Abstract
The Na,K-ATPase is the membrane “pump” that generates the Na+ and K+ gradients across the plasma membrane that drives many physiological processes. This enzyme is highly sensitive to inhibition by cardiotonic steroids, most notably the digitalis/ouabain class of compounds, which have been used for centuries to treat congestive heart failure and arrhythmias. The amino acids that constitute the ouabain-binding site are highly conserved across the evolutionary spectrum. This could be fortuitous or could result from this site being conserved because it has an important biological function. New physiological approaches using genetically engineered mice are being used to define the biological significance of the “receptor function” of the Na,K-ATPase and its regulation by potential endogenous cardiotonic steroid-like compounds. These studies extend the reach of earlier studies involving the biochemical purification of endogenous regulatory ligands.
Keywords: hypertension, blood pressure regulation, endogenous ouabain, endogenous cardiac glycosides, pregnancy, ACTH, volume expansion
INTRODUCTION
The Na,K-ATPase is a complex membrane protein that utilizes ATP to transport three Na+ ions out of cells and two K+ ions in against their concentration gradients. The enzyme generates an electrical gradient across the plasma membrane and maintains the resting potential of cells, which is particularly important for the function of electrically excitable tissues, such as muscle and brain. The Na+ gradient produced drives many transport processes through cotransporters such as those for the sodium glucose cotransporter and exchangers such as the Na+/Ca2+ exchanger. The Na+ gradient produced by the enzyme also drives amino acid and vitamin transport into cells. The Na,K-ATPase also generates the Na+ gradient that is critical for the reabsorption of Na+ and water from the glomerular filtrate in the nephron and absorption of fluid from the lungs and intestine. The Na,K-ATPase is also a key player in maintaining osmotic regulation of cells.
The Na,K-ATPase plays a pivotal role in many biological processes and its regulation at the protein and enzymatic levels is complex. This enzyme is composed of three subunits, α, β, and a member of the FXYD family (1–4). There are four isoforms of the α subunit (α1, α2, α3, and α4) and three for the β subunit (β1, β2, and β3) (1–4), which adds to the diversity of Na,K-ATPase function. Each of these subunits is coded for by separate genes. The FXYD subunits add additional diversity as there are seven such proteins (5–21). It is possible that the Na,K-ATPase is always associated with one member of the FXYD family of proteins but this has not been established. The α subunit is responsible for the catalytic function of the enzyme, and the β subunit, although required for enzymatic activity, is responsible for maturation and insertion of the Na,K-ATPase into the plasma membrane (22–25). This subunit also influences Na,K-ATPase activity (25, 26). Members of the FXYD proteins modulate enzymatic activity in their own specific manner by increasing or decreasing Na,K-ATPase activity and by being tissue specific (7–21).
The α1 isoform is ubiquitously expressed, whereas the α2 isoform is expressed mainly in skeletal, heart, and smooth muscle, brain, lung, and adipocytes (1–3). The α3 isoform occurs mainly in neurons (1–3) and ovaries (A. Moseley & J. B. Lingrel, unpublished data), as well as in developing hearts of rat (27) and in adult human heart (28, 29). This isoform also occurs in white blood cells (30). The α4 isoform of the Na,K-ATPase is found in sperm, where it is synthesized at the spermatogonia stage (31, 32), and is required for sperm motility (33, 34).
An interesting feature of Na,K-ATPase is the highly conserved nature of the ouabain-binding site, suggesting that this site plays a significant physiological role. Therefore, it is important to determine if this is the case and, if so, whether there is a naturally occurring ligand. If the ouabain-binding site is important, then how does it function?
In addition to its transport function, the Na,K-ATPase plays a signaling role (35), which represents a separate function from its role of transporting Na+ and K+ across the plasma membrane (36). A portion of the Na,K-ATPase is nontransporting and is located in the caveolae (35). When ouabain binds to the enzyme in caveolae, it activates Src, which is normally bound to the Na,K-ATPase in caveolae (35). Src activation, in turn, activates other downstream signaling pathways. Transactivation of the epidermal growth factor receptor (EGFR) also occurs when ouabain binds to Na,K-ATPase (35). Because Na,K-ATPase is acting as a receptor, only a few molecules of ouabain need to bind as signaling pathways are amplified (35). The signaling pathways regulate early response genes associated with cell growth and also regulate cell motility and a number of metabolic pathways (36–44).
Because of the complex roles that Na,K-ATPase plays, it is extremely important to understand any receptor/ligand interactions that occur and the physiological consequences of these interactions. This review focuses on the role of the highly conserved cardiotonic steroid–binding site of the Na,K-ATPase in regulating physiological processes.
CONSERVATION OF THE CARDIOTONIC STEROID/OUABAIN-BINDING SITE DURING EVOLUTION
The cardiotonic steroid–binding site of the Na,K-ATPase is often called the ouabain-binding site, as this is the cardiotonic steroid most often used in the laboratory. This binding site is highly conserved in diverse organisms such as Drosophila (45), toad (46), frog (47), rodent (48–51), sheep (51), guinea pig (51), marmoset (49), and human (52, 53). The ouabain sensitivity of the Na,K-ATPase is determined by the α subunit. The Na,K-ATPase of almost all species is sensitive to all ouabain, although there are exceptions. Two exceptions are monarch butterflies (54) and leaf beetles (55), which eat plant material containing cardiotonic steroids and thus have developed a ouabain-insensitive Na,K-ATPase. In this instance, there is an Asn122His substitution in the α isoform, and this change is thought to be responsible for the ouabain resistance. This is one of the amino acid sites that is responsible for the relative insensitivity of the α1 isoform in mouse and rat (56). Some toads have a ouabain-resistant Na,K-ATPase, and they also contain a substitution at position 122. Nevertheless, even in toad, where there is a ouabain-resistant form of the Na,K-ATPase, a sensitive Na,K-ATPase occurs as well (46).
A notable exception to the evolutionary conservation of ouabain sensitivity of Na,K-ATPase is in mouse and rat, where one of the four α isoforms is relatively resistant to ouabain (48, 51). The α1 isoform exhibits low affinity to ouabain, although the α2, α3, and α4 isoforms of these animals are quite sensitive to this compound. The Kds for ouabain of the rat Na,K-ATPase containing the α2, -3, and -4 isoforms are 115 nM, 1.6 nM, and 312 nM, respectively (50, 32). It is difficult to determine the Kd for the mouse and rat α1 isoform because of its low affinity for ouabain, but in terms of IC50, the rat α1 isoform has an IC50 of 48,000 nM, whereas the IC50 of the rat α2 and α3 isoforms are 58 nM and 6.7 nM, respectively (50). Other studies give somewhat different values, but overall there is close agreement among the laboratories that have determined Kds for ouabain. The Kd values vary from species to species and in humans, where all four α1 isoforms are ouabain-sensitive, the Kd values are in the nM range (52, 53).
ENDOGENOUS NA,K-ATPASE LIGANDS
The finding of a substance present in animals that inhibits the Na,K-ATPase suggested that the binding site of this enzyme may play a physiological role. This has been an active area of research, and many reviews summarize the findings dealing with endogenous ligands that inhibit the Na,K-ATPase (57–62).
A number of early reports suggested that an endogenous material might regulate renal Na+ excretion; however, its site of action was unknown. Of particular importance were the studies of de Wardener and colleagues (63), who showed that when normal dogs were transfused with blood from volume-expanded animals, natriuresis occurred in the recipient dogs. From these studies it was concluded that a natriuretic material occurred in the circulation and was increased by volume expansion. These studies were followed by others demonstrating that volume-expanded animals contain a material in their plasma and urine that could cause natriuresis when introduced into other animals (64–71). Although the nature of this material was unknown, one possibility was that it was atrial natriuretic factor. Further studies at this time demonstrated that Na+ transport was inhibited in toad bladder by plasma extracts from volume-expanded dogs (72). This finding eventually led to the demonstration that tissue and plasma extracts from volume-expanded animals inhibit Na,K-ATPase activity (73, 74).
Other investigators studying hypertension proposed that an endogenous ligand inhibited Na,K-ATPase activity in vascular tissue (75–77), causing vasoconstriction and an increase in blood pressure. In 1977, Blaustein (78) proposed a unifying concept of how an endogenous compound could cause vasoconstriction through inhibition of the Na,K-ATPase. The increase in intracellular Na+ caused by the inhibition of this enzyme resulted in an increase in intracellular Ca2+ through the Na+/Ca2+ exchanger, which is also located in the plasma membrane (62). The increased Ca2+, in turn, caused increased smooth muscle contraction and thus an increased blood pressure through vasoconstriction.
The key finding that volume-expanded dogs contained a material in the plasma that cross-reacted with digoxin antibodies suggested that digoxin-like compounds occurred in these animals. Furthermore, plasma extracts that contained the material reacting with the digoxin antibodies inhibited Na,K-ATPase (79).
A role of the central nervous system in regulating blood pressure was defined using rat models of hypertension, and an endogenous Na,K-ATPase ligand was implicated. When a high-NaCl diet was provided to Dahl-sensitive hypertensive or spontaneously hypersensitive animals, an increase in the Na+ concentration of the cerebral spinal fluid was observed (80–85). Several days later there was an increase in sympathoexcitatory response, and endogenous cardiotonic steroids increased in both cerebral spinal fluid and brain. An increase in sympathetic activity, blood pressure, and heart rate also occurred when ouabain was injected directly into the intracerebroventricular region of the brain. Thus, endogenous cardiotonic steroids such as ouabain could play a role in regulating blood pressure and heart rate through the central nervous system. When Digibind®, a commercial preparation containing the purified Fab fragment of the sheep antidigoxin antibody, which cross-reacts with other cardiotonic steroids, was injected into the brain, the increase in sympathetic hyperreactivity, hypertension, and increased heart rate was prevented (84). This finding suggested that digoxin, ouabain, or another cross-reacting material is involved in blood pressure control. Mice lacking one copy of the α2 isoform of the Na,K-ATPase exhibit a rise in pressor response when cerebrospinal fluid Na+ concentration is increased (86). As there is a reduction in the α2 Na,K-ATPase in these mice, they should respond similarly to inhibition of the Na,K-ATPase by an endogenous ligand, both of which decrease Na,K-ATPase activity. This response supports the hypothesis that an endogenous ligand acts on the Na,K-ATPase and inhibits its activity.
The search for endogenous inhibitors of the Na,K-ATPase identified a number of potential compounds. Hamlyn and coworkers (87) purified a compound from human plasma that appeared to be ouabain. The material has been extensively studied by mass spectroscopy and, on the basis of comparison to various isomers (88), is concluded to be the naturally occurring ouabain rather than an isomer (88). Further studies showed that ouabain or a ouabain-like material is synthesized by the adrenal gland and in tissue culture cells derived from this gland (89–95), although not all steps in the synthesis of ouabain have been defined. Rhamnose, the sugar moiety of ouabain, has been found in rabbit skin (96), which supports the contention that ouabain could be synthesized in mammals.
A second compound, marinobufagenin (97, 98), has also been found to be present in the plasma of dog and rat following volume expansion (99, 100). Marinobufogenin is a bufadenolide, whereas ouabain and digoxin are cardenolides. Cardenolides have a five-member lactone ring, and bufadenolides have a six-member lactone ring. Although most studies have concentrated on endogenous ouabain and endogenous marinobufagenin, other cardiotonic steroids such as the cardenolide digoxin (101) and bufadenolides elocinobufagin and 19-norbufalin have also been identified in animals (101, 102). Although it is attractive to postulate that the endogenous material is either ouabain or marinobufogenin or both, the actual endogenous ligand that interacts with the Na,K-ATPase is yet to be identified.
When Digibind®, an antibody reacting with cardenolides, was administered to rats with DOCA-salt–induced hypertension, blood pressure was decreased, implicating a Digibind®-reacting material in salt-induced hypertension (103). Digibind® also prevented ACTH (adrenocorticotropic hormone)-induced hypertension (104) as well as increased blood pressure in a reduced renal mass model of hypertension (105). Similarly, Digibind® when administered to the central nervous system also reduced salt-induced hypertension (106, 107). Antibodies against either ouabain or marinobufogenin also reduced blood pressure in salt-sensitive Dahl rats (99, 108). These studies make a strong case for the existence of endogenous ligands and suggest that changes in their concentrations during volume expansion could regulate natriuresis or vasoconstriction by interacting with the Na,K-ATPase.
CARDIOTONIC STEROIDS IN SIGNALING
A new function of the Na,K-ATPase has come to light more recently largely through the work of Zijian Xie, Amir Askari, and their collaborators (35, 36, 109, 110): a signaling role for the Na,K-ATPase (37–44). This work followed the finding of a number of laboratories that low doses of ouabain, which were insufficient to inhibit enough enzyme to alter intercellular Na+ and K+ levels, affected a number of biological processes such as growth and gene expression (37–44). The elegant work of Xie and his coworkers has defined the signaling pathway (109–117). Src interacts with the Na,K-ATPase (111) in the caveolae (111, 112), and when ouabain binds, Src is activated, thereby initiating a cascade of signaling events.
Na,K-ATPase binds to the N terminus of caveolin-1—presumably one reason why some of the Na,K-ATPase is found in caveolae. When ouabain binds to the Na,K-ATPase, the EGFR is transactivated (115, 116) and additional signaling occurs that activate downstream targets including She, Grb, Ras, Raf, MEK, and ERK. Signaling through Src is supported by the finding that in a cell-free system, the addition of ouabain alters the Na,K-ATPase Src complex that activates Src activity (111). The Na,K-ATPase ouabain complex is eventually internalized (116) similar to other receptors when they interact with their ligands. Approximately 50% of the Na,K-ATPase is estimated to occur in caveolae in LLC-PK1 cells (117, 118).
A critical experiment examined the stimulation of tyrosine kinases by ouabain in membrane preparations that either did or did not contain caveolae (112). Ouabain increased tyrosine kinase activity in the caveolae, but not in noncaveolar membrane preparations. Another observation that distinguished ion transport function and signaling was the finding that the signaling activity is retained in a purified signaling complex following addition of vanadate, an inhibitor of Na,K-ATPase enzymatic activity. Thus, it is not the inhibition of the enzyme that activates signaling but rather the binding of ouabain to its binding site (111). Na,K-ATPase with a substitution in the ATP-binding site, which eliminates the enzyme’s transport function but does not alter ouabain binding, retains the signaling function of the Na,K-ATPase (119). These studies provide very strong evidence that signaling through Na,K-ATPase is an important function of this enzyme and is independent of changes in intracellular Na+, K+, and Ca2+ ion concentrations through Na,K-ATPase inhibition.
In 2001, Aizman et al. (44) also demonstrated a signaling role of the Na,K-ATPase. It was found that the Na,K-ATPase interacts with the inositol 1,4,5-triphosphate (IP3) receptor in epithelial cells and that ouabain binding to Na,K-ATPase alters this interaction, resulting in synchronized Ca2+ oscillations. These are slow oscillations that activate NF-kB. The interaction of ouabain with the Na,K-ATPase-IP3R receptor occurs with the signaling Na,K-ATPase located in the signaling microdomain. Ankyrin B, a cytoskeletal protein, is also a component of the IP3 receptor with which the Na,K-ATPase interacts. It has further been shown that the N-terminal tail of the Na,K-ATPase interacts with the N-terminal portion of the IP3 receptor and that ankyrin B interacts with this region of the Na,K-ATPase as well.
The novel finding of a signaling role for the Na,K-ATPase provides a completely new dimension to the function of this enzyme. This new role of the Na,K-ATPase must be considered in the context of the traditional view that endogenous cardiotonic steroids have their effect by enhancing contraction in cardiac or smooth muscle myocytes because of an increase in intracellular Ca2+. This effect occurs through inhibition of the Na,K-ATPase with the subsequent increase in Ca2+ through the Na+/Ca2+ exchanger. Higher Ca2+ can increase contraction, but for this to occur, sufficient Na,K-ATPase must be inhibited to significantly raise intracellular Na+ concentrations, even if this occurs only in a limited space in the cytoplasm (120–122). On the contrary, the signaling pathway would be activated at much lower concentrations of endogenous ligands and could play an important role in contractility. However, it is possible that signaling is only involved in processes such as growth, hypertrophy, ischemia, and yet unidentified processes. Therefore, the interaction of endogenous ligands with the ouabain-binding site of Na,K-ATPase could have diverse functions depending on the concentrations of the ligand.
PHYSIOLOGICAL ROLE OF THE CARDIOTONIC STEROID/OUABAIN-BINDING SITE OF THE NA,K-ATPASE
New Approach for Determining the Importance of the Ouabain-Binding Site
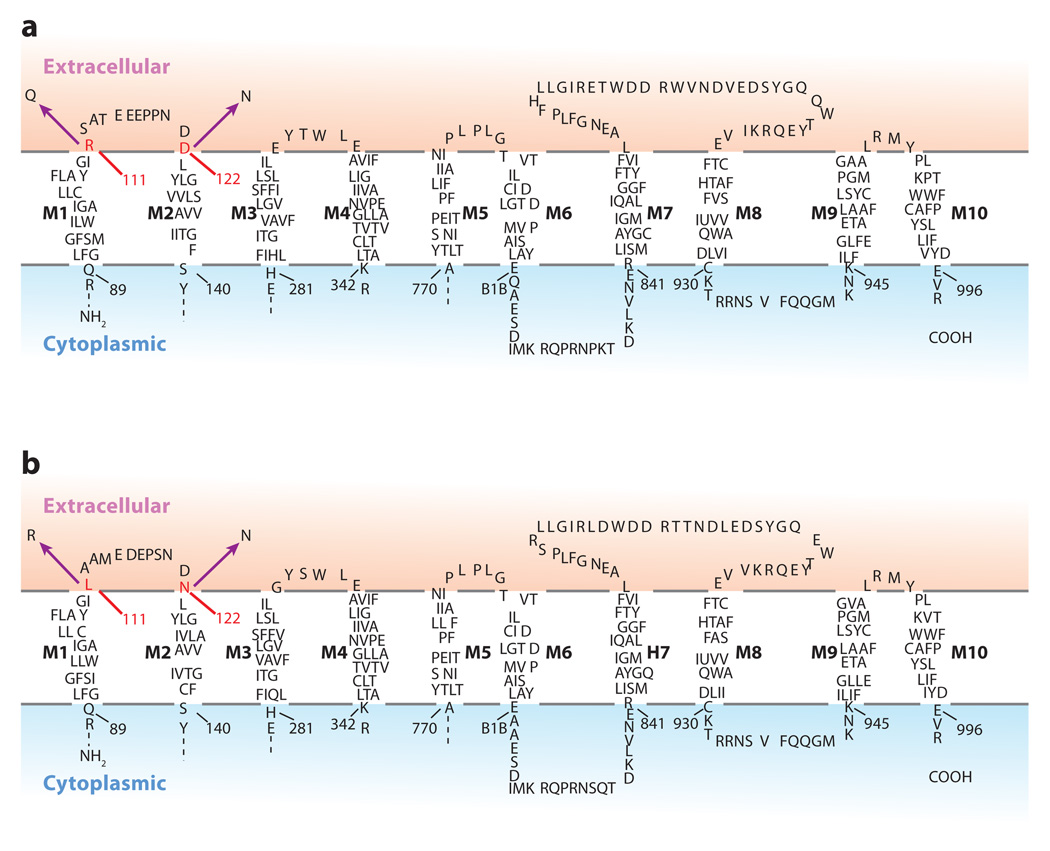
Amino acids at various regions in the Na,K-ATPase influence ouabain sensitivity; however, of particular importance are two amino acids on the cell surface located on either side for the first extracellular domain (Figure 1). These amino acids (56) are responsible for the differential ouabain sensitivity of the relatively ouabain-resistant α1 isoform of the Na,K-ATPase of rat and mouse compared to the ouabain-sensitive α2, α3, and α4 isoforms of these animals. In rat the ouabain-sensitive α2 isoform contains Gln at position 111 and Asn at 122, whereas Arg and Asp occur at these positions in the α1-resistant isoform (Figure 1a). In mouse the ouabain-sensitive α2 isoform contains Leu at position 111, and similar to rat, Asn occurs at position 122 (Figure 1b). In general, sensitive α isoforms have uncharged amino acids at these positions, whereas the resistant isoforms have charged amino acids (123). As both the ouabain-sensitive and -insensitive Na,K-ATPases are active in ion transport, the opportunity opened to develop mice in which the ouabain-sensitive α2 isoform is made resistant to ouabain by substituting the amino acids in the sensitive isoform with those present in the resistant α1 isoform (124, 125).
Figure 1.
Transmembrane and extracellular amino acid sequence of the α1 and α2 isoforms of the mouse Na,K-ATPase. The amino acids responsible for the differential sensitivity of the α1 and α2 isoforms are shown in red. The substitutions introduced into the mouse α1 and α2 isoforms that change their sensitivity to ouabain are shown in black. (a) The amino acid sequence of the α1 isoform and amino acid substitutions that convert this relatively ouabain-resistant isoform to one that is sensitive. The amino acids introduced are those occurring in the ouabain-sensitive human α1 isoform. (b) The mouse α2 isoform and the amino acid substitutions that convert this ouabain-sensitive isoform to one that is relatively ouabain resistant.
The amino acid substitutions introduced into the α1 and α2 isoforms of mice are shown in Figure 1. The mouse α1 isoform, which is relatively resistant to ouabain, is made sensitive by substituting Arg 111 and Asp 122 with those in the human α1 ouabain–sensitive isoform, i.e., Gln 111 and Asn 122. Mice with a ouabain-sensitive α2 isoform were made ouabain-resistant by introducing Arg and Asp at positions 111 and 122 as they occur in the ouabain-resistant α1 isoform. Thus, mice were bred in which the α1 Na,K-ATPase, which is normally resistant to ouabain, is sensitive and the α2 isoform, which is normally sensitive to ouabain, is resistant. These animals are designated α1S/Sα2S/S and α1R/Rα2R/R, whereas wild-type mice are α1R/Rα2S/S. By crossing these animals, α1S/Sα2R/R mice can also be produced.
Development of these animals makes it possible to test whether the ouabain-binding site plays a significant physiological role. That is, if mice with a ouabain-resistant α2 isoform are compared to mice with the wild-type ouabain-sensitive α2 isoform, a physiological alteration should occur if the ouabain-binding site plays a biological role. In the same way, if the α1 isoform, which is normally resistant to ouabain in mice, is made sensitive, these animals could exhibit an altered function. This approach has been helpful in determining if the ouabain-binding site plays a physiological role and in investigating whether endogenous ligands occur and interact with the ouabain-binding site in vivo.
CONSEQUENCE OF CHANGING THE OUABAIN SENSITIVITY OF THE α1 AND α2 ISOFORMS OF THE NA,K-ATPASE
Animals Under Normal Laboratory Conditions
Mice with an altered ouabain sensitivity were born in normal Mendelian ratios, and no observable differences were observed under normal laboratory conditions between the wild-type mice, α1R/Rα2S/S, and the genetically altered animals, α1R/Rα2R/R and α1S/Sα2R/R. The parameters measured included systolic blood pressure, heart rate, growth rate, size of litters, and heart cross section of area (126, 127). It was concluded from these studies that reversed ouabain sensitivity in either the α1 or α2 isoforms of the Na,K-ATPase had no apparent effect in mice maintained under normal laboratory conditions.
Role of the Cardiotonic Steroid–Binding Site in ACTH Hypertension
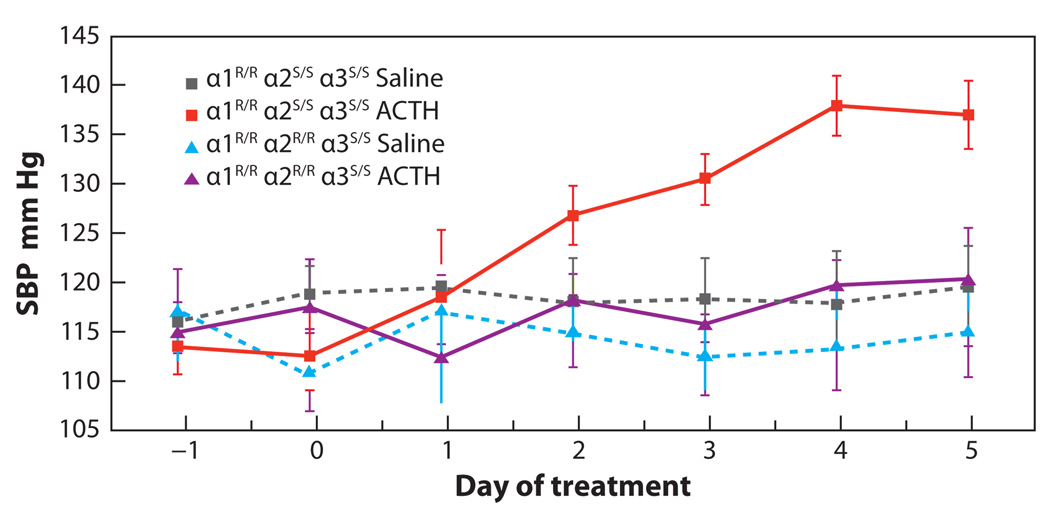
The lack of observable differences in the physiological parameters between the α1R/Rα2R/R and α1S/Sα2R/R mice and wild-type mice, α1R/Rα2S/S, maintained under normal laboratory conditions could be expected, as potential endogenous ligands such as ouabain, which interacts with the Na,K-ATPase, may increase only under certain conditions. The fact that endogenous cardiotonic steroids increase during ACTH-induced hypertension (128, 129) enabled us to determine whether the ouabain-binding site of the α2 isoform exhibited an effect on this form of hypertension. The results of these studies (126, 127) are shown in Figure 2. ACTH was administered to mice over a period of five days, during which time blood pressure increased significantly (Figure 2). When ACTH was administered to mice with a resistant α2 isoform (α1R/Rα2R/R) hypertension did not occur (126, 127). This result clearly demonstrated that ACTH-induced hypertension requires the ouabain-sensitive binding site of the α2 isoform.
Figure 2.
ACTH-induced hypertension is dependent on the ouabain-binding site of α2 isoform of the Na,K-ATPase. Systolic blood pressure (SBP) was measured in wild-type mice α1R/Rα2S/S and genetically engineered mice with an ouabain-resistant α2 isoform, α1R/Rα2R/R, before and during treatment with ACTH. Mice with either of these two genotypes were also injected with saline to serve as controls. Although animals with an α1R/Rα2S/S genotype exhibited an increase in blood pressure following administration of ACTH, those with the α1R/Rα2R/R genotype failed to increase blood pressure following ACTH administration. The blood pressure of mice with either of the two genotypes did not increase with saline administration. These studies clearly demonstrate that the ouabain-binding site of the α2 isoform of the Na,K-ATPase is required for ACTH-induced hypertension in mice. This figure is modified from figure 1a of Reference 126.
Blood pressure in these studies was measured by tail cuff, which induces some anxiety in mice, although telemetry was used with similar results (130). These findings clearly demonstrated that the ouabain-binding site plays a physiological role, at least in ACTH-induced hypertension. As part of these studies, endogenous cardiotonic steroids were measured using Digibind®, an antibody that interacts with a variety of cardiotonic steroids, albeit preferentially with digoxin. As expected, cardiotonic steroids increased approximately threefold in animals to which ACTH was administered. When Digibind® was administered to mice with ACTH-induced hypertension, no increase in blood pressure occurred. This result suggests that the presence of an endogenous ligand that is recognized by the Digibind® antibody plays a role in ACTH-induced hypertension. The ligand could be ouabain or another related cardiotonic steroid. The mechanism of the ACTH effect is unknown, but an increase in both cardiac performance and vascular tone is thought to be involved (128).
As indicated above, the α1 isoform of the Na,K-ATPase in mice is relatively resistant to ouabain, and thus hypertension was induced by ACTH in animals where the relatively ouabain-resistant α1 isoform was made ouabain sensitive, α1S/Sα2R/R (126, 127). These animals developed marked hypertension following ACTH-induced administration. These results are interesting and can be viewed in at least two ways. First, the α1 isoform is sensitive to ouabain in human, and therefore these findings may indicate that the α1 isoform in human plays a significant role in hypertension, at least in ACTH-induced hypertension. Second, although the α1 isoform is normally resistant and would not respond to an endogenous ligand, the fact that it does respond when it is made sensitive indicates that it must be recognizing an endogenous ligand. This conclusion then can be viewed as an indicator of the presence of an endogenous ligand in ACTH-induced hypertension that interacts with the ouabain-binding site of Na,K-ATPase. These studies provide a very strong case for the significance of the ouabain-binding site in Na,K-ATPase and implicate an endogenous ligand interacting with this site.
Importance of the Ouabain-Binding Site During Pregnancy
During pregnancy, blood pressure drops and begins to return to normal at the end of pregnancy (131, 132). This fluctuation occurs in mouse as well as in human. Endogenous cardiotonic steroids are suggested to be involved in the regulation of blood pressure during pregnancy. To examine this hypothesis further, blood pressure measurements were carried out using wild-type α1R/Rα2S/S and α1R/Rα2R/R mice (N. Oshiro & J.B. Lingrel, unpublished data). A marked difference in blood pressure occurs between these two phenotypes. In wild-type mice, blood pressure drops following pregnancy and returns to near normal just before delivery. In contrast, animals with the ouabain-resistant α2 isoform have a lower blood pressure during the first and third trimesters compared to wild-type animals. This indicates that the ouabain-binding site of the α2 isoform in Na,K-ATPase plays a role in the regulation of blood pressure during pregnancy. Pregnancy in humans and mice is associated with changes in the cardiovascular system that include an increase in plasma volume and cardiac output. Maternal vasodilatation that occurs during pregnancy causes a decrease in blood pressure. The difference in blood pressure between the wild-type α1R/Rα2S/S and the α1R/Rα2R/R mice could be the result of production of an endogenous ligand during pregnancy to increase blood pressure by inhibiting the Na,K-ATPase and thus increase Ca2+ through effects on the Na+/Ca2+ exchanger, which, in turn, would increase vascular contractility. This sequence of events would counteract the decrease in blood pressure that occurs normally. If the endogenous ligand cannot interact with the α2 isoform, blood pressure cannot be increased, and therefore a lower blood pressure occurs during pregnancy in the α2 ouabain-resistant animals.
The Role of the α2 Isoform of the Na,K-ATPase in Skeletal Muscle
The role of the ouabain sensitivity of the α2 isoform has also been examined in skeletal muscle (133). This sensitivity is of considerable importance, as the α2 isoform is the predominant isoform of Na,K-ATPase in this tissue. Of particular interest is the finding that the α1R/Rα2R/R mice perform better in response to physical exercise challenges than do wild-type animals, α1R/Rα2S/S. The explanation for this observation is not clear and will require further study, but these findings suggest that the ouabain-binding site of the Na,K-ATPase does indeed play a physiological role in skeletal muscle.
Mice with a Ouabain-Sensitive α1 Isoform of Na,K-ATPase Exhibit Increased Na+ Excretion
Mice with a ouabain-sensitive α1 isoform similar to that in humans were examined in terms of their response to NaCl loading. Animals with a ouabain-sensitive α1 isoform, α1S/Sα2R/R, responded with increased natriuresis compared to wild-type mice α1R/Rα2S/S (134). These studies are compatible with an increase in Na,K-ATPase ligand during salt loading, a process that inhibits the Na,K-ATPase and results in increased excretion of Na+. In support of a ouabain-like material that would bind to the newly created ouabain-binding site in α1, Digibind®, an antidigoxin antibody fragment that cross-reacts with ouabain-like compounds, prevented the increased natriuresis. These studies are likely to serve as a model for kidney function in human, as the α1 isoform in human is ouabain sensitive. In addition, these studies strongly suggest the existence of an endogenous ligand that interacts with the ouabain-binding site of the Na,K-ATPase.
UNANSWERED QUESTIONS AND FUTURE DIRECTIONS
Early studies suggested the existence of a ligand that interacts with the Na,K-ATPase and is implicated in both natriuresis and vascular contractility. Although the identity of such ligand(s) is not entirely clear, mounting evidence indicates that the ouabain-binding site of the Na,K-ATPase plays a physiological role, strongly suggesting that a natural ligand exists and is functional. Studies to date have demonstrated a physiological role for the ouabain-binding site of both the α1 and α2 isoforms of the Na,K-ATPase, but a role for this site on the α3 isoform has not yet been explored.
The demonstration that the ouabain-binding site Na,K-ATPase plays a functional role will undoubtedly raise interest in the continued search for and characterization of compounds that serve as the natural ligand(s) binding to the ouabain-binding site of this enzyme. The determination that antibodies that bind ouabain or marinobufagenin prevent certain types of hypertension similar to making the Na,K-ATPase ouabain resistant supports the contention that such compounds are endogenous Na,K-ATPase ligands. However, it is also possible that multiple ligands exist and that some of these could even be proteins that bind to the ouabain-binding site. Antibodies that bind the ouabain-binding site are known to either inhibit or enhance Na,K-ATPase activity (135, 136).
The major challenge will be to determine how the Na,K-ATPase ligand interaction exerts its effect. For example, in the case of ACTH-induced hypertension or blood pressure regulation during pregnancy, do endogenous ligands reach concentrations that inhibit enough Na,K-ATPase to increase intracellular levels of Na+ and thus Ca2+? Does intracellular Ca2+ increase sufficiently to increase vascular contraction, or does it function through signaling to alter blood pressure? The concentrations of the endogenous ligands studied so far are present in nanomolar levels and therefore may not inhibit sufficient Na,K-ATPase to effectively alter intracellular Na+, as would be required to increase intracellular Ca2+ concentrations through the Na+/Ca2+ exchanger, which, in turn, would be expected to increase vascular smooth muscle contraction. Changes in Na+ are suggested (120–122) to occur in localized regions of the cell, and thus low concentrations of an endogenous ligand could increase vascular contraction and regulate blood pressure through inhibition of the Na,K-ATPase. This suggestion is a reasonable possibility. However, as the Na,K-ATPase acts as a signaling receptor, at least in cell studies, it is also possible that this is the mode of action by which low concentrations of endogenous Na,K-ATPase ligands exert their effects. These two potential functions of the ouabain-binding site of the Na,K-ATPase are diagrammed in Figure 3a,b. Figure 3a describes the consequences of having either pharmacological concentrations of endogenous ligand or amounts of ligand sufficient to inhibit enough Na,K-ATPase to influence either cellular or subcellular amounts of Na+. The rise in intracellular Na+ would increase Ca2+ through the Na+/Ca2+ exchanger. This model would fit with many of the existing data.
Figure 3.
(a) Pharmacological activities of cardiotonic steroids such as ouabain. Pharmacological levels of ouabain inhibit the Na,K-ATPase to such an extent that intracellular Na+ increases, which, in turn, raises intracellular Ca2+ through the Na+/Ca2+ exchanger. The increase in Ca2+ then increases vascular muscle contraction. Physiological levels of endogenous ligands, which raise the concentration of intracellular Na+ and thus Ca2+, would be expected to act similarly. These concentrations of ouabain would also activate Na,K-ATPase-mediated signaling pathways. (b) Stress, volume, pregnancy. The effect of physiological levels of endogenous ouabain or ouabain-like compounds. With physiological levels of endogenous ligand such as endogenous ouabain or marinobufagenin, only the signaling pathway may be activated, as only a few molecules of Na,K-ATPase are needed to bind to the ligand as signaling responses are amplified. As only a few molecules of Na,K-ATPase are inhibited, this binding is unlikely to raise intracellular Na+ levels sufficiently to increase intracellular Ca2+ and activate vascular contraction. Still possible is that local concentrations of Na+ increase sufficiently with low levels of endogenous Na,K-ATPase ligands to alter vascular tone, as described (120–122).
An alternative possibility (Figure 3b) is that the signaling function of Na,K-ATPase is responsible for the finding that the ouabain-binding site plays a role in various physiological processes. In this model, an endogenous ligand such as ouabain would bind a few molecules of Na,K-ATPase located in caveolae and initiate signaling, most notably the activation of Src, which, in turn, activates a number of signaling pathways. Only a few molecules are required to activate signaling pathways as they are amplified. This model is particularly attractive, as the levels of endogenous ligands observed so far are relatively low. Whereas the signaling mechanism of Na,K-ATPase is well understood in cell systems, the role of signaling must now be explored in whole animals. Such investigation is possible using genetically modified animals in which the ligand receptor interaction is prevented, as described above, or by developing new gene-targeted mice in which signaling is abolished. Levels of endogenous ligands that inhibit the Na,K-ATPase would most certainly initiate the signaling pathway as well. Also possible is the use of both pathways. The challenge will be to explore in detail the contributions of these two mechanisms.
Additional challenges relate to an understanding of the physiological functions of Na,K-ATPase receptor-ligand interactions. For example, endogenous ligands that interact with the Na,K-ATPase are known to increase in response to dietary salt loading (137, 138), in certain forms of hypertension (139), and in congestive heart failure (140–142) and myocardial infarction (143). Also, ouabain-like compounds have been shown to be elevated during stress (144, 145) and even during normal exercise (144, 146). Thus, mild inhibition of Na,K-ATPase activity and/or modulation of its signaling function via receptor-ligand interactions have the potential to regulate physiological responses in health and disease. In the future, studies will need to assess both the importance of these receptor-ligand interactions in the various physiological processes that have been implicated as well as the relative roles of Na,K-ATPase inhibition or activation of signaling pathways in the underlying mechanisms.
SUMMARY POINTS
The ouabain-binding site of the Na,K-ATPase plays a physiological role.
An endogenous ligand for the Na,K-ATPase must exist.
Whether the endogenous ligand acts through a change in intracellular Na+ or through a signaling mechanism is unknown.
ACKNOWLEDGMENTS
I thank Gary Shull, Anil Menon, Tara Rindler, and Naomi Oshiro for reading the manuscript and for their helpful discussions and Bill Nienaber for his help with the illustrations. My research is supported by NIH grants 5 RO1 HL 28573 and 5 RO1 HL 66062.
Glossary
- Src
V-src sarcoma (Schmidt-Ruyspin A-2 viral oncogene homolog avian)
- EGFR
epidermal growth factor receptor
- She
SH2 domain–containing protein E
- Grb
growth factor receptor–bound Protein 10
- Raf
V-Raf-1 murine leukemia viral oncogene homolog 1
- MEK
mitogen-activated protein kinase kinase 1–interacting protein 1
- ERK
extracellular signal–regulated kinase
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochem. Biophys. Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 2.Lingrel JB, Kuntzweiler T. Na+,K+-ATPase. J. Biol. Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- 3.Kaplan JH. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 4.Malik N, Canfield VA, Beckers MC, Gross P, Levenson R. Identification of the mammalian Na,K-ATPase β-subunit. J. Biol. Chem. 1996;271:22754–22758. doi: 10.1074/jbc.271.37.22754. [DOI] [PubMed] [Google Scholar]
- 5.Feschenko MS, Donnet C, Wetzel RK, Asinovski NK, Jones LR, Sweadner KJ. Phospholemman, a single-span membrane protein, is an accessory protein of Na,K-ATPase in cerebellum and choroid plexus. J. Neurosci. 2003;23:2161–2169. doi: 10.1523/JNEUROSCI.23-06-02161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweadner KJ, Arystarkhova E, Donnet C, Wetzel RK. FXYD proteins as regulators of the Na,K-ATPase in the kidney. Ann. NY Acad. Sci. 2003;986:382–387. doi: 10.1111/j.1749-6632.2003.tb07218.x. [DOI] [PubMed] [Google Scholar]
- 7.Sweadner KJ. Phospholemman: a new force in cardiac contractility. Circ. Res. 2005;97:510–511. doi: 10.1161/01.RES.0000184616.84960.7a. [DOI] [PubMed] [Google Scholar]
- 8.Beguin P, Wang X, Firsov D, Puoti A, Claeys D, et al. The γ subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J. 1997;16:4250–4260. doi: 10.1093/emboj/16.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therien AG, Karlish SJ, Blostein R. Expression and functional role of the γ subunit of the Na,K-ATPase in mammalian cells. J. Biol. Chem. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- 10.Therien AG, Goldshleger R, Karlish SJD, Blostein R. Tissue-specific distribution and modulatory role of the γ subunit of the Na,K-ATPase. J. Biol. Chem. 1997;272:32628–32634. doi: 10.1074/jbc.272.51.32628. [DOI] [PubMed] [Google Scholar]
- 11.Zouzoulas A, Dunham PB, Blostein R. The effect of the gamma modulator on Na/K pump activity of intact mammalian cells. J. Membr. Biol. 2005;204:49–56. doi: 10.1007/s00232-005-0746-7. [DOI] [PubMed] [Google Scholar]
- 12.Wetzel RK, Pascoa JL, Arystarkhova E. Stress-induced expression of the gamma subunit (FXYD2) modulates Na,K-ATPase activity and cell growth. J. Biol. Chem. 2004;279:41750–41757. doi: 10.1074/jbc.M405622200. [DOI] [PubMed] [Google Scholar]
- 13.Arystarkhova E, Sweadner KJ. Splice variants of the gamma subunity (FXYD2) and their significance in regulation of the Na,K-ATPase in kidney. J. Bioenerg. Biomem. 2005;37:381–386. doi: 10.1007/s10863-005-9475-y. [DOI] [PubMed] [Google Scholar]
- 14.Beguin P, Crambert G, Guennoun S, Garty H, Horisberger JD, Geering K. Chif, a member of the FXYD protein family, is a regulator of Na,K-ATPase distinct from the gamma-subunit. EMBO J. J. 2001;20:3993–4002. doi: 10.1093/emboj/20.15.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zouzoulaas A, Therien AG, Scanzano R, Deber CM, Blostein R. Modulation of Na,K-ATPase by the gamma subunit. J. Biol. Chem. 2003;278:40437–40441. doi: 10.1074/jbc.M308610200. [DOI] [PubMed] [Google Scholar]
- 16.Bibert S, Roy S, Schaer D, Horisberger JD, Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J. Biol. Chem. 2008;283:476–486. doi: 10.1074/jbc.M705830200. [DOI] [PubMed] [Google Scholar]
- 17.Bibert S, Roy S, Schaer D, Felley-Bosco E, Geering K. Structural and functional properties of two human FXYD3 (Mat-8) isoforms. J. Biol. Chem. 2006;281:39142–39151. doi: 10.1074/jbc.M605221200. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Crambert G, Thuillard D, Roy S, Schaer D, et al. Role of the transmembrane domain of FXYD7 in structural and functional interactions with Na,K-ATPase. J. Biol. Chem. 2005;280:42738–42743. doi: 10.1074/jbc.M508451200. [DOI] [PubMed] [Google Scholar]
- 19.Delprat B, Schaer D, Roy S, Wang J, Puel JL, et al. FXYD6 is a novel regulator of Na,K-ATPase expressed in the inner ear. J. Biol. Chem. 2007;282:7450–7456. doi: 10.1074/jbc.M609872200. [DOI] [PubMed] [Google Scholar]
- 20.Crambert G, Li C, Claeys D, Geering K. FXYD3 (Mat-8), a new regulator of Na,K-ATPase. Mol. Biol. Cell. 2005;16:2363–2371. doi: 10.1091/mbc.E04-10-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geering K. FXYD proteins: new regulators of Na,K-ATPase. Am. J. Physiol. Ren. Physiol. 2006;290:241–250. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 22.McDonough AA, Geering K, Farley RA. The sodium pump needs its β subunit. FASEB J. 1990;4:1598–1605. doi: 10.1096/fasebj.4.6.2156741. [DOI] [PubMed] [Google Scholar]
- 23.Geering K. The functional role of the β-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991;285:189–193. doi: 10.1016/0014-5793(91)80801-9. [DOI] [PubMed] [Google Scholar]
- 24.Beguin P, Hasler U, Beggah A, Horisberger JD, Geering K. Membrane integration of Na,K-ATPase α-subunits and β-subunit assembly. J. Biol. Chem. 1998;273:24921–24931. doi: 10.1074/jbc.273.38.24921. [DOI] [PubMed] [Google Scholar]
- 25.Hasler U, Wang X, Crambert G, Beguin P, Jaisser F, et al. Role of β-subunit domains in the assembly, stable expression, intracellular routing, and functional properties of Na,K-ATPase. J. Biol. Chem. 1998;273:30826–30835. doi: 10.1074/jbc.273.46.30826. [DOI] [PubMed] [Google Scholar]
- 26.Lutsenko S, Kaplan JH. An essential role for the extracellular domain of the Na,K-ATPase β-subunit in cation occlusion. Biochemistry. 1993;32:6737–6743. doi: 10.1021/bi00077a029. [DOI] [PubMed] [Google Scholar]
- 27.Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic α isoform and β subunit mRNAs. J. Biol. Chem. 1988;263:10436–10442. [PubMed] [Google Scholar]
- 28.McDonough AA, Velotta JB, Schwinger RH, Philipson KD, Farley RA. The cardiac sodium pump: structure and function. Basic Res. Cardiol. 2002;97:119–124. doi: 10.1007/s003950200024. [DOI] [PubMed] [Google Scholar]
- 29.Shamraj OI, Grupp IL, Grupp G, Melvin D, Gradoux N, et al. Characterisation of Na/K-ATPase, its isoforms, and the inotropic response to ouabain in isolated failing human hearts. Cardiovasc. Res. 1993;27:2229–2237. doi: 10.1093/cvr/27.12.2229. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman JF, Wickrema A, Potapova O, Milanick M, Yingst DR. NA pump isoforms in human erythroid progenitor cells and mature erythrocytes. Proc. Natl. Acad. Sci. USA. 2002;99:14572–14577. doi: 10.1073/pnas.222539999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamraj OI, Lingrel JB. A putative fourth Na,K-ATPase α-subunit gene is expressed in testis. Proc. Natl. Acad. Sci. USA. 1994;91:12952–12956. doi: 10.1073/pnas.91.26.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo AL, James PF, Lingrel JB. Characterization of the fourth α isoform of the Na,K-ATPase. J. Membr. Biol. 1999;169:39–44. doi: 10.1007/pl00005899. [DOI] [PubMed] [Google Scholar]
- 33.Woo AL, James PF, Lingrel JB. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J. Biol. Chem. 2000;275:20693–20699. doi: 10.1074/jbc.M002323200. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez G, Nguyen A-NT, Timmerberg B, Tash JS, Blanco G. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod. 2006;12:565–576. doi: 10.1093/molehr/gal062. [DOI] [PubMed] [Google Scholar]
- 35.Pierre SV, Zijian Xie. The Na,K-ATPase receptor complex. Cell Biochem. Biophys. 2006;46:303–315. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 36.Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 1996;271:10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 37.Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J. Mol. Cell Cardiol. 1997;29:429–437. doi: 10.1006/jmcc.1996.0320. [DOI] [PubMed] [Google Scholar]
- 38.Gao J, Wymore RS, Wang Y, Gaudette GR, Krukenkamp IB, et al. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J. Gen. Physiol. 2002;119:297–312. doi: 10.1085/jgp.20028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong XH, Komiyama Y, Nishimura N, Masuda M, Takahashi H. Nanomolar level of ouabain increases intracellular calcium to produce nitric oxide in rat aortic endothelial cells. Clin. Exp. Pharmacol. Physiol. 2004;31:276–283. doi: 10.1111/j.1440-1681.2004.03995.x. [DOI] [PubMed] [Google Scholar]
- 40.Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. J. Eur. Biochem. 2004;271:1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 41.Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J. Biol. Chem. 2003;278:28160–28166. doi: 10.1074/jbc.M303768200. [DOI] [PubMed] [Google Scholar]
- 42.Abramowitz J, Dai C, Hirschi KK, Dmitrieva RI, Doris PA, et al. Ouabain- and marionbufagin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell line, A7r5. Circulation. 2003;108:3048–3053. doi: 10.1161/01.CIR.0000101919.00548.86. [DOI] [PubMed] [Google Scholar]
- 43.Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, et al. Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J. Membr. Biol. 2004;198:147–158. doi: 10.1007/s00232-004-0670-2. [DOI] [PubMed] [Google Scholar]
- 44.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc. Natl. Acad. Sci. USA. 2001;98:13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lebovitz RM, Takeyasu K, Fambrough DM. Molecular characterization and expression of the (Na+ + K+)-ATPase alpha-subunit in Drosophila melanogaster. EMBO J. 1989;8:193–202. doi: 10.1002/j.1460-2075.1989.tb03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris JF, Ismail-Beigi F, Butler VP, Jr, Gati I, Lichtstein D. Ouabain—sensitive Na+,K+-ATPase activity in toad brain. Comp. Biochem. Physiol. 1997;118:599–606. doi: 10.1016/s0300-9629(96)00465-3. [DOI] [PubMed] [Google Scholar]
- 47.Liu AY-C, Bentley PJ. A comparison of the toxicity of ouabain in vivo and in vitro in the frog, Rana pipiens, and the toad, Bufo marinus. Comp. Gen. Pharmacol. 1971;2:476–478. doi: 10.1016/0010-4035(71)90045-0. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz A, Lindenmayer GE, Allen JC. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol. Rev. 1976;27:3–134. [PubMed] [Google Scholar]
- 49.Abeywardena MY, McMurchie EJ, Russell GR, Charnock JS. Species variation in the ouabain sensitivity of cardiac Na+/K+-ATPase. Apossible role for membrane lipids. Biochem. Pharmacol. 1984;33:3649–3654. doi: 10.1016/0006-2952(84)90152-7. [DOI] [PubMed] [Google Scholar]
- 50.O’Brien WJ, Lingrel JB, Wallick ET. Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch. Biochem. Biophy. 1994;310:32–39. doi: 10.1006/abbi.1994.1136. [DOI] [PubMed] [Google Scholar]
- 51.Akera T, Larsen FS, Brody TM. The effect of ouabain on sodium-and potassium-activated adenosine triphosphatase from the hearts of several mammalian species. J. Pharmacol. Exp. Ther. 1969;170:17–26. [PubMed] [Google Scholar]
- 52.Wang J, Velotta JB, McDonough AA, Farley RA. All human Na+−K+-ATPase α-subunit isoforms have a similar affinity for cardiac glycosides. Am. J. Physiol. Cell Physiol. 2001;281:1336–1343. doi: 10.1152/ajpcell.2001.281.4.C1336. [DOI] [PubMed] [Google Scholar]
- 53.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, et al. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 54.Holzinger F, Frick C, Wink M. Molecular basis for the insensitivity of the monarch (Danaus plexippus) to cardiac glycosides. FEBS Lett. 1992;314:477–480. doi: 10.1016/0014-5793(92)81530-y. [DOI] [PubMed] [Google Scholar]
- 55.Labeyrie E, Dobler S. Molecular adaptation of Chrysochus leaf beetles to toxic compounds in their food plants. Mol. Biol. Evol. 2004;2:218–221. doi: 10.1093/molbev/msg240. [DOI] [PubMed] [Google Scholar]
- 56.Price EM, Lingrel JB. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- 57.deWardener HE. Sodium transport inhibitors and hypertension. J. Hypertens. 1996;14:S9–S18. [PubMed] [Google Scholar]
- 58.Buckalew VM. Endogenous digitalis-like factors, an historical overview. Front. Biosci. 2005;10:2325–2423. doi: 10.2741/1701. [DOI] [PubMed] [Google Scholar]
- 59.Haupert GT. Physiological inhibitors of Na,K-ATPase: concept and status. Prog. Clin. Biol. Res. 1988;268:297–320. [PubMed] [Google Scholar]
- 60.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs. 2007;7:173–189. doi: 10.2165/00129784-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 61.Graves SW, Williams GH. Endogenous digitalis-like natriuretic factors. Annu. Rev. Med. 1987;38:433–444. doi: 10.1146/annurev.me.38.020187.002245. [DOI] [PubMed] [Google Scholar]
- 62.Blaustein MP, Zhang J, Chen L, Hong S, Hema R, et al. The pump, the exchanger, and endogenous ouabain signaling mechanisms that link salt retention to hypertension. Hypertension. 2009;53:291–298. doi: 10.1161/HYPERTENSIONAHA.108.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Wardener HE, Mills IH, Clapham WF, Hayter CJ. Studies on the efferent mechanism of the sodium diuresis which follows the administration of intravenous saline in the dog. Clin. Sci. 1961;21:249–258. [PubMed] [Google Scholar]
- 64.Cort JH, Lichardus B. The nature of the renal response to the carotid-sinus pressor reflex. In: Williams PC, editor. Hormones and the Kidney. New York: Academic; 1963. pp. 25–39. [Google Scholar]
- 65.Cort JH. Electrolytes, Fluid Dynamics and the Nervous System. New York: Academic; 1965. [Google Scholar]
- 66.Cort JH, Dousa T, Pliska V, Lichardus B, Safarova J, et al. Saluretic activity of blood during carotid occlusion in the cat. Am. J. Physiol. 1968;215:921–927. doi: 10.1152/ajplegacy.1968.215.4.921. [DOI] [PubMed] [Google Scholar]
- 67.Krück F. Influence of humoral factors on renal tubular sodium handling. Nephron. 1969;6:205–216. doi: 10.1159/000179729. [DOI] [PubMed] [Google Scholar]
- 68.Sealy JE, Kirschmann JD, Laragh JH. Natriuretic activity in plasma and urine of salt loaded man or sheep. J. Clin. Investig. 1969;48:2210–2224. doi: 10.1172/JCI106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viskoper JR, Czaczes JW, Schwartz N, Ullman TD. Natriuretic activity of a substance isolated from human urine during the excretion of salt load. Nephron. 1971;8:540–548. doi: 10.1159/000179959. [DOI] [PubMed] [Google Scholar]
- 70.Brown PR, Koutsaimanis KG, deWardener HE. Effect of urinary extracts from salt-loaded man on urinary sodium excretion by the rat. Kidney Int. 1972;2:1–5. doi: 10.1038/ki.1972.62. [DOI] [PubMed] [Google Scholar]
- 71.Pearce JW, Veress AT. Concentration and bioassay of natriuretic activity in the blood of volume expanded rats. Can. J. Physiol. Pharmacol. 1975;53:742–747. [Google Scholar]
- 72.Buckalew VM, Martinez FJ, Green WE. The effect of dialysates and ultrafiltrates of plasma of saline-loaded dogs on toad bladder sodium transport. J. Clin. Investig. 1970;49:926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer HJ, Gonick HC, Krück F. Natriuretic hormone. Klin. Wochenschr. 1972;50:893–897. doi: 10.1007/BF01487918. [DOI] [PubMed] [Google Scholar]
- 74.Gonick HC, Kramer HJ, Paul W, Lu E. Circulating inhibitor of sodium-potassium activated adenosine triphosphatase after expansion of extracellular fluid volume in rats. Clin. Sci. Mol. Med. 1977;53:329–334. doi: 10.1042/cs0530329. [DOI] [PubMed] [Google Scholar]
- 75.Haddy FJ. Local control of vascular resistance as related to hypertension. Arch. Intern. Med. 1974;133:916–931. [Google Scholar]
- 76.Haddy FJ. Potassium and blood vessels. Life Sci. 1975;16:1489–1497. doi: 10.1016/0024-3205(75)90065-x. [DOI] [PubMed] [Google Scholar]
- 77.Haddy FJ, Overbeck HW. The role of humoral agents in volume-expanded hypertension. Life Sci. 1976;19:935–947. doi: 10.1016/0024-3205(76)90284-8. [DOI] [PubMed] [Google Scholar]
- 78.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation and hypertension: a reassessment and a hypothesis. Am. J. Physiol. 1977;232:165–173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 79.Gruber KA, Whitaker JM, Buckalew VM. Endogenous digitalis-like substance in plasma of volume-expanded dogs. Nature. 1980;287:743–745. doi: 10.1038/287743a0. [DOI] [PubMed] [Google Scholar]
- 80.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. Curr. Opin. Cardiol. 2006;21:295–304. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- 81.Huang BS, Van Vliet BN, Leenen FHH. Increases in CSF (Na+) precede the increases in blood pressure in Dahl S rats and SHR on high salt diet. Am. J. Physiol. Heart. Circ. Physiol. 2004;281:1881–1889. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 82.Leenen FHH, Harmsen E, Yu H. Dietary sodium and central vs peripheral ouabain-like activity in Dahl salt-sensitive vs salt-resistant rats. Am. J. Physiol. 1994;267:1916–1920. doi: 10.1152/ajpheart.1994.267.5.H1916. [DOI] [PubMed] [Google Scholar]
- 83.Huang BS, Wang H, Leenen FHH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs -resistant rats. Am. J. Physiol. Heart Circ. Physiol. 2001;281:1881–1889. doi: 10.1152/ajpheart.2001.281.5.H1881. [DOI] [PubMed] [Google Scholar]
- 84.Huang BS, Harmsen E, Yu H, Leenen FH. Brain ouabain-like activity and the sympathoexcitatory and pressor effects of central sodium in rats. Circ. Res. 1992;71:1059–1066. doi: 10.1161/01.res.71.5.1059. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi H, Matsuzawa M, Okabayashi H, Suga K, Ikegaki I, et al. Evidence for digitalis-like substance in the hypothalamopituitary axis in rats: implications in the central cardiovascular regulation associated with excess intake of sodium. Jpn. Circ. J. 1987;51:1199–1207. doi: 10.1253/jcj.51.1199. [DOI] [PubMed] [Google Scholar]
- 86.Hou X, Theriault SF, Dostanic-Larson I, Mosely A, Lingrel JB, et al. Enhanced pressor response to increased CSF sodium concentration and to central angiotensin I in heterozygous α2 Na,K-ATPase knockout mice. Am. J. Phys. Regul. Integr. Comp. Physiol. 2009;296:R1427–R1438. doi: 10.1152/ajpregu.00809.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. USA. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong BC, Kim S, Kim TS, Corey EJ. Synthesis and properties of several isomers of the cardioactive steroid ouabain. Tetrahedron. Lett. 2006;47:2711–2115. [Google Scholar]
- 89.Hamlyn JM, Lu Z, Manunta P, Ludens JH, Kimura K, et al. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin. Exp. Hypertens. 20:523–533. doi: 10.3109/10641969809053230. [DOI] [PubMed] [Google Scholar]
- 90.Qazzaz HM, El-Masri MA, Valdes RJ. Secretion of a lactone-hydrogenated ouabain-like effector of sodium, potassium-adenosine triphosphatase activity by adrenal cells. Endocrinology. 2000;141:3200–3209. doi: 10.1210/endo.141.9.7664. [DOI] [PubMed] [Google Scholar]
- 91.Perrin A, Brasmes B, Chambaz EM, Defaye G. Bovine adrenocortical cells in culture synthesize an ouabain-like compound. Mol. Cell Endocrinol. 1997;126:7–15. doi: 10.1016/s0303-7207(96)03964-0. [DOI] [PubMed] [Google Scholar]
- 92.Doris PA, Hayward-Lester A, Bourne D, Stocco DM. Ouabain production by cultured adrenal cells. Endocrinology. 1996;137:533–539. doi: 10.1210/endo.137.2.8593799. [DOI] [PubMed] [Google Scholar]
- 93.Laredo J, Hamilton BP, Hamlyn JM. Ouabain is secreted by bovine adrenocortical cells. Endocrinology. 1994;135:794–797. doi: 10.1210/endo.135.2.8033829. [DOI] [PubMed] [Google Scholar]
- 94.Hamlyn JM, Lu Z, Manunta P, Ludens JH, Kimura K, et al. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin. Exp. Hypertens. 1998;20:523–533. doi: 10.3109/10641969809053230. [DOI] [PubMed] [Google Scholar]
- 95.Lichstein D, Steinitz M, Gati I, Samuelov S, Deutsche J, Orly J. Biosynthesis of digitalis-compound in rat adrenal cells: hydroxycholesterol as a precursor. Life Sci. 1998;62:2109–2126. doi: 10.1016/s0024-3205(98)00186-6. [DOI] [PubMed] [Google Scholar]
- 96.Malawista I, Davidson AE. Isolation and identification of rhomnose from rabbit skin. Nature. 1961;192:871–872. doi: 10.1038/192871a0. [DOI] [PubMed] [Google Scholar]
- 97.Lichstein D, Gati J, Babila T, Haver E, Katz U. Effect of salt acclimation on digitalis-like compounds in the toad. Biochim. Biophys. Acta. 1991;1073:65–68. doi: 10.1016/0304-4165(91)90183-h. [DOI] [PubMed] [Google Scholar]
- 98.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha 1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 99.Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc. Res. 1996;31:296–305. [PubMed] [Google Scholar]
- 100.Fedorova OV, Doris PA, Bagrov AY. Endogenous marionbufagenin-like factor in acute plasma volume expansion. Clin. Exp. Hypertens. 1998;20:581–593. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 101.Komiyama Y, Nichimura N, Dong XH, Hirose S, Kosaka C, et al. Liquid chromatography mass spectrometric analysis of ouabain-like factor in biological fluid. Hypertens. Res. 2000;23:S21–S27. doi: 10.1291/hypres.23.supplement_s21. [DOI] [PubMed] [Google Scholar]
- 102.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Krep H, Price DA, Soszynski P, Tao QF, Graves SW, et al. Volume sensitive hypertension and the digoxin-like factor: reversal by a Fab directed against digoxin in DOCA-salt hypertensive rats. Am. J. Hypertens. 1995;8:921–927. doi: 10.1016/0895-7061(95)00181-N. [DOI] [PubMed] [Google Scholar]
- 104.Li M, Wen C, Whitworth JA. Hemodynamic effects of the Fab fragment of digoxin antibody (Digibind) in corticotrophin (ACTH)-induced hypertension. Am. J. Hypertens. 1997;10:332–336. doi: 10.1016/s0895-7061(96)00318-4. [DOI] [PubMed] [Google Scholar]
- 105.Kaide JI, Ura N, Torii T, Nakagawa M, Takada T, Shimamoto K. Effects of digoxin-specific antibody fab fragment (Digibind) on blood pressure and renal water-sodium metabolism in 5/6 reduced renal mass hypertensive rats. Am. J. Hypertens. 1999;12:611–619. doi: 10.1016/s0895-7061(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 106.Huang BS, Leenen FH. Brain ‘ouabain’ mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ. Res. 1994;74:586–595. doi: 10.1161/01.res.74.4.586. [DOI] [PubMed] [Google Scholar]
- 107.Huang BS, Leenen FH. Brain ‘ouabain,’ sodium, and arterial baroreflex in spontaneously hypertensive rats. Hypertension. 1995;25:814–817. doi: 10.1161/01.hyp.25.4.814. [DOI] [PubMed] [Google Scholar]
- 108.Gomez-Sanchez EP, Gomez-Sanchez CE, Fort C. Immunization of Dahl SS/jr rats with an ouabain conjugate mitigates hypertension. Am. J. Hypertens. 1994;7:591–596. doi: 10.1093/ajh/7.7.591. [DOI] [PubMed] [Google Scholar]
- 109.Xie Z, Askari A. Na+,K+-ATPase as a signal transducer. Eur. J. Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 110.Xie Z. Ouabain interaction with cardiac Na,K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell Mol. Biol. 2001;47:383–390. [PubMed] [Google Scholar]
- 111.Tian J, Cai T, Yuan Z, Wang H, Liu L, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H, Haas M, Liang M, Cai T, Tian J, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 113.Liu J, Liang M, Liu L, Malhotra D, Xie Z, et al. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 114.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 115.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002;277:18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 116.Liu J, Kesiry R, Periyasami SM, Malhotra D, Xie Z, et al. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 117.Liang M, Tian J, Liu L, Pierre S, Liu J, et al. Identification of a pool of non-pumping Na,K-ATPase. J. Biol. Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 118.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem. Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 119.Liang M, Cai T, Tian J, Qu W, Xie Z-J. Functional characterization of Src-interacting Na,K-ATPase using RNA interference assay. J. Biol. Chem. 2006;281:19709–19719. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]
- 120.Juhaszova M, Blaustein MP. Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann. NY Acad. Sci. 1997;834:524–536. doi: 10.1111/j.1749-6632.1997.tb52310.x. [DOI] [PubMed] [Google Scholar]
- 121.Lee MY, Song H, Nakai J, Ohkura M, Kotlikoff MI, et al. Local subplasma membrane Ca+ signals detected by a tethered CA2+ sensor. Proc. Natl. Acad. Sci. USA. 2006;103:13232–13237. doi: 10.1073/pnas.0605757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J. Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Price EM, Rice DA, Lingrel JB. Structure-function studies of Na,K-ATPase: site directed mutagenesis of the border residues from the H1-H2 extracellular domain of the α subunit. J. Biol. Chem. 1990;265:6638–6641. [PubMed] [Google Scholar]
- 124.Dostanic I, Lorenz JN, Schultz JEJ, Grupp IL, Neumann JC, et al. The α2 isoform of Na,K-ATPase mediates ouabain-induced cardiac inotropy in mice. J. Biol. Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. [DOI] [PubMed] [Google Scholar]
- 125.Dostanic I, Schultz JEJ, Lorenz JN, Lingrel JB. The α1 isoform of NaK-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J. Biol. Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 126.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc. Natl. Acad. Sci. USA. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, et al. Physiological role of the α1-and α2-isoforms of the Na+−K+-ATPase and biological significance of their cardiac glycoside binding site. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:524–528. doi: 10.1152/ajpregu.00838.2005. [DOI] [PubMed] [Google Scholar]
- 128.Wen C, Fraser T, Li M, Turner SW, Whitworth JA. Haemodynamic mechanisms of corticotrophin (ACTH)-induced hypertension in the rat. J. Hypertens. 1999;17:1715–1723. doi: 10.1097/00004872-199917120-00008. [DOI] [PubMed] [Google Scholar]
- 129.Whitworth JA, Zhang Y, Mangos G, Kelly JJ. Species variability in cardiovascular research: the example of adrenocorticotrophin-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2006;33:887–891. doi: 10.1111/j.1440-1681.2006.04460.x. [DOI] [PubMed] [Google Scholar]
- 130.Lorenz JN, Loreaux EL, Dostanic-Larson I, Lasko V, Schnetzer JR. ACTH-induced hypertension is dependent on the ouabain-binding site of the α2-Na+-K+-ATPase subunit. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H273–H280. doi: 10.1152/ajpheart.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fedorova OV, Kolodkin NI, Agalakova NI, Namikas AR, Bzhelyansky A, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J. Hypertens. 2005;23:835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 132.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, et al. Discovery of a spontaneous genetics mouse model of preeclampsia. Hypertension. 2002;39:337–342. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 133.Radzyukevich TL, Lingrel JB, Heiny JA. The cardiac glycoside binding site on the Na,K-ATPase α2 isoform plays a role in the dynamic regulation of active transport in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2008;106:2565–2570. doi: 10.1073/pnas.0804150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain sensitive α1 Na,K-ATPase enhances natriuretic response to saline load. J. Am. Soc. Nephrol. 2008;19:1947–1954. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arystarkhova E, Gasparian M, Modyanov NN, Sweadner KJ. Na,K-ATPase extracellular surface probes with a monoclonal antibody that enchances ouabain binding. J. Biol. Chem. 1992;267:13694–13701. [PubMed] [Google Scholar]
- 136.Xu KY. Dual activity of the H1–H2 domain of the (Na+K)-ATPase. Biochem. Biophys. Res. Commun. 2008;377:469–473. doi: 10.1016/j.bbrc.2008.09.137. [DOI] [PubMed] [Google Scholar]
- 137.Delva P, Degan M, Capra C, Fallo F, Mantero F, et al. Plasma ouabain-like activity in essential hypertensive patients and in subjects with primary aldosteronism. Miner. Electrolyte Metab. 1989;15:315–320. [PubMed] [Google Scholar]
- 138.Bagrov AY, Agalakova NI, Kashkin VA, Fedorova OV. Endogenous cardiotonic steroids and differential patterns of sodium pump inhibition in NaCl-loaded salt sensitive and normotensive rats. Am. J. Hypertens. 2009;5:559–563. doi: 10.1038/ajh.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamlyn JM, Ringel R, Schaeffer J, Levinson PD, Hamilton BP, et al. A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 140.Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, et al. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86:420–425. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 141.Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, et al. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J. Hypertens. 2002;20:1189–1194. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 142.Leenen FH, Huang BS, Yu H, Yuan B. Brain ‘ouabain’ mediates sympathetic hyperactivity in congestive heart failure. Circ. Res. 1995;77:993–1000. doi: 10.1161/01.res.77.5.993. [DOI] [PubMed] [Google Scholar]
- 143.Bagrov AYa, Fedorova OV, Maslova MN, Roukoyaykina NI, Ukhanova MV, et al. Endogenous plasma Na,K-ATPase inhibitory activity and digoxin like immunoreactivity after acute myocardial infarction. Cardiovasc. Res. 1991;25:371–377. doi: 10.1093/cvr/25.5.371. [DOI] [PubMed] [Google Scholar]
- 144.Goto A, Yamada K, Nahoshi H, Terano Y, Omata M. Stress-induced elevation of oubain-like compound in rat plasma and adrenal. Hypertension. 1995;26:1173–1176. doi: 10.1161/01.hyp.26.6.1173. [DOI] [PubMed] [Google Scholar]
- 145.Fedorova OV, Anderson DE, Lakatta EG, Bagrov AY. Interaction of NaCl and behavioral stress on endogenous sodium pump ligands in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R352–R358. doi: 10.1152/ajpregu.2001.281.1.R352. [DOI] [PubMed] [Google Scholar]
- 146.Bauer N, Muller-Ehmsen J, Kramer U, Hambarchin N, Zobel C, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]