Cyclic nucleotide second messengers represent a cornerstone signal transduction mechanism in all domains of life. Cyclic adenosine 3’,5’-monophosphate (cAMP) and cyclic guanosine 3’,5’-monophosphate (cGMP) are the most important nucleotide messengers in eukaryotes. For several decades, bacteria have been known to use cAMP to control a variety of processes, from utilization of alternative sugars to motility and virulence. However, until now, the involvement of cGMP in bacterial signaling has been at best controversial (reviewed in Linder, 2010). This controversy is now over. Carl Bauer from Indiana University and his colleagues in this issue of Molecular Microbiology provide unambiguous evidence that the Alphaproteobacterium Rhodospirillum centenum synthesizes cGMP and uses it for regulation of a developmental process via the cGMP-dependent transcription factor. Among nucleotide messengers, cGMP represented the last bastion of eukaryotic exclusivity; and that bastion has now fallen.
Over the last few years, the universe of bacterial cyclic nucleotide messengers has been rapidly expanding (Fig. 1). A few years ago, a new cyclic nucleotide (more precisely, cyclic dinucleotide), cyclic dimeric GMP (c-di-GMP) discovered in Moshe Benziman’s lab at Hebrew University in the mid-80s (Ross et al., 1987), rose from obscurity to the limelight of a ubiquitous second messenger (Ryjenkov et al., 2005). c-di-GMP is now known to control a transition in the Proteobacteria from a single-cell motile state to a surface-attached multicellular state. In the multicellular state, c-di-GMP plays a central role in formation and dissolution of biofilms by regulating — via diverse mechanisms — production of extracellular polysaccharides, adhesive proteins, pili and flagella (Wolfe and Visick, 2009).
Fig. 1.
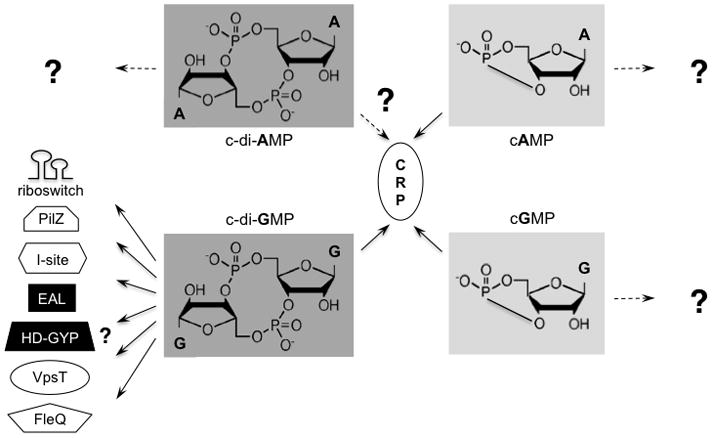
A scheme depicting major known cyclic mono- and dinucleotide signaling mechanisms in Bacteria. The protein domains (or proteins) that bind cNMP or c-di-NMP are shown. Black background of protein domains indicates the lack of enzymatic activities usually associated with these domains (for details, see Gomelsky, 2009). In the chemical structures, A stands for adenine, and G stands for guanine.
In 2008, we have learned from Karl-Peter Hopfner’s group at Ludwig Maximilians University in Munich about yet another cyclic dinucleotide, c-di-AMP. At present little is known about c-di-AMP (Fig. 1), except that it is involved in DNA-damage dependent cell cycle control in Bacillus subtilis (Witte et al., 2008) and that it is essential for viability of the food-borne pathogen Listeria monocytogenes (Woodward et al., 2010). However, based on the broad distribution of diadenylyl cyclases in Bacteria and Archaea, it is clear that c-di-AMP is poised to join its fellow cyclic nucleotide messengers as a ubiquitous and important signaling molecule (Römling, 2008).
While in eukaryotes the role of cGMP in signal transduction can hardly be overestimated, the involvement of this molecule in bacterial regulation has been murky. Early on, the bacterial cGMP-signaling field has had several false starts. For instance, intracellular cGMP levels in Escherichia coli and Bacillus lichenijormis were estimated to be approximately two orders of magnitude lower than levels of known second messengers like cAMP and c-di-GMP, and some reports linking cGMP to physiological functions (Black et al., 1980) proved to be irreproducible. To my knowledge, there is no credible evidence that E. coli or Bacilli involve cGMP in signaling. However, evidence has been accumulating that some “bugs” might do so. For example, biologically relevant levels of cGMP have been measured in the cyanobacterium Synechocystis sp. PCC6803, and these levels change in response to environmental conditions (Cadoret et al., 2005). Furthermore, the catalytic domain of the Synechocystis nucleotidyl cyclase, Cya2, has higher specific activity for synthesizing cGMP than cAMP (Rauch et al., 2008). Interestingly, Cya2 as well as all eukaryotic guanylyl cyclases are closely related to type III adenylyl cyclases, and, in fact, mutations in just a few residues can change substrate specificities in these cyclases (Sunahara et al., 1998; Ryu et al, 2011). Given this, why has evolution not changed these residues in bacterial adenylyl cyclases, and why have bacteria not exploited cGMP to diversify their signaling repertoire? The answer from the Marden and colleagues work is that we were simply not looking thoroughly enough. For the first time, these researchers present compelling evidence (i) that R. centenum makes cGMP via a bona fide guanylyl cyclase, (ii) that cGMP is essential for encystment (cyst formation) in this bacterium, and (iii) that cGMP works via a specific transcription factor.
The path that led the Bauer’s group to identifying a cGMP signaling system began with a search for encystment regulators in R. centenum. This bacterium and some other Proteobacteria form cysts (dormant cells) to survive such unfavorable conditions as desiccation. The researchers used a mini-Tn5 mutagenesis on the hypercyst mutant (Berleman et al., 2004) to identify suppressors impaired in encystment. They found two transposon insertions in a gene encoding a class III nucleotidyl cyclase. Peculiarly, four genes downstream of the cyclase gene was a gene encoding a CRP homolog. CRP is a cAMP-dependent transcription factor best known as an activator of the lac operon as well as other operons involved in sugar catabolism (Zubay et a., 1970). It has not escaped the authors’ attention that such gene proximity was suggestive of possible interactions between the two gene products. Indeed, deletion of either gene impaired encystment, which supported the authors’ expectation.
To address the question of what product was formed by cyclase, the researchers measured intracellular and extracellular levels of cAMP and cGMP during the encystment process. Interestingly, they found that wild type cells accumulated and excreted nanomolar amounts of cGMP but not cAMP, while the cyclase mutant no longer did so. Furthermore, cGMP (but not cAMP) added exogenously at micromolar levels restored encystment in the cyclase mutant, but not in the CRP-like mutant. These data clearly indicated that cGMP was the signaling molecule involved in regulating encystment, and that the cyclase and the CRP-like protein were the major players positioned, respectively, upstream and downstream of cGMP. The authors of the study then overexpressed the cyclase and the CRP-like genes in E. coli, purified the proteins and assayed their activities in vitro. The in vitro tests confirmed that the enzyme functions as a guanylyl cyclase. It was noted that small amounts of cAMP were produced under certain conditions, however, clearly this was a minor side activity. The differential scanning fluorimetry and isothermal titration calorimetry experiments performed on the CRP-like protein revealed that this protein specifically binds cGMP, but not cAMP, with the affinity that was similar to the affinity of the E. coli CRP for cAMP. Therefore, in R. centenum, the cGMP signaling story is very solid.
To convince the most critical skeptics (or, possibly, themselves) that cGMP was a bona fide second messenger that is not limited to one “bug”, the researchers went a step further. They investigated whether fellow cyst-forming Alphaproteobacteria, Azospirillum brasilense and Sinorhizobium meliloti, also excrete cGMP during encystment. Sure enough, they did. Marden et al. noted that genomes of several other cyst-forming species have guanylyl cyclase gene clusters homologous to the one from R. centenum. Therefore, cGMP appears to be the regulator of encystment in a number of species.
Can one predict cGMP-dependent processes in bacteria that don’t have that particular gene cluster? A careful bioinformatic analysis of the sequences and structural models of type III nucleotidyl cyclases might help. However, this analysis has to be careful indeed because the residues believed to be important for substrate binding in class III nucleotidyl cyclases (Sunahara et al., 1998; Ryu et al., 2011) are not absolutely conserved in the R. centenum cyclase. Hence, we do not completely understand how to distinguish a bacterial guanylyl cyclase from an adenylyl cyclase. A simultaneous analysis of nucleotidyl cyclases and their potential CRP partners, of which at least one is now known to bind cGMP, might be more reliable than the analysis of cyclases in isolation. However, CRP proteins are not that simple either. The CRP/FNR protein family to which they belong continues to expand the realm of functions. We have known that proteins belonging to this family can bind cAMP, heme (to sense CO), and Fe-S clusters (to sense O2, oxidative and nitroxidative stresses). Recently, we learned that some CRP members also bind and respond to c-di-GMP (Fig. 1; Leduc and Roberts, 2009; see also Gomelsky, 2009). Therefore, we should not underappreciate the sequence and substrate flexibility among these veteran signal transduction factors. To better understand cGMP binding, a structure of the cGMP-bound CRP homolog would definitely be helpful.
What could we project from the findings reported in this exciting study? There is little doubt that the number and diversity of biological processes regulated by cGMP will increase as this molecule continues to be discovered in new bacterial species. Nobody really knows how big the bacterial cGMP signaling universe will end up being. If cGMP can be synthesized only by a subset of type III nucleotidyl cyclases (which is the case in eukaryotes and apparently in bacteria), this universe will probably be smaller than the c-di-GMP or cAMP universes. However, the history of previously underappreciated cyclic nucleotide messengers teaches us to be prepared for surprises on the upside. The story of cGMP in eukaryotes can serve as another guide. There, cGMP controls diverse processes (e.g. vision, muscle contractile functions, platelet aggregation, gamet development, and water and electrolyte homeostasis) via several molecular mechanisms (e.g. protein kinases, cGMP-gated ion channels, and cNMP phosphodiesterases) (Schmidt et al., 2009). Would one expect bacterial cGMP signaling to be less diverse? Doubtfully. After all, cGMP is just a second messenger, and as such, it can be adapted to deliver its message to diverse outputs and via different mechanisms.
First cAMP, then c-di-GMP, later c-di-AMP, and now cGMP; the family of bacterial cyclic mono- and dinucleotide second messengers has grown. Bacteria can find the use of them all!
Acknowledgments
Work on cyclic nucleotide second messengers in my laboratory is supported by National Science Foundation (MCB 0645876), National Institutes of Health (NCRR P20RR016474), United States Department of Agriculture (AFRI 2010-65201-20599) and Cooperative State Research Education Extension Service Grant via Agricultural Experiment Station. I am thankful to Dan Wall for critical reading of the manuscript.
References
- Berleman JE, Hasselbring BM, Bauer CE. Hypercyst mutants in Rhodospirillum centenum identify regulatory loci involved in cyst cell differentiation. J Bacteriol. 2004;186:5834–5841. doi: 10.1128/JB.186.17.5834-5841.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr RW, Haddox MK, Goldberg ND. Cyclic Guanosine 3’:5’ monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1976;249:4326–433. [PubMed] [Google Scholar]
- Black RA, Hobson AC, Adler J. Involvement of cyclic GMP in intracellular signaling in the chemotactic response of Escherichia coli. Proc Natl Acad Sci U S A. 1980;77:3879–83. doi: 10.1073/pnas.77.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret JC, Rousseau B, Perewoska I, Sicora C, Cheregi O, et al. Cyclic nucleotides, the photosynthetic apparatus and response to a UV–B stress in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 2005;280:33935–33944. doi: 10.1074/jbc.M503153200. [DOI] [PubMed] [Google Scholar]
- Gomelsky M. C-di-GMP-binding CRP-like protein: a spectacular new role for a veteran signal transduction actor. J Bacteriol. 2009;191:6785–6787. doi: 10.1128/JB.01173-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc JL, Roberts G. Cyclic di-GMP allosterically inhibits the CRP-like protein (Clp) of Xanthomonas axonopodis pv. citri. J Bacteriol. 2009;191:7121–7122. doi: 10.1128/JB.00845-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JU. cGMP production in bacteria. Mol Cell Biochem. 2010;334:215–219. doi: 10.1007/s11010-009-0321-0. [DOI] [PubMed] [Google Scholar]
- Rauch A, Leipelt M, Russwurm M, Steegborn C. Crystal structure of the guanylyl cyclase Cya2. Proc Natl Acad Sci USA. 2008;105:15720–15725. doi: 10.1073/pnas.0808473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1:pe39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- Ross P, Weinhouse H, Aloni Y, Michaeli D, Weinberger-Ohana P, Mayer R, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanilic acid. Nature. 1987;325:279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: Insights into the biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem. 2010 Oct 28; doi: 10.1074/jbc.M110.177600. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Hofmann F, Stasch JP, editors. Handb Exp Pharmacol. Vol. 191. Springer-Verlag; Berlin Heidelberg: 2009. cGMP: generators, effectors and therapeutic implications; p. 583. [PubMed] [Google Scholar]
- Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem. 1998;273:16332–16338. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- Witte G, Hartung S, Büttner K, Hopfner KP. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 2008;30:167–178. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Wolfe A, Visick K, editors. The second messenger cyclic di-GMP. ASM Press; Washington, DC: 2010. p. 356. [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G, Schwartz D, Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci USA. 1970;66:104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


