Abstract
Objective
Little is known about long-term cognitive side effects of adjuvant chemotherapy for breast cancer. We thus examined incidence of dementia diagnoses in older women diagnosed with breast cancer, stratified by types of chemotherapy regimen.
Methods
We identified patients with incident dementia diagnoses through Medicare claims linked to the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) tumor registry data. The study population (n = 6,932) consisted of women at least 68 years of age, who were diagnosed with early-stage breast cancer from 1994 through 2002 in one of the SEER areas and received chemotherapy as part of their cancer treatment. Excluded were women with a diagnosis of dementia within the 3 years prior to their cancer diagnosis.
Results
Our sample comprised mostly white women. The mean age was 74. Fifty-seven percent were estrogen receptor positive. Over 70% had no comorbidity. The use of taxol and anthracycline-based treatments increased from mid-1990s to early 2000. Increasing age at cancer diagnosis, Black ethnicity, living in a census tract with lower level of education, and increasing number of comorbidities were associated with new claims of dementia diagnoses after chemotherapy. There was no significant association between types of chemotherapy agents and risk of subsequent dementia diagnoses.
Conclusion
No association was found between types of adjuvant chemotherapy agents for breast cancer and risk of new dementia diagnoses. Our findings suggest that concerns about post-chemotherapy dementia should not be a major factor in determining type of adjuvant chemotherapy regimen to prescribe for older women with breast cancer.
Keywords: Breast cancer, Oncology, Dementia, Chemotherapy, Aging
Introduction
Studies show an association between adjuvant chemotherapy for breast cancer and cognitive impairment [1–11]. Cognitive changes after chemotherapy have been described as “chemo-brain.” Meta-analyses suggest that the common chemotherapy-related cognitive changes include visual or verbal memory impairments and executive dysfunction [12–15]. One study of breast cancer and lymphoma patients noted that 39% of chemotherapy patients (N = 71) exhibited cognitive decline, versus 14% of controls (N = 57) [16]. Another longitudinal controlled study (n = 46, mean age of 43 years) showed that women with breast cancer who received chemotherapy plus tamoxifen had greater 1-year decline in visual memory and verbal working memory compared to women who received chemotherapy alone [17]. Women who received neither chemotherapy nor tamoxifen had improved 1-year scores on their cognitive functions [17].
One study of patients with a mean of 2 years since the completion of last chemotherapy treatment showed that impaired cognitive function was seen in 32% of those treated with high-dose chemotherapy, in 17% of the patients treated with standard-dose chemotherapy, and in 9% of the control group [18]. The control group consisted of women with stage I breast cancer who did not receive chemotherapy [18]. Subsequently, Schagen et al. showed greater cognitive deterioration in high-dose chemotherapy patients compared with low-dose chemotherapy [19]. Over 4 years of follow-up, women in both groups (high-dose and low-dose chemotherapy) showed cognitive improvement after 4 years, suggesting transient cognitive changes or practice effects [20, 21]. These studies were in women younger than 65. Several had small sample size [16–21]. It is not clear if chemotherapy-associated cognitive impairment differs by types of chemotherapeutic agents or whether older patients will be at higher risk.
An understanding of the cognitive risks of different chemo agents is especially important in the patients aged 65 and older. Most patients living with cancer are in that age group. The patients aged 65 and older are at high risk of having pre-cancer cognitive impairment, so chemotherapeutic agent with the lowest cognitive side effects may be preferred in this population. In particular, we need to examine chemotherapy agents (e.g., methotrexate, 5-Fluorouracil (5-FU), and taxanes) with known toxic effects on peripheral nervous system [22–27]. The central nervous system in the older cancer patients may be particularly vulnerable to these agents. To our knowledge, there are no population-based studies specifically comparing the cognitive side-effects of different breast cancer chemotherapy agents. Studies have shown specific CNS toxicities associated with individual treatment regimens (e.g., cyclophosphamide, methotrexate, fluorouracil, and paclitaxel), but none has compared the risk of cognitive impairment associated with each drug [22–24, 28]. It is particularly important to understand the central nervous system impact of paclitaxel because of its well-known side effects of peripheral neuropathy [24, 25]. Selection biases (e.g., not giving chemotherapy to patients with cancer and serious comorbidities) preclude us from looking at effect of chemotherapy versus non-chemotherapy on dementia, but these biases should be less when comparing different chemotherapy regimens.
We thus did an observational study of older women getting adjuvant chemotherapy after a diagnosis of breast cancer. We examined incidence of dementia diagnoses as outcome, using a diagnosis of dementia as a surrogate for cognitive dysfunction. Our goal is to know if the risk of subsequent dementia diagnoses varied by types of chemotherapy drug. We identified patients with incident dementia diagnoses through Medicare claims linked to the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) tumor registry data [29]. We hypothesized that incident dementia would vary by specific chemotherapy drugs. The SEER-Medicare data registry is population-based database that offers a unique opportunity to examine association of chemotherapy with incident dementia across a large population [29–31].
Methods
The current study was reviewed and approved by the Institutional Review Board of the University of Texas Medical Branch.
Data
The source of data for this study was the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database [29–37]. The SEER Program supports population-based cancer registries that covered 14% of the U.S. population from 1992 to 1999 and 25% of the population (with an expanded set of registries) from 2000 to the present. The linked database contains tumor registry data, Medicare enrollment information and claims for Medicare enrollees who (1) were diagnosed with any cancer (except non-melanoma skin cancer) from 1973 to 2002 in one of the SEER geographic areas and (2) linked to the Medicare enrollment file. This study used the Medicare claims data for physician services, inpatient stays, institutional outpatient care and durable medical equipment. The linked database also includes selected socio-economic characteristics for each cancer case's census tract and zip code.
Study population
The study population consisted of women at least 68 years of age, who were diagnosed with stage I, II, or III breast cancer from 1994 through 2002 in one of the SEER areas and received chemotherapy as part of their cancer treatment. Receipt of chemotherapy was determined from Common Procedural Terminology (CPT) J codes in the women's claims files for physician services, institutional outpatient care and durable medical equipment. The included codes were J9000 to J9999. We excluded the codes of J9202 (goserelin), J9209 (mesna), J9212 to J9214 (interferon), and J9217, J9218 (leuprolide acetate) because these drugs are not cytotoxic chemotherapeutic agents. A woman with breast cancer was defined as receiving chemotherapy if there was a chemotherapy claim within 6 months after breast cancer diagnosis for any of the following: Group A: Anthracycline (doxorubicin—J9000, J9001, J9010, mitoxantrone—J9293, daunorubicin—J9150, J9151, or epirubicin—J9180), Group B: CMF (cyclophosphamide—J9070, J9080, J9090-J9097, methotrexate—J9250, J9260, and 5-fluorouracil—J9190), Group C: Taxane (docetaxel—J9170, paclitaxel—J9265), and Group D: Other. Because cyclophosphamide can be given as oral medication and only injection medications are available in current Medicare claims, we coded as Group C patients who only had methotrexate and 5-fluorouracil.
Women were excluded if they (1) had a diagnosis of dementia within the 3 years prior to their cancer diagnosis and/or (2) did not have full coverage of Medicare Parts A and B or were members of an HMO over the period from 3 years before to 1 year after breast cancer diagnosis. The ICD–9-CM codes used to identify Alzheimer's disease and related dementias prior to breast cancer diagnosis are those codes evaluated by Taylor et al. [31]: 331.0 to 331.2; 331.7; 290.0; 290.1; 290.10 to 290.13; 290.20; 290.21; 290.3; 290.40 to 290.43; 294.0; 294.1; 294.8; 797. A woman was considered to have had a prior diagnosis of dementia if she had one of these codes on a physician, outpatient, or inpatient claim in the 3 years prior to her breast cancer diagnosis.
The minimum age was defined as 68 in order to have at least 3 years of Medicare claims prior to diagnosis to identify and exclude subjects diagnosed with dementia before they were diagnosed with breast cancer. Women who did not have full Medicare coverage or were members of an HMO were also excluded since their claims may not be complete for the 3 years prior to diagnosis (n = 31,497 exclusions). After these exclusions, there were 43,099 women aged 68 years and older with Stage I, II, or III breast cancer at the time of cancer diagnosis (1994–2002) and with full Medicare coverage (enrolled in Medicare Parts A and B) from 3 years before diagnosis through 1 year after diagnosis. The final study population of breast cancer cases with chemotherapy as defined above was 6,932.
Measures of patient and census tract characteristics
Patient demographic characteristics obtained from the SEER data include age (in years) at diagnosis and race (Hispanic/non-Hispanic white, black, other). Level of comorbidity (none, one, two or more comorbidities) was assessed with Medicare claims data over the 12 months prior to breast cancer diagnosis using Klabunde's adaptation of the Charlson's Comorbidity Index (with the exclusion of dementia) [35]. Tumor characteristics obtained from SEER data include year of diagnosis, American Joint Committee on Cancer (AJCC) stage of breast cancer at diagnosis (I, II, or III), histologic grade (well differentiated, moderately differentiated, poorly differentiated, or undifferentiated), tumor size (in centimeters), lymph nodes involved (negative, number of nodes), and estrogen receptor status (negative, positive). Census tract characteristics include the percentage of adults living in a census tract with less than a high school degree and percentage living in a census tract with income below the poverty line.
Measures of treatment characteristics
Use of different chemotherapy regimens was defined as described above in the definition of the study population. Initial course of surgical therapy was ascertained from the SEER data's site specific codes. For cases diagnosed from 1994 to 1997, breast conserving surgery was coded as 10, 18, 20, or 28 and mastectomy as 30, 38, 40, 48, 50, 58, 60, 68, or 90. For cases diagnosed from 1998 to 2002, codes 10–17 defined breast conserving surgery and codes 30–61 and 80 defined mastectomy.
Identification of patients with new diagnoses of dementia after chemotherapy
We used Taylor et al.'s list of diagnosis codes described above to identify all breast cancer cases with new diagnoses of dementia after chemotherapy [31]. A patient with a dementia diagnosis in any position of the listed diagnoses on an outpatient or physician claim after the date of the first chemotherapy treatment was considered to have incident dementia.
Statistical analyses
As described above, only women who received chemotherapy were included in the analyses. Percents of patients receiving the 4 groups of chemotherapy agents comprising: Anthracycline, CMF, Taxane, and Others were reported. Each patient could have multiple groups of medication in the descriptive analyses. In the Kaplan–Meier analysis, if they received multiple groups of medication, they were assigned to a group based on the following hierarchy: Taxane, CMF, Anthracycline, and Others. The Kaplan–Meier method was then used to generate survival curves of incident dementia for patients in each chemotherapy group. A Cox proportional hazard model was used to evaluate the association between receipt of each type of chemotherapy and incident dementia, with adjustment for demographic characteristics, lymph node involvement, tumor size, histologic grade, and other patient and tumor characteristics. The reference group for each chemotherapy agent was its own non-regimen in the Cox proportional hazard model. For example, the reference group for anthracycline comprised patients who did not receive any anthracycline in the 6 months course of their cancer treatment post-diagnosis. All analyses were performed with the use of SAS version 9.1.
Results
Table 1 lists the percentages of patients who received each chemotherapy agent by year of diagnosis, age, AJCC stage, ER status, comorbidity, grade, and surgery type. Many patients received more than one agent. Our sample comprised mostly white women. The mean age was 74. Fifty-seven percent were ER positive. Over 70% had no comorbidity. The use of taxol and anthracycline-based treatments increased from mid-1990s to early 2000, while the use of CMF treatment decreased.
Table 1.
Baseline characteristics of older women with breast cancer by types of chemotherapy agents
| Number of patients | Anthracycline (%) | CMF (%) | Taxane (%) | Othera (%) | |
|---|---|---|---|---|---|
| Overall | 6,932 | 50.7 | 44.1 | 15.4 | 12.4 |
| Year of Dx | |||||
| 1994 | 377 | 32.4 | 62.9 | 0.5 | 24.9 |
| 1995 | 376 | 35.9 | 61.2 | 1.1 | 15.2 |
| 1996 | 392 | 38.8 | 61.0 | 3.6 | 13.5 |
| 1997 | 503 | 44.3 | 55.6 | 4.2 | 15.9 |
| 1998 | 613 | 38.5 | 52.5 | 8.7 | 17.3 |
| 1999 | 645 | 52.9 | 41.7 | 17.7 | 12.6 |
| 2000 | 1,386 | 56.5 | 36.7 | 25.7 | 11.7 |
| 2001 | 1,373 | 56.2 | 38.8 | 17.3 | 8.6 |
| 2002 | 1,267 | 59.0 | 34.6 | 20.9 | 8.5 |
| Age at Dx, years | |||||
| 68–74 | 4,543 | 56.0 | 40.4 | 16.1 | 11.8 |
| 75–84 | 2,303 | 41.0 | 51.2 | 13.7 | 13.1 |
| 85+ | 86 | 23.3 | 48.8 | 24.4 | 24.4 |
| Race | |||||
| White | 6,076 | 50.6 | 43.9 | 15.5 | 12.3 |
| Black | 487 | 52.2 | 43.7 | 15.0 | 12.3 |
| Other | 362 | 49.5 | 47.0 | 13.8 | 13.8 |
| AJCC stage | |||||
| I | 1,185 | 34.4 | 54.2 | 6.5 | 16.8 |
| II | 4,583 | 51.1 | 45.5 | 14.4 | 10.4 |
| III | 1,164 | 65.5 | 28.3 | 28.3 | 15.8 |
| ER status | |||||
| Positive | 3,767 | 51.7 | 42.3 | 15.1 | 12.6 |
| Negative | 2,106 | 48.2 | 48.0 | 15.2 | 11.8 |
| Unknown | 1,059 | 51.9 | 42.9 | 17.0 | 12.7 |
| Comorbidity | |||||
| 0 | 5,144 | 52.7 | 42.6 | 15.9 | 12.3 |
| 1 | 1,273 | 46.6 | 47.1 | 13.8 | 13.0 |
| >1 | 515 | 40.0 | 51.5 | 14.8 | 12.2 |
| Grade | |||||
| Well | 599 | 43.2 | 48.4 | 13.9 | 12.4 |
| Moderately | 2,318 | 51.3 | 43.7 | 14.9 | 11.4 |
| Poorly | 3,107 | 53.1 | 42.7 | 16.3 | 12.3 |
| Undifferentiated | 176 | 49.4 | 44.3 | 14.8 | 15.3 |
| Unknown | 732 | 44.7 | 47.7 | 14.5 | 15.4 |
| Surgery type | |||||
| None | 174 | 60.3 | 25.3 | 28.7 | 21.3 |
| BCS | 2,503 | 46.9 | 46.9 | 11.8 | 13.1 |
| Mastectomy | 4,255 | 52.5 | 43.2 | 16.9 | 11.6 |
Out of 858 patients receiving “Other” chemotherapy, the medications were not classified for 111 (12.9%) of them (only having J9999). A total of 396 of them (46.2%) received Cyclophosphamide without Methotrexate sodium and Flurouracil, 56 received either Methotrexate or Flurouracil, 76 received Trastuzumab, 75 received Carboplatin, 37 received Vincristine, 24 received Vinorelbine, 23 received Etoposide, 14 received Fludarabine, 12 received Floxuridine, 11 received Vinblastine, and 46 received other medications
A Cox model was performed to assess the risks of dementia subsequent to specific chemotherapy regimens. Cox proportional hazard models for the likelihood of developing dementia are shown in Table 2. Significant predictors of incident dementia after chemotherapy included increasing age at diagnosis, black ethnicity, lower education level, and increasing number of comorbidities. There was no association between type of chemotherapy and incidence of dementia. Lymph node involvement, tumor size, tumor grade, and surgery were also not significantly associated with post-chemotherapy dementia. The reference for each chemotherapy agent was its own non-regimen in the Cox proportional model. We also examined the data with a hierarchical model and once again found no differences.
Table 2.
Hazard ratios of incident dementia diagnoses by demographics, cancer characteristics, comorbidities and types of chemotherapy for breast cancer in women aged >67
| Variable | Hazard ratio | 95% CI |
|---|---|---|
| Age at Dx, years | ||
| Age | 1.11 | (1.09,1.13) |
| Race | ||
| White | 1 | 1 |
| Black | 1.49 | (1.15,1.92) |
| Other | 1.00 | (0.70,1.42) |
| Unknown | 1.89 | (0.46,7.85) |
| EDU* | ||
| ≤9.12 | 1 | 1 |
| 9.13–15.79 | 1.08 | (0.88,1.34) |
| 15.80–24.55 | 1.09 | (0.87,1.38) |
| 24.56+ | 1.24 | (0.94,1.64) |
| Unknown | ||
| POV* | ||
| ≤3.97 | 1 | 1 |
| 3.98–7.16 | 1.01 | (0.82,1.24) |
| 7.17–13.05 | 1.03 | (0.81,1.30) |
| 13.06+ | 0.93 | (0.70,1.24) |
| Unknown | 1.29 | (0.83,1.99) |
| Lymph node | ||
| Negative | 1 | 1 |
| “1–3” | 1.09 | (0.91,1.30) |
| “4–9” | 1.12 | (0.91,1.38) |
| “10+” | 1.09 | (0.83,1.42) |
| Unknown | 1.15 | (0.86,1.53) |
| Tumor size, cm | ||
| 0–2.0 | 1 | 1 |
| 2.1–5.0 | 1.03 | (0.89,1.20) |
| >5 | 1.03 | (0.80,1.33) |
| Unknown | 1.11 | (0.82,1.50) |
| Estrogen receptor | ||
| Negative | 1 | 1 |
| Positive | 0.89 | (0.75,1.04) |
| Unknown | 1.04 | (0.84,1.29) |
| Comobidities | ||
| 0 | 1 | 1 |
| 1 | 1.36 | (1.15,1.61) |
| 2+ | 1.67 | (1.32,2.10) |
| Grade | ||
| Well | 1 | 1 |
| Moderately | 1.18 | (0.90,1.56) |
| Poorly | 1.19 | (0.90,1.56) |
| Undifferentiated | 0.98 | (0.57,1.69) |
| Unknown | 0.97 | (0.70,1.36) |
| Surgerya | ||
| Mastectomy | 1 | 1 |
| BCS | 0.95 | (0.82,1.11) |
| Chemotherapy | ||
| Anthracycline | 0.99 | (0.77,1.30) |
| cmf | 1.02 | (0.78,1.33) |
| Taxane | 0.99 | (0.79,1.25) |
| Other | 1.10 | (0.88,1.39) |
Group A: Anthracycline (Doxorubicin, 60 mg/m2 every 3 weeks, or epirubicin, 100 mg/m2 every 3 weeks), Group B: CMF (cyclophosphamide, methotrexate, and 5-fluorouracil), Group C: Taxane (typically, Cyclophosphamide + Doxorubicin + Taxel), and Group D: Other
Patients who did not have either Mastectomy or BCS were excluded
EDU*: Census tract education, % adult with < 12 years education
POV*: Census tract poverty, % living below poverty line
Figure 1 presents Kaplan–Meier survival curves of incident dementia for older patients who received chemotherapy for breast cancer stratified by type of chemotherapy. Up to 6 years post-chemotherapy, all patients have similar survival curves. We chose to look beyond 6 years because taxane is a relatively new chemotherapy agent. This allowed us to capture any taxane-related outcomes since there are less long-term data available on taxane-based regimen. Even though the Kaplan-Meier survival curves after 6 years showed higher rates of dementia diagnoses for taxanes, the Cox proportional hazard models showed no significant difference in incidence of dementia diagnoses among the 4 groups. The P-value from log rank test is 0.4281; the small sample size of taxane at the longer follow-up lack sufficient power to detect any significant difference in the Cox model. The departure of taxane on Kaplan–Meier curves after 6 years was only based on 34 patients. From the figure, only 7 patients remained at the 8th year.
Fig. 1.
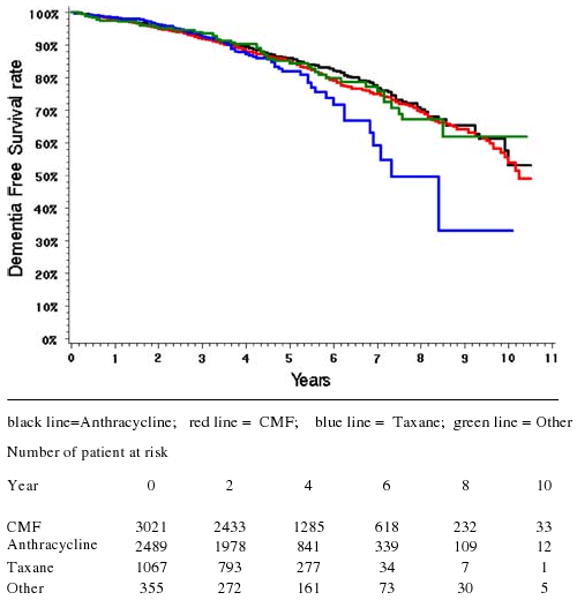
Kaplan–Meier survival curves showing incidence of dementia diagnoses in older women who received chemotherapy for breast cancer stratified by type of chemotherapy. Note: Group A: Anthracycline (Doxorubicin, 60 mg/m2 every 3 weeks, or epirubicin, 100 mg/m2 every 3 weeks), Group B: CMF (cyclophosphamide, methotrexate, and 5-fluorouracil), Group C: Taxane (typically, Cyclophosphamide + Doxorubicin + Taxel), and Group D: Other. The time period before diagnosis of breast cancer was calculated by months because data on days before diagnosis were not available from SEER data
Discussion
The results of this study can be summarized as follows. We found that increasing age at cancer diagnosis, black ethnicity, living in a census tract with level of lower education, and increasing number of comorbidities were associated with new claims of dementia diagnoses after chemotherapy. We did not find any association between types of chemotherapy agents and risk of subsequent dementia diagnoses. Past studies showed association between chemotherapy use and cognitive impairment [1–21]. Outcome of the current study is incident dementia diagnosis. Medicare claims underestimate mild cognitive impairment and milder dementia. Past studies have described subtle cognitive decline before and after chemotherapy in cancer patients [12–15]. Such cognitive changes are not captured in claims data. This may in part explain the lack of association between chemotherapy and incident dementia in our study. Older women are less likely to receive chemotherapy [34], so the older cancer patients undergoing chemotherapy likely represent a selected group of healthier cancer patients who may have fewer risk factors for dementia.
Brain dysfunction in older patients with cancer reflects multiple etiologic pathways: anxiety, stress, depression, aging, cancer, inflammation, paraneoplastic syndrome, hormonal manipulation, radiotherapy, surgery, non-cancer drugs, e.g., opiates and anti-cholinergics, preexisting vascular risk factors for brain infarcts, and chemotherapy [8–20]. The current study was an observational study of women getting chemotherapy. We looked at incident dementia as outcome to see if risk of subsequent dementia varied by type of chemotherapy. It did not. Our data suggest the need for studies that can do more comprehensive cognitive testing, especially in older women where the data are limited. The non-significant trend for increased incidence of dementia diagnoses in women receiving taxane suggests a need for longer follow-up of more women on taxane. Long-term cognitive evaluation of older cancer patients who have received chemotherapy may allow early detection and treatment of any cognitive decline related to cancer or its treatment. The cognitive evaluation is especially important in older Blacks and those with multiple comorbidities who are already at high risk of developing dementia [38]. Cardiovascular risk factors (e.g., hypertension and diabetes) that are common in Blacks have been shown to increase the risk of vascular and Alzheimer's dementia [38–40].
Limitations of our study include concerns about validity and completeness of claims data identifying patterns and toxicities of cancer care. Several studies showed good internal and external validity regarding the use of Medicare claims data for outcomes research in breast cancer [34–37]. Due to inherent selection biases for treatment and diagnosis, retrospective data from SEER must be evaluated with caution. It is possible that the permanent cognitive effects of chemotherapy may be too subtle to be captured by fee for service claims data. One issue is the accuracy and completeness of using Medicare Claims in identifying patients with incident dementia [31, 41, 42]. The population of patients with incident cognitive impairment and mild dementia after chemotherapy differs from the population of patients who have been given a diagnosis of dementia by their physicians. Our study focus is on the latter population. This population likely under-represents individual with incident mild cognitive impairment or milder dementia [41].
Taylor et al. examined sensitivity of using up to 5 years of Medicare claims in 417 patients known to have a clinical diagnosis of dementia [31]. With 3 years of data from the physician supplier and hospital outpatient claims files, investigators were able to correctly identify 87% of patients with dementia using ICD-9-CM dementia codes [31]. Because Medicare claims likely under-report patients with early dementia [41], our findings largely relate to incidence of moderate to severe dementia diagnoses after breast cancer chemotherapy. It is thus possible that chemotherapy might be associated with new-onset of early dementia or mild cognitive impairment, but Medicare claims are just not capturing these cognitive changes.
Another limitation of our study is lack of information on hormonal adjuvant therapy. Medicare claims data provide injection medication data only. Hormonal adjuvant medications (e.g., tamoxifen and aromatase inhibitors such as anastrozole) are typically given to postmenopausal women with hormone receptor-positive breast cancer. Although past studies showed association between anti-estrogen therapy and risk of cognitive impairment [4, 17], our data did not show any difference in incidence of dementia diagnoses between subjects with estrogen receptor-positive tumors and those with estrogen receptor-negative tumors.
Because our analysis is based on the experiences of older breast cancer patients enrolled in fee-for-service Medicare, our findings may not be applicable to younger patients or patients enrolled in Medicare HMOs. Our study has several strengths, including its large sample size, availability of inpatient and outpatient data, information on specific chemotherapy agents, and a wide geographic representation of the US.
In summary, the data show that older women, Blacks, and those with multiple comorbidities are at high risk of new-onset dementia after chemotherapy for breast cancer. Type of chemotherapy agents is not associated with risk of incident dementia. Our study on chemotherapy-associated dementia generated from Medicare claims diagnoses may not reflect findings from prospective studies using detailed neuropsychological measures. Though past studies have described subtle cognitive decline before and after chemotherapy in cancer patients, our findings suggest that concerns about risk of post-chemotherapy dementia should not be a major determinant of which type of adjuvant chemotherapy regimen to prescribe for women with breast cancer. The data on association between comorbidities and subsequent dementia diagnoses suggest that clinicians, patients, and caregivers discuss potential risk of post-chemotherapy dementia diagnoses in the context of co-existing co-morbidities (e.g., diabetes and hypertension) and other risk factors for vascular dementia.
Acknowledgments
James S. Goodwin was supported by Grants P50 CA105631 and R01CA104949 from the National Cancer Institute. Mukaila A. Raji was supported in part by Public Health Service Grant No P30-AG-024832-01. Lynsey Proctor Tamborello received funding from the UTMB Stjepcevich Scholars Program for Medical Students. The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
Contributor Information
Mukaila A. Raji, Email: muraji@utmb.edu, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
Lynsey P. Tamborello, Email: ljprocto@utmb.edu, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
Yong-Fang Kuo, Email: yokuo@utmb.edu, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
Hyunsu Ju, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
Jean L. Freeman, Email: jfreeman@utmb.edu, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
Dong D. Zhang, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA
Sharon H. Giordano, Email: sgiordan@mdanderson.org, Department of Breast Medical Oncology, The University of Texas M.D. Anderson Cancer Center, Houston, TX 77230, USA.
James S. Goodwin, Email: jsgoodwi@utmb.edu, Department of Internal Medicine, Sealy Center on Aging, University of Texas Medical Branch, 301 University Blvd., Rt. 0460, Galveston, TX 77555-0460, USA.
References
- 1.Early Breast Cancer Trialists' CollaborativeGroup (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–34. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldhirsch A, William C, Wood WC, Richard D, Gelber RD, Alan S, et al. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Clin Oncol. 2003;21(17):3357–65. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 4.Scherwath A, Mehnert A, Schleimer B, et al. Neuropsychological function in high- risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: evaluation of long-term treatment effects. Ann Oncol. 2006;17:415–23. doi: 10.1093/annonc/mdj108. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson RJ, Ahles TA. Low neuropsychological performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep. 2003;3(3):215–22. doi: 10.1007/s11910-003-0081-2. [DOI] [PubMed] [Google Scholar]
- 6.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-oncology. 1995;4:61–6. doi: 10.1002/pon.2960040108. [DOI] [Google Scholar]
- 7.Crivellari D, Bonetti M, Castiglione-Gertsch M. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: The International Breast Study Group Trial VII. J Clin Oncol. 2001;18:1412–22. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 8.Hurria A, Brogan K, Panageas KS, Pearce C, Norton L, Jaku-bowski A, et al. Patterns of toxicity in older patients with breast cancer receiving adjuvant chemotherapy. Breast Cancer Res Treat. 2005;92:151–6. doi: 10.1007/s10549-005-1410-8. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–9. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, Ahles TA, Ganz PA, van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–9. doi: 10.1200/JCO.2004.08-094. [DOI] [PubMed] [Google Scholar]
- 11.Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs M, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat. 2006;98(3):343–8. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- 12.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–63. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 13.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–82. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 14.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104:2222–33. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 15.Fallet MG, Sanfilippo A, Maruff P, Wlh L, Phillip KA. The nature and severity of cognitive impairment associated with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–93. doi: 10.1200/JCO.20.2.485. [DOI] [PubMed] [Google Scholar]
- 17.Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology. 2006;15:422–30. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 18.van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–8. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 19.Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85(3):640–50. doi: 10.1002/(SICI)1097-0142(19990201)85:3<640∷AID-CNCR14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn S, Rodenhuis S, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002;13:1387–97. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- 21.Schagen SB, Muller MJ, Boogerd W, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–5. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 22.Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49:92–104. doi: 10.1159/000069773. [DOI] [PubMed] [Google Scholar]
- 23.Wefel JS, Kayle AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691–6. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keime-Guibert F, Napolitano MJY, Delattre JY. Neurological complications of radiotherapy and chemotherapy. J Neurol. 1998;245(11):695–708. doi: 10.1007/s004150050271. [DOI] [PubMed] [Google Scholar]
- 25.Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MHN, Olver IN, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17(8):2355–64. doi: 10.1200/JCO.1999.17.8.2355. [DOI] [PubMed] [Google Scholar]
- 26.Vardy J, Wong K, Yi Q, Park A, Maruff P, Wagner L, et al. Assessing cognitive function in cancer patients. Support Care Cancer. 2006;14(11):1111–8. doi: 10.1007/s00520-006-0037.6. [DOI] [PubMed] [Google Scholar]
- 27.Downie FP, Fan HG, Houédé-Tchen N, Yi Q, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psychooncology. 2006;15(10):921–30. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- 28.Schiff D, Wen P. Central nervous system toxicity from cancer therapies. Hematol Oncol Clin N. 2006;20:1377–99. doi: 10.1016/j.hoc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2003), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission.
- 30.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER cancer statistics review, 1975-2000. Bethesda, MD: USA National Cancer Institute; 2004. p. 748. [Google Scholar]
- 31.Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55:929–37. doi: 10.1016/S0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 32.Potosky AL, Warren JL, Riedel MA, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40 Suppl:IV-62–8. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 33.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for services research using a linked medicare-tumor registry database. Med Care. 1993;31:732–48. doi: 10.1097/00005650-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Goodwin JS. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006 Jun 20;24(18):2750–6. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 35.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40:26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 36.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 37.Du X, Goodwin JS. Increase of chemotherapy use in older women with breast carcinoma from 1991 to 1996. Cancer. 2001;92(4):730–7. doi: 10.1002/1097-0142(20010815)92:4<730∷ AID-CNCR1376>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris Y, Gorelick PB, Freels S, Billingsley M, Brown N, Robinson D. Neuroepidemiology of vascular and Alzheimer's dementia among African-American women. J Natl Med Assoc. 1995;87(10):741–5. [PMC free article] [PubMed] [Google Scholar]
- 39.Tang MX, Cross P, Andrews H, Jacobs DM, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Kivipelto M, et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 41.Newcomer R, Clay T. Misclassification and selection bias when identifying Alzheimer's disease solely from Medicare claims records. J Am Geriatr Soc. 1999;47:215–9. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor DH, Jr, Sloan FA, Doraiswamy PM. Marked increase in Alzheimer's disease identified in Medicare claims records between 1991 and 1999. J Gerontol A Biol Sci Med Sci. 2004;59:762–6. doi: 10.1093/gerona/59.7.m762. [DOI] [PubMed] [Google Scholar]


