Abstract
Objective
Rheumatoid arthritis (RA) is a debilitating autoimmune disease. Smoking is an important environmental factor in a subset of RA patients. Furthermore, the role of the cholinergic anti-inflammatory pathway in autoimmune inflammation is increasingly being realized. Nicotine is a major component of cigarette smoke and it also stimulates the α7-nicotinic acetylcholine receptors. Therefore, defining the mechanisms underlying the immunomodulatory effects of nicotine on arthritis is of high relevance. We have addressed this using the rat adjuvant-induced arthritis model of human RA.
Methods
Lewis rats were immunized s.c. with heat-killed M. tuberculosis H37Ra (Mtb) for disease induction. Rats were treated with nicotine i.p. either before (pretreatment) or after (posttreatment) the onset of AA. Control rats received the vehicle (buffer) in place of nicotine. The severity of arthritis was assessed and graded. The draining lymph node cells (LNC) were tested for T cell proliferative and cytokine responses against the disease-related antigen, mycobacterial heat-shock protein 65 (Bhsp65). The sera were tested for anti-cyclic citrullinated peptide antibodies (a-CCP) and anti-Bhsp65 antibodies.
Results
Nicotine-pretreatment aggravated arthritis, whereas nicotine posttreatment suppressed the disease. This altered severity of AA directly correlated with the levels of the aCCP antibodies, of the Th1/Th17 cytokines, and of the corresponding dendritic cell-derived cytokines. The majority of these effects on cellular responses could be replicated in vitro.
Conclusion
Nicotine-induced modulation of AA involves specific alterations in the disease-related cellular and humoral immune responses in AA. These results are of significance in advancing our understanding of the pathogenesis of RA.
Introduction
Rheumatoid arthritis (RA) is characterized by synovial inflammation, joint destruction, and disability (1–3). It is a multifactorial disease involving an interplay between genetic and environmental factors (1, 4, 5). Among the environmental factors, cigarette smoking shows a strong association with RA in a subset of patients (1, 4, 6, 7). The pro-inflammatory cytokines such as tumor necrosis factor- (TNF-α), interleukin-1β (IL-1β), IL-17 and interferon- (IFN-γ) play a critical role in RA pathogenesis. Over the past decade, the role of the vagus nerve and the cholinergic anti-inflammatory pathway in modulating inflammation induced by microbial components (e.g., lipopolysaccharide (LPS)) and immune-mediated events has increasingly been realized (8–12). The experimental approaches employed in these studies included vagotomy and stimulation of the α7-nicotinic acetylcholine receptors (α7nAChRs) by nicotine.
Nicotine is a major component of cigarette smoke and therefore, study of the cholinergic anti-inflammatory pathway in RA is of relevance. The α7nAChR receptors are expressed on a variety of immune cells including monocytes, macrophages, T and B lymphocytes, dendritic cells (DCs) and fibroblasts, which are found in the inflamed synovial tissue of RA patients (9, 13–15). However, the effects of nicotine on immune cells are incompletely characterized with conflicting conclusions. One set of studies has provided evidence that nicotine promotes inflammation (16–20). For example, nicotine induced the expression of costimulatory molecules, adhesion molecules and major histocompatibility complex (MHC) class II molecules on DCs; enhanced the ability of DCs to activate T cells; increased the secretion of proinflammatory cytokine IL-12 by DCs (16); upregulated LPS-stimulated IL-6 production by gingival fibroblasts (19); and facilitated the release of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) from maturing DCs (20). On the contrary, another set of studies has shown that nicotine is a key mediator of the cholinergic anti-inflammatory pathway (10–12, 21), and that nicotine suppresses the activity of immune cells (22–24). In fact, animals treated with nicotine showed a significant reduction not only in the antibody response, but also in T-cell proliferation (25) and secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-8, and IL-12 (9, 26–28). Similarly, nicotine treatment prevented the relapse of ulcerative colitis in patients (29). Thus, both pro-inflammatory and anti-inflammatory activities have been attributed to nicotine. However, the precise immunological changes induced by nicotine have not been fully defined. A couple of reports on nicotine-induced suppression of experimental arthritis are focused mostly on TNF-α (30, 31). Considering the emerging significance of IL-17 in RA, it is imperative to examine the mechanistic association between nicotine-induced modulation of arthritis and IL-17.
We have addressed the above-mentioned issues using the rat adjuvant-induced arthritis (AA) model of human RA by employing a nicotine-pretreatment and a nicotine-posttreatment regimen. We demonstrate for the first time in an experimental model of RA that nicotine pretreatment can exacerbate arthritis (AA); a couple of studies have reported only suppressive effect of nicotine (30, 31), which was also observed by us but only when using the post-treatment regimen. Our study also is the first one in the AA model to show the presence of anti-cyclic citrullinated peptide (aCCP) antibodies during the course of the natural disease, as well as the modulation of their levels by nicotine treatment. Our results also unravel the precise immunological changes in IL-17 and other cytokines underlying the dual role of nicotine in AA. These results are of significance in advancing our understanding of the pathogenesis of RA.
Materials and Methods
Animals
Male Lewis (LEW/SsNHsd) (RT-11) rats, 5 to 6-wk-old, were obtained from Harlan Sprague Dawley (Indianapolis, IN) and then maintained in the animal care facility of the University of Maryland School of Medicine Baltimore, MD. All experimental procedures performed on these rats were in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Induction and evaluation of adjuvant arthritis (AA)
AA was induced in Lewis rats by injecting them s.c. at the base of the tail with 200 µl of heat-killed M. tuberculosis H37Ra (Mtb) (Difco, Detroit, Michigan) (2 mg/rat) in mineral oil. All animals were examined regularly for signs of arthritis, and the severity of the disease was graded on a scale of 0 to 4 for each paw based on the level of erythema, swelling, and induration (32). Total arthritic score per rat was derived from the sum of individual scores of 4 paws. Histopathological sections of hind paws were examined for hyperplasia of synovial membrane, infiltration by mononuclear cells, and cartilage and bone damage (33). The disease course of AA consists of the following phases: incubation (Inc), onset (Ons), peak (Pk), and recovery (Rec) phase.
Treatment of Lewis rats with nicotine
Nicotine [(−)-nicotine hydrogen tartrate salt, minimum 98% TLC] was obtained from Sigma-Aldrich (St. Louis, MO). Pretreatment regimen: a daily i.p. injection of nicotine (0.625, 1.25, or 2.5 mg/kg·day, 200 µl/rat) was started on d -7 prior to Mtb challenge, and then continued for another 7 d. Posttreatment regimen: injection (i.p.) of the indicated amounts of nicotine was initiated at the onset of AA. In both the regimen, control rats were injected i.p. with PBS on the days corresponding to those of nicotine injection in experimental rats. The dose and timing of nicotine administration used in this study did not produce any significant adverse effects in rats.
Determining the effect of nicotine on T cell proliferation
a) Proliferation of splenic T cells (non-adherent cells) of naïve rats
In nicotine-posttreatment in vitro group, the cells were cultured (5×105 cells/well) in HL-1 serum-free medium (Lonza, Walkersville, MD) with or without nicotine (10−7 to 10−4 mol/L), concanavalin A (Con A) (Sigma) (2.5 µg/ml), or Con A plus nicotine for 48 hr before adding 3[H]-thymidine (1 uCi/well, ICN Biomedicals, Irvine, CA) for another 18 hr. In nicotine-pretreatment in vitro group, the cells were treated with nicotine for 12 hr followed by Con A stimulation. The level of radioactivity was detected and results presented as a stimulation index (SI) (34).
b) Proliferation of lymph node cells (LNC) of nicotine-treated rats
The draining lymph nodes were harvested from nicotine-treated rats with AA on d 19 (Pk phase) after Mtb injection and cultured in HL-1 medium in a 96-well plate (5×105 cells/well) for 48 hr at 37°C with or without endotoxin-free mycobacterial hsp65 (Bhsp65) (5 µg/ml) (32). PPD-and Con A- were used as positive controls, whereas ovalbumin (Ova; Sigma) served as a negative control. The results were presented as SI.
Cytokine assays
a) Measurement of cytokines produced by naïve splenic DCs treated with nicotine and Mtb sonicate in vitro
Splenic adherent cells containing DC (34) were seeded in a 6-well tissue culture plate (5×106 cells/well). For nicotine posttreatment in vitro, aliquots of cells were cultured for 6 hr at 37°C with medium alone, with sonicated Mtb (10 µg/ml) alone in medium, or with Mtb sonicate plus nicotine (10−7 to 10−5 mol/L) in medium. [Mtb sonicate contained the whole lysate after ultrasonic treatment by Sonic Dismembrator Model 100 (Fisher Scientific, Pittsburgh, PA) with 3 pulse.] For nicotine pretreatment in vitro, the adherent cells were cultured in the presence of nicotine for 12 hr before stimulation with Mtb for 6 hr. Thereafter, in both regimen, the cells were suspended in Trizol and the total RNA was analyzed for cytokine expression by qPCR (35).
b) Assays for cytokine secretion by splenic DC and LNC of nicotine-treated rats
Spleen and LNC were harvested from rats on d 19 after Mtb injection. Splenic DCs were cultured for 6 hr with or without sonicated Mtb, whereas the LNC were cultured with Bhsp65 or medium alone for 24 hr prior to cytokine testing by qPCR.
c. Assays for cytokines produced by synovium-infiltrating cells (SIC)
SIC were harvested from the inflamed joints on d 19 after Mtb injection and then lysed. The supernate was tested for total protein content using DC protein assay kit II (Bio-Rad, Hercules, CA) and for IL-1β and TNF-α using ELISA (eBioscience, San Diego, CA).
Measurement of serum nitric oxide (NO) levels
Serum was collected from nicotine-pretreated, Mtb-immunized rats at the following time points: before nicotine treatment (d −7), just before Mtb injection (d 0), and d 7 (Inc), d 14 (Ons), d 21 (Pk) and d 28 (Rec) after Mtb injection. For nicotine-posttreated, Mtb-immunized rats, serum was collected on d 0, 7, 14, 21, and 28. These serum samples were diluted (1:3) and 85 µl of the diluted samples were assayed using a nitric oxide colorimetric assay kit (BioVision, Mountain View, CA). Absorbance was measured at 540 nm using a microplate ELISA reader (Molecular Devices, Sunnyvale, CA).
Determination of the levels of total antibodies and their isotypes in serum
Serum samples were collected as described above for the nitric oxide assay. These sera were tested for the levels of total antibodies against cyclic citrullinated peptide (CCP) and Bhsp65, as well as isotypes of antibodies against Bhsp65. The levels of aCCP in diluted (1:75) sera were measured using plates coated with CCP (CCP3 IgG ELISA kit, INOVA Diagnostics, San Diego, CA) and labeled anti-rat Ig secondary antibody. The results were expressed in relative units (RU) after correction for serum dilution and 26.67 RU (= 20 U) was set as the cut-off value. Assay for antibodies against Bhsp65 was performed as described elsewhere (32). Horseradish peroxidase (HRP)-conjugated mouse anti-rat total Ig, anti-IgG1, or anti-IgG2a (1:1500) (Zymed, San Francisco, CA) was used as a secondary antibody. The results were expressed as OD450 nm.
Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). Group differences were analyzed by a two-tailed Student’s t test for two groups, or by one-way ANOVA with Bonferroni adjustment for several groups. A p value of less than 0.05 was considered significant.
Results
Administration of nicotine before induction of AA exacerbates the disease, whereas nicotine injection after onset of AA attenuates the disease in Lewis rats
We first tested whether the administration of nicotine alone to naïve Lewis rats without Mtb challenge had any arthritic activity or adverse effects. No signs of systemic toxicity or clinical arthritis were observed following the i.p. injection of nicotine (0.625–2.5 mg/kg·day) to rats for 21 days, and these rats exhibited normal joint histology (data not shown).
In subsequent experiments, we injected nicotine to Mtb-immunized Lewis rats following the pretreatment and posttreatment regimen (Figure 1). We observed that treatment of Lewis rats with nicotine starting as soon as the signs of AA were evident alleviated the disease, whereas nicotine administration before the onset of AA exaggerated the severity of arthritis. The results representing the aggravating/ suppressive effect of nicotine at 1.25 mg/kg·day are shown (Figure 2A, 2B). The optimal test dose of 1.25 mg/kg·day was selected after a series of preliminary experiments using 3 doses of nicotine (0.625, 1.25, 2.5 mg/kg·day) (data not shown).
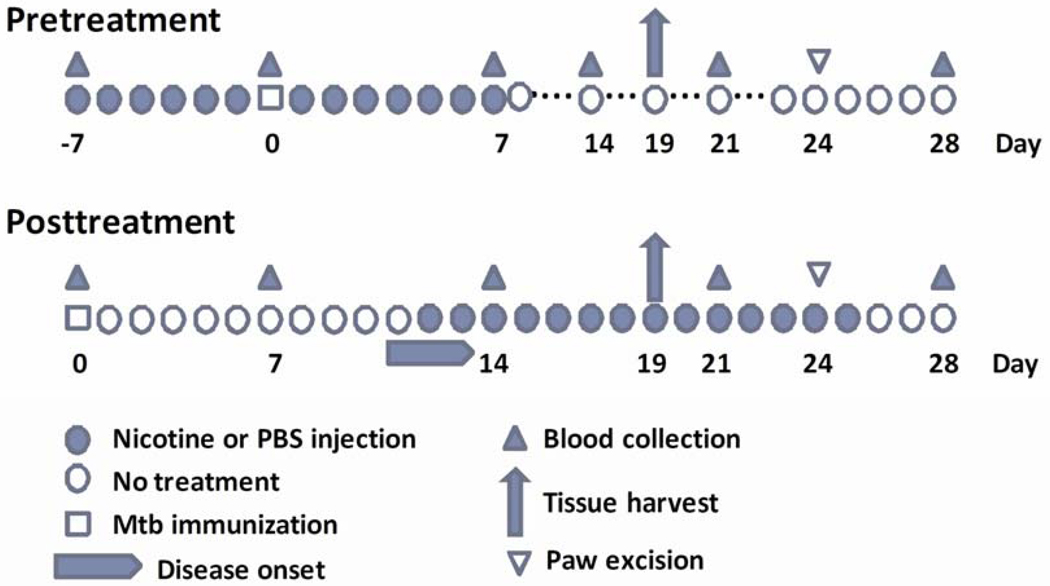
Figure 1. Pre- and post-treatment regimen of nicotine administration to rats in vivo and sample collection.
Lewis rats were treated with nicotine (1.25 mg/kg·day) i.p. either beginning before Mtb injection and then continued for another week after the injection (Pretreatment), or beginning after the onset of arthritis in Mtb-immunized rats (Posttreatment). Blood samples and tissues were harvested for testing at the indicated time points during the course of AA.
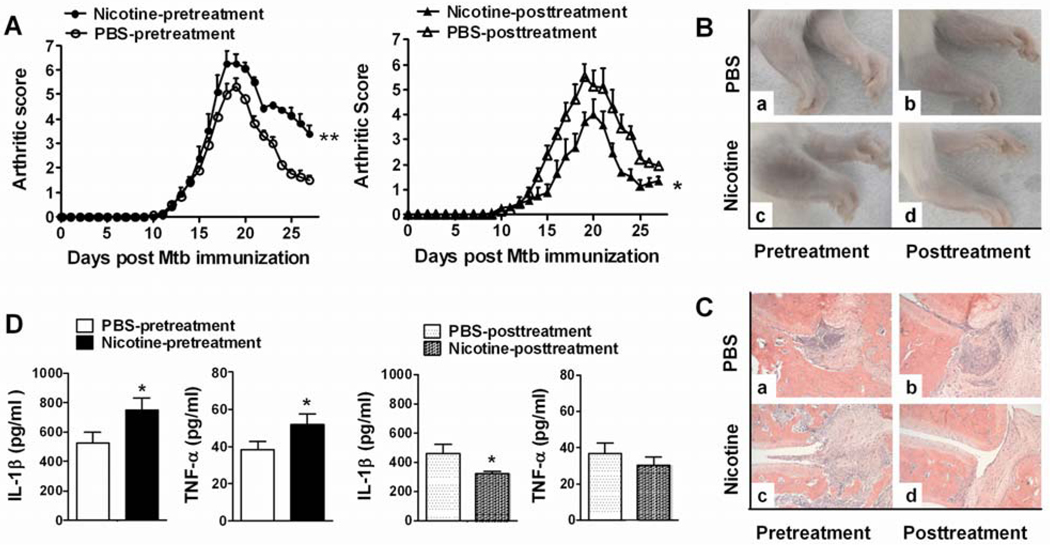
Figure 2. Nicotine-pretreatment exaggerates, whereas nicotine-posttreatment attenuates AA in Lewis rats.
(A) Arthritis scores of nicotine-pretreated (left) and nicotine-posttreated (right) rats during the course of AA. (B) Photographs and (C) histological sections of hind paws of nicotine-pretreated (c) and nicotine-posttreated (d) arthritic rats and the corresponding controls (a, b). (D) The level of IL-1β and TNF-α in synovium-infiltrating cells (SIC) from the joints of nicotine-pretreated (left) and nicotine-posttreated (right) rats with AA. Statistical data are mean ± SEM, n= 3–4, *p<0.05. The results of one of 2–4 reproducible experiments are shown.
The effect of nicotine on the severity of arthritis was further validated by histopathological examination of arthritic paws (Figure 2C). The control groups of rats revealed characteristic synovial cell lining hyperplasia, mononuclear cell infiltration, and focal destruction of cartilage and bone (Figure 2C-a, b). In comparison, the paw sections of nicotine-pretreated arthritic rats showed significantly increased severity of arthritis (Figure 2C-c). However, the paws of nicotine-posttreated arthritic rats exhibited markedly reduced signs of inflammation and joint damage (Figure 2C-d).
We also tested the levels of pro-inflammatory cytokines (TNF-α and IL-1β) in the inflamed synovial tissue and of another mediator of inflammation, NO in the serum of nicotine-treated arthritic rats. The levels of TNF-α and IL-1β were reduced in nicotine-posttreated rats, but enhanced in nicotine-pretreated rats (Figure 2D). A similar trend was observed for NO when tested at different time points post Mtb injection, but the difference was significant in nicotine-posttreatment group at d 21 and d 28 but not in nicotine-pretreatment group (data not shown).
Effects of nicotine on T cell proliferative and cytokine responses of Lewis rats with AA
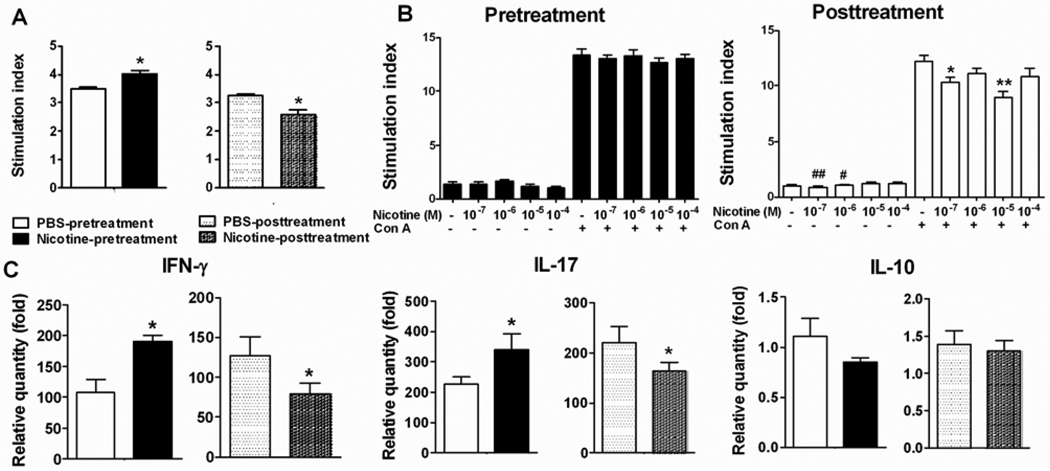
We determined the Bhsp65-specific T cell proliferative response of the draining LNC of Mtb-immunized Lewis rats treated with nicotine/ PBS using the pretreatment or posttreatment regimen. Increased T cell proliferation was observed in nicotine-pretreated rats, while reduced proliferation was evident in nicotine-posttreated rats (Figure 3A). To confirm the in vivo effects of nicotine under controlled in vitro conditions, we tested the effect of nicotine in vitro using splenic lymphocytes of naive rats stimulated with the mitogen, Con A. Nicotine pretreatment had no effect on T cell proliferation, whereas nicotine posttreatment suppressed T cell proliferation (Figure 3B).
Figure 3. Nicotine modulates T cell proliferation and cytokine response.
(A) The effect of nicotine treatment on LNC proliferative response against Bhsp65 in nicotine pre-/post-treated rats with AA. (B) The effect of nicotine on Con A-induced splenic T cell proliferative response of naïve rats. (C) The effect of nicotine treatment on cytokines produced by Bhsp65-stimulated LNC of rats with AA. The results of a representative experiment from 2–3 separate experiments with similar results are shown. Statistical data are mean ± SEM (n= 3). * p<0.05, ** p<0.01 (vs. cells cultured with Con A in Figure 3B); # <0.05, ## p<0.01 (vs. cells cultured in medium). Figure 3A and 3C have a common legend.
We further examined the effect of nicotine treatment of rats with AA on the expression of IFN-γ and IL-17 in their draining LNC. The expression of IFN-γ and IL-17 in Bhsp65-primed LNC of nicotine-pretreated arthritic rats was increased by 70% and 50%, respectively on d 19 compared to that of controls (Figure 3C). In contrast, a reduction in the expression of IFN-γ and IL-17 by 40% and 20%, respectively was observed in nicotine-posttreated rats. However, no significant change in the expression of anti-inflammatory cytokine IL-10 was observed in either group of rats.
Nicotine influences the production of cytokines by splenic DCs of arthritic Lewis rats
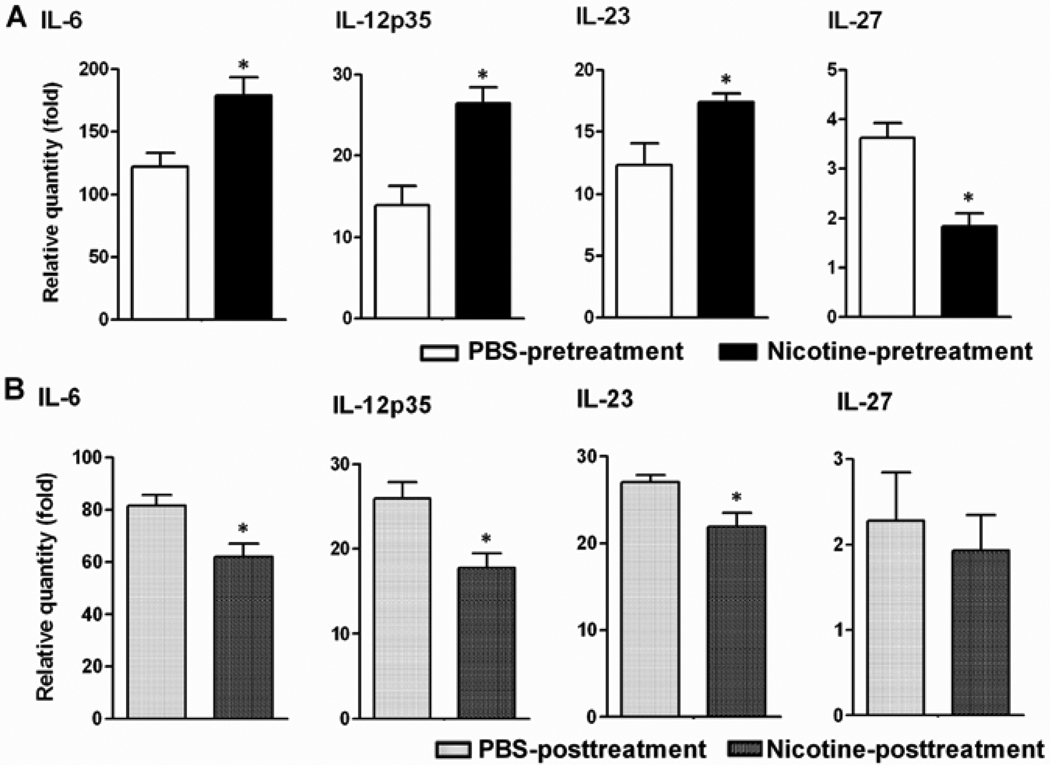
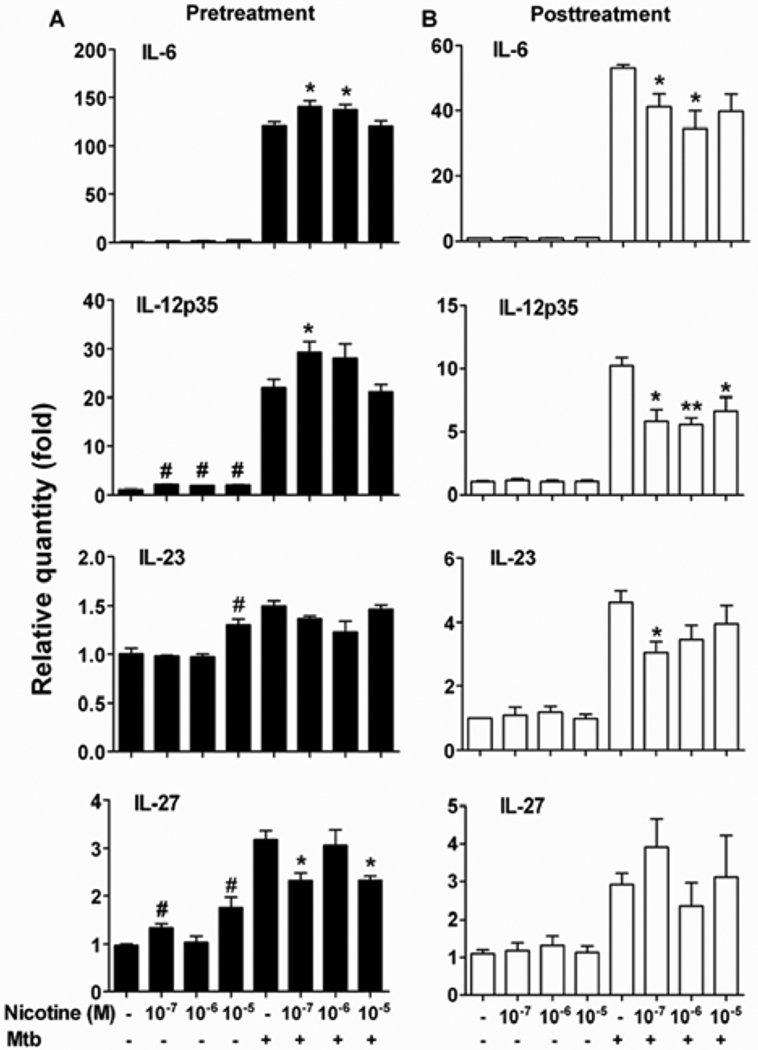
In view of the nicotine-induced changes on Th1/Th17 response (Figure 3C), we tested the effects of nicotine on DCs for the expression of specific cytokines that control the differentiation of these T cell subsets, namely IL-12p35 for Th1, and IL-6, IL-23 and TGF-β for Th17. Arthritic rats subjected to nicotine pre- or post-treatment were sacrificed on d 19, and their splenic DCs were cultured with Mtb sonicate (10 µg/ml) in vitro. DCs of nicotine-pretreated rats showed a significant increase in IL-6, IL-12p35 and IL-23 expression but a decrease in IL-27 (Figure 4A). On the contrary, DCs of nicotine-posttreated rats revealed reduced expression of IL-6, IL-12p35 and IL-23 (Figure 4B), without any change in IL-27.
Figure 4. Nicotine differentially regulated the proinflammatory and anti-inflammatory cytokine expression in splenic DCs from arthritic rats.
(A) Nicotine-induced changes in the expression of different cytokines by DCs of nicotine-pretreated arthritic rats. (B) Changes in cytokine production by DCs of nicotine-posttreated rats with AA. Representative data (n= 3) from one of 3–4 separate experiments with similar results are shown. *p<0.05.
We further confirmed the in vivo effects of nicotine under controlled in vitro conditions by testing the cytokine expression in DCs exposed to both nicotine and Mtb components (Mtb sonicate) in vitro. We treated DCs of naïve Lewis rats with nicotine in vitro (10−7 to 10−5 mol/L) for 12 hr before stimulating them with Mtb sonicate (10 µg/ml) to mimic the in vivo condition of nicotine-pretreated rats with AA. Similarly, we treated DCs with nicotine after stimulating them with Mtb sonicate in vitro to simulate the in vivo condition of nicotine-posttreated arthritic rats. DCs subjected to nicotine pretreatment showed increased expression of IL-6, and IL-12p35, but decreased expression of IL-27 (Figure 5A). In comparison, DCs exposed to nicotine-posttreatment revealed reduced expression of IL-6, IL-12p35, and IL-23 (Figure 5B). The levels of other cytokines tested (IL-12p40 and TGF-β) did not change significantly (data not shown).
Figure 5. Nicotine-induced changes in cytokine production by splenic DCs of naïve rats.
Dose-dependent change in the expression of different cytokines in DCs subjected to 12 hr-nicotine pretreatment in vitro (A) or nicotine-posttreatment in vitro (B). DCs were stimulated in vitro with Mtb sonicate where indicated. The results of a representative experiment (n= 3) out of two are shown. Statistical data are mean ± SEM, * p<0.05, ** p<0.01 (vs. cell cultured with Mtb stimulation), and # p<0.05 (vs. cell cultured in medium alone).
Taken together, the results of the in vivo and in vitro experiments revealed that the production of pro-inflammatory cytokines by DCs was enhanced by nicotine pretreatment but inhibited by nicotine posttreatment.
Arthritic rats develop anti-CCP- (aCCP-) and anti-Bhsp65 antibodies, and the levels of these antibodies are significantly modulated by nicotine treatment
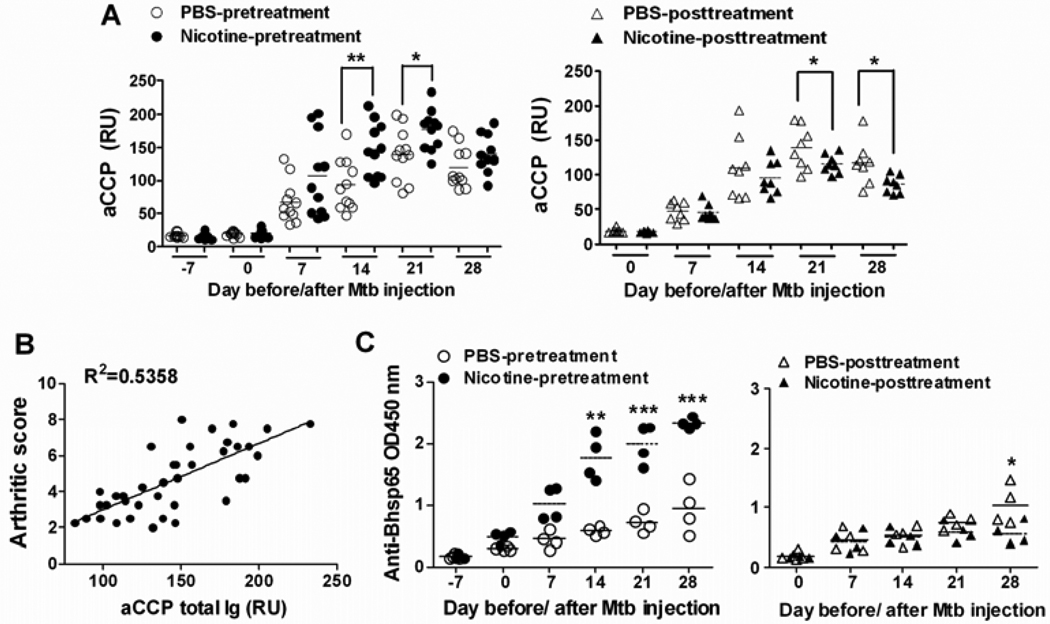
Taking into consideration the role of antibodies in the pathophysiology of arthritis, we next determined whether nicotine treatment influenced the antibody response in rats with AA. We monitored the levels of serum antibodies to CCP as well as Bhsp65, the disease-related antigen, at the indicated time points during the course of AA. All the arthritic rats had detectable aCCP total Ig beginning d 7 after Mtb injection. The levels of aCCP antibodies increased gradually and reached the highest level at peak phase of AA, followed by a decline at recovery phase of the disease (Figure 6A). Intriguingly, the direction of change (increase/decrease) in the aCCP antibody levels matched well with the severity of the disease (Figure 6B), with R2 values of 0.5358 (p < 0.0001).
Figure 6. Nicotine treatment modulates aCCP- and anti-Bhsp65 antibody responses in arthritic rats.
(A) Serum levels of aCCP total Ig (RU) in nicotine-pretreated and nicotine-posttreated arthritic rats. The collective results of 2–3 different experiments (n= 3–4 for each group) are shown. (B) Linear regression analysis of anti-CCP total Ig (RU) levels on d 21 versus the corresponding arthritic scores, using data from both the pretreatment and the posttreatment groups of rats; R2 values of 0.5358 (p < 0.0001). (C) The levels of total Ig against Bhsp65 (OD450 nm); representative data (n= 4) from one of 3 repeat experiments are shown. In sections A and C, the mean values of each group are indicated by horizontal lines. (*p<0.05, ** p<0.01, and *** <0.001)
Compared with controls, the level of total Ig against Bhsp65 was significantly higher in nicotine-pretreated rats beginning d 7 after Mtb injection (Figure 6C). In nicotine-posttreated rats with AA, significant difference was observed only on d 28, with decreased level of anti-Bhsp65. In nicotine-pretreated rats, levels of both Bhsp65-specific IgG1 and IgG2a were elevated, particularly the former, resulting in increased IgG2a/IgG1 ratio on d 7 and d 14 compared with controls (data not shown). However, nicotine-posttreated AA rats developed lower levels of IgG2a but similar level of IgG1 compared with controls, which resulted in a significantly lower IgG2a/IgG1 ratio on d 21 and d 28. The changes in IgG2a (data not shown) matched with the changes in IFN-γ response (Figure 3C) in nicotine-treated rats.
As the baseline control, we also determined the level of above-mentioned antibodies in rats (n= 3) treated for 21 d with PBS or nicotine alone. There was no detectable aCCP or anti-Bhsp65 antibody response (data not shown) in these rats. This also matched with the absence of any signs of arthritis in nicotine treated naïve rats (data not shown).
Discussion
The objective of this study was to determine how nicotine, one of the key substances in cigarette smoke, affected the course of arthritis in Lewis rats, and to delineate the immunological mechanisms underlying these effects. Our results showed that nicotine had a profound effect on the severity of AA. A difference in the timing of nicotine injection in reference to disease induction yielded opposite outcomes. Nicotine-induced modulation of AA involved specific effects on Th1 and Th17 responses, changes in aCCP and anti-Bhsp65 antibodies, and alteration in the levels of NO, a biochemical mediator of inflammation. Nicotine binds to α7nAChRs, which are expressed on multiple subsets of leukocytes and fibroblasts. These cells are known to play an important role in the initiation and development of RA (1–3). It is noteworthy that naive rats treated with nicotine alone for 21 d showed no signs of arthritis. Thus, the observed change in disease severity in nicotine-treated arthritic rats apparently was owing to the interplay between nicotine and the disease-related immune events.
To the best of our knowledge, this study offers the first report in the AA model on the nicotine-induced modulation of the disease, as well as on the aggravation of arthritis in any experimental model of RA. The enhanced severity of AA in our study was observed following a pretreatment regimen using nicotine i.p. This contrasts with the reports of suppression of arthritis in the CIA following administration of nicotine either via drinking water (30, 31) or via i.p. injection (31). However, the suppression of arthritis by nicotine posttreatment in AA in our study was also observed in the CIA model (31). In regard to the relationship between smoking and RA, most epidemiological studies suggest smoking as a strong environmental risk factor for RA (5). It is not clear why exposure to smoke or nicotine led to suppression of arthritis in most of the above-mentioned animal model-based studies, whereas the opposite is observed in a majority of RA patient-based studies. One explanation could be that nicotine only represents one component of cigarette smoke. Also, the route of intake of nicotine (as a chemical or in smoke) might influence the disease process differentially.
To define the immunologic basis of nicotine-induced modulation of AA, we tested the antigen (Bhsp65)-specific cell-mediated- as well as antibody-based immune responses in nicotine-treated rats. We observed enhanced T cell proliferation in nicotine-pretreated rats, but reduced response in nicotine-posttreated rats. Furthermore, nicotine-pretreated arthritic rats displayed upregulation of IFN-γ as well as IL-17, whereas nicotine-posttreated rats revealed downregulation of both these cytokines. Notably, the level of the anti-inflammatory cytokine IL-10 was unaffected. Furthermore, DCs of nicotine-treated arthritic rats showed altered levels of IL-6, IL-12p35, IL-23, which are critical for the differentiation of Th1 (IL-12p35) and Th17 (IL-6 and IL-23) cells. Moreover, IL-27 level was significantly lower in rats with more severe AA, but its level was unaffected in rats with reduced AA. The effect of nicotine on DCs has also been examined by other investigators, who reported a suppression (21), enhancement (16), or no effect (36) on pro-inflammatory cytokine production. Our results suggest that the differential effect of nicotine on cytokine production by DCs was the result of the timing of nicotine treatment (pretreatment vs. posttreatment) in relation to the onset of arthritis. The role of IL-27 in autoimmune diseases is not fully clear as both pathogenic (directly or indirectly) (37, 38) and protective (39, 40) roles have been assigned to this cytokine. Our results associate a lower level of IL-27 to the increased severity of AA by nicotine-pretreatment, assigning a regulatory role to this cytokine in AA. In regard to the target organ, the joints, nicotine treatment of rats also induced changes in the cytokines (TNF-α and IL-1β) produced by the synovium-infiltrating cells in the joints, and these alterations correlate well with the severity of arthritis in nicotine pre-treated as well as nicotine-posttreated arthritic rats. A similar correlation was found for NO.
Antibodies play an important role in the pathogenesis of arthritis and other rheumatic diseases (1, 2, 41). The presence of anti-citrullinated protein/peptide antibodies (ACPA) is associated with increased radiological progression and structural damage in RA (42), and they serve as a reliable diagnostic marker for RA (42, 43). We measured the temporal kinetics of anti-cyclic citrullinated peptide (aCCP) antibody during the course of AA. The levels of aCCP paralleled that of the disease severity in untreated arthritic rats. Furthermore, nicotine-posttreated rats with attenuated disease had reduced aCCP, whereas nicotine-pretreated rats with aggravated disease had increased level of aCCP. This is the first report demonstrating the presence of aCCP during the natural course of AA in Lewis rats, as well as the alteration of aCCP levels by nicotine treatment. However, our results of aCCP in AA differ from that of aCCP in the CIA model (30). Either a low frequency (13%) of aCCP (IgG) (30) or no aCCP at all (44) was reported in mice with CIA. In comparison, in our study, all rats (100%) with established AA had detectable aCCP (total Ig), and 79% of rats were positive for aCCP even before the appearance of signs of arthritis. This discrepancy might be attributable to the different species (mice/ rat) used in the two animal models, to the detection of total Ig versus IgG against CCP, and to the timing of blood collection (aCCP level dropped after acute phase of AA in our study). Furthermore, aCCP have been shown to be pathogenic in CIA (45, 46), but a similar characterization of aCCP in AA remains to be done. As the level of aCCP correlates with the severity of AA in our study, we suggest that aCCP might serve as a biomarker for AA.
Nicotine treatment of rats with AA not only affected aCCP but it also had a significant effect on antibodies specific to the disease-related antigen, Bhsp65 (32, 47). Rats with AA subjected to a therapeutic (posttreatment) regimen of nicotine showed reduced level of anti-Bhsp65 as well as IgG2a: IgG1 ratio largely because of decreased IgG2a. In contrast, rats that received a disease-aggravating (pretreatment) regimen of nicotine had higher level of both the anti-Bhsp65 antibodies as well as the IgG2a: IgG1. This altered ratio was primarily due to increase in IgG2a, and it correlated with the change in IFN-γ expression.
The timing of nicotine administration was a critical variable in the opposite outcomes regarding disease severity observed in this study. We suggest that the differential outcome of nicotine pre- versus post-treatment might be owing to differences/changes in one or more of the following: a) the number and/or sensitivity of the α7nAChRs and other nicotine-binding receptors expressed on different cell types (9, 13–15, 48) in the peripheral lymphoid tissues and the joints; b) the levels and/or activity of various molecular mediators of inflammation (e.g., NF-kB, COX-2, PGE2, LTB4, etc.) (20, 28); c) the levels of pro- versus anti-inflammatory cytokines, and anti-Bhsp65 and anti-CCP antibodies; d) the molecules directing cell migration into the joints (e.g., adhesion molecules, chemokines and their receptors); and e) the mediators of angiogenesis. Taken together, the cells/tissues exposed to various inflammatory stimuli (as after the onset of AA) might respond differently to nicotine than those not yet exposed to that microenvironment (as during the incubation phase of AA). Nicotine might be perceived as an agonist (or stimulatory) for certain reactions or events at one stage of AA but as an antagonist (or regulatory) at another stage of AA. This is reminiscent of certain cytokines (e.g., IFN-γ and TNF-α) that have been shown to exhibit both pro- and anti-inflammatory effects in AA and other models of autoimmunity (49).
We used male Lewis rats to be consistent with our previous studies in the AA model (32–35, 49). Studies in different animal (rodent) models of autoimmunity have revealed that disease susceptibility and immune responses of females may differ from that of the males. Also reported are strain- and gender-related differences in the stress response (50). The cholinergic system is a part of the organism’s stress response, and nicotine stimulates nicotinic acetylcholine receptors. Therefore, it is conceivable that the effects of nicotine on female Lewis rats might differ quantitatively and/or qualitatively from that of male rats. However, this aspect of nicotine action remains to be tested experimentally.
Acknowledgements
This work was supported by RO3AI076942 grant from the National Institutes of Health and the Other Tobacco Related Diseases Research Grant (OTRD) from the University of Maryland School of Medicine, Baltimore, MD. We wish to thank Shivaprasad H. Venkatesha, Steva A. Komeh-Nkrumah and Siddaraju M. Nanjundaiah for their helpful suggestions.
References
- 1.Lipsky PE. Rheumatoid arthritis. In: Kasper D, Braunwald E, Fauci A, Hauser S, Longo D, Jameson J, editors. Harrison's Principles of Internal Medicine. 16th edition ed. New York: McGraw-Hill; 2005. pp. 1968–1977. [Google Scholar]
- 2.Gorman CL, Cope AP. Immune-mediated pathways in chronic inflammatory arthritis. Best Pract Res Clin Rheumatol. 2008;22:221–238. doi: 10.1016/j.berh.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hahn B. A pathophysiologic approach to the clinical management of arthritis and pain: current and future implications. J Clin Rheumatol. 2004;10:S3–S4. doi: 10.1097/01.rhu.0000130683.61892.b2. [DOI] [PubMed] [Google Scholar]
- 4.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. Autoimmun Rev. 2010;9:A288–A292. doi: 10.1016/j.autrev.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 7.Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 10.Bonaz B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology. 2007;133:1370–1373. doi: 10.1053/j.gastro.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 11.Gullahorn L, Lippiello L, Karpman R. Smoking and osteoarthritis: differential effect of nicotine on human chondrocyte glycosaminoglycan and collagen synthesis. Osteoarthritis Cartilage. 2005;13:942–943. doi: 10.1016/j.joca.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Green JT, Thomas GA, Rhodes J. Nicotine: therapeutic potential for the treatment of ulcerative colitis. Expert Opin Investig Drugs. 1997;6:17–22. doi: 10.1517/13543784.6.1.17. [DOI] [PubMed] [Google Scholar]
- 13.Sato KZ, Fujii T, Watanabe Y, et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999;266:17–20. doi: 10.1016/s0304-3940(99)00259-1. [DOI] [PubMed] [Google Scholar]
- 14.Skok MV, Grailhe R, Agenes F, Changeux JP. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–2336. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Westman M, Engstrom M, Catrina AI, Lampa J. Cell specific synovial expression of nicotinic alpha 7 acetylcholine receptor in rheumatoid arthritis and psoriatic arthritis. Scand J Immunol. 2009;70:136–140. doi: 10.1111/j.1365-3083.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 16.Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation. 2003;107:604–611. doi: 10.1161/01.cir.0000047279.42427.6d. [DOI] [PubMed] [Google Scholar]
- 17.Furie MB, Raffanello JA, Gergel EI, Lisinski TJ, Horb LD. Extracts of smokeless tobacco induce pro-inflammatory changes in cultured human vascular endothelial cells. Immunopharmacology. 2000;47:13–23. doi: 10.1016/s0162-3109(99)00181-2. [DOI] [PubMed] [Google Scholar]
- 18.Totti N, 3rd, McCusker KT, Campbell EJ, Griffin GL, Senior RM. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science. 1984;223:169–171. doi: 10.1126/science.6318317. [DOI] [PubMed] [Google Scholar]
- 19.Wendell KJ, Stein SH. Regulation of cytokine production in human gingival fibroblasts following treatment with nicotine and lipopolysaccharide. J Periodontol. 2001;72:1038–1044. doi: 10.1902/jop.2001.72.8.1038. [DOI] [PubMed] [Google Scholar]
- 20.Vassallo R, Kroening PR, Parambil J, Kita H. Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol Immunol. 2008;45:3321–3329. doi: 10.1016/j.molimm.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinet E, Yoshida K, Nouri-Shirazi M. Nicotinic environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs) Immunol Lett. 2004;95:45–55. doi: 10.1016/j.imlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Nouri-Shirazi M, Tinajero R, Guinet E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs) Immunol Lett. 2007;109:155–164. doi: 10.1016/j.imlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 25.Geng Y, Savage SM, Johnson LJ, Seagrave J, Sopori ML. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol Appl Pharmacol. 1995;135:268–278. doi: 10.1006/taap.1995.1233. [DOI] [PubMed] [Google Scholar]
- 26.Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Zuo X, Zhou Y, et al. The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J Clin Immunol. 2010;30:213–220. doi: 10.1007/s10875-009-9346-0. [DOI] [PubMed] [Google Scholar]
- 28.Sugano N, Shimada K, Ito K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252:25–28. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- 29.Guslandi M. Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis. 1999;14:261–262. doi: 10.1007/s003840050221. [DOI] [PubMed] [Google Scholar]
- 30.Lindblad SS, Mydel P, Jonsson IM, Senior RM, Tarkowski A, Bokarewa M. Smoking and nicotine exposure delay development of collagen-induced arthritis in mice. Arthritis Res Ther. 2009;11:R88. doi: 10.1186/ar2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Maanen MA, Lebre MC, van der Poll T, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 32.Kim HR, Kim EY, Cerny J, Moudgil KD. Antibody responses to mycobacterial and self heat shock protein 65 in autoimmune arthritis: epitope specificity and implication in pathogenesis. J Immunol. 2006;177:6634–6641. doi: 10.4049/jimmunol.177.10.6634. [DOI] [PubMed] [Google Scholar]
- 33.Tong L, Moudgil KD. Celastrus aculeatus Merr. suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res Ther. 2007;9:R70. doi: 10.1186/ar2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durai M, Kim HR, Bala KK, Moudgil KD. T cells against the pathogenic and protective epitopes of heat-shock protein 65 are crossreactive and display functional similarity: novel aspect of regulation of autoimmune arthritis. J Rheumatol. 2007;34:2134–2143. [PubMed] [Google Scholar]
- 35.Kim HR, Rajaiah R, Wu QL, et al. Green tea protects rats against autoimmune arthritis by modulating disease-related immune events. J Nutr. 2008;138:2111–2116. doi: 10.3945/jn.108.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol. 2008;181:1536–1547. doi: 10.4049/jimmunol.181.2.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173:1171–1178. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–930. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 39.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 40.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 41.Cai C, La Cava A. Mimicking self-antigens with synthetic peptides in systemic autoimmune rheumatic diseases. Curr Clin Pharmacol. 2009;4:142–147. doi: 10.2174/157488409788184936. [DOI] [PubMed] [Google Scholar]
- 42.Uysal H, Nandakumar KS, Kessel C, et al. Antibodies to citrullinated proteins: molecular interactions and arthritogenicity. Immunol Rev. 2010;233:9–33. doi: 10.1111/j.0105-2896.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 43.Pruijn GJ, Wiik A, van Venrooij WJ. The use of citrullinated peptides and proteins for the diagnosis of rheumatoid arthritis. Arthritis Res Ther. 2010;12:203. doi: 10.1186/ar2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vossenaar ER, Nijenhuis S, Helsen MM, et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum. 2003;48:2489–2500. doi: 10.1002/art.11229. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn KA, Kulik L, Tomooka B, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uysal H, Bockermann R, Nandakumar KS, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med. 2009;206:449–462. doi: 10.1084/jem.20081862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulmansky R, Cohen CJ, Szafer F, et al. Resistance to adjuvant arthritis is due to protective antibodies against heat shock protein surface epitopes and the induction of IL-10 secretion. J Immunol. 2002;168:6463–6469. doi: 10.4049/jimmunol.168.12.6463. [DOI] [PubMed] [Google Scholar]
- 48.De Rosa MJ, Dionisio L, Agriello E, Bouzat C, Esandi Mdel C. Alpha 7 nicotinic acetylcholine receptor modulates lymphocyte activation. Life Sci. 2009;85:444–449. doi: 10.1016/j.lfs.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Kim EY, Moudgil KD. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol Lett. 2008;120:1–5. doi: 10.1016/j.imlet.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg EM. Neuroendocrine regulation of autoimmune/inflammatory disease. J Endocrinol. 2001;169:429–435. doi: 10.1677/joe.0.1690429. [DOI] [PubMed] [Google Scholar]