in patients treated with RI alone (n=67)
| Response to RI | ||
|---|---|---|
| Variable | OR (95% CI) | P-value |
| Patient characteristics | ||
| Age at diagnosis | 0.95 (0.92,0.99) | 0.006 |
| Transplanted organ (kidney vs. non-kidney) | 1.615 (0.59,4.45) | 0.354 |
| Late (≥1 yr) vs. Early (<1yr) | 0.889 (0.48,1.66) | 0.711 |
| >1 previous allograft | 8.996 (1.01,79.92) | 0.049 |
| Pathologic characteristics | ||
| Monomorphic vs. Polymorphic | 0.433 (0.15,1.26) | 0.124 |
| EBV-positive vs. EBV-negative | 0.762 (0.24,2.39) | 0.641 |
| Clinical Presentation | ||
| B symptoms | 0.478 (0.17,1.34) | 0.161 |
| Stage | 0.609 (0.39,0.95) | 0.0306 |
| Single vs. Multiple Sites | 0.31 (0.09, 1.05) | 0.061 |
| Bulky disease | 0.103 (0.01,0.87) | 0.037 |
| Extranodal disease | 0.65 (0.21,1.99) | 0.451 |
| Anorexia as initial symptom | 0.27 (0.09,0.84) | 0.024 |
| Lab abnormalities | ||
| Abnormal LDH | 0.424 (0.14,1.27) | 0.126 |
| Anemia | 0.635 (0.15,2.64) | 0.531 |
| Thrombocytopenia | 0.951 (0.28,3.25) | 0.935 |
| Management | ||
| Partial vs. Complete RI | 0.921 (0.28, 3.03) | 0.893 |
| Multivariate Analysis | ||
| Bulky disease | 0.09 (0.008,0.97) | 0.048 |
| Stage | 0.58 (0.33,1.003) | 0.051 |
| Age at diagnosis | 0.94 (0.89,0.98) | 0.002 |
Odds ratios (ORs) are presented with 95% confidence intervals (CI) for predictors of response. P-values<0.05 are in bold. Additional variables that were not found to be predictors of response include demographic parameters, presenting symptoms other than anorexia, specific organ involvement (including BM and CNS), hepatitis serostatus and additional lab abnormalities (data not shown).
Table 5.
Predictors of mortality in patients treated with RI alone (n=67)
| Survival | ||
|---|---|---|
| RI alone (n=67) | ||
| Variable | HR (95% CI) | P-value |
| Patient characteristics | ||
| Age at diagnosis | 1.059 (1.03,1.09) | <0.0001 |
| Year of diagnosis (<2000 vs. ≥2000) | 0.961 (0.49, 1.88) | 0.907 |
| Transplanted organ (kidney vs. non-kidney) | 0.635 (0.32,1.26) | 0.191 |
| Hep. B | 1.033 (0.35, 3.02) | 0.952 |
| Hep. C | 3.75 (1, 14.06) | 0.05 |
| Hep. B or Hep. C | 1.281 (0.42, 3.92) | 0.665 |
| Late (≥1 yr) vs. Early (<1yr) | 1.137 (0.77,1.68) | 0.519 |
| Pathologic characteristics | ||
| Monomorphic vs. Polymorphic | 1.159 (0.60,2.25) | 0.662 |
| EBV-positive vs. EBV-negative | 0.781 (0.38,1.61) | 0.502 |
| Clinical presentation | ||
| B symptoms | 2.04 (1.01,4.10) | 0.046 |
| Weight loss | 2.03 (1.08,3.82) | 0.028 |
| Dyspnea | 2.68 (1.17,6.13) | 0.02 |
| Stage | 1.269 (0.96,1.67) | 0.09 |
| Single vs. Multiple sites | 1.815 (0.83, 3.97) | 0.136 |
| Bulky disease | 1.06 (0.44,2.56) | 0.9 |
| Extranodal disease | 1.675 (0.77,3.65) | 0.194 |
| CNS involvement | 1.378 (0.18, 10.2) | 0.75 |
| BM involvement | 3.404 (1.05, 11) | 0.041 |
| Liver involvement | 2.79 (1.26,6.20) | 0.012 |
| Management | ||
| Partial vs. Complete RI | 0.981 (0.45,2.15) | 0.96 |
| Lab abnormalities | ||
| Renal failure | 2.05 (0.99,4.23) | 0.052 |
| LDH > ULN | 1.821 (0.88,3.78) | 0.108 |
| LDH > 2.5 × ULN | 5.97 (2.51,14.23) | <0.0001 |
| Anemia | 2.227 (0.78,6.33) | 0.133 |
| Thrombocytopenia | 0.676 (0.33,1.40) | 0.29 |
Hazard ratios (HRs) are presented with 95% confidence intervals (CI) for predictors of survival. P-values<0.05 are in bold. Additional variables that were not found to predict survival include other demographic parameters, presenting symptoms, lab abnormalities and organ involvement other than listed in the table (data not shown).
Figure 2.
Proportions of responding (Complete Response + Partial Response) and non-responding (Stable Disease + Progressive Disease) patients with PTLD following treatment with RI alone analyzed according to clinical and pathologic characteristics. Odds ratios indicate the difference in likelihood of response between subgroups and P-values indicate significance level in a logistic regression model. * P<0.05. A. Representative variables from Table 4. B. Prediction of response to RI using a summary of 3 independent adverse factors: Age≥50, advanced stage (3–4 vs. 1–2) & bulky disease (mass>7cm).
After identifying variables that predicted response in univariate analysis, we applied a stepwise logistic regression model (Table 4). Independent predictors for response to RI were younger age (OR 0.94 per year), early stage (OR 0.58) and non-bulky disease (OR 0.09). Only 1/10 patients with bulky disease had a response (CR) to RI alone. Curiously, bulky disease did not predict overall survival, implying that these patients can be salvaged with 2nd-line therapy.
We combined the three most significant predictors for lack of response to RI (stage, bulky disease and age over 50) into a single variable (Figure 2B). As expected, the combined variable predicted response very well (OR 0.223, p=0.001). Response rates to RI alone were 77%, 54% and 15% in patients with 0, 1 and 2–3 adverse factors respectively.
Survival analysis was performed in 67 patients managed with RI alone (Table 5). Significant predictors of poor survival were age, B symptoms, weight loss, dyspnea, hepatitis C, bone marrow and liver involvement as well as very high LDH (greater than 2.5 × ULN). The extent of increase in serum LDH levels had a very strong correlation with survival; the median survival for patients with LDH>2.5 × ULN was shorter than 2 months.
Seven risk factors that were identified as significant prognostic factors in our survival analysis were used to stratify patients for subgroup analysis (Figure 3). The 3-year OS estimates were 100%, 79%, 32% and 7.5% for patients with 0,1,2 and greater than 2 risk factors respectively (p<0.0001 in a log-rank test across strata).
Figure 3.
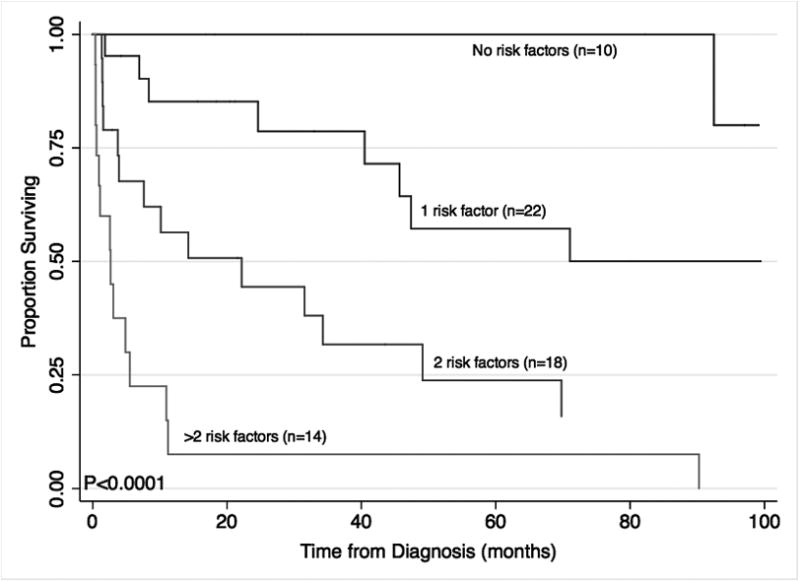
Overall survival of patients with PTLD who were treated with reduction of immunosuppression (RI) alone as initial therapy stratified using a survival model that includes 7 prognostic factors: Age≥50, serum LDH>2.5×ULN, hepatitis C, liver involvement, bone marrow involvement, B symptoms, dyspnea at presentation. Survival estimates are plotted using the Kaplan-Meier method. P-values represent a logrank test across strata. Total n=64 patients with complete data.
Discussion
PTLD is a major cause of morbidity and mortality after solid organ transplantation. Despite advances in immunosuppression, the risk of PTLD remains significant, affecting 1–30% of transplant recipients(23–27). In this study we aimed to determine the outcome of the common initial step in the management of PTLD – withdrawal of immunosuppression. It follows common wisdom established more than 20 years ago(5): if PTLD is caused by the unchecked proliferation of EBV-transformed lymphocytes under immunosuppressive therapy, then withdrawal of immunosuppression will allow the anti-tumor effects of the immune system to recover, resulting in tumor regression.
Our study demonstrates a response rate of 45% in patients selected for treatment with RI alone, with the majority being complete responses. Only 4 relapsed/refractory patients never received 2nd-line therapy, illustrating that most patients have the potential for second-line salvage if they fail RI.
Our second cohort included 30 patients who underwent surgical excision of a skin lesion, gastrointestinal mass or a graft containing PTLD, rendering them free of disease prior to adjuvant RI, intended to prevent relapse. We think of lymphoid proliferative disorders as systemic diseases and normally favor systemic therapy. Several reports have utilized surgery in PTLD(28–32), and here we describe a large group of patients, in which surgical treatment followed by RI resulted in successful control of the disease and most often permanent cure (only 27% relapsed). We did not identify a sufficient number of patients who underwent surgery without adjuvant RI and whether RI was necessary is a question that cannot be answered by this study.
The safety of RI has been a major concern. We demonstrated a 32% acute rejection rate with RI-containing regimens. Some of these patients recovered and others underwent a second transplantation following resolution of their PTLD (Figure S1). Keeping in mind that cytotoxic chemotherapy agents are very effective immunosuppressants, 2nd-line therapy after failure of RI can salvage an acute rejection episode. If allograft rejection becomes irreversible, a second transplant is a valid option, as demonstrated in our study and others(33, 34). Interestingly, the extent of RI failed to predict rejection, but RI strategies were unbalanced across organs and firm conclusions cannot be drawn.
Should we attempt RI alone in all patients presenting with PTLD? This study provides significant insight. First, some presumed risk factors — such as EBV negativity or monomorphic PTLD — did not show association with response. As opposed to previously published work, our study included multiple allograft types and a significant number of patients with EBV-negative disease (30% of the RI alone cohort), allowing us to demonstrate that RI can be effective in EBV-negative PTLD(35) and across all histologic subtypes and allograft types, including “high-risk” organs such as heart and lung. Second, lack of response is predictable. In the current study, we identified bulky disease, advanced stage and older age as adverse factors that predict failure to respond. Patients who lacked these factors had a 77% chance of response to RI alone. The validity of these conclusions is limited by the retrospective nature of our study and the lack of data availability for some of the important variables. Prospective studies are needed to clarify the role of previously reported biomarkers of response, such as quantitative PCR for EBV(36) and analysis of EBV-specific cytotoxic T-lymphocytes(37).
Finally, our survival analysis identified several strong predictors of poor survival, which generally reconcile with known risk factors in lymphoid malignancies (age, high LDH, B symptoms, BM involvement). These findings partially overlap previous PTLD studies, which identified age, BM and CNS involvement, LDH, performance status, and high-risk histology (Burkitt-like, T-cell) as important prognostic factors(22, 32, 38–41). EBV status, stage and late PTLD have been identified by some studies (22, 40) but not by others (38, 42), which illustrates the heterogeneity in study design, size and population (pediatric vs. adult, organ-focused vs. mixed). Our study focused on adult patients treated with RI alone and had a baseline imbalance towards monomorphic PTLD and lack of CNS involvement. Our survival analysis may therefore not apply to all PTLD patients.
Approximately 35% of patients in this study were diagnosed before the availability of rituximab, which can be used either alone or in combination with chemotherapy resulting in response rates of 50–80%(9, 19, 43–45). Rituximab can spare patients the risks of cytotoxic chemotherapy, but still has serious side effects, mainly infusion reactions, immune suppression and other idiosyncratic reactions(46). A recent retrospective report demonstrated favorable outcome and 3-year OS of 73% with the use of rituximab early in therapy, but all patients in that series also underwent RI initially(19). The relative contributions of RI and rituximab to the high response rate in that study are unknown. Similarly, other reports on rituximab for PTLD have focused on 2nd-line therapy(8, 44, 45, 47). Considering the poor survival that was demonstrated in our study for patients who never underwent RI, including patients who received rituximab (Figure 1), our study underscores the importance of RI either alone or in conjunction with rituximab. Notably, our survival analysis failed to demonstrate a difference in survival between patients treated with RI alone before and after the year 2000 (p=0.91). A similar analysis performed on our entire patient cohort (n=153) also showed a non-significant difference (p=0.39), implying that the introduction of rituximab may not have resulted in a significant improvement in outcome.
Our survival analysis demonstrated two subgroups of low-risk patients, in which treatment with RI alone resulted in 3-year overall survival rates of 100% and 79%, respectively. These outcomes are similar or better compared to the recent report about rituximab(19), allowing us to hypothesize that RI alone may be sufficient in low-risk patients. It is also notable that rituximab did not seem to protect against rejection (Table 3).
Our study was not powered to differentiate between RI strategies. Complete and partial withdrawals of immunosuppression resulted in similar rates of response and rejection and in similar survival hazards, but were used in different allograft types and possibly different clinical scenarios, making it difficult to draw any conclusions on a better strategy. More specific information about RI strategies and organ-specific rates of rejection has been published(48, 49).
Our observations support initial therapy with RI and close monitoring in low-risk patients. Patients who have an initial response can often be observed with frequent imaging and fine-tuning of the immunosuppressive regimen. With this strategy, many patients avoided cytotoxic chemotherapy and the associated risk of complications. Conventional chemotherapy often requires dose reductions due to underlying organ dysfunction and is associated with a poor outcome; patients in our study who required chemotherapy at any stage of their treatment had a median survival of 19 months.
Certain limitations of this study should be acknowledged. The retrospective observational nature of this study is prone to confounding and may lead to a bias in our estimates of the effects of RI and the predictors of these effects. Sample size limitations may result in failure to detect significant predictors of response or survival. In addition, our conclusions may not apply to the pediatric population, which has special characteristics and a higher incidence of primary EBV infection. Our cohort did not include a sufficient number of patients with CNS involvement or rare subtypes of PTLD such as T-cell or Hodgkin-like disease. Our conclusions may not be applicable to such patients.
To summarize, in our retrospective analysis, RI alone as initial therapy for PTLD has a high response rate and can lead to a favorable outcome. Relapse is uncommon in patients who experience a complete response to RI. Rejection is common but manageable by further adjustment of immunosuppressive therapy, second-line therapy including cytotoxic chemotherapy and in some cases a second transplant. RI can also be used efficiently and safely for patients in the adjuvant setting following resection of PTLD lesions. Older age, bulky disease and advanced stage (particularly stage IV) are associated with poor response to RI. Low-risk patients should be considered for treatment with RI alone and can expect a favorable outcome.
Supplementary Material
Detailed description of management and allograft outcome on 67 patients who underwent RI alone as initial therapy for PTLD.
Acknowledgments
The authors wish to thank Oren Litvin (Columbia University) for his assistance with preparation of the figures for this manuscript.
Funding Sources: This work was supported by National Institutes of Health grants CA16520 (S.V. and D.F.H.), CA117879 (D.L.P.) & HL069286 (E.A.S.). R.R. is a fellow of the Institute for Translational Medicine and Therapeutics, University of Pennsylvania.
Footnotes
Presented in part at the 14th European Hematology Association Congress, Berlin, Germany, June 2009, and the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 2009.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Frey NV, Tsai DE. The management of posttransplant lymphoproliferative disorder. Med Oncol. 2007;24(2):125–36. doi: 10.1007/BF02698031. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 3.Yang J, Tao Q, Flinn IW, Murray PG, Post LE, Ma H, et al. Characterization of Epstein-Barr virus-infected B cells in patients with posttransplantation lymphoproliferative disease: disappearance after rituximab therapy does not predict clinical response. Blood. 2000 December 15;96(13):4055–63. [PubMed] [Google Scholar]
- 4.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas.[see comment] N Engl J Med. 2004 Mar 25;350(13):1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984 Mar 17;1(8377):583–7. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai DE, Stadtmauer EA, Canaday DJ, Vaughn DJ. Combined radiation and chemotherapy in posttransplant lymphoproliferative disorder. Med Oncol. 1998 Dec;15(4):279–81. doi: 10.1007/BF02787213. [DOI] [PubMed] [Google Scholar]
- 7.Benkerrou M, Jais JP, Leblond V, Durandy A, Sutton L, Bordigoni P, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood. 1998 Nov 1;92(9):3137–47. [PubMed] [Google Scholar]
- 8.Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. 2006 Mar;6(3):569–76. doi: 10.1111/j.1600-6143.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- 9.Faye A, Van Den Abeele T, Peuchmaur M, Mathieu-Boue A, Vilmer E. Anti-CD20 monoclonal antibody for post-transplant lymphoproliferative disorders. Lancet. 1998 Oct 17;352(9136):1285. doi: 10.1016/S0140-6736(05)70493-1. [DOI] [PubMed] [Google Scholar]
- 10.Loren AW, Tsai DE. Post-transplant lymphoproliferative disorder. Clin Chest Med. 2005 Dec;vii26(4):631–45. doi: 10.1016/j.ccm.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Swinnen LJ, Mullen GM, Carr TJ, Costanzo MR, Fisher RI. Aggressive treatment for postcardiac transplant lymphoproliferation. Blood. 1995 Nov 1;86(9):3333–40. [PubMed] [Google Scholar]
- 12.Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009 Jul 10;27(20):3354–62. doi: 10.1200/JCO.2008.20.0857. [DOI] [PubMed] [Google Scholar]
- 13.Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001 Apr 27;71(8):1076–88. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 14.Issa N, Amer H, Dean PG, Kremers WK, Kudva YC, Rostambeigi N, et al. Posttransplant Lymphoproliferative Disorder Following Pancreas Transplantation. Am J Transplant. 2009 Jun 10; doi: 10.1111/j.1600-6143.2009.02691.x. [DOI] [PubMed] [Google Scholar]
- 15.Aversa SM, Stragliotto S, Marino D, Calabrese F, Rigotti P, Marchini F, et al. Post-transplant lymphoproliferative disorders after heart or kidney transplantation at a single centre: presentation and response to treatment. Acta Haematol. 2008;120(1):36–46. doi: 10.1159/000155234. [DOI] [PubMed] [Google Scholar]
- 16.Swinnen LJ, LeBlanc M, Grogan TM, Gordon LI, Stiff PJ, Miller AM, et al. Prospective study of sequential reduction in immunosuppression, interferon alpha-2B, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation. 2008 Jul 27;86(2):215–22. doi: 10.1097/TP.0b013e3181761659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel H, Vogl DT, Aqui N, Shaked A, Olthoff K, Markmann J, et al. Posttransplant lymphoproliferative disorder in adult liver transplant recipients: a report of seventeen cases. Leuk Lymphoma. 2007 May;48(5):885–91. doi: 10.1080/10428190701223275. [DOI] [PubMed] [Google Scholar]
- 18.Chen JM, Barr ML, Chadburn A, Frizzera G, Schenkel FA, Sciacca RR, et al. Management of lymphoproliferative disorders after cardiac transplantation. Ann Thorac Surg. 1993 Sep;56(3):527–38. doi: 10.1016/0003-4975(93)90893-m. [DOI] [PubMed] [Google Scholar]
- 19.Evens AM, David KA, Helenowski I, Nelson B, Kaufman D, Kircher SM, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. Feb 20;28(6):1038–46. doi: 10.1200/JCO.2009.25.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzig KA, Juffs HG, Norris D, Brown AM, Gill D, Hawley CM, et al. A single-centre experience of post-renal transplant lymphoproliferative disorder. Transpl Int. 2003 Jul;16(7):529–36. doi: 10.1007/s00147-003-0596-0. [DOI] [PubMed] [Google Scholar]
- 21.Gill D, Juffs HG, Herzig KA, Brown AM, Hawley CM, Cobcroft RG, et al. Durable and high rates of remission following chemotherapy in posttransplantation lymphoproliferative disorders after renal transplantation. Transplant Proc. 2003 Feb;35(1):256–7. doi: 10.1016/s0041-1345(02)03796-x. [DOI] [PubMed] [Google Scholar]
- 22.Caillard S, Lelong C, Pessione F, Moulin B. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006 Nov;6(11):2735–42. doi: 10.1111/j.1600-6143.2006.01540.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JI. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine (Baltimore) 1991 Mar;70:137–60. doi: 10.1097/00005792-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Finn L, Reyes J, Bueno J, Yunis E. Epstein-Barr virus infections in children after transplantation of the small intestine. Am J Surg Pathol. 1998 Mar;22(3):299–309. doi: 10.1097/00000478-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Shroff R, Trompeter R, Cubitt D, Thaker U, Rees L. Epstein-Barr virus monitoring in paediatric renal transplant recipients. Pediatr Nephrol. 2002 Sep;17(9):770–5. doi: 10.1007/s00467-002-0931-1. [DOI] [PubMed] [Google Scholar]
- 26.Sokal EM, Antunes H, Beguin C, Bodeus M, Wallemacq P, de Ville de Goyet J, et al. Early signs and risk factors for the increased incidence of Epstein-Barr virus-related posttransplant lymphoproliferative diseases in pediatric liver transplant recipients treated with tacrolimus. Transplantation. 1997 Nov 27;64(10):1438–42. doi: 10.1097/00007890-199711270-00011. [DOI] [PubMed] [Google Scholar]
- 27.Shahinian VB, Muirhead N, Jevnikar AM, Leckie SH, Khakhar AK, Luke PP, et al. Epstein-Barr virus seronegativity is a risk factor for late-onset posttransplant lymphoroliferative disorder in adult renal allograft recipients. Transplantation. 2003 Mar 27;75(6):851–6. doi: 10.1097/01.TP.0000055098.96022.F7. [DOI] [PubMed] [Google Scholar]
- 28.Foroncewicz B, Mucha K, Usiekniewicz J, Chmura A, Kryst P, Soldacki D, et al. Posttransplant lymphoproliferative disorder of the lung in a renal transplant recipient treated successfully with surgery. Transplant Proc. 2006 Jan–Feb;38(1):173–6. doi: 10.1016/j.transproceed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Hauke R, Smir B, Greiner T, Bierman P, Tarantolo S, Anderson J, et al. Clinical and pathological features of posttransplant lymphoproliferative disorders: influence on survival and response to treatment. Ann Oncol. 2001 Jun;12(6):831–4. doi: 10.1023/a:1011131700811. [DOI] [PubMed] [Google Scholar]
- 30.Mucha K, Foroncewicz B, Niemczyk K, Ziarkiewicz-Wroblewska B, Stanislawek-Sut O, Zieniewicz K, et al. Tonsil enlargement after liver transplantation in adults--reason enough for tonsillectomy? Two cases of tonsillar posttransplantation lymphoproliferative disease. Liver Transpl. 2007 Jun;13(6):918–23. doi: 10.1002/lt.21177. [DOI] [PubMed] [Google Scholar]
- 31.Beynet DP, Wee SA, Horwitz SS, Kohler S, Horning S, Hoppe R, et al. Clinical and pathological features of posttransplantation lymphoproliferative disorders presenting with skin involvement in 4 patients. Arch Dermatol. 2004 Sep;140(9):1140–6. doi: 10.1001/archderm.140.9.1140. [DOI] [PubMed] [Google Scholar]
- 32.Trofe J, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. Am J Transplant. 2005 Apr;5(4 Pt 1):775–80. doi: 10.1111/j.1600-6143.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- 33.Hanto DW. Retransplantation After Post-Transplant Lymphoproliferative Diseases (PTLD): When is it Safe? Am J Transplant. 2004;11;4:1733–4. doi: 10.1111/j.1600-6143.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson SR, Cherikh WS, Kauffman HM, Pavlakis M, Hanto DW. Retransplantation After Post-Transplant Lymphoproliferative Disorders: An OPTN/UNOS Database Analysis. Am J Transplant. 2006;11;6:2743–9. doi: 10.1111/j.1600-6143.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 35.Reshef R, Luskin M, Morgans A, Pfanzelter N, Bloom R, Brozena S, et al., editors. American Transplant Congress. Boston, MA: Wiley-Blackwell Publishing, Inc; 2009. May 30 – June 3, EBV-Negative PTLD: 20 Year Experience in a Large Transplant Center. [Google Scholar]
- 36.Tsai DE, Douglas L, Andreadis C, Vogl DT, Arnoldi S, Kotloff R, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008 May;8(5):1016–24. doi: 10.1111/j.1600-6143.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 37.Guppy AE, Rawlings E, Madrigal JA, Amlot PL, Barber LD. A quantitative assay for Epstein-Barr Virus-specific immunity shows interferon-gamma producing CD8+ T cells increase during immunosuppression reduction to treat posttransplant lymphoproliferative disease. Transplantation. 2007 Dec 15;84(11):1534–9. doi: 10.1097/01.tp.0000290232.65830.e7. [DOI] [PubMed] [Google Scholar]
- 38.Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, et al. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007 Nov 1;25(31):4902–8. doi: 10.1200/JCO.2006.10.2392. [DOI] [PubMed] [Google Scholar]
- 39.Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005 Mar;37(2):954–5. doi: 10.1016/j.transproceed.2004.12.130. [DOI] [PubMed] [Google Scholar]
- 40.Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001 Feb 1;19(3):772–8. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 41.Choquet S, Mamzer BM, Hermine O, Porcher R, Nguyen QS, Davi F, et al. Identification of prognostic factors in post-transplant lymphoproliferative disorders. Recent Results Cancer Res. 2002;159:67–80. doi: 10.1007/978-3-642-56352-2_9. [DOI] [PubMed] [Google Scholar]
- 42.Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, et al. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005 Jan 27;79(2):244–7. doi: 10.1097/01.tp.0000144335.39913.5c. [DOI] [PubMed] [Google Scholar]
- 43.Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier JL, Niaudet P, et al. Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med. 1991 May 23;324(21):1451–6. doi: 10.1056/NEJM199105233242102. [DOI] [PubMed] [Google Scholar]
- 44.Milpied N, Vasseur B, Parquet N, Garnier JL, Antoine C, Quartier P, et al. Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol. 2000;11(Suppl 1):113–6. [PubMed] [Google Scholar]
- 45.Choquet S, Leblond V, Herbrecht R, Socie G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006 Apr 15;107(8):3053–7. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 46.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008 Aug 7;359(6):613–26. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 47.Oertel SH, Verschuuren E, Reinke P, Zeidler K, Papp-Vary M, Babel N, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD) Am J Transplant. 2005 Dec;5(12):2901–6. doi: 10.1111/j.1600-6143.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 48.Aull MJ, Buell JF, Trofe J, First MR, Alloway RR, Hanaway MJ, et al. Experience with 274 cardiac transplant recipients with posttransplant lymphoproliferative disorder: a report from the Israel Penn International Transplant Tumor Registry. Transplantation. 2004 Dec 15;78(11):1676–82. doi: 10.1097/01.tp.0000144333.19106.58. [DOI] [PubMed] [Google Scholar]
- 49.Issa N, Amer H, Dean PG, Kremers WK, Kudva YC, Rostambeigi N, et al. Posttransplant lymphoproliferative disorder following pancreas transplantation. Am J Transplant. 2009 Aug;9(8):1894–902. doi: 10.1111/j.1600-6143.2009.02691.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description of management and allograft outcome on 67 patients who underwent RI alone as initial therapy for PTLD.