Abstract
Primary graft dysfunction (PGD) is the leading cause of early post-transplant morbidity and mortality after lung transplantation. Clara cell secretory protein (CC16) is produced by the non-ciliated lung epithelium and may serve as a plasma marker of epithelial cell injury. We hypothesized that elevated levels of CC16 would be associated with increased odds of PGD. We performed a prospective cohort study of 104 lung transplant recipients. Median plasma CC16 levels were determined at three time points: pre-transplant and 6 and 24 hours post transplant. The primary outcome was the development of grade 3 PGD within the first 72 hours after transplantation. Multivariable logistic regression was performed to evaluate for confounding by donor and recipient demographics and surgical characteristics. Twenty-nine patients (28%) developed grade 3 PGD within the first 72 hours. The median CC16 level 6 hours after transplant was significantly higher in patients with PGD (13.8 ng/ml (IQR 7.9, 30.4 ng/ml)) than in patients without PGD (8.2 ng/ml (IQR 4.5, 19.1 ng/ml)), p = 0.02. Elevated CC16 levels were associated with increased odds of PGD after lung transplantation. Damage to airway epithelium or altered alveolar permeability as a result of lung ischemia and reperfusion may explain this association.
Keywords: Primary Graft Dysfunction, Lung transplantation, Clara Cell Secretory Protein
INTRODUCTION
Lung transplantation has become an accepted therapy for many forms of advanced lung disease, with 2,708 transplant procedures reported to the Registry of the International Society for Heart and Transplantation in 2007 (1). However, both short- and long-term outcomes lag significantly behind those of other solid organ transplants. From 1994–2007, median survival after lung transplantation was only approximately 5.7 years compared to 10 years after heart transplantation (1, 2).
Primary graft dysfunction (PGD) remains the leading cause of early post-transplantation morbidity and mortality, although the exact incidence is unclear; reports indicate 10–25% of lung transplant recipients are affected (3–7). Thought to be a form of ischemia reperfusion injury, severe PGD leads to worse physical function, increased risk of BOS, and increased risk of death (5, 8–11).
Biomarker studies have suggested important mechanistic pathways in clinical PGD (12–19). Injury to the distal airway epithelium is a common finding in PGD and acute lung injury (ALI). Clara Cell Secretory Protein (CCSP), also known as CC16, is produced by the non-ciliated lung epithelium and circulating plasma levels may reflect lung epithelial cell injury. The function of Clara cells appears to be the protection and repair of respiratory epithelium, detoxification of toxins and production of surfactant proteins (20). CC16, the primary protein produced by these cells, has already been identified as a potential marker of airway injury in other pathologic conditions, including ALI. A previous study showed that lower median CC16 levels were associated with ALI compared to controls with cardiogenic pulmonary edema (21). Serum CC16 levels were also decreased in patients with bronchiolitis obliterans after lung and allogeneic stem-cell transplantation (22, 23). Brief exposure to smoke inhalation increases CC16 concentrations (24).
We hypothesized that elevated levels of plasma CC16 would be associated with the development of high grade PGD after lung transplantation. Additionally, we aimed to identify associations between pre-operative plasma CC16 levels and common diagnoses leading to transplantation.
MATERIALS AND METHODS
Selection of Patients
The Lung Transplant Outcomes Group (LTOG) cohort in the United States is an ongoing multi-center, prospective cohort of lung transplant patients that has been previously described (13–15). In this study, we included subjects from two sites, the University of Pennsylvania and Columbia, enrolled between October 2006 and May 2008. Blood samples were collected before transplant and at 6 and 24 hours after allograft reperfusion. Plasma samples were centrifuged within 30 minutes and then stored at −80°C for subsequent analysis. Clinical data were collected prospectively for all patients as described in detail elsewhere (6). The Institutional Review Boards at each site approved this study. Informed consent was obtained from each patient enrolled in the cohort.
Determination of PGD Grade
The severity of PGD was graded according to the International Society for Heart and Lung Transplantation criteria (25). Chest radiographs were examined by two trained physicians to assess for the presence and severity of PGD with adjudication. These assessments were done immediately after transplantation (T0) and at 24, 48, and 72 hours post-transplant and scores were reported at each time point. All patients with PGD had radiographs revealing diffuse alveolar infiltrates involving the lung allograft(s); the native lung was spared in patients undergoing single lung transplant. The severity of PGD was graded by determination of the PaO2/FiO2 ratio. Grade 3 PGD was defined as PaO2/FiO2 <200 with exclusion of secondary causes (6, 13, 15, 25, 26). The primary outcome in this study was defined as grade 3 PGD at any time point within 72 hours post transplant. Sensitivity analysis was performed using grade 3 PGD present at the 72 hour time point post-transplant as the outcome.
Measurement of Plasma CC16 levels
Plasma CC16 concentrations were measured in duplicate using a commercially available sandwich enzyme-linked immunosorbent assay (Biovendor; Candler, NC). The intraassay coefficient of variation for this assay was 2.95%.
Statistical Analysis
Continuous variables were compared using two sample t-tests or Wilcoxon rank sum tests as appropriate. Proportions across the two groups were compared using two-group proportion tests while characteristics with greater than two variables were assessed with Kruskal-Wallis rank tests. Spearman rank correlation was used to evaluate the relationship of total ischemic time as a continuous variable with CC16 concentration and Wilcoxon rank sum tests to assess the association of ischemic time as a categorical variable to CC16 level. Ischemic time was additionally categorized as high or low using a cutoff of 330 minutes (27). Fractional polynomial fitting was used to evaluate the linearity of the relationship between CC16 and predicted probability of PGD. The relationship of PGD with CC16 at 6 hours after transplant was evaluated using logistic regression. Previous studies of ALI used plasma and BAL sample CC16 levels measured after the development of radiographic and physiologic changes (21). An a priori defined analysis stratified by pre-transplant diagnosis was performed. Additionally, the interaction of CC16 with pre-transplant pulmonary diagnosis leading to transplantation (idiopathic pulmonary fibrosis (IPF) vs. other) was assessed using interaction terms. We assessed donor and recipient age, race, and sex, total ischemic time, cardiopulmonary bypass, pulmonary artery systolic pressure, packed red blood cell and platelet transfusion, and transplant type individually as potential confounders (defined as > 20% change in beta coefficient upon addition of a covariate to the model). Based on the number of events in the cohort, a maximum of three covariates could be included in a multivariable model (28). Generalized estimating equations (GEE) were utilized to assess differences in CC16 profiles over time between PGD and non-PGD subjects. A sensitivity analysis of the time course of CC16 levels with the development of PGD was performed by subdividing the cohort into “early PGD” (PGD in the first 24 hours, “not early PGD” (all patients without PGD in the first 24 hours), “later PGD” (PGD developing after 24 hours), and “no PGD”. For all of the statistical analysis, a p-value < 0.05 was considered statistically significant; p-value < 0.1 was considered significant for logistic regression interaction terms. The Bonferroni correction was utilized to account for multiple comparisons. Reported CC16 concentrations are median values. All statistical analysis was done using STATA 10.1 (STATA Corp., College Station, TX).
RESULTS
There were 104 patients in the study sample. Sixty-six had pre-transplant samples, 104 had samples at 6 hours post-reperfusion, and 98 had samples at 24 hours post-reperfusion. Twenty-nine (28%) developed grade 3 PGD within the first 72 hours post-transplant. Table 1 shows recipient, donor, and surgical characteristics for the study sample. The distribution of pre-transplant diagnoses was different between the two groups (p=0.01), with more pulmonary fibrosis in the PGD group and more cystic fibrosis (CF) in the non-PGD group. In addition, intra-operative cardiopulmonary bypass was used more frequently and intra-operative PASP was significantly higher in patients who developed grade 3 PGD than in those without PGD.
Table 1.
Donor and recipient characteristics stratified by Primary Graft Dysfunction Status
| Variables | PGD (n = 29) | No PGD (n = 75) | p-value |
|---|---|---|---|
| Recipient | |||
| Age, yr | 53 (49, 58) | 52 (49, 55) | 0.6 |
| Female Gender | 41% | 51% | 0.4 |
| Race | 0.04 | ||
| Caucasian | 72% | 89% | |
| African American | 24% | 8% | |
| Hispanic | 0% | 3% | |
| Asian | 3% | 0% | |
| Donor | |||
| Age, yr | 39 (33, 44) | 36 (32, 39) | 0.4 |
| Female Gender | 41% | 53% | 0.3 |
| Race | 0.3 | ||
| Caucasian | 69% | 56% | |
| African American | 17% | 27% | |
| Hispanic | 10% | 8% | |
| Asian | 3% | 5% | |
| Other | 0% | 3% | |
| Recipient Diagnosis | 0.01 | ||
| Chronic Obstructive Pulmonary Disease | 28% | 37% | |
| Cystic Fibrosis | 7% | 24% | |
| Pulmonary Fibrosis | 41% | 35% | |
| Pulmonary Arterial Hypertension | 7% | 1% | |
| Sarcoidosis | 14% | 1% | |
| Transplant Type, single | 17% | 36% | 0.06 |
| Use of Cardiopulmonary Bypass | 66% | 39% | 0.01 |
| Time on Bypass, min | 218 (187, 250) | 230 (209, 250) | 0.5 |
| Ischemic Time, min | 305 (285, 325) | 322 (286, 357) | 0.4 |
| Pulmonary Artery Systolic Pressure, mmHg | 47 (42, 51) | 40 (38, 41) | 0.008 |
| Packed Red Blood Cells, ml | 464 (206, 722) | 456 (277, 635) | 0.9 |
| Fresh Frozen Plasma, ml | 820 (542, 1099) | 698 (482, 914) | 0.5 |
| Platelets, ml | 296 (0, 662) | 141 (27, 255) | 0.3 |
Donor and recipient characteristics stratified by Primary Graft Dysfunction Status
PGD is defined as any grade 3 PGD during first 72 hours.
Continuous variables are expressed as means with 95% confidence intervals, while dichotomous and categorical variables are expressed as percentages, which may not exactly total 100% because of rounding.
PGD subjects had higher median CC16 levels at 6 hours after reperfusion than did subjects without PGD (13.8 ng/ml vs. 8.2 ng/ml, uncorrected p=0.01, corrected p=0.04) (Figure 1). There was no significant difference between PGD and non-PGD median CC16 levels measured pre-transplant (9.4 ng/ml vs. 5.7 ng/ml, p=0.08) or at 24 hours (8.5 ng/ml vs. 6.9 ng/ml, p=0.7). The results were similar when we defined PGD at the 72 hour time point.
Figure 1.
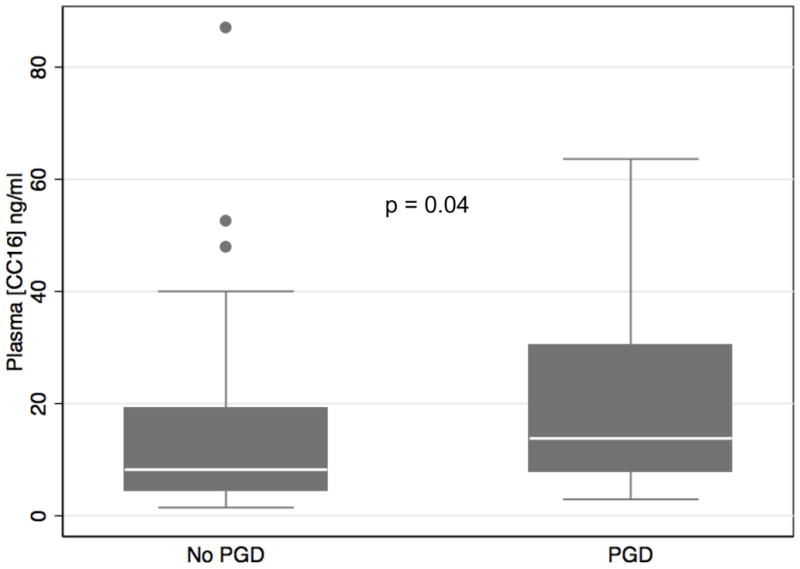
Plasma CC16 concentration 6 hours after transplant in all patients stratified by the development of Grade 3 PGD any time in the first 72 hours. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the interquartile range. Solid circles represent outliers. P-value reported is from Wilcoxon rank sum test and is corrected for multiple comparisons.
The median pre-transplant CC16 concentration was 5.0 ng/ml (IQR 2.92, 6.93) in patients with underlying chronic obstructive pulmonary disease (COPD), 4.25 ng/ml (IQR 2.57, 5.64) in patients with CF, and 13.87 ng/ml (IQR 6.1, 19.15) in patients with IPF (Figure 2). Pre-operative CC16 concentrations were higher in IPF patients than in patients with CF or COPD regardless of transplant type, while post-operative levels at 6 and 24 hours were not statistically different across predisposing diagnosis.
Figure 2.
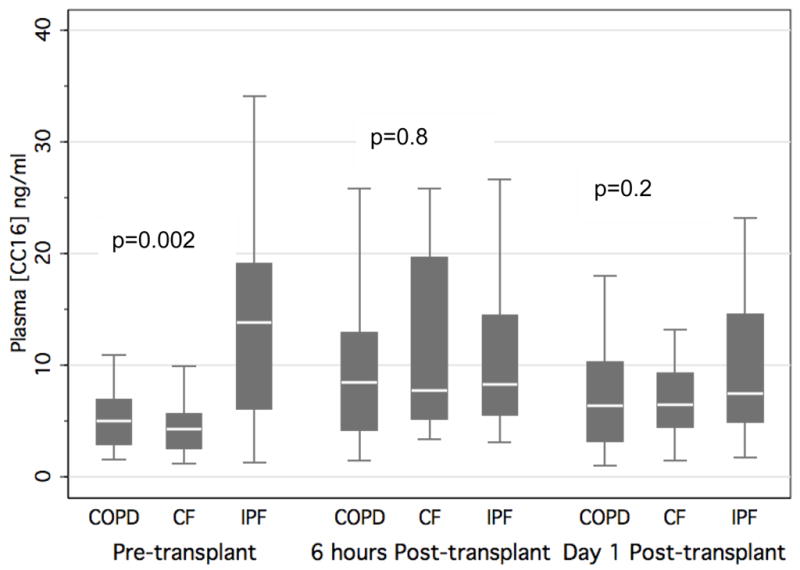
Plasma CC16 concentration pre-transplant, 6 hours post-transplant, and post-operative day 1 stratified by the three most common diagnoses leading to transplant in all patients. P-values reported are for Kruskal-Wallis equality-of-populations rank test.
Horizontal line indicates median concentration. The upper and lower limits of the box indicate the interquartile range.
Longitudinal GEE analysis of biomarker profiles across all time points identified a significant association between CC16 level over time and PGD in the overall cohort (β= 0.4, 95%CI 0.1, 0.6, p=0.01). There was a linear relationship between CC16 level at 6 hours after transplantation and the predicted probability of PGD. The OR for PGD was 1.6 for every 15 ng/ml increase in CC16 at 6 hours (p=0.03). The odds ratio for developing PGD was determined per 15 ng/ml change based on the standard deviation of plasma CC16 concentrations. There was no association between CC16 levels pre-transplant (OR=2.1, 95% CI 0.9, 4.9) or at 24 hours (OR=1.2, 95% CI 0.8, 1.8) and PGD.
When stratified by pre-transplant diagnosis, GEE analysis demonstrated a significant association among non-IPF patients (β=0.8, 95%CI 0.4, 1.3, p<0.001) but not for IPF patients (β=−0.1, 95%CI −0.6, 0.3, p=0.5). In logistic regression models, the p for interaction for CC16 level and IPF diagnosis was significant at 6 hours (p=0.1) and at 24 hours (p=0.05). While the median CC16 levels at 6 hours after transplant in IPF patients with PGD (13.8 ng/ml, IQR 6.3, 16.5) and those without (8.0 ng/ml, IQR 5.5, 14.5) were similar in magnitude to the values of the overall cohort, the difference did not meet statistical significance in this subpopulation (p=0.3). Some of this discrepancy is explained by the wider interquartile range for the non-IPF patients with PGD and by the effect of the outliers in the IPF subpopulation (Figure 3). Additionally, the OR for PGD for every 15 ng/ml increase in CC16 at 6 hours was 2.1 (95%CI 1.2–3.7, p=0.01) in non-IPF patients and 1.1 (95%CI 0.6–2.0, p=0.7) in IPF patients. For these reasons, we focused on patients without IPF in multivariate analyses using the 6 hour CC16 level (Table 2). The association of 6 hour CC16 levels and PGD remained significant when individually controlling for recipient and donor race, sex and age, cardiopulmonary bypass, transplant type, packed red blood cell or platelet transfusion, and ischemic time (all p<0.04; Table 2). Intra-operative pulmonary artery systolic pressure attenuated the relationship between CC16 level and PGD (p=0.05).
Figure 3.
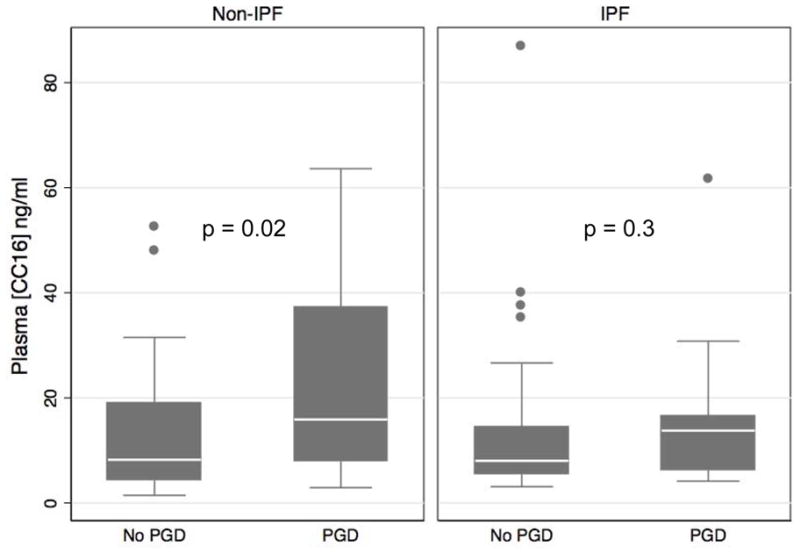
Plasma CC16 concentration 6 hours after transplant in IPF or Non-IPF patients stratified by the development of Grade 3 PGD any time in the first 72 hours. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the interquartile range. Solid circles represent outliers. P-value reported is from Wilcoxon rank sum test.
Table 2.
Odds ratios for the development of Primary Graft Dysfunction in logistic regression models
| Model | Odds Ratio per 15 ng/ml increase in [CC16] (95% CI) | p-value |
|---|---|---|
| Unadjusted Base Model (n=103) | 1.6 (1.03, 2.3) | 0.03 |
| Non-IPF only (n=67) | 2.1 (1.2, 3.7) | 0.01 |
| IPF only (n=36) | 1.1 (0.6–2.0) | 0.7 |
| Non-IPF Adjusted for: | ||
| Cardiopulmonary Bypass (n=67) | 2.0 (1.1, 3.6) | 0.02 |
| Transplant Type (n=67) | 2.1 (1.2, 3.8) | 0.01 |
| Recipient Age (n=67) | 2.0 (1.2, 3.6) | 0.02 |
| Recipient Sex (n=67) | 2.1 (1.2, 3.7) | 0.01 |
| Recipient Race/Ethnicity (n=67) | 1.9 (1.04, 3.5) | 0.04 |
| Donor Age (n=60) | 2.2 (1.2, 4.3) | 0.01 |
| Donor Sex (n=65) | 2.0 (1.1, 3.6) | 0.02 |
| Donor Race/Ethnicity (n=64) | 2.2 (1.2, 4.0) | 0.01 |
| Total ischemic time (n=66) | 2.1 (1.2, 3.9) | 0.01 |
| PASP (n=66) | 1.8 (1.0–3.4) | 0.05 |
| Packed Red Blood Cells (n=43) | 2.3 (1.1, 4.8) | 0.02 |
| Platelets (n=47) | 2.3 (1.2, 4.4) | 0.02 |
Odds ratios for the development of Primary Graft Dysfunction in logistic regression models per 15 ng/ml increase in [CC16].
IPF = Idiopathic Pulmonary Fibrosis
PASP = Pulmonary Arterial Systolic Pressure
OR determined using 6 hour [CC16]
Given previous identification of a correlation between allograft ischemia and PGD and the transient association of CC16 level with PGD, we further assessed the association between total ischemic time and CC16 level. Spearman rank correlation revealed no association of CC16 level measured at 6 or 24 hours after transplant and total ischemic time when subjects were categorized as single or bilateral transplant (p>0.3 for all comparisons). Similarly, when the total ischemic time was categorized as high or low, there was no association between CC16 concentration at 6 or 24 hours and ischemic time category for single or bilateral transplant recipients (p>0.1 for all comparisons).
We assessed the relationship between CC16 levels measured at 6 hours and the timing of onset of PGD. Twenty-one patients developed “early PGD” after transplant and had a median CC16 concentration of 14.7 ng/ml (IQR 7.9, 37.3). There was a statistically significant difference in median CC16 level when comparing these 21 “early PGD” patients to the “not early PGD” group (8.5 ng/ml, IQR 4.9, 19.2), p=0.03. We then further analyzed the “not early PGD” group and identified 7 patients who developed “later PGD”. There was no statistically significant difference between the median CC16 concentration in the “later PGD” group (10.7 ng/ml, IQR 7.9, 30.1) and the “no PGD” group (8.2 ng/ml, IQR 4.5, 19.1), p=0.2.
DISCUSSION
Elevated post-operative CC16 levels were significantly associated with the risk of developing severe PGD. Longitudinal analysis also demonstrated significant differences in CC16 profiles over time between PGD and non-PGD subjects. This relationship was not seen in patients with IPF, a disease associated with baseline elevation of serum CC16 levels. In non-IPF patients, the association of elevated CC16 levels 6 hours post-transplant was independent of multiple donor, recipient, and surgical characteristics. This is the first study to evaluate the potential role of CC16 as a biomarker for the subsequent development of PGD and may provide additional insight into the role of lung epithelial injury in the pathogenesis of PGD and subsequent development of long-term complications such as BOS.
Rat models of lipopolysaccharide-induced acute lung injury have shown elevated plasma and decreased bronchoalveolar lavage fluid CC16 levels to be associated with acute lung injury (29). However, human studies of patients with acute lung injury have shown mixed results. For example, one study reported that CC16 levels were significantly lower in patients with ALI compared to those with cardiogenic pulmonary edema (21). In contrast, elevated CC16 levels were found in patients with ALI developing after ventilator-associated pneumonia (30). Our findings suggest that peri-transplant injury to the respiratory epithelium and subsequent disruption of the alveolar capillary barrier increases permeability and results in leakage of CC16 into the plasma. However, to further assess this would require concurrent measurement of CC16 in bronchoalveolar lavage fluid, measurements that were beyond the scope of the current evaluation as concurrent post-transplant BAL samples were not prospectively collected on the study subjects.
CC16 concentrations measured 24 hours after reperfusion were not associated with the presence or development of PGD, indicating that release or secretion of CC16 may be closely associated temporally with the ischemia-reperfusion injury initiated intra-operatively. Epithelial cell damage resulting in Clara Cell dropout early after reperfusion is a possible explanation for these findings. In vitro, both CC16 and phospholipase-A2 (PLA2), a potent pro-inflammatory protein, were induced by tissue necrosis factor-alpha (TNF-α). CC16 inhibits PLA2 function, suggesting an anti-inflammatory role (31–33). An alternative explanation to epithelial cell injury is that CC16 production may actually be upregulated in response to ischemia-reperfusion injury in the allograft, perhaps in an attempt to modulate inflammatory cascades. It is also unknown what the effect of high dose corticosteroids, administered intra-operatively, and other immunosuppressive medications have on the function or activity of CC16.
Plasma levels of CC16 were not associated with increased odds of severe PGD in IPF; patients with IPF had a higher baseline CC16 level compared to others. Small sample size prevented a subgroup analysis of IPF subjects to evaluate the differential effect of the remaining IPF native lung on CC16 levels in single lung transplantation. The baseline elevation in CC16 in IPF may have masked any temporal changes related to transplantation. Indeed, other serum biomarkers have performed differently in lung recipients with IPF compared to other causes of end stage lung disease when evaluated prior to lung transplantation. Surfactant protein-D differentiates IPF from acute respiratory distress syndrome, chronic beryllium disease, progressive systemic sclerosis, and normal controls (34). Endothelin-1 levels in bronchoalveolar lavage fluid were found to be significantly higher in patients with IPF, fibrosing alveolitis in systemic sclerosis, and sarcoidosis compared with COPD, occupational lung disease, and healthy controls (35). Matrix metalloproteinase-1 and -7 have been shown to differentiate IPF from COPD, hypersensitivity pneumonitis, and sarcoidosis (36). While the sample size of IPF patients with PGD was small and may have limited our ability to detect statistically significant differences in CC16 concentrations compared to non-PGD IPF patients, the interaction term for IPF diagnosis in the multivariable regression analysis was significant and the direction and magnitude of the association between CC16 and PGD was also identified in the unadjusted base model. Pre-transplant CC16 levels in IPF patients were similar to post-transplant levels in non-IPF PGD patients, suggesting that significant epithelial injury may also be a component of IPF. A future direction of study is a more detailed investigation of the association of CC16 and PGD in larger numbers of non-IPF patients.
In the only other study of CC16 in lung transplant patients, CC16 levels in serum and BAL measured at the time of screening bronchoscopy greater than 2 weeks after transplant were decreased in patients with bronchiolitis obliterans syndrome (BOS) (23). PGD has been shown to be a risk factor for later BOS (10, 11, 37, 38). While the function is not completely defined, CC16 is thought to have an immunomodulatory role in the lung. Additionally, CC16 has anti-oxidant activity in rat and mice models of ozone exposure and oxidant stress (39). Oxidant stress has been implicated in the pathogenesis of both PGD and BOS (40, 41). The independent associations of elevated CC16 levels with PGD and later decreased levels with BOS provides an intriguing possible pathogenic connection between the two conditions.
There were several limitations to our study. First, there was the potential for misclassification of PGD grade. In this study, PGD grade was determined by two investigators according to a well-validated scoring system. Altering the definition of severe PGD from any grade 3 PGD by the 72 hour time point to presence of grade 3 at 72 hours did not significantly alter the differences in median CC16 levels between PGD and non-PGD patients. The potential for bias due to loss to follow up was reduced in this study because the outcomes of interest were determined within three days of transplant. There was also potential for bias secondary to missing data. Some of the subjects were not fully characterized by clinical or surgical covariates, although these values appear to be missing at random, limiting the potential for bias. While we hypothesize that epithelial cell damage leads to increased CC16 serum concentrations, the absence of concurrent BAL fluid measurements limits the conclusions that can be drawn from these data. We were also unable to conclude a causal relationship between CC16 and PGD although this study does suggest a potential mechanistic role for CC16 in the clinical syndrome of PGD. We were able to identify concurrence of CC16 elevation and the development of PGD but the ability to demonstrate a predictive role for PGD was limited by the small number of subjects who developed PGD greater than 24 hours after reperfusion and because the presence or absence of PGD at the exact time the plasma sample was taken is unknown. Further evaluation of CC16 as a potential predictive biomarker is an avenue for future study. While we lacked separate measurements for warm and cold ischemic times, we saw no effect when adjusting for total ischemic time. Finally, there is the potential for unmeasured confounders, including occult bacterial colonization of the allograft, to give a false positive result. However, patients in this cohort were prospectively enrolled with careful characterization of clinical and surgical characteristics. In addition, we performed bivariable logistic regression to assess for confounding by previously documented clinical and surgical independent risk factors for PGD.
In summary, we identified increased postoperative CC16 plasma levels to be strongly associated with severe PGD. This study provides increasing support for the possible role of significant epithelial cell injury in the pathogenesis for severe PGD and builds on several prior reports of the role of epithelial injury in PGD (14, 42). These findings should be further investigated by prospectively evaluating CC16 levels in BAL fluid and plasma of patients with and without severe PGD over time until the development of BOS.
Acknowledgments
This study was funded by NIH grants HL 087115, HL086919, HL081332 and HL088263.
ABBREVIATIONS
- PGD
Primary graft dysfunction
- CC16
Clara Cell Secretory Protein
- CCSP
Clara Cell Secretory Protein
- ALI
Acute lung injury
- LTOG
Lung Transplant Outcomes Group
- IPF
Idiopathic pulmonary fibrosis
- COPD
Chronic obstructive pulmonary disease
- CF
Cystic fibrosis
- BOS
Bronchiolitis obliterans syndrome
- TNF-α
Tissue necrosis factor-alpha
- PLA2
Phospholipase-A2
- PASP
Pulmonary Arterial Systolic Pressure
- GEE
Generalized Estimating Equations
Footnotes
DISCLOSURE: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult lung and heart-lung transplantation report--2009. J Heart Lung Transplant. 2009;28(10):1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009;28(10):1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. J Heart Lung Transplant. 2005;24(10):1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal N, Kaiser LR, et al. Primary Graft Failure Following Lung Transplantation. Chest. 1998;114(1):51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 7.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009;6(1):39–46. doi: 10.1513/pats.200808-082GO. [DOI] [PubMed] [Google Scholar]
- 8.Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127(1):161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, et al. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–195. doi: 10.1016/j.athoracsur.2008.03.073. discussion 196–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 11.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 12.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Respir Crit Care Med. 2008;177(6):660–668. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–1015. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, et al. Antitype V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181(8):5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krenn K, Klepetko W, Taghavi S, Lang G, Schneider B, Aharinejad S. Recipient vascular endothelial growth factor serum levels predict primary lung graft dysfunction. Am J Transplant. 2007;7(3):700–706. doi: 10.1111/j.1600-6143.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawut SM, Okun J, Shimbo D, Lederer DJ, De Andrade J, Lama V, et al. Soluble p-selectin and the risk of primary graft dysfunction after lung transplantation. Chest. 2009;136(1):237–244. doi: 10.1378/chest.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 21.Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattsson J, Remberger M, Andersson O, Sundberg B, Nord M. Decreased serum levels of clara cell secretory protein (CC16) are associated with bronchiolitis obliterans and may permit early diagnosis in patients after allogeneic stem-cell transplantation. Transplantation. 2005;79(10):1411–1416. doi: 10.1097/01.tp.0000158354.39635.ab. [DOI] [PubMed] [Google Scholar]
- 23.Nord M, Schubert K, Cassel TN, Andersson O, Riise GC. Decreased serum and bronchoalveolar lavage levels of Clara cell secretory protein (CC16) is associated with bronchiolitis obliterans syndrome and airway neutrophilia in lung transplant recipients. Transplantation. 2002;73(8):1264–1269. doi: 10.1097/00007890-200204270-00013. [DOI] [PubMed] [Google Scholar]
- 24.Bernard A, Hermans C, Van Houte G. Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup Environ Med. 1997;54(1):63–65. doi: 10.1136/oem.54.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 26.King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, et al. Reperfusion Injury Significantly Impacts Clinical Outcome after Pulmonary Transplantation. Ann Thorac Surg. 2000;69(6):1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 27.Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly JF, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171(7):786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 29.Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A. Clara cell specific protein (CC16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. Am J Respir Crit Care Med. 2000;161(5):1624–1630. doi: 10.1164/ajrccm.161.5.9812157. [DOI] [PubMed] [Google Scholar]
- 30.Determann RM, Millo JL, Waddy S, Lutter R, Garrard CS, Schultz MJ. Plasma CC16 levels are associated with development of ALI/ARDS in patients with ventilator-associated pneumonia: a retrospective observational study. BMC Pulm Med. 2009;9:49. doi: 10.1186/1471-2466-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson O, Nordlund-Moller L, Barnes HJ, Lund J. Heterologous expression of human uteroglobin/polychlorinated biphenyl-binding protein. Determination of ligand binding parameters and mechanism of phospholipase A2 inhibition in vitro. J Biol Chem. 1994;269(29):19081–19087. [PubMed] [Google Scholar]
- 32.Lesur O, Bernard A, Arsalane K, Lauwerys R, Begin R, Cantin A, et al. Clara cell protein (CC-16) induces a phospholipase A2-mediated inhibition of fibroblast migration in vitro. Am J Respir Crit Care Med. 1995;152(1):290–297. doi: 10.1164/ajrccm.152.1.7541278. [DOI] [PubMed] [Google Scholar]
- 33.Yao XL, Levine SJ, Cowan MJ, Logun C, Shelhamer JH. Tumor necrosis factor-alpha stimulates human Clara cell secretory protein production by human airway epithelial cells. Am J Respir Cell Mol Biol. 1998;19(4):629–635. doi: 10.1165/ajrcmb.19.4.3129. [DOI] [PubMed] [Google Scholar]
- 34.Greene KE, King TE, Jr, Kuroki Y, Bucher-Bartelson B, Hunninghake GW, Newman LS, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19(3):439–446. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 35.Reichenberger F, Schauer J, Kellner K, Sack U, Stiehl P, Winkler J. Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung. 2001;179(3):163–174. doi: 10.1007/s004080000058. [DOI] [PubMed] [Google Scholar]
- 36.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, et al. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8(11):2454–2462. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26(10):1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clinical and Experimental Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 40.Behr J, Maier K, Braun B, Schwaiblmair M, Vogelmeier C. Evidence for oxidative stress in bronchiolitis obliterans syndrome after lung and heart-lung trnsplantation: The Munich Lung Transplant Group. Transplantation. 2000;69(9):1856–1860. doi: 10.1097/00007890-200005150-00020. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch J, Elssner A, Mazur G, Maier K, Bittmann I, Behr J, et al. Bronchiolitis obliterans syndrome after (Heart-) Lung Transplantation. American Journal of Critical Care Medicine. 1999;160(5):1640–1646. doi: 10.1164/ajrccm.160.5.9902012. [DOI] [PubMed] [Google Scholar]
- 42.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159(3):980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]


