Abstract
The atomic force microscope is broadly used to study the morphology of cells1–5 but it can also probe the mechanics of cells. It is now known that cancerous cells may have different mechanical properties than normal cells6–8 but the reasons for these differences are poorly understood9. Here we report quantitatively the differences between normal and cancerous human cervical epithelial cells by considering the brush layer on the cell surface. These brush layers, which consist mostly of microvilli, microridges, and cilia are important for interacting with the environment. Deformation force curves obtained from cells in vitro are processed according to the 'brush on soft cell model 10. We found that normal cells have brushes with one length while cancerous cells displayed long and short brushes with significantly different densities. The observed differences suggest that brush layers should be taken into account when characterizing the cell surface by mechanical means.
Cancerous cells differ from normal cells in terms of cell growth, morphology, cell–cell interaction, organization of the cytoskeleton, and interactions with the extracellular matrix11–13. The atomic force microscope (AFM) can be used to detect most of these changes9,14 but one of the main challenges in understanding these differences is the lack of statistically sound quantitative data. To quantify the mechanical properties of cells, AFM probes with a well-defined geometry are needed, which should not be too sharp to avoid non-linear cell responses15,16. For example, micron-sized silica spheres have been used successfully to study the mechanics of cells4,5 (see the Supporting information for advantages of these probes). In addition, it is also important to consider the presence of brush-type structures on the cell surface such as molecules that are grafted on the cell membrane and membrane corrugations like microvilli and microridges. Here we show quantitatively the differences between the brush layers on the surfaces of normal and cancerous human cervical epithelial cells.
While expected, the difference in surface of cancerous and normal cells has still a lot of unknowns. For example, a difference in cilia on the surface of cancerous and normal cells has been found just recently17. Cilia that protrude from the apical/lumenal surface of polarized cells, act as a sensor of environmental cues. Similarly, microvilli are important for the cells to interact with the environment. Molecular entropic brushes are known to surround neurofilaments to maintain interfilament spacing2,18. Furthermore, molecular brushes on living cells, composed of the glycocalyx layer and the pericellular molecular coating19,20 are known to be responsible for cell-cell interaction, cell migration, differentiation, and proliferation21,22. The size of the pericellular coating was shown20,23 to correlate with the degree of invasiveness of cancer (although it's still not clear whether the brush size or molecular composition, or maybe both, play the major role). Therefore, our reported difference in the brush layers may have a large biological significance.
Collected curves of the displacement of the AFM cantilever versus vertical position of the scanner (raw force data) are shown in Fig. 1. As expected14, cancerous cells show higher variability of force behaviour. However, one can see no clear difference between the curves collected on normal and cancerous cells.
Fig. 1.
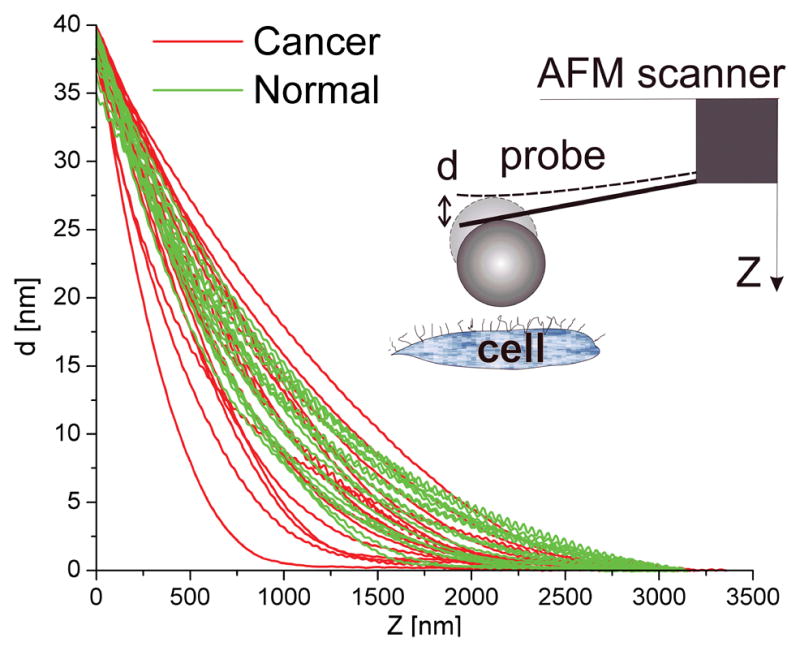
Raw force curves of normal and cancerous cells. Plot shows the deflection of the cantilever, d, versus the vertical position of the scanner, Z. Curves were collected from 35–40 normal and cancerous cells. Each curve represents an average of 30–50 individual curves measured over relatively flat regions of each cell (incline < 10–150; can be the top and/or side of the cell). Data from two cells were discarded because they deviated too far from the others. This is not unusual because some cells may be dead, damaged or in mitosis, which can change the mechanical characteristics of the cell 9,30.
As we observed with confocal and electron microscopy (see below), both cancerous and normal cells do show the clear presence of a brush. Thus, we should use a brush-on-soft-surface model described in 10 to process the collected force curves. Having processed the data shown in Fig. 1 (see the Supplementary Materials for detail), one obtains both the Young’s modulus of the cell body, Fig. 2a,b, and the long-range repulsive force associated with the brush, Fig. 2c. Interestingly, the difference in rigidity between normal and cancerous cells is not statistically significant. In contrast with the raw force curves in Fig. 1, the forces associated with the brush show a clear difference between cancerous and normal cells. It is quite intriguing to note that the variability of the force curves corresponding to the brush layer is rather small and about the same for both cancerous and normal cells. The cancer variability seen in the raw force curves seems to be entirely accumulated in the values of the Young’s moduli of the cell body.
Fig. 2.
Processed raw force data of Fig. 1. Derived distributions of the Young’s moduli of (a) normal and (b) cancerous cell body. (c) Forces between the AFM probe and cell brush (see Supporting Materials for details). Force shown in logarithmic scale is plotted against the distance between the probe and cell body, h. The slower force decay at large distances in cancerous cells is highlighted by the circle. Force curves for cancerous and normal cells are clearly different. Only one force curve for a normal cell is in the region of the cancerous cell and approximately two cancerous force curves overlapped with normal cells.
One can easily recognize a rather explicit exponential behaviour of the force dependence of the brush10,24 (straight line in the logarithmic scale used in Fig. 2c). There is one more intriguing feature that one can see in Fig. 2c. Almost all cancerous force dependences demonstrate straight lines with two slopes (highlighted by a circle in Fig. 2c).
In the case of one-slope force dependence for normal cells, the force between a spherical AFM probe of radius R and the brush grafted can be described by the following equation25
| (1) |
where L is the equilibrium thickness of the brush layer, N is the effective surface density of the brush constituents (grafting density), and T is the temperature of the medium. Eq. (1) is a good description of a brush for 0.1 < h/L < 0.8 .
To describe the force dependences of cancerous cells, in particular, the feature circled in Fig. 2c, we assume that the probe-cell force is created by two brushes with different lengths described as follows:
| (2) |
Here, the indexes of N and L correspond to the first and second brushes. Eq. (2) is definitely an approximation. It is out of applicability if the distance h is between the lengths L1 and L2, as well as greater than the larger L. However, the exponential functions vanish quickly, leaving rather small corrections. Similar approximation was already used previously26. Secondly, the forces we are fitting are the values averaged over the surface of each cell. Therefore, the grafting densities N1 and N2 should be treated as a sort of average density.
Fig. 3 shows representative examples of fitting normal and cancerous force curves with eqs. (1) and (2), respectively. One can see a rather good fit. For normal cells, the force drops to zero more rapidly than predicted by eq. (1) for h > L, which is expected for a brush. In contrast, the cancerous cell forces lie above the fitting line for large h. This indicates that cancerous cells demonstrate even a weak third brush (can be seen in SEM images, SI Fig. 7). However, it is too close to the limit of AFM sensitivity to make quantitative conclusions.
Fig. 3.
Representative force curves due to brush on (a) normal and (b) cancerous cells. Solid lines are the model, eq. (1) for normal, and (2) for cancerous cells. Double-brush behavior of cancerous cells is seen. An additional dash-line shown for larger h in (b) demonstrates the presence of a weak third brush on cancerous cells.
The results of processing the force data for both normal and cancerous cells through eqs. (1) and (2) are compiled in SI Tables 1 and 2, and presented as histograms in Fig. 4 (top). One can see a clear difference between normal and cancerous cell brushes. While normal cells have a single-length brush, cancerous cells have double-length brush; the long length is about the size of normal cell brush (there is no statistically significant difference) but the shorter length is approximately 5 times smaller (the difference is statistically significant). The grafting density of the long brush is almost one half that of normal cells, but the short brush is 2 times denser than the brush of normal cells. All graphing densities are statistically different. For illustration, Fig. 4 (bottom) shows a graphical sketch of the brushes having the derived parameters.
Fig. 4.
Brush parameters for cells derived from the force curves of Fig. 2. (Top): Distribution histograms of brush lengths and grafting density are shown. Normal cells have single-length brush of ~2.4μm with the grafting density of ~300 “molecules”/μm2, while cancerous cells have a brush with two characteristic lengths of 0.45μm and 2.6μm with grafting densities of ~640 and 180 “molecules”/μm2, respectively. (Bottom): A cartoon of cells with brushes drawn to the scale by using the derived brush parameters shown in the top histograms for normal (left) and cancerous (right) cells. A rare long third brush is also shown for cancerous cells.
To understand the nature of the detected brushes, optical (fluorescent confocal) and electron (SEM and TEM) microscopy were used, Fig. 5. The sizes of the observed surface corrugations, microvilli and microridges are comparable with the large brush lengths derived from the AFM data. SEM images, Fig. 5a, show higher variability of brush sizes and a longer brush of cancerous cells as compared to normal cells. The longer brush of normal cells appears quite dense on the SEM images, and can be seen as a brush of appropriate size only in confocal and TEM-type images, Fig. 5b,c. The smaller dense brush derived from the AFM data is seen on the TEM-type images, Fig. 5c. It consists of shorter microvilli and microridges (see also SI Figs.5,6).
Fig. 5.
Visualization of brush. (a) Side view scanning electron microscopy images of cancerous and normal cells. Colours are artificial and used to highlight the cells. Scale bars are 5 μm. (b) Confocal images of the cells showing the brush associated with membrane corrugations. To distinguish the cell surface brush and filopodia developed on the cell culture dish surface, we also show 3D cross-sections of cells The arrows indicate vertical direction pointing out of the Petri dish. The scale bars are 5 μm. (c) Transmission electron microscopy type images of thin cross-sections of the cell edges. Scale bars are 2 μm.
Comparing Fig. 5 with the graphical sketch of Fig. 4 (bottom), one should remember that Fig. 4 shows average brush parameters. Therefore, the graphics are smoother than the actual cell. Secondly, the graphing density of the “molecules”, being an effective parameter, is not necessarily directly related to the number of membrane protrusions per unit area. To find such relation, a new model will be developed. This also illustrates the difference in the type of data collected by the AFM versus other microscopy.
Our study shows a quantitative difference between cancerous and normal human cervical epithelial cells found in vitro with an AFM method. The standard AFM procedure of processing the collected force data (treating cells as homogeneous medium) did not show a noticeable difference between these two kinds of cells. However, when processing with a more realistic brush-on-soft-surface model, one can see an unambiguous difference. Although some difference between cancerous and normal cells can be seen using other microscopy techniques (see supporting information for representative sets of SEM and TEM-type images), it is rather difficult to describe this difference unambiguously and quantitatively. In order to get the amount of data about the brush similar to the obtained here with the AFM, one would need to do TEM tomography of each cell and calculate the brush length. Even that would not be directly comparable to the AFM data because AFM measures mechanical response, not just geometry. Adding the fact that AFM works with viable cells, which decreases the amount of work needed for sample preparation and eliminates artifacts, one can conclude that AFM is a unique technique to study the cell surface. Thus, the observed difference herein may suggest a new dimension in consideration of cancerous cells, and their characterization by means of forces and mechanical parameters. This may lead to a new way of looking at cancer, and its possible detection.
Materials and Methods
Cell cultures
We used primary cultures of human cervical epithelial cells prepared from tissues collected from transformation zone of cervix from three cancer patients and three healthy individuals. The cell isolation was performed by a two-stage enzymatic digestion using dispase to remove the epithelium and then trypsin to disperse the individual epithelial cell27. Normal and cancerous cell cultures were maintained in keratinocyte serum free medium (KSFM, Invitrogen, Carlsbad, CA) under the same experimental conditions. KSF-M is a well defined and widely used medium for growth of epithelial cells. The epithelial cells adhered tightly to the bottom of the plastic dish, and the 60mm cell culture dishes were mounted on the chuck of the AFM with a double sticky tape. Cells were grown to approximately 10% of confluency before using them for scanning (to avoid induction of squamous differentiation of normal cells). All scanning and measurements related to rigidity were performed on proliferating viable cells maintained to room temperature in Hank’s balanced salt solution (HBSS) within 2–3 hours after removal of growth medium.
Atomic force microscopy
A Nanoscope Dimension 3100 (Digital Instruments/Veeco, Inc., Santa Barbara, CA) atomic force microscope (AFM) was used in the present study. A standard cantilever holder for operation in liquids was employed. To collect sufficient statistics, the force-volume mode of operation was utilized. For details, see Methods in SI Text.
Dye staining of glycoconjugates of the pericellular brushes
We used the cationic dye Alcian blue (Sigma-Aldrich, St. Louis, MO, USA) to study glycoconjugates of the pericellular brushes of normal and cancerous cells. Using the critical electrolyte concentration method28, one can also distinguish weakly and strongly charged proteoglycan groups in the cell glycocalix29. For details, see Methods in SI Text.
Confocal Microscopy
A confocal microscope (Nikon C1, 10mW 488nm and 514 nm argon laser, 100x 1.4 NA CFI VC oil immersed objective) was used. The cells were attached to a LabTek slide (cell suspension in growth medium was placed to the slide for a couple of days). To visualize the cell and cytoplasmic membrane corrugations, 20 μL of 5% ethanol aqueous solution of Nile Red fluorescent dye was added to 1mL of buffer. Within 15 minutes after placing the cells in this buffer, viable cells were imaged directly in that buffer.
Electron Microscopy
Cells were fixed with Karnovsky'sfixative at 4°C for 2 days, post-stained with 1%osmium tetroxide, further stained with 0.67% aqueous ruthenium tetroxide. SEM imaging was done on freeze-dried samples. For TEM-type of imaging, cells were further dehydrated with absolute ethanol and embedded in Spurr epoxy (firm formulation). Thinsections (20–50nm) were viewed using electron back-scattering SEM mode. FEI Phenom SEM was used in this study.
Data analysis
The data have been fitted to the analytical expression using the Levenberg-Marquardt and Simplex algorithms. The error of the fit is one standard deviation. When calculating “error-bars” for average values, individual standard deviations are discarded as small, and RMS value of mean values is calculated. So, the “error-bars” for average values should be called “variability bar”. Statistically significant difference was defined using t-test statistics at p<0.05 level.
Supplementary Material
Acknowledgments
We gratefully acknowledge funding for this work from grants: NSF CBET 0755704 (IS), NCI 1R15CA126855-01 (CDW), and operational funds of Nanoengineering and Biotechnology Laboratories Center (NABLAB). Tissue was obtained from the Cooperative Human Tissue Network. We are thankful to Profs. Minko and Katz for useful discussions.
Footnotes
Author Contributions I.S. conceived and designed the experiments; S.I., R.M.G., V.S.R., and I.S. performed AFM and confocal measurements; C.D.W. and I.S. performed EM measurements; R.M.G. performed critical electrolyte study; R.M.G., V.S.R., and I.S. analyzed data; S.I., C.D.W., and I.S. co-wrote the paper.
Author Information Reprints and permissions information is available at www.nature.com/reprints. Correspondence and requests for materials should be addressed to I.S. (isokolov@clarkson.edu).
Supplementary information accompanies this paper at www.nature.com/naturenanotechnology. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Habelitz S, et al. Peritubular dentin lacks piezoelectricity. Journal of dental research. 2007;86:908–911. doi: 10.1177/154405910708600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Hoh JH. Modulation of repulsive forces between neurofilaments by sidearm phosphorylation. Biochemical and Biophysical Research Communications. 2004;324:489–496. doi: 10.1016/j.bbrc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 3.Sokolov I, Subba-Rao V, Luck LA. Change in Rigidity in the Activated Form of the Glucose/ Galactose Receptor from E-coli: A Phenomenon That Will Be Key to the Development of Biosensors. Biophysical Journal. 2006;90:1055–1063. doi: 10.1529/biophysj.105.060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokolov I, Iyer S, Woodworth CD. Recover of Elasticity of Aged Human Epithelial Cells In-Vitro. Nanomedicine: Nanotechnology, Biology and Medicine (Nanomedicine) 2006;2:31–36. doi: 10.1016/j.nano.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Berdyyeva TK, Woodworth CD, Sokolov I. Human epithelial cells increase their rigidity with ageing in vitro: direct measurements. Physics in Medicine and Biology. 2005;50:81–92. doi: 10.1088/0031-9155/50/1/007. [DOI] [PubMed] [Google Scholar]
- 6.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nature Nanotechnology. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 7.Goldmann WH, et al. Differences in elasticity of vinculin-deficient F9 cells measured by magnetometry and atomic force microscopy. Exp Cell Res. 1998;239:235–242. doi: 10.1006/excr.1997.3915. [DOI] [PubMed] [Google Scholar]
- 8.Lekka M, et al. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur Biophys J Biophy. 1999;28:312–316. doi: 10.1007/s002490050213. [DOI] [PubMed] [Google Scholar]
- 9.Sokolov I. In: Cancer Nanotechnology–Nanomaterials for Cancer Diagnosis and Therapy. Nalwa HS, Webster T, editors. APS; 2007. pp. 43–59. [Google Scholar]
- 10.Sokolov I, Iyer S, Subba-Rao V, Gaikwad RM, Woodworth CD. Detection of surface brush on biological cells in vitro with atomic force microscopy. Applied Physics Letters. 2007;91 023902-023901-023903. [Google Scholar]
- 11.Han JD, Rubin CS. Regulation of cytoskeleton organization and paxillin dephosphorylation by cAMP. Studies on murine Y1 adrenal cells. J Biol Chem. 1996;271:29211–29215. doi: 10.1074/jbc.271.46.29211. [DOI] [PubMed] [Google Scholar]
- 12.Yang IH, Co CC, Ho CC. Alteration of human neuroblastoma cell morphology and neurite extension with micropatterns. Biomaterials. 2005;26:6599–6609. doi: 10.1016/j.biomaterials.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Berdyyeva T, Woodworth CD, Sokolov I. Visualization of cytoskeletal elements by the atomic force microscope. Ultramicroscopy. 2005;102:189–198. doi: 10.1016/j.ultramic.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoelson B, Dimitriadis EK, Cai H, Kachar B, Chadwick RS. Evidence and implications of inhomogeneity in tectorial membrane elasticity. Biophysical journal. 2004;87:2768–2777. doi: 10.1529/biophysj.104.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitriadis EK, Horkay F, Bechara K, Chadwick RS. Issues concerning the measurement of elastic properties at microscopic scales with the AFM. Biophysical journal. 2002;82:56A–56A. [Google Scholar]
- 17.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown HG, Hoh JH. Entropic exclusion by neurofilament sidearms: A mechanism for maintaining interfilament spacing. Biochemistry. 1997;36:15035–15040. doi: 10.1021/bi9721748. [DOI] [PubMed] [Google Scholar]
- 19.Cohen M, Klein E, Geiger B, Addadi L. Organization and adhesive properties of the hyaluronan pericellular coat of chondrocytes and epithelial cells. Biophysical journal. 2003;85 :1996–2005. doi: 10.1016/S0006-3495(03)74627-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones LM, Gardner MJ, Catterall JB, Turner GA. Hyaluronic acid secreted by mesothelial cells: a natural barrier to ovarian cancer cell adhesion. Clin Exp Metastasis. 1995;13 :373–380. doi: 10.1007/BF00121913. [DOI] [PubMed] [Google Scholar]
- 21.Toole B. In: Cell Biology of the Extracellular Matrix. Hay E, editor. Plenum Press; 1982. pp. 259–294. [Google Scholar]
- 22.Zimmerman E, Geiger B, Addadi L. Initial stages of cell-matrix adhesion can be mediated and modulated by cell-surface hyaluronan. Biophysical journal. 2002;82:1848–1857. doi: 10.1016/S0006-3495(02)75535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Underhill CB, Chen L. Hyaluronan on the surface of tumor cells is correlated with metastatic behavior. Cancer research. 1995;55:428–433. [PubMed] [Google Scholar]
- 24.Israelachivili J. Intermolecular and Surface Forces. 2. Academic. Press; 1992. [Google Scholar]
- 25.Butt HJ, et al. Steric forces measured with the atomic force microscope at various temperatures. Langmuir. 1999;15:2559–2565. [Google Scholar]
- 26.Emerson RJt, Camesano TA. Nanoscale investigation of pathogenic microbial adhesion to a biomaterial. Appl Environ Microbiol. 2004;70:6012–6022. doi: 10.1128/AEM.70.10.6012-6022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodworth CD, Doniger J, Dipaolo JA. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989;63:159–164. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuprun V, Santi P. Crystalline arrays of proteoglycan and collagen in the tectorial membrane. Matrix Biol. 1996;15:31–38. doi: 10.1016/s0945-053x(96)90124-9. [DOI] [PubMed] [Google Scholar]
- 29.Lim DJ, Rueda J. Distribution of glycoconjugates during cochlea development. A histochemical study. Acta Otolaryngol. 1990;110:224–233. doi: 10.3109/00016489009122541. [DOI] [PubMed] [Google Scholar]
- 30.Matzke R, Jacobson K, Radmacher M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat Cell Biol. 2001;3:607–610. doi: 10.1038/35078583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






