Abstract
Functional imaging studies have reported with remarkable consistency hyperactivity in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and caudate nucleus of patients with Obsessive-Compulsive Disorder (OCD). These findings have often been interpreted as evidence that abnormalities in cortico-basal ganglia-thalamo-cortical loops involving the OFC and ACC are causally related to OCD. This interpretation remains controversial, however, because such hyperactivity may represent either a cause or a consequence of the symptoms. This article analyzes the evidence for a causal role of these loops in producing OCD in children and adults. The article first reviews the strong evidence for anatomical abnormalities in these loops in patients with OCD. These findings are not sufficient to establish causality, however, because anatomical alterations may themselves be a consequence rather than a cause of the symptoms. The article then reviews three lines of evidence that, despite their own limitations, permit stronger causal inferences: the development of OCD following brain injury, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection, and neurosurgical lesions that attenuate OCD. Converging evidence from these various lines of research supports a causal role for the cortico-basal ganglia-thalamo-cortical loops that involve the OFC and ACC in the pathogenesis of OCD in children and adults.
Obsessive-Compulsive Disorder (OCD) is a debilitating illness (Koran, 2000; Koran, Thienemann, & Davenport, 1996) that has a lifetime prevalence of 2–3% in nearly every country for which epidemiological data are available (Bland, Newman, & Orn, 1988; Horwath & Weissman, 2000; Karno, Golding, Sorenson, & Burnam, 1988; Robins et al., 1984; Sasson et al., 1997; Weissman et al., 1994). It affects an estimated 50 million people worldwide (Sasson et al., 1997). OCD is typically characterized by the presence of both obsessions and compulsions, although the presence of either obsessions or compulsions alone is sufficient to make the diagnosis of OCD (DSM-IV). Obsessions are recurrent, persistent, and intrusive ego-dystonic thoughts, impulses, or images; compulsions are repetitive behaviors or mental acts that are executed with the goal of preventing or reducing distress, or preventing some dreaded event or situation (DSM-IV).
Childhood- Versus Adult-Onset OCD
The age of onset of OCD seems to have a bimodal distribution, with a peak in childhood (at approximately 10 years of age) and another in early adulthood (Geller, 2006; Geller, Biederman, Jones, Shapiro et al., 1998). Estimates of the prevalence of OCD among adolescents are of approximately 2 – 3.5% (Flament et al., 1988; Valleni-Basile et al., 1994; Zohar et al., 1992). Approximately 40% of cases of childhood-onset OCD continue into adulthood, and that number increases to 60% when subthreshold presentations of OCD are also considered (Stewart et al., 2004). Conversely, one-third to one-half of adults with OCD have the onset of their symptoms in childhood or adolescence (Eichstedt & Arnold, 2001).
Childhood- and adult-onset OCD differ in several ways. Compared to adult-onset OCD, childhood-onset OCD is more prevalent among males (Eichstedt & Arnold, 2001; Geller, Biederman, Jones, Shapiro et al., 1998), is more familial (Bellodi, Sciuto, Diaferia, Ronchi, & Smeraldi, 1992; do Rosario-Campos et al., 2005; Nestadt et al., 2000; Pauls, Alsobrook, Goodman, Rasmussen, & Leckman, 1995), and is associated with a higher prevalence of tic disorders, both in the patients with OCD themselves and in their first-degree relatives (Chabane et al., 2005; Grados et al., 2001; Pauls et al., 1995). These findings have led some authors to suggest that childhood-onset OCD is a distinct, “tic-related” subtype of OCD (Eichstedt & Arnold, 2001; S. Taylor, 2005). The distinction between childhood- and adult-onset OCD may also have treatment implications: adults with childhood-onset OCD respond less to treatment with clomipramine than do adults with adult-onset OCD, independent of illness duration or severity (Ackerman, Greenland, Bystritsky, Morgenstern, & Katz, 1994).
Most brain imaging studies that include adults with OCD use mixed samples that include adults with childhood- and adult-onset OCD. Given the evidence that brain activity differs in adults with childhood- versus adult-onset OCD (Busatto et al., 2001), such heterogeneous samples may compromise statistical power and confound findings. In addition, many studies report only the mean and standard deviation of age of illness onset, which are inadequate to characterize what are likely to be bimodal distributions. Consequently, knowing whether findings apply to all adults with OCD or only to those with childhood- or adult-onset OCD is impossible for most existing studies that include adults with OCD.
In addition, few studies have compared the neural correlates of OCD across children and adults, severely limiting our knowledge of their similarities and differences. One of the aims of this review is to begin to redress this gap by systematically evaluating the similarities and differences in findings from studies of the neural bases of pediatric and adult OCD. We should note, however, that differences in findings across these age groups may be due to several factors. We list four, non-exhaustive possibilities. First, any such differences may be a consequence of comparing children with OCD with mixed samples that include adults with childhood-onset OCD and adults with adult-onset OCD. If childhood-onset OCD is a distinct biological subtype, this comparison confounds age or developmental effects with the effects of illness subtype. Second, such differences may be a consequence of compensatory behavioral, cognitive, or affective responses, which may be more developed – or simply different – in adults. Third, such differences may reflect the differences in phenomenology between pediatric and adult OCD (Geller, Biederman, Jones, Park et al., 1998). Fourth, such differences may be a consequence of brain development, affecting either the neural systems directly involved in the symptoms of OCD or closely related systems.
Functional Neuroimaging of OCD
Much of our understanding of the pathophysiology of OCD has been derived from functional neuroimaging studies. Broadly speaking, these can be divided into four categories, according to the experimental paradigm used (Saxena & Rauch, 2000): (a) resting studies, which compare some measure of brain activity at rest in patients with OCD and controls; (b) symptom provocation studies, which compare brain activity before and after the provocation of symptoms (e.g., through contact with something dirty in patients with obsessions about germs and cleanliness); (c) pre-/post-treatment studies, which compare brain activity at rest before and after treatment with medication or psychotherapy; and (d) cognitive activation studies, which compare brain activity in patients with OCD and controls as they perform a cognitive task. Combinations of these categories are also possible: one can, for example, image cognitive activation or symptom provocation before and after treatment (Lazaro et al., 2008; Nakao et al., 2005b), or image cognitive activation before and after symptom provocation.
Three brain areas – the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), and the head of the caudate nucleus – have been consistently implicated in a large number of resting, symptom provocation, and pre/post-treatment studies of adults with OCD. These areas (a) are hyperactive at rest in adults with OCD relative to healthy controls, (b) become more active with symptom provocation, and (c) no longer show hyperactivity at rest following successful treatment with either medication or cognitive-behavioral therapy (Baxter, Clark, Iqbal, & Ackermann, 2001; Saxena, Bota, & Brody, 2001; Saxena, Brody, Schwartz, & Baxter, 1998; Saxena & Rauch, 2000; Schwartz, 1998; Whiteside, Port, & Abramowitz, 2004). These findings have generally been interpreted as evidence that abnormalities in these or closely related areas cause OCD (e.g., Baxter et al., 2001; Saxena et al., 2001; Saxena et al., 1998; Saxena & Rauch, 2000).
Two studies have compared resting blood flow before and after pharmacological treatment in children with OCD (Castillo et al., 2005; Diler, Kibar, & Avci, 2004). One of these studies (Diler et al., 2004) also compared resting blood flow in the children with OCD before treatment with resting blood flow in a group of healthy controls. The findings of this study were largely consistent with the findings in adults, including hyperactivity at rest in the caudate and ACC of treatment-naïve children, which declined following treatment. The other study (Castillo et al., 2005) failed to detect any differences between pre- and post-treatment scans. We are not aware of any symptom provocation studies in children with OCD.
The vast majority of functional imaging studies of OCD through the end of the 1990s used resting, symptom provocation, or pre/post-treatment designs. A comprehensive review of imaging studies of OCD that was published in 2001 listed 17 resting studies, 7 symptom provocation studies, 10 pre/post-treatment studies, and only 4 cognitive activation studies (Saxena et al., 2001). The situation has shifted in recent years, with the publication of a large number of cognitive activation studies in adults with OCD, using a wide variety of cognitive tasks (with an emphasis on executive function tasks). The tasks used have included the Go/NoGo (Maltby, Tolin, Worhunsky, O’Keefe, & Kiehl, 2005; Roth et al., 2007), Stroop (Harrison et al., 2006; Nakao et al., 2005a; van den Heuvel, Veltman, Groenewegen, Witter et al., 2005), Eriksen Flanker (Fitzgerald et al., 2005), Multi-Source Interference (Yucel et al., 2007), Stop Signal (Woolley et al., 2008), Numeric Conflict (Viard et al., 2005), Reversal Learning (Remijnse et al., 2006), Tower of London (van den Heuvel, Veltman, Groenewegen, Cath et al., 2005), Spatial N-Back (van der Wee et al., 2003), Continuous Performance (Ursu, Stenger, Shear, Jones, & Carter, 2003), Task Switching (Gu et al., 2008), Word Generation (Pujol et al., 1999), and Serial Reaction Time (Rauch et al., 1997; Rauch et al., 2007; Rauch et al., 2001) tasks. The findings of these studies have generally been consistent with the findings of resting, symptom provocation, and pre/post-treatment studies, highlighting functional disturbances in the OFC, ACC, basal ganglia, and related areas (Menzies et al., 2008). Rather than attempting to survey this large and varied literature, though, we wish to highlight a general difficulty with interpreting cognitive activation studies that applies across tasks.
Typically, a given cognitive task only activates those areas involved in the cognitive processes required to perform the task. Whether and how the cognitive processes involved in the tasks that have been used to study OCD may relate to the symptoms of OCD is not always obvious. Cognitive activation studies therefore often have less face validity than resting, symptom provocation, or pre/post-treatment studies for addressing the neural bases of obsessive-compulsive symptoms. For example, most cognitive activation studies of OCD have used inhibitory control tasks. Patients with OCD exhibit behavioral deficits in at least some inhibitory control tasks (Chamberlain, Blackwell, Fineberg, Robbins, & Sahakian, 2005), and imaging studies that employ those tasks are useful to determine the neural correlates of those deficits. However, the relevance of those studies to understanding the symptoms of OCD depends on the unproven assumption that those symptoms relate directly to a deficit in inhibitory control. The symptoms of OCD have indeed been suggested to be a consequence of deficient inhibitory control, with obsessions arising from a failure to inhibit intrusive thoughts, and compulsions arising from a failure to inhibit certain behaviors (Chamberlain et al., 2005). Deficits in inhibitory control have, however, been reported for several psychiatric disorders, including attention deficit/hyperactivity disorder (Barkley, 1997; Nigg, 2001), bipolar disorder (Larson, Shear, Krikorian, Welge, & Strakowski, 2005; Quraishi & Frangou, 2002; L. J. Robinson et al., 2006), schizophrenia (Crawford, Bennett, Lekwuwa, Shaunak, & Deakin, 2002; Weisbrod, Kiefer, Marzinzik, & Spitzer, 2000), and addiction (Li & Sinha, 2008; Yucel & Lubman, 2007). The presence of deficits in inhibitory control in so many disorders with such widely varying phenotypes suggests caution in interpreting such deficits as the root cause of OCD.
Despite these limitations, cognitive activation studies can certainly be informative. Early cognitive activation studies, for example, found that healthy controls recruited the striatum during performance of an implicit (habit) learning task, whereas patients with OCD recruited the hippocampus, despite similar behavioral performances across the groups (Rauch et al., 1997; Rauch et al., 2001).1 These findings suggested that patients with OCD might use hippocampus-dependent (declarative) learning to overcome deficits in striatum-dependent (implicit) learning. If so, the performance of patients with OCD should suffer more than that of healthy controls when a secondary task that taxes working memory is introduced, and this was confirmed experimentally (Deckersbach et al., 2002). These studies show that even in the absence of obvious differences in behavioral performance, cognitive activation studies may highlight differences in neural activity that reflect compensatory strategies. On the other hand, the pathogenic relation, if any, of deficits in implicit (habit) learning and the symptoms of OCD remains unclear. Even if compulsions are maladaptive, exaggerated habits (Graybiel & Rauch, 2000), why patients with OCD would have difficulty using the striatal habit-learning system (Packard & Knowlton, 2002) to learn other habits remains unclear. Post-hoc, one can speculate that in patients with OCD the striatal habit-learning system is “overloaded” by compulsions, making it impossible for them to learn other habits. However, from the idea that OCD prominently involves exaggerated habits (the compulsions), one could equally plausibly have predicted the opposite pattern of findings – that the habit learning system in OCD should be especially powerful and effective. This highlights again the difficulties with interpreting the relevance of cognitive tasks and cognitive activation studies for the pathogenesis of OCD.
Cortico-Basal Ganglia-Thalamo-Cortical Loops and the Pathophysiology of OCD
The OFC and ACC are intimately connected to the basal ganglia via cortico-basal ganglia-thalamo-cortical (CBGTC) loops (Alexander, DeLong, & Strick, 1986; Mega & Cummings, 2001; Middleton & Strick, 2001b). Several CBGTC loops exist, and they seem to run largely parallel courses through the basal ganglia (Alexander et al., 1986; Mega & Cummings, 2001; Middleton & Strick, 2001b). Each of these loops receives inputs from multiple cortical areas and then projects back to one of its cortical areas of origin, thereby partly closing the loop (Alexander et al., 1986). No consensus exists in the literature about exactly how many such loops there are, but a particularly influential formulation proposes the existence of five loops, whose target areas are (a) the supplementary motor area, (b) the frontal eye fields, (c) the dorsolateral prefrontal cortex (DLPFC), (d) the lateral OFC; and (e) the ACC (Alexander et al., 1986). A more recent formulation adds two additional loops with target areas in the medial OFC and in the inferotemporal/posterior parietal cortex, and suggests that each of the loops may actually be further subdivided into multiple parallel loops (Middleton & Strick, 2001b). The main site in the striatum through which the CBGTC loops involving the OFC and ACC run is the head of the caudate nucleus (Alexander et al., 1986; Mega & Cummings, 2001; Middleton & Strick, 2001b). The findings from the resting, symptom provocation, and pre/post-treatment studies described above may therefore be interpreted as implicating the CBGTC loops involving the OFC and ACC (henceforward referred to as OFC/ACC CBGTC loops) in OCD.
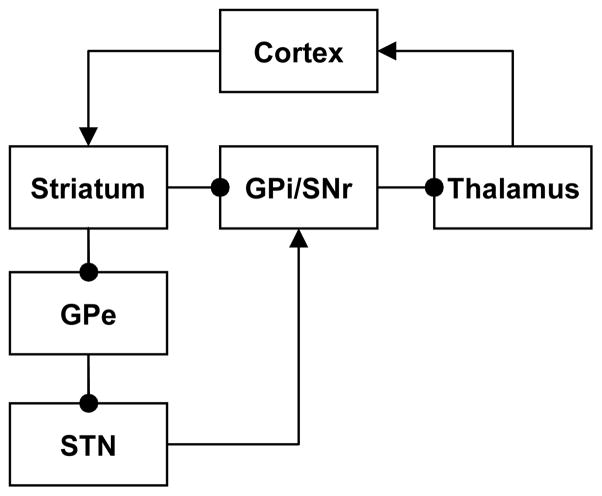
An influential idea based on these observations is that OCD results from an imbalance between the so-called “direct” and “indirect” pathways through the basal ganglia (Figure 1; Baxter et al., 2001; Saxena et al., 2001; Saxena et al., 1998; Saxena & Rauch, 2000). The net effect of the direct pathway is excitatory and the net effect of the indirect pathway is inhibitory (Figure 1), so these pathways are sometimes said to form positive and negative feedback loops, respectively. This simple model of opposing direct and indirect pathways has proven extremely useful in understanding hyperkinetic and hypokinetic movement disorders, such as Huntington’s and Parkinson’s diseases, respectively (Wichmann & DeLong, 1996). The idea is that excessive relative activity in the direct pathway disinhibits cortical motor programs, producing hyperkinetic symptoms, such as the chorea in Huntington’s disease. Conversely, excessive relative activity in the indirect pathway inhibits cortical motor programs, producing hypokinetic symptoms, such as the difficulty initiating movement in Parkinson’s disease.
Figure 1.
Classical conceptualization of the anatomy of CBGTC loops in terms of direct and indirect pathways (Albin, Young, & Penney, 1989; DeLong, 1990). The direct pathway runs from cortex to the striatum, then directly to the globus pallidus internal segment (GPi) and substantia nigra pars reticulata (SNr), then to the thalamus, and finally back to cortex. The indirect pathway runs from cortex to the striatum, then to the globus pallidus external segment (GPe), then to the subthalamic nucleus (STN), then to the GPi/SNr, then to the thalamus, and finally back to cortex. Arrows represent excitatory (glutamatergic) connections and circles represent inhibitory (GABAergic) connections. The direct pathway contains an even number of inhibitory connections (2), so its net effect from cortex back to cortex is excitatory. The indirect pathway contains an odd number of inhibitory connections (3), so its net effect from cortex back to cortex is inhibitory.
These symptoms are in the motor domain, but different symptoms may occur if the balance of the direct and indirect pathways is compromised in non-motor CBGTC loops. In particular, excessive relative activity in the direct pathway in OFC/ACC CBGTC loops has been suggested to result in a positive feedback loop in which obsessive thoughts become “trapped” (Baxter et al., 2001; Saxena et al., 2001; Saxena et al., 1998; Saxena & Rauch, 2000). Consistent with this idea, the prevalence of obsessive-compulsive symptoms is significantly greater in Huntington’s disease than in the general population (Anderson, Louis, Stern, & Marder, 2001; Beglinger et al., 2007; De Marchi & Mennella, 2000), and some case reports describe the onset of OCD following the onset of Huntington’s disease (Cummings & Cunningham, 1992; Scicutella, 2000).
This theory of OCD is not without difficulties, though. In particular, it does not explain why patients with OCD should have specific obsessions, as opposed to obsessing about everything. This can be unpacked into two, related questions. The first is why one finds great similarity in the contents of obsessions cross-culturally (Sasson et al., 1997); the second is why each individual patient tends to obsess only about a small subset of the larger set of common obsessions. One suggestion has been that “in persons with OCD, a response bias exists toward stimuli relating to socioterritorial concerns about danger, violence, hygiene, order, and sex – the themes of most obsessions in patients with OCD – mediated by orbitofrontal circuits” (Saxena & Rauch, 2000). However, the OFC responds to a variety of both positive and negative emotional stimuli, as well as to neutral stimuli that have been previously paired with positive or negative outcomes (Elliott & Deakin, 2005; Elliott, Dolan, & Frith, 2000; Kringelbach & Rolls, 2004; O’Doherty, 2007; Rolls, 1996, 1999, 2004; Schultz, Tremblay, & Hollerman, 2000; Zald & Kim, 2001). Why, then, “socioterritorial concerns” in particular should become locked in the OCD positive feedback loop is unclear. This suggestion also fails to explain why some patients with OCD will, for example, obsess about germs and wash compulsively, whereas others may obsess about whether they have locked the doors to their house and check the locks compulsively.
Searching for the Pathogenesis of OCD
As noted above, the findings that in patients with OCD the OFC, ACC, and caudate nucleus are hyperactive at rest, become more active under symptom provocation, and show less activity following treatment, have generally been interpreted as evidence that hyperactivity in these areas generates the symptoms of OCD (e.g., Baxter et al., 2001; Saxena et al., 2001; Saxena et al., 1998; Saxena & Rauch, 2000). Several alternative interpretations of these findings are, however, equally plausible. We know, for example, that when healthy controls are exposed to pictures depicting OCD-relevant scenes (specifically, pictures that are washing-relevant, checking-relevant, and hoarding-relevant) and are asked to imagine related scenarios (e.g., “imagine that you must come into contact with what’s shown in the following pictures without washing yourself afterward”), they activate regions similar to those that have been found hyperactive in OCD (Mataix-Cols et al., 2003). One possible interpretation of these findings is that rather than being the origin of obsessional content, these regions become hyperactive as a normal consequence of the obsessional content (Shafran & Speckens, 2005). We also know that the OFC and ACC are involved in emotion regulation (Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner & Gross, 2005), so again, hyperactivity in these areas may be a consequence of an attempt by patients with OCD to regulate their anxiety, rather than being the cause of their symptoms.
A related, though slightly different idea is that hyperactivation of the OFC, ACC, and caudate nucleus simply reflects the need to inhibit compulsive behavior during the scan (Peterson, 2003). This idea is supported by the finding that the ACC and the head of the caudate nucleus are activated when patients with Tourette’s syndrome (TS) voluntarily inhibit their tics (Peterson et al., 1998). The genetic, phenomenological, and pathophysiological similarities between TS and OCD (Fineberg, Saxena, Zohar, & Craig, 2007; Marsh, Leckman, Bloch, Yazgan, & Peterson, 2008) suggest that excessive activity in these areas in patients with OCD might reflect the inhibition of compulsions. Further support for this idea comes from the findings that the OFC, ACC, and caudate are activated in a variety of tasks that require the suppression of a prepotent response (Peterson, 2003). Weighing against this thesis are human lesion studies suggesting that response inhibition may be localized to the right inferior frontal cortex, rather than in the OFC, ACC, or caudate (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Aron, Robbins, & Poldrack, 2004). The evidence from functional imaging for an involvement of the OFC in response inhibition, as studied using the Go/NoGo task, also remains inconclusive, with block designs typically showing OFC activation, but event-related designs failing to show such activation (Elliott & Deakin, 2005).
Other authors have similarly suggested that OFC hyperactivation may reflect patients’ efforts to inhibit their symptoms during the scanning procedure (Adler et al., 2000; Roth et al., 2007), remaining agnostic as to whether that inhibition is targeted specifically at obsessions, compulsions, anxiety, or possibly all of them. Consistent with the idea that OFC activity plays an inhibitory role, keeping the obsessions, compulsions, or anxiety in check, greater activation of the OFC during symptom provocation is associated with a smaller increase in reported symptoms with the provocation (Adler et al., 2000; Rauch et al., 1994). If OFC hyperactivity played a role in causing, rather than in inhibiting symptoms, then greater activation of the OFC during symptom provocation should be associated with a greater, not a lesser, increase in symptoms.
Our goal is not to suggest that one of these various interpretations is the correct one. Instead, we wish to emphasize that all are consistent with the findings that the OFC, ACC, and caudate nucleus are hyperactive at rest, become more active under symptom provocation, and show less activity following treatment. For example, if the activation of these areas reflects an attempt to inhibit symptoms, such activation could be expected to be higher at rest in patients with OCD than in healthy controls, to increase with symptom provocation, when such inhibition becomes more necessary, and to decrease after treatment, when such inhibition becomes less necessary.
The problem with interpreting these activations as the cause of the symptoms is that these functional imaging findings are inherently correlational: they demonstrate only that activity in these areas correlates with a symptomatic state in OCD. One might hope that anatomical imaging studies would resolve the difficulties in establishing a causal role for these areas in OCD. However, much evidence suggests that repeatedly engaging in a class of behaviors or cognitive processes can change brain structure (Lazar et al., 2005; Mechelli et al., 2004; Pascual-Leone, 2001; Schlaug, 2001; Schlaug, Norton, Overy, & Winner, 2005). Thus, even anatomical differences in the brains of patients with OCD may be a consequence rather than a cause of the disorder. This highlights the usefulness of studying patients with OCD as close to symptom onset as possible, when such epiphenomenal changes may be less prominent. OCD is, however, often diagnosed long after the onset of symptoms, making this strategy relatively impractical. Furthermore, subclinical patterns of thinking and behavior that may long precede clinically-significant symptoms (Rasmussen & Eisen, 2002) may also conceivably produce epiphenomenal changes in brain structure that could be apparent by the time of symptom onset.
The problem is that, like functional imaging studies, anatomical studies only provide information about the correlates of the disorder. Several valuable animal models of OCD have been developed in efforts to study causal mechanisms in OCD more directly (Joel, 2006; Korff & Harvey, 2006). These have, however, been criticized because they only model repetitive behaviors, whereas compulsive behaviors in OCD are intimately tied to obsessions (Shafran & Speckens, 2005) – and we have no way of assessing whether animals have obsessions.
Other lines of evidence may, however, help establish causality in humans. We will explore three such lines of evidence. First, certain brain lesions due to accidents, stroke, and other naturalistic causes seem to cause OCD. Second, some cases of OCD and of related psychiatric disorders, such as TS, seem to develop as a consequence of an autoimmune reaction in which antibodies to Group-A beta-hemolytic streptococcus attack and damage the basal ganglia (Hoekstra & Minderaa, 2005; Leonard & Swedo, 2001; T. K. Murphy, Husted, & Edge, 2006; Snider & Swedo, 2004); these are usually termed “Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections” (PANDAS). Third, several neurosurgical procedures make localized lesions in attempts to provide symptom-relief in severe, treatment-refractory cases of OCD.
Each of these lines of evidence has important limitations in establishing a causal role for abnormalities in specific brain regions in producing OCD. First, studies of OCD as a consequence of brain injury typically involve a small number of subjects who have diffuse lesions that vary greatly across subjects. Furthermore, demonstrating that lesions that affect certain brain circuits result in OCD does not, per se, prove a pathogenic role for those circuits in most cases of OCD. Second, the validity of the PANDAS construct remains controversial (Harris & Singer, 2006; Kurlan, 2004; Kurlan & Kaplan, 2004; Singer & Loiselle, 2003), with substantial difficulties involved in establishing a causal relation between infection with Group-A beta-hemolytic streptococcus and the development of neuropsychiatric disorders (and additional difficulties in proving that the possible autoimmune reaction affects the basal ganglia). Third, neurosurgical studies lack appropriate controls, and the mechanisms by which they attenuate OCD symptoms are not fully understood. Moreover, an improvement in symptoms following certain neurosurgical lesions does not, per se, prove that the circuits that were lesioned caused the symptoms (just as having a gastric bypass surgery and losing weight as a result does not prove that the cause of excess weight was an enlarged stomach).
Although each of the aforementioned lines of evidence – anatomical imaging, OCD as a consequence of brain injury, PANDAS, and neurosurgery – in isolation is insufficient to establish causality, their convergence with functional imaging studies in implicating OFC/ACC CBGTC loops in OCD would provide some reassurance that those loops may indeed play a causal role in OCD. We will therefore review each of these lines of evidence in turn.
Anatomical and Spectroscopy Studies in Adults with OCD
Studies Based on Regions of Interest
Volumetric studies measure the volumes of specific regions of interest (ROIs) in the brain. Proton magnetic resonance spectroscopy (1H-MRS) studies measure the concentrations of certain metabolites in ROIs. N-acetyl-aspartate (NAA), one of those metabolites, has been of particular interest in studies of disease processes because decreases in NAA levels may indicate neuronal loss or dysfunction (Barker, 2001; Dager & Steen, 1992; Maier, 1995). NAA levels may be more sensitive than volumetric measures to detect neuronal abnormalities (Bartha et al., 1998; Cendes, Andermann, Preul, & Arnold, 1994; Ebisu, Rooney, Graham, Weiner, & Maudsley, 1994).
The vast majority of anatomical imaging studies of OCD have been conducted after the early functional imaging findings implicated the OFC, ACC, and caudate nucleus in the pathophysiology of OCD. Those studies were therefore strongly influenced by the theory that OFC/ACC CBGTC loops are centrally involved in the pathogenesis of OCD, and have therefore tended to look specifically at brain regions that are part of those loops: the OFC, ACC, caudate nucleus or striatum, and thalamus. Fewer studies have focused specifically on the globus pallidus, another key component of those loops.
Orbitofrontal cortex
Several studies have reported bilateral reductions in OFC volumes in adults with OCD (Atmaca et al., 2006; Atmaca, Yildirim, Ozdemir, Tezcan, & Poyraz, 2007; Szeszko et al., 1999); one study reported a volume reduction only in the left OFC (Kang et al., 2004). A study that measured specifically the volume of the medial OFC also found it to be reduced bilaterally in adults with OCD (Cardoner et al., 2007). Another study that divided the OFC into anterior and posterior regions reported a volume reduction in the left anterior OFC, but not in the left posterior or right anterior or posterior OFC (Choi et al., 2004). Both left and right OFC volumes have been found to correlate inversely with the severity of OCD symptoms (Atmaca et al., 2007; Kang et al., 2004) and to be larger post-treatment in patients who responded to a trial of selective serotonin reuptake inhibitors than in treatment-naïve or treatment-refractory patients (Atmaca et al., 2006). In summary, considerable evidence suggests that OFC volumes are significantly reduced in adults with OCD, with greater reductions accompanying more severe symptoms.
Anterior cingulate cortex
Several 1H-MRS studies have reported decreased levels of NAA in the ACC of adults with OCD (Ebert et al., 1997; Jang et al., 2006; Sumitani, Harada, Kubo, & Ohmori, 2007; Yucel et al., 2007). Furthermore, treatment with the selective serotonin reuptake inhibitor citalopram increases levels of NAA in the ACC (and other regions of prefrontal cortex) in adults with OCD (Jang et al., 2006), although no significant correlation was detected between increases in NAA and decreases in symptom severity. Several studies have compared ACC volumes in adults with OCD and normal controls, but have generally failed to detect significant differences (Atmaca et al., 2006; Atmaca et al., 2007; Grachev et al., 1998; Kellner et al., 1991; Riffkin et al., 2005; Szeszko et al., 1999), even when the ACC has been subdivided into anterior and posterior regions (Kang et al., 2004).
To the extent that decreased levels of NAA are a more sensitive indicator of neuronal loss or dysfunction than are traditional volumetric measurements (Bartha et al., 1998; Cendes et al., 1994; Ebisu et al., 1994), these findings may indicate the presence of subtle neuronal abnormalities in the ACC of adults with OCD. The fact that the reductions in NAA are reversible with administration of citalopram (Jang et al., 2006) suggests that those reductions may reflect reversible abnormalities either in metabolic processes or in neuropil (axonal or dendritic arbors and synapses), rather than reductions in the number of neurons (Barker, 2001).
Striatum
Studies comparing caudate volumes in adults with OCD and healthy controls have yielded highly variable findings. Although most studies have not detected significant differences (Atmaca et al., 2006; Atmaca et al., 2007; Aylward et al., 1996; Kang et al., 2004; Kellner et al., 1991; Riffkin et al., 2005), two studies have reported decreased caudate volumes in OCD (Luxenberg et al., 1988; D. Robinson et al., 1995). One study reported increases in the volume of the head of the right caudate in adults with OCD (Scarone et al., 1992), but two studies failed to detect significant differences in volumes of the head of the caudate between adults with OCD and healthy controls (Bartha et al., 1998; Kellner et al., 1991). One study reported increased ventral striatum volumes in adults with OCD (Cardoner et al., 2007). Studies using 1H-MRS have been slightly more consistent, with two studies reporting decreased levels of NAA in the striatum of adults with OCD (Bartha et al., 1998; Ebert et al., 1997), but a third study failing to detect such differences (Sumitani et al., 2007).
The variability in findings for the caudate may be, in part, a consequence of the heterogeneous nature of the circuits that traverse it. Different portions of the caudate participate in different CBGTC loops, and disturbances in only one or two of those loops (e.g., the ones involving the OFC and ACC) may not be of sufficient magnitude to yield differences in overall caudate volume. In fact, different portions of the caudate could differ from healthy controls both in the direction and magnitude of the effect. Thus, the overall volume of the caudate could be normal (Atmaca et al., 2006; Atmaca et al., 2007; Aylward et al., 1996; Kang et al., 2004; Kellner et al., 1991; Riffkin et al., 2005) or even reduced (Luxenberg et al., 1988; D. Robinson et al., 1995) in adults with OCD, whereas the volumes of the head of the caudate (Scarone et al., 1992) and ventral striatum (Cardoner et al., 2007) could be increased. Volume increases in the head of the caudate and ventral striatum, if confirmed, would provide additional evidence for the involvement of OFC/ACC CBGTC loops in OCD, since these are the regions of the striatum through which such loops run.
Globus pallidus
Most components of the OFC/ACC CBGTC loops – the OFC, ACC, striatum, and thalamus – have been relatively well investigated in ROI studies. However, two other key components of these loops – the globus pallidus and the subthalamic nucleus – remain largely unstudied. Volumes of the subthalamic nucleus have not been assessed because this is a small, poorly demarcated structure that is exceedingly difficult to measure. The few studies that have measured volumes of the globus pallidus or overall volumes of the lenticular nuclei in adults with OCD generally failed to detect differences from healthy controls (Choi et al., 2007; Jenike et al., 1996; Luxenberg et al., 1988).
Thalamus
Volumes of the thalamus have been reported to be larger in treatment-naïve adults with OCD than in healthy controls (Atmaca et al., 2006; Atmaca et al., 2007). Patients who responded to medication treatment had smaller thalami post-treatment than either treatment-naïve or treatment-refractory patients, with no differences detected between the latter two groups (Atmaca et al., 2006). Patients were not scanned before and after treatment, though, so whether treatment responders had smaller thalami to begin with or as a consequence of treatment is unclear. Nevertheless, a study that scanned children with OCD before and after medication treatment did detect medication-induced reductions in thalamic volume that moreover correlated with symptom improvement (Gilbert et al., 2000), suggesting that the findings of smaller thalami in treatment-responding adults may also have been a consequence of treatment. Thalamic volumes were found to correlate positively with symptom severity in both treatment-naïve and treatment-refractory patients (Atmaca et al., 2006; Atmaca et al., 2007).
Two studies failed to detect differences in volumes of the thalamus between adults with OCD and healthy controls (Kang et al., 2004; Kwon et al., 2003). However, approximately 70% of the patients in one of these studies (Kang et al., 2004) had previously been treated with medication (although all were medication-free for at least 4 weeks at the time of study), and the medication history and medication status of patients in the other study (Kwon et al., 2003) were not reported. Given the evidence that medication treatment may reduce thalamic volumes in patients with OCD, the failure of these studies to detect differences between adults with OCD and controls could conceivably be due to the effects of medication.
Other areas
Other areas that strongly influence the OFC/ACC CBGTC loops, by virtue of their projections to the same regions of the striatum as the OFC or ACC, include the superior temporal gyrus and hippocampus (Alexander et al., 1986; Middleton & Strick, 2001b). These areas have received less attention in studies of OCD. Nevertheless, volume reductions have been reported for both the superior temporal gyrus (Choi et al., 2006) and hippocampus (Kwon et al., 2003) in adults with OCD. Two studies failed to detect volumetric abnormalities in the hippocampus of adults with OCD (Jenike et al., 1996; Szeszko et al., 1999), but one of these (Jenike et al., 1996) had a small sample and failed to find differences in most areas, and the other (Szeszko et al., 1999) found a trend for less asymmetry in hippocampus volumes in adults with OCD.
An area that is closely related, anatomically and functionally, to the OFC and ACC is the amygdala (Amaral, Price, Pitkanen, & Carmichael, 1992; Carmichael & Price, 1995; Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000; Rolls, 1999; Zald & Kim, 2001). The amygdala also projects strongly to the mediodorsal nucleus of the thalamus (Amaral et al., 1992), the final relay station before the OFC/ACC CBGTC loops project back to the cortex (Alexander et al., 1986; Middleton & Strick, 2001b), and it is therefore critically positioned to influence the output of these loops. The amygdala’s key role in mediating normal fear and anxiety (LeDoux, 2000, 2007; Phelps & LeDoux, 2005) and its prominent involvement in anxiety disorders (Bremner, 2004; Miller, Taber, Gabbard, & Hurley, 2005; Rauch, Shin, & Wright, 2003) highlight its potential relevance for understanding the pathophysiology of OCD, a disorder in which anxiety plays a crucial role (Foa, Steketee, & Ozarow, 1985; Rachman & Hodgson, 1980). The few studies that have examined volumes of the amygdala in adults with OCD have, however, yielded inconsistent findings, with one study reporting bilateral reductions in amygdala volume (Szeszko et al., 1999) and another reporting an increase in left amygdala volume (Kwon et al., 2003).
Summary
Volumetric studies in adults with OCD provide convincing evidence for reduced OFC volumes, suggestive evidence for increased thalamic and normal ACC volumes, and inconsistent evidence for caudate or striatum volumes. 1H-MRS studies in adults with OCD provide convincing evidence for decreased NAA levels in the ACC and suggestive evidence for decreased NAA levels in the striatum. Together, these findings point to abnormalities in volumes or NAA levels in all components of the OFC/ACC CBGTC loops that have been studied in detail. Abnormalities in closely related areas, such as the hippocampus, amygdala, and superior temporal gyrus, have also been reported. The findings from volumetric and 1H-MRS studies of adults with OCD are summarized in Table 1.
Table 1.
Summary of volumetric, VBM, and 1H-MRS findings in children and adults with OCD
| Children | Adults | ||
|---|---|---|---|
| ROI Volumes | OFC | − − − − −a −b | |
| ACC | + + | = = = = = = = | |
| Striatumc | = −d | = = = = = =e =e +e +f − − | |
| GP | − | = = =g | |
| Thalamus | + | = = + + | |
| STG | = | − | |
| Amygdala | = = | + − | |
| Hippocampus | = | = = − | |
| VBM | OFC | + − ±h | |
| ACC i | − | − − − | |
| Striatum | +f +j +j | ||
| Thalamus | + + | ||
| STG | + − | ||
| Amygdala | + | ||
| Insula | + + − − | ||
| Cerebellum | + − | ||
| 1H-MRS | ACC | * * * * | |
| Striatum | * * = | ||
| Thalamusk | * * * * | ||
Note. In a given cell, each symbol (+, −, =, or *) represents one study. Symbols in different cells may refer to the same study.
+: increased volumes or increased gray matter density in patients
−: decreased volumes or decreased gray matter density in patients
=: no significant differences between patients and healthy controls
*: abnormal concentrations of neurometabolites (NAA, choline, or creatine/phosphocreatine) in patients
1H-MRS: proton magnetic resonance spectroscopy; ACC: anterior cingulate cortex; GP: globus pallidus; OFC: orbitofrontal cortex; ROI: region-of-interest; STG: superior temporal gyrus; VBM: voxel-based morphometry.
Medial OFC.
Left anterior OFC.
Studies refer to the caudate unless otherwise noted.
Decreased putamen but not caudate volumes.
Head of the caudate.
Ventral striatum.
Lenticular nuclei.
Gray matter increases and decreases were reported for differing regions of OFC.
All gray matter decreases were reported for both the ACC and the medial frontal gyrus.
Putamen.
All studies refer to the medial thalamus.
Limitations of Region-of-Interest Approaches
ROI approaches suffer from two serious limitations. First, because manual delimitation of ROIs is laborious, ROI studies often analyze only a small number of regions for which there are prior suspicions of abnormality, which limits the opportunity to find abnormalities in unanticipated regions. Automated methods to delimit ROIs partly alleviate this problem, but they are significantly less accurate than manual methods. Second, anatomical ROIs may not correspond to functionally meaningful units because gross anatomical structures or landmarks do not always reflect anatomical connectivity or cytoarchitecture. For example, several CBGTC loops run through the caudate nucleus (Alexander et al., 1986; Middleton & Strick, 2001b) and each of those loops also partly seems to run through the putamen (Haber, 2003). Considering each nucleus – caudate or putamen – as a whole misses both the distinctions between different loops within the nucleus and the fact that portions of one nucleus should be considered together with portions of the other. A meta-analysis of PET and SPECT studies of OCD that found reliable differences between patients with OCD and normal controls in the orbital gyrus and the head of the caudate nucleus, but not in the OFC or caudate nucleus as a whole (Whiteside et al., 2004), illustrates the importance of moving towards finer anatomical distinctions in the study of OCD.
These limitations are addressed by two techniques: voxel-based morphometry and tensor-based morphometry. Voxel-based morphometry is a popular whole-brain approach that gives voxel-by-voxel measurements (Ashburner & Friston, 2000). It remains controversial, however, because errors in spatial normalization may confound all subsequent computations, resulting in unreliable findings (Bookstein, 2001). Tensor-based morphometry is used to analyze local morphological changes on the surface of cortical or subcortical structures (a procedure often termed “surface analysis”) or to measure cortical thickness. This approach gives highly localized, point-by-point measurements. Surface analyses, however, still require the prior delimitation of ROIs, as it is within a previously-delimited ROI that surface analysis gives detailed, point-by-point measurements.
Cortical Thickness
The findings from the only extant study of cortical thickness in OCD (Shin et al., 2007) are consistent with a pathophysiological model that emphasizes disturbances in the OFC, but also suggest abnormalities in a number of areas that are not traditionally considered involved in the pathophysiology of OCD. This study found cortical thinning in several areas of the left hemisphere of adults with OCD, including not only the OFC but also the ventrolateral prefrontal, middle frontal, precentral, superior temporal, parahippocampal, and lingual cortices. No abnormalities were detected in the ACC or anywhere in the right hemisphere. The findings from this study should be interpreted with care because most patients were medicated. Nevertheless, the finding of thinning in the superior temporal cortex is consistent with the volume reductions that have been reported for the superior temporal gyrus (Choi et al., 2006), and the finding of thinning in the parahippocampal cortex may point to broader abnormalities in the tissue surrounding the hippocampus that would be consistent with the volume reductions that have been reported for the hippocampus proper (Kwon et al., 2003).
Surface Analysis
Only two studies, both by the same group and in adults, have used surface analysis to analyze subcortical structures in OCD. One of these studies focused on the hippocampus and reported shape changes concentrated on the hippocampus head (Hong et al., 2007). These results are consistent with the previous report by the same group of volumetric abnormalities in the hippocampus of adults with OCD (Kwon et al., 2003). The other study focused on the basal ganglia and found outward deformities concentrated in the dorsal anterior caudate nucleus bilaterally and, to a lesser extent, in the ventral lateral part of the left putamen (Choi et al., 2007). These results are surprising, given that these are not the striatal regions through which the OFC/ACC CBGTC loops run (Alexander et al., 1986; Haber, 2003; Lehericy et al., 2004; Middleton & Strick, 2001b). The results of these surface analysis studies need to be interpreted with care because neither study corrected for multiple comparisons, and in both studies the majority of patients had a history of medication (although all were medication-free for at least 4 weeks prior to scanning). Surface analysis techniques are also sufficiently new that replication by other laboratories would be important.
Voxel-Based Morphometry
Several studies have used voxel-based morphometry (VBM) to compare gray matter in adults with OCD and healthy controls (J. J. Kim et al., 2001; Pujol et al., 2004; Valente et al., 2005; Yoo et al., 2008). Findings from these studies have been generally consistent with the pathohpysiological model of OCD that emphasizes the OFC and ACC and their loops through the basal ganglia. Both increases (J. J. Kim et al., 2001; Valente et al., 2005) and decreases (Pujol et al., 2004; Valente et al., 2005) in gray matter have been reported for differing subregions of the OFC, highlighting the potential value of a finer level of morphological analysis than is typically achieved in ROI-based studies. Decreases in gray matter have been consistently reported in the ACC and surrounding medial frontal gyrus (Pujol et al., 2004; Valente et al., 2005; Yoo et al., 2008). Increases in gray matter have generally been reported in the striatum (Pujol et al., 2004; Valente et al., 2005; Yoo et al., 2008). These increases have included the ventral striatum (Pujol et al., 2004), which is consistent with the known neuroanatomy of OFC/ACC CBGTC loops, but also the putamen (Valente et al., 2005; Yoo et al., 2008), which is less clearly consistent with the standard pathophysiological model of OCD. Increases in gray matter have also been reported in the thalamus (J. J. Kim et al., 2001; Yoo et al., 2008). VBM studies have also reported abnormalities in other areas that have been found abnormal in volumetric studies or in the only study of cortical thickness in OCD to date (Shin et al., 2007) and that relate closely to OFC/ACC CBGTC loops. For example, VBM studies have reported both increases (J. J. Kim et al., 2001) and decreases (Yoo et al., 2008) in gray matter in the superior temporal gyrus, as well as increases in gray matter in the parahippocampal gyrus, extending to the amygdala (Valente et al., 2005).
The only other areas for which abnormalities have been found in at least two VBM studies in adults with OCD are the insula (J. J. Kim et al., 2001; Pujol et al., 2004; Valente et al., 2005; Yoo et al., 2008) and cerebellum (J. J. Kim et al., 2001; Pujol et al., 2004). The insula is contiguous with the posterior OFC and is heavily interconnected with both the OFC and the ACC (Mesulam & Mufson, 1982; Mufson & Mesulam, 1982; Ongur & Price, 2000). The cerebellum and frontal cortex are interconnected in parallel cortico-cerebellar loops (R. M. Kelly & Strick, 2003; Middleton & Strick, 1997, 2000, 2001a), and the cerebellum can also influence the striatum via a disynaptic connection through the thalamus (Hoshi, Tremblay, Feger, Carras, & Strick, 2005). The potential involvement of the insula and cerebellum in OCD may therefore be consistent with the pathophysiological model that emphasizes disturbances in the OFC/ACC CBGTC loops and related areas in OCD.
Although abnormalities in several other areas have been reported in single VBM studies, they have not been replicated across studies. While this does not exclude the possible involvement of other areas, it does provide some measure of reassurance that the main structural abnormalities in adult OCD are concentrated along the OFC/ACC CBGTC loops and related areas. The findings from VBM studies of adults with OCD are summarized in Table 1.
Anatomical and Spectroscopy Studies in Children with OCD
Studies Based on Regions of Interest
Orbitofrontal cortex
We are not aware of any volumetric studies of the OFC in pediatric OCD. This is a major gap in the literature, given that reduced volumes in the OFC are the most consistent finding in the volumetric literature on adult OCD.
Anterior cingulate cortex
One study reported larger volumes of the ACC in treatment-naïve children with OCD than in matched controls, with larger ACC volumes accompanying more severe symptoms (Rosenberg & Keshavan, 1998). Another study reported increased volumes of gray matter, but not of white matter, in the ACC of medication-naïve children with OCD (Szeszko, MacMillan, McMeniman, Chen et al., 2004). These findings stand in contrast to those in adults with OCD, which have generally failed to detect volumetric abnormalities in the ACC. A possible explanation for this difference in findings across age groups is that the ACC may develop differently in patients with OCD and healthy controls. One cross-sectional study reported a nearly significant correlation between age and ACC volumes in healthy children but not in children with OCD (Rosenberg & Keshavan, 1998). If this finding is confirmed, ideally in a longitudinal study, it could mean that ACC volumes are larger in children with OCD but then stay relatively constant with advancing age, whereas ACC volumes in healthy children might be smaller but then increase with age, resulting in similar ACC volumes in patients with OCD and healthy controls by adulthood.
Striatum
Two studies detected no significant differences in caudate volumes between children with OCD and matched controls (Rosenberg et al., 1997; Szeszko, MacMillan, McMeniman, Chen et al., 2004), although one of these studies reported smaller putamen volumes in children with OCD (Rosenberg et al., 1997). The finding of a putamen abnormality is somewhat surprising from the perspective of the pathophysiological model of OCD that emphasizes the OFC/ACC CBGTC loops, given that those loops run mostly (though not exclusively) through the caudate; replication of this finding would therefore be important. If confirmed, this finding would also emphasize the need for volumetric studies of the putamen in adults with OCD, as virtually all volumetric studies of the striatum in adults with OCD have focused on the caudate.
Globus pallidus
One study reported smaller globus pallidus volumes in medication-naïve children with OCD compared with matched healthy controls (Szeszko, MacMillan, McMeniman, Chen et al., 2004). This finding stands in contrast to those in adults with OCD, which have generally failed to detect volumetric abnormalities in the globus pallidus.
Thalamus
One study reported increased thalamic volumes in medication-naïve children with OCD that decreased to normal levels following treatment with the selective serotonin reuptake inhibitor paroxetine (Gilbert et al., 2000). Furthermore, the reduction in thalamic volumes with treatment correlated with the improvement in OCD symptoms. The finding of increased thalamic volumes in children with OCD is consistent with similar findings in adults. The finding that paroxetine treatment in children with OCD reduces thalamic volumes is also consistent with the indirect evidence that medication reduces thalamic volumes in adults with OCD. Another study found no differences in volumes of the thalamus measured before and after successful cognitive-behavioral therapy (Rosenberg, Benazon, Gilbert, Sullivan, & Moore, 2000), suggesting that the reduction in volumes of the thalamus seen with paroxetine treatment is not necessary for symptom improvement. This study, however, did not include a control group, so the possibility that the children with OCD in this study might not have significantly enlarged thalami prior to treatment cannot be excluded.
Several studies by the same group using 1H-MRS have reported abnormalities in the medial thalamus of treatment-naïve children with OCD. The first such study detected reduced ratios of NAA to choline and NAA to creatine/phosphocreatine + choline levels in the medial but not the lateral thalami of treatment-naïve children with OCD (Fitzgerald, Moore, Paulson, Stewart, & Rosenberg, 2000). The findings were interpreted as indicative of reduced NAA levels in the medial thalami in the patient group, with the caveat that an increase in choline could produce the same results. Indeed, subsequent studies from the same group did report increases in choline (Rosenberg, Amponsah, Sullivan, MacMillan, & Moore, 2001; Smith et al., 2003) and creatine/phosphocreatine levels (Mirza et al., 2006) in the medial thalami of children with OCD, suggesting that the earlier findings reflected elevated levels of choline and creatine/phosphocreatine rather than decreased levels of NAA. In fact, measurement of absolute NAA concentrations in the medial thalamus did not reveal differences between children with OCD and matched controls (Rosenberg et al., 2001). The significance of elevated choline and creatine/phosphocreatine levels in the medial thalamus remains to be fully elucidated. Nevertheless, it is noteworthy that these abnormalities occur in the medial thalamus, where the mediodorsal nucleus – the primary nucleus of the thalamus in OFC/ACC CBGTC loops – is located.
Other areas
The only study that measured volumes of the superior temporal gyrus and hippocampus in children with OCD did not detect significant differences from healthy controls (Rosenberg & Keshavan, 1998). This is in partial contrast to the findings in adults, which tentatively suggest that these structures may be abnormal in adults with OCD. Two studies failed to detect significant differences in amygdala volumes between children with OCD and healthy controls (Rosenberg & Keshavan, 1998; Szeszko, MacMillan, McMeniman, Lorch et al., 2004), although one of these studies found that children with OCD, unlike healthy controls, had significantly larger left than right amygdalae (Szeszko, MacMillan, McMeniman, Lorch et al., 2004). Abnormalities in other areas – e.g., increased NAA levels in the left but not right DLPFC of children with OCD (Russell et al., 2003) and decreased pituitary volumes in boys but not girls with OCD (MacMaster et al., 2006) – have been reported sporadically, but need to be replicated.
Summary
Significantly fewer volumetric or 1H-MRS studies exist for children than for adults with OCD. Nevertheless, two findings receive convergent support from more than one study of children with OCD: increased volumes of the ACC and abnormal metabolite concentrations in the thalamus. Findings that were obtained in only one study and require replication include reduced putamen and globus pallidus volumes and increased thalamus volume. The findings from volumetric and 1H-MRS studies of children with OCD are summarized in Table 1.
The findings of increased thalamic volumes in children with OCD echo similar findings in adults. The findings of increased ACC volumes in children with OCD, however, are in contrast to the findings in adults with OCD, which have generally failed to detect volumetric abnormalities in the ACC, although they are consistent with several 1H-MRS studies that have reported decreased NAA levels in the ACC of adults with OCD. The finding of reduced globus pallidus volumes in children with OCD is also in contrast to the findings of the few existing volumetric studies of the globus pallidus in adults with OCD. Volumetric studies in adults with OCD have generally not measured the putamen, so whether reduced putamen volumes are found in adults with OCD remains unclear.
Most extant volumetric and 1H-MRS studies of OCD have focused on comparing volumes or metabolite levels between patients with OCD and healthy controls, paying little to no attention to how the volumes or metabolite levels change with age in persons with and without OCD. This information is, however, vitally important for a developmental perspective on the pathogenesis of OCD, as well as to understand how the abnormalities reported in children with OCD ultimately evolve to the abnormalities reported in adults with OCD, at least for adults with childhood-onset OCD. Similarly, studies of adults with OCD should distinguish better whether their findings relate to adult- or childhood-onset OCD, or to both.
Voxel-Based Morphometry
No cortical thickness or surface analysis studies of children with OCD have been reported in the literature. However, two VBM studies have been recently published (Carmona et al., 2007; Gilbert et al., 2008). One of these (Gilbert et al., 2008) reported decreased gray matter in the left ACC and in the medial frontal gyrus bilaterally in children with OCD, consistent with similar findings in adults (Pujol et al., 2004; Valente et al., 2005; Yoo et al., 2008) and with the hypothesized involvement of the ACC in OCD. The consistency of the findings of decreased gray matter in the medial frontal gyrus in children and adults underscores the need to consider not only the ACC proper, but also the surrounding medial frontal gyrus as a potential locus of abnormality in OCD.
The other VBM study in children with OCD (Carmona et al., 2007) also reported a large cluster of decreased gray matter in the cingulate cortex, although that finding did not remain significant after correcting for multiple comparisons. Other large clusters of decreased gray matter were found in the middle frontal gyrus, with the cluster in the right middle frontal gyrus remaining significant after correcting for multiple comparisons. Additional clusters were also reported, but they were substantially smaller and none remained significant after correcting for multiple comparisons. Additional studies are necessary to determine whether the finding in the middle frontal gyrus is reproducible, especially because of the 18 children with OCD, 11 had comorbid tic disorder and 10 were on medication.
The only consistent finding between the two existing VBM studies of children with OCD is reduced gray matter in the ACC. Although this is consistent with similar findings in VBM studies of adults with OCD, it contrasts with the findings of volumetric studies in children with OCD, which have generally found increased ACC volumes (Rosenberg & Keshavan, 1998; Szeszko, MacMillan, McMeniman, Chen et al., 2004). A possible explanation for this discrepancy comes from the observation that, depending on the details of the VBM procedure used, VBM may characterize gray matter density rather than volume (Good et al., 2001). In fact, one of the studies (Gilbert et al., 2008) states precisely that its findings concern gray matter densities, not volumes, and that this may explain their discrepancy with prior volumetric studies. The findings from VBM studies of children with OCD are summarized in Table 1.
PANDAS
Another potential line of evidence for the involvement of the basal ganglia in OCD comes from the study of PANDAS. This designation refers to childhood-onset OCD or tic disorders with abrupt onset or an episodic symptom course, in which symptom onset or exacerbation is temporally associated with infection with Group-A beta-hemolytic streptococcus (GAS; Swedo et al., 1998). Several authors have suggested that PANDAS are autoimmune disorders caused by antibodies to GAS that interact with the basal ganglia, in particular the striatum (Hoekstra & Minderaa, 2005; Leonard & Swedo, 2001; T. K. Murphy, Husted et al., 2006; Snider & Swedo, 2004). Controversy remains, however, regarding not only this causal hypothesis but the validity of the PANDAS construct itself (Harris & Singer, 2006; Kurlan, 2004; Kurlan & Kaplan, 2004; Singer & Loiselle, 2003).
GAS infection was first observed to produce Sydenham’s chorea, an illness associated with rheumatic fever and characterized primarily by involuntary rapid, jerky movements, which are, however, often accompanied by other motor, behavioral, and emotional problems, including obsessive-compulsive symptoms and OCD (Swedo et al., 1993; Swedo et al., 1989). Later observations identified putative cases of abrupt childhood-onset OCD or TS that followed GAS infections but did not meet criteria for Sydenham’s chorea (Allen, Leonard, & Swedo, 1995; Swedo et al., 1998). Establishing a clear and definitive causal role for GAS infections in these cases has, however, proven difficult, in part because GAS infections are so common in childhood (T. K. Murphy, Sajid, & Goodman, 2006).
Experimental evidence suggests but does not prove a causal role for GAS in the onset or exacerbation of OCD or TS. Several studies have reported elevated anti-basal ganglia antibodies in the sera of patients with TS or OCD (Dale, Heyman, Giovannoni, & Church, 2005; Kiessling, Marcotte, & Culpepper, 1994; Rizzo, Gulisano, Pavone, Fogliani, & Robertson, 2006; Singer et al., 1998; Wendlandt, Grus, Hansen, & Singer, 2001), although at least one study failed to detect differences in anti-basal ganglia antibodies between patients with PANDAS and healthy controls (Singer et al., 2004). One study reported that immunomodulatory treatment of PANDAS led to improvements in both OCD and TS symptoms (Perlmutter et al., 1999), although this study has been criticized on multiple grounds (Singer, 1999). Two studies have reported that antibiotic prophylaxis and treatment was effective in reducing symptoms in PANDAS (M. L. Murphy & Pichichero, 2002; Snider, Lougee, Slattery, Grant, & Swedo, 2005), although these studies should be considered preliminary because neither of them included a placebo group. Finally, infusion of sera from TS patients who have elevated antineuronal antibodies into the striatum of rats has been reported to produce an increase in stereotypies (Hallett, Harling-Berg, Knopf, Stopa, & Kiessling, 2000; J. R. Taylor et al., 2002), although these findings have not always been replicated (Loiselle, Lee, Moran, & Singer, 2004; Singer et al., 2005).
The caudate, putamen, and globus pallidus are enlarged in patients with PANDAS (Giedd, Rapoport, Garvey, Perlmutter, & Swedo, 2000), just as they are in Sydenham’s chorea (Giedd et al., 1995). Changes in basal ganglia volumes are, however, commonly found in OCD and TS, so the possibility that the volumetric changes found in PANDAS subjects simply reflect changes associated with TS or OCD, independently of an association with GAS infection, cannot be excluded. Stronger evidence for a causal link between an autoimmune reaction and enlarged basal ganglia volumes would be obtained if immunomodulatory treatment was shown to reduce the size of enlarged basal ganglia. A case study of an adolescent boy with exacerbation of OCD symptoms after a streptococcal pharyngitis showed precisely that, and the reduction in basal ganglia volumes was also accompanied by a reduction in symptom severity (Giedd, Rapoport, Leonard, Richter, & Swedo, 1996). However, larger controlled studies are needed to confirm these findings.
To summarize, the existing evidence suggests but does not prove that a GAS infection may, in some cases, trigger an autoimmune response that attacks the basal ganglia, leading to OCD, TS, Sydenham’s chorea, or a combination of these disorders. The specific CGBTC loops affected may determine the neuropsychiatric presentation, with disturbances in motor loops causing TS or Sydenham’s chorea and disturbances in OFC/ACC CBGTC loops causing OCD.
OCD as a Consequence of Brain Injury
The onset of OCD following focal brain lesions can provide valuable clues regarding the anatomical bases of OCD. In principle, simply because a lesion in a given brain region can produce OCD, that does not necessarily imply that all or even some cases of non-lesion-related OCD involve impairments in that region. Nevertheless, if lesion studies highlight the same general circuits that have been implicated in anatomical and functional imaging studies of OCD, the case for a causal role for those circuits in the pathogenesis of OCD becomes stronger.
Multiple reports describe cases of OCD following lesions confined to the basal ganglia (Carmin, Wiegartz, Yunus, & Gillock, 2002; Chacko, Corbin, & Harper, 2000; Laplane et al., 1989; Lopez-Rodriguez, Gunay, & Glaser, 1997; Rodrigo Escalona, Adair, Roberts, & Graeber, 1997; Weilburg et al., 1989; Weiss & Jenike, 2000); two describe cases following lesions confined to the OFC (K. W. Kim & Lee, 2002; Ogai, Iyo, Mori, & Takei, 2005); a few describe cases following lesions involving broader expanses of frontal cortex (Swoboda & Jenike, 1995; Ward, 1988; Weiss & Jenike, 2000); and a few others describe cases following more widespread lesions, which nonetheless also involve the frontal lobes (typically including the OFC) or the basal ganglia (Berthier, Kulisevsky, Gironell, & Lopez, 2001; Gamazo-Garran, Soutullo, & Ortuno, 2002; Max et al., 1995). In addition, two case reports describe improvements in pre-existing OCD after hemorrhage in the basal ganglia (Fujii, Otsuka, Suzuki, Endo, & Yamadori, 2005; Yaryura-Tobias & Neziroglu, 2003).
The consistency of the brain areas involved in these case reports and those implicated in anatomical and functional imaging studies of patients with OCD suggests a causal role for the frontal cortices – in particular, the OFC – and the basal ganglia in the pathogenesis of OCD. Some caution is warranted when interpreting the findings of case reports, however, for three main reasons. First, the number of subjects involved is relatively small, even when considering the findings of all available case reports. Second, the stress associated with having a brain lesion could, by itself, aggravate or even precipitate some cases of OCD. In fact, greater psychosocial adversity is associated with the development of obsessive-compulsive symptoms following traumatic brain injury (Grados et al., 2008). Third, the possibility of reporting bias cannot be excluded, given that several of the cases mentioned above were reported after theories of the involvement of the OFC and respective loops through the basal ganglia had appeared in the literature, and clinicians may have been more willing to report cases consistent with that theoretical framework. To the extent that childhood- and adult-onset OCD may have differing etiologies, these cases may also bear mostly on adult-onset illness, as the vast majority of them involve adults (often older adults).
A large, prospective study assessed new-onset obsessive-compulsive symptoms in 80 children and adolescents (ages 6–18) following severe traumatic brain injury (Grados et al., 2008). This study reported a high prevalence of new-onset obsessions or compulsions one year after injury, with 21 out of the 80 subjects reporting obsessions or compulsions at that time but not before the lesion. Conversely, 5 subjects who had obsessions or compulsions before the lesion no longer had them one year after the lesion. Medial prefrontal and temporal lobe lesions were associated with new-onset obsessions, but this effect disappeared when obsessions and compulsions were considered conjointly. OFC lesions seemed to be associated with fewer symptoms, suggesting that anatomical integrity of the OFC is required for the onset of OCD. This is consistent with neurosurgical treatments for OCD that disrupt the OFC or its connections, but contrasts with the case reports that have implicated lesions of the OFC in the onset of OCD (K. W. Kim & Lee, 2002; Ogai et al., 2005). A possible explanation for this discrepancy is that lesions to certain subregions of the OFC may cause OCD, whereas lesions to other subregions may prevent its expression.
This study suffered from three important limitations. First, it used an ROI approach that examined only the areas that have traditionally been implicated in OCD (OFC, medial prefrontal cortex, basal ganglia, and thalamus), plus the temporal lobe. Second, symptoms were assessed one year after injury, and their onset or disappearance could, at least in some cases, be due simply to the passing of time. The non-inclusion of a group of matched non-injured controls in the study precludes any inferences regarding the extent to which the changes in symptoms were caused by the injury. Third, scanning and symptom assessment occurred 9 months apart. In addition, the focus of the study was on obsessive-compulsive symptoms, not full-blown OCD. In fact, only 2 out of the 80 children had new-onset OCD following the lesion.
In summary, several reports describe cases of OCD following lesions of the basal ganglia or frontal cortex (with an emphasis on the OFC); two reports describe cases of improvement in OCD after lesions of the basal ganglia. A larger, prospective study (Grados et al., 2008) also suggests a role for the OFC in obsessive-compulsive symptoms following brain injury, although in that study lesions to the OFC were inversely related to symptom severity. The same study also reported an association between lesions to the medial prefrontal cortex (which included the ACC) and obsessions. All of these findings are consistent with the hypothesis that the pathogenesis of OCD involves CBGTC loops, in particular the loops involving the OFC and possibly the ACC.
Despite the convergence of findings from lesion studies with those from anatomical and functional imaging studies, the correct way to conceptualize the relation of lesion findings with functional imaging findings remains elusive. For example, we have seen above that the OFC is often hyperactive in (non-lesion-related) OCD. Do we interpret this as evidence that activity of the OFC is somehow causally related to the symptoms of OCD, and therefore expect a lesion of the OFC to improve symptoms, given that a lesioned OFC would not be active? Or do we interpret the imaging data as evidence that the OFC is working in overdrive to try to inhibit or control the expression of OCD symptoms, and therefore expect a lesion of the OFC to worsen the symptoms? Or do we interpret the hyperactivity in OFC not as a sign of heightened function but rather of dysfunction in that region, and therefore expect that lesions of the OFC might produce OCD, by making the OFC dysfunctional? Different versions of all of these hypotheses can be found, implicitly or explicitly, in the literature. Existing lesion studies do not, however, help us discriminate between these alternatives, given that lesions of the OFC have been associated with both increases and decreases in OCD symptoms. More research is needed to address these questions.
Another question, raised specifically by findings of the prospective study described above (Grados et al., 2008), is whether the temporal lobe may also be involved in the pathogenesis of OCD. In support of this possibility, temporal lobe epilepsy has been associated with OCD and obsessive-compulsive symptoms (Kroll & Drummond, 1993; Monaco et al., 2005). In addition, several studies have reported anatomical abnormalities in the superior temporal gyrus in patients with OCD (Choi et al., 2006; J. J. Kim et al., 2001; Shin et al., 2007; Yoo et al., 2008). One possibility, which we have already discussed, is that the temporal lobe – in particular the superior temporal gyrus – is involved in OCD via its connections with the regions of the striatum that are part of the OFC/ACC CBGTC loops (Alexander et al., 1986). However, additional research is needed to test this hypothesis.
Neurosurgical Lesions
Neurosurgical lesions are sometimes made to attenuate symptoms in extremely severe cases of OCD that do not respond to psychotherapy and medication. Four types of neurosurgical lesions are used: anterior capsulotomy, subcaudate tractotomy, cingulotomy, and limbic leucotomy (for review, see, e.g., Greenberg, Murphy, & Rasmussen, 2000; Greenberg et al., 2003; Mindus, Rasmussen, Lindquist, & Noren, 2001). All are small bilateral lesions that interrupt white matter tracts that connect the OFC or ACC with subcortical structures involved in CBGTC loops. Their success, though modest, provides additional converging evidence for the involvement of the OFC, ACC, and their associated CBGTC loops in the pathophysiology of OCD.
The lesions in anterior capsulotomy are placed in the anterior limb of the internal capsule. Such lesions are believed to interrupt the connections between the OFC and the mediodorsal nucleus of the thalamus (Greenberg et al., 2000; Mindus et al., 2001), which is the thalamic nucleus with densest reciprocal connections with the OFC and the primary nucleus in the thalamus involved in CBGTC loops that connect to the OFC (Fuster, 1997; Ongur & Price, 2000). The lesions in subcaudate tractotomy are placed below and immediately anterior to the head of the caudate nucleus. They seem to be restricted to white matter, as they are anterior to the substantia innominata and rarely include the cortex (Malhi & Bartlett, 1998; Newcombe, 1975). These lesions are believed to disconnect the OFC from the thalamus, basal ganglia, and limbic system (Feldman, Alterman, & Goodrich, 2001; Malhi & Bartlett, 1998). They have also been shown to produce degeneration in the anterior limb of the internal capsule, which can be traced back to the mediodorsal nucleus of the thalamus (Mindus et al., 2001). The lesions in cingulotomy are placed in the cingulate bundle (a.k.a. cingulum). The mechanisms by which cingulotomy reduces the severity of OCD symptoms are unknown, but may involve the fibers that connect the ACC and the caudate nucleus (Rauch et al., 2000). Limbic leucotomy refers simply to a combination of subcaudate tractotomy and cingulotomy.
Evaluating the success of these procedures is complicated by the small number of subjects in each study and the obvious ethical difficulties with conducting randomized double-blind studies involving neurosurgical lesions (Earp, 1979). In addition, different studies use different criteria (e.g., remission of symptoms, percent improvement over pre-operative symptom severity) to evaluate the success of these procedures. Nevertheless, estimated success rates are generally approximately 50% or more for all procedures (Jenike, 1998; Mindus et al., 2001). Similar rates are, however, often seen in open trials of medication or psychotherapy, and typically drop substantially in blinded, controlled treatment trials.
The fact that these procedures primarily target fiber bundles that connect the OFC or ACC with related subcortical structures – notably, with the thalamus and basal ganglia – provides additional evidence for the involvement of these circuits in the pathogenesis of OCD. Such evidence remains circumstantial, however, for three reasons. First, all of these procedures were developed largely empirically between 30 and 60 years ago (Bingley, Leksell, Meyerson, & Rylander, 1977; D. Kelly et al., 1973; Knight, 1964; Richardson, 1973; Talairach, Hecaen, David, Monnier, & Ajuriaguerra, 1949; Whitty, Duffield, Tov, & Cairns, 1952), often based on neuroanatomical theories that are now outdated. Consequently, in most cases we still do not fully know all of the neuroanatomical connections that they affect. Second, the improvement in symptoms is often gradual following surgery, occurring over a time span of weeks to months (Jenike, 1998). This suggests that the anatomical changes underlying such improvement may be a secondary consequence of the lesion, possibly involving retrograde degeneration or a neuroplastic response. Indeed, volume reductions in distant structures have been observed after localized neurosurgical lesions (Rauch et al., 2000; Taren, Curtis, & Gebarski, 1994). Third, the fact that a lesion in a given circuit improves symptoms does not necessarily imply that an abnormality at that lesion site or even in that circuit caused the symptoms. In fact, the same neurosurgical procedures used for the treatment of OCD are also used for a variety of other conditions, including other anxiety disorders (Cosgrove & Rauch, 2003), depression (Cosgrove & Rauch, 2003; Malhi & Bartlett, 2000), and intractable pain (Wilkinson, Davidson, & Davidson, 1999). For all these reasons, whether these procedures target directly and specifically the circuits involved in the pathogenesis of OCD remains unclear.
Development of OFC/ACC CBGTC Circuits
Knowledge of the normal development of OFC/ACC CBGTC circuits may provide clues to the neurodevelopmental processes that go awry in patients with OCD. Although brain weight and volume nearly reach their adult values by 5–6 years of age (Courchesne et al., 2000; Dekaban & Sadowsky, 1978; Giedd, Snell et al., 1996; Kretschmann, Kammradt, Krauthausen, Sauer, & Wingert, 1986; Reiss, Abrams, Singer, Ross, & Denckla, 1996; Sahni, Jit, & Sodhi, 1998), the brain continues to develop throughout childhood, adolescence, and early adulthood. The total volume of gray matter starts to decrease after around 5 years of age (Courchesne et al., 2000; Pfefferbaum et al., 1994; Reiss et al., 1996; Sowell et al., 2003), with cortical thickness and gray matter density decreasing in much of the cortex (Gogtay et al., 2004; Sowell et al., 2004). In contrast, white matter volume increases throughout childhood, adolescence, and early adulthood (Courchesne et al., 2000; Giedd et al., 1999; Matsuzawa et al., 2001; Pfefferbaum et al., 1994; Reiss et al., 1996; Sowell et al., 2003). In general, sensorimotor areas mature earlier and higher-order areas, including the OFC, mature later (Benes, 1989; Gogtay et al., 2004; Yakovlev & Lecours, 1967). During the years in which childhood-onset OCD typically starts, the prefrontal cortex (PFC), basal ganglia, and thalamus are undergoing major developmental changes. Furthermore, some of those changes seem to affect primarily boys, which may explain the higher prevalence of OCD among boys than girls. This section reviews these developmental changes and their possible relation to the pathogenesis of OCD.
Development of the PFC
The PFC grows markedly between 5 and 14 years of age (Kanemura, Aihara, Aoki, Araki, & Nakazawa, 2003; Sowell et al., 2004; Thompson et al., 2000). Such growth seems to include the most ventral aspects of the PFC (Sowell et al., 2004; Thompson et al., 2000), possibly including the OFC. However, the studies that have mapped the growth of the PFC did not include the ventral surface of the brain, so the growth patterns of the OFC remain to be elucidated. Some evidence suggests that the fastest growth in the PFC occurs between 8 and 14 years of age (Kanemura et al., 2003), the period during which most cases of childhood-onset OCD begin, raising the possibility that abnormalities in that growth could cause childhood-onset OCD – a hypothesis that is consistent with the findings of reduced OFC volume in patients with OCD. The PFC, including the OFC, continues to mature through adulthood (Gogtay et al., 2004), however, so abnormalities in later stages of maturation of the OFC could conceivably cause adult-onset OCD.
The growth of the PFC during childhood and adolescence may be largely driven by the development of white matter (Barnea-Goraly et al., 2005; Reiss et al., 1996). In fact, gray matter density and cortical thickness in PFC seem to decrease during childhood, adolescence, and young adulthood (Gogtay et al., 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sowell et al., 2004). Furthermore, postmortem studies indicate that the density of synapses and the sizes of neuronal cell bodies and dendritic fields in PFC increase only until approximately 2 to 3 years of age (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; Koenderink, Uylings, & Mrzljak, 1994; Petanjek, Judas, Kostovic, & Uylings, 2008). Whether this means that OCD is caused primarily by abnormalities in the development of white matter or in the regressive processes that fine-tune synaptic organization in OFC is unknown.
Future studies should address more directly the normal development of the OFC. Several of the MRI studies that have mapped cortical development did not map the inferior surface of the brain (Sowell et al., 2003; Sowell et al., 2004; Thompson et al., 2000) and postmortem developmental studies have generally focused on the lateral PFC (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997; Koenderink et al., 1994; Petanjek et al., 2008). Our knowledge of the development of the OFC is therefore indirect, and the extent to which the OFC and the lateral and dorsal PFC share similar developmental trajectories is unknown. One study reported that while the volume of white matter in dorsal PFC increased throughout childhood and adolescence, the volume of white matter in the OFC decreased (Reiss et al., 1996), raising the possibility that these structures may develop differently. Even within the OFC, decreases in gray matter density throughout childhood and adolescence seem to follow medial-to-lateral and anterior-to-posterior gradients (Gogtay et al., 2004). A better understanding of the development of the OFC in healthy children and in children with OCD could substantially advance our understanding of the pathogenesis of this disorder.
Current knowledge of the development of the ACC is similarly limited. Volumes of the cingulate seem to change less during childhood and adolescence than do volumes of other regions such as the frontal or medial temporal lobes (Sowell, Trauner, Gamst, & Jernigan, 2002). Gray matter density also seems to change less across the lifespan in the ACC than in many other cortical regions (Sowell et al., 2003). Furthermore, cortical thickness and size of the dorsal ACC seem to change relatively little between 5 and 11 years of age, although cortical thickness and size of the ventral ACC may increase in that age range (Sowell et al., 2004). Together, these findings may suggest that anatomical development is less prominent in the ACC than in other portions of the OFC/ACC CBGTC loops during the years in which OCD typically begins. On the other hand, the volume of the right ACC has been reported to increase during childhood and adolescence (Casey et al., 1997; Marquardt et al., 2005). Furthermore, larger volumes of the right ACC have been associated with better performance on an attentional task in children and adolescents (Casey et al., 1997). Some authors have argued that development of the ACC during infancy, childhood, and adolescence may underlie the improvements in self-regulatory capacities that characterize these periods (Posner & Rothbart, 1998; Posner, Rothbart, Sheese, & Tang, 2007). Whether abnormalities in the development of the ACC may lead to deficits in the self-regulation of intrusive thoughts, images, or feelings, or to the well-documented deficits in inhibitory control in OCD (Chamberlain et al., 2005) is unknown.
Development of the Basal Ganglia and Thalamus
The volume of the caudate nucleus has generally been reported to decrease during childhood and adolescence (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997; Giedd, Snell et al., 1996; Jernigan, Trauner, Hesselink, & Tallal, 1991). Some evidence suggests that such decreases may be most pronounced between 4 and 12 years of age (Giedd, Snell et al., 1996), and marked tissue loss has been reported in the head of the caudate between 7 and 13 years of age (Thompson et al., 2000). These age ranges are consistent with the ages during which childhood-onset OCD typically begins, raising the possibility that abnormal development of the caudate could cause childhood-onset OCD. Volumes of the lenticular nuclei (Giedd et al., 1997; Giedd, Snell et al., 1996; Jernigan et al., 1991; Reiss et al., 1996; Sowell et al., 2002) and thalamus (Jernigan et al., 1991; Reiss et al., 1996; Sowell et al., 2002) also decrease throughout childhood and adolescence. In addition, white matter tracts within the basal ganglia and between the basal ganglia and thalamus also develop during childhood and adolescence (Barnea-Goraly et al., 2005). Together with the findings of maturation of frontal cortex during childhood and adolescence (Barnea-Goraly et al., 2005; Gogtay et al., 2004; Kanemura et al., 2003; Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Reiss et al., 1996; Sowell et al., 1999; Sowell et al., 2004; Sowell et al., 2002; Thompson et al., 2000), these results may reflect the integrated development of CBGTC loops. OCD may conceivably be caused by abnormalities in the development of one or more of the key structures or white matter tracts in these loops.
Sex Differences in the Development of CBGTC Circuits
Many studies have reported sexual dimorphisms in the human brain (Gilmore et al., 2007; Goldstein et al., 2001; Luders et al., 2005; Rabinowicz, Dean, Petetot, & de Courten-Myers, 1999; Sowell et al., 2007). These tend to occur in regions that have high concentrations of sex steroid receptors during brain development (Goldstein et al., 2001), suggesting a role for gonadal hormones in causing such dimorphisms. Sex differences in brain structure and development during childhood and adolescence may provide an explanation for the significantly higher prevalence of childhood-onset OCD in boys than in girls. In fact, existing evidence suggests that both the caudate and the OFC – two key components of the OFC/ACC CGBTC loops – differ markedly and develop differently in boys and girls.
Male brains are, on average, larger than female brains across all age groups (Caviness, Kennedy, Richelme, Rademacher, & Filipek, 1996; Courchesne et al., 2000; Giedd et al., 1997; Giedd, Snell et al., 1996; Goldstein et al., 2001; Lenroot et al., 2007; Reiss et al., 1996; Sowell et al., 2007). Comparisons of volumes of brain structures between males and females are therefore usually performed after adjusting for overall brain volume. When this is done, girls have greater caudate volumes than boys (Caviness et al., 1996; Giedd et al., 1997; Giedd, Snell et al., 1996; Sowell et al., 2002). In addition, the decline in volumes of the caudate and lenticular nuclei during childhood and adolescence may be restricted to boys (Giedd et al., 1997; Giedd, Snell et al., 1996; Sowell et al., 2002). Childhood and adolescence may therefore be particularly critical for the development of the basal ganglia in boys, which may explain why boys are more susceptible than girls to OCD and other disorders of CBGTC circuitry such as TS and attention-deficit/hyperactivity disorder.
Potential sexual dimorphisms in the human OFC during childhood or adolescence remain to be studied. In adulthood, women have larger OFC volumes than men (Goldstein et al., 2001), and in a sample spanning childhood to old age, females had thicker OFC cortices than males (Sowell et al., 2007). These anatomical differences have functional implications, as males perform better on average than females on tasks that rely on OFC function (Overman, 2004). Such performance differences seem to apply across most age groups, and have been found even in children younger than 3 years of age (Overman, 2004). Similar findings have been reported in monkeys, with male infant monkeys performing better than female infant monkeys on an object discrimination reversal task, which is known to depend on the OFC (Clark & Goldman-Rakic, 1989). The OFC also appears to develop earlier in male monkeys than in female monkeys (Clark & Goldman-Rakic, 1989; Goldman, Crawford, Stokes, Galkin, & Rosvold, 1974). In short, the structure, function, and development of the OFC seem to differ between males and females. The contribution of these differences to the differing susceptibilities of boys and girls to OCD remains, however, to be elucidated.
Several lines of evidence suggest that these differences in OFC structure, function, and development in males and females are caused by the effects of gonadal hormones on the OFC during development. First, the OFC has high affinity for androgen binding (Clark, MacLusky, & Goldman-Rakic, 1988) and high aromatase activity (MacLusky, Clark, Naftolin, & Goldman-Rakic, 1987; MacLusky, Naftolin, & Goldman-Rakic, 1986) in the developing monkey, suggesting that it may be particularly susceptible to the influence of gonadal hormones. Second, treating female infant monkeys with androgen early in postnatal life abolishes the performance differences between male and female monkeys in the object discrimination reversal task (Clark & Goldman-Rakic, 1989). Third, female rats have more extensive dendritic fields in the OFC than do male rats, but those differences are abolished by neonatal gonadectomy (Kolb, Pellis, & Robinson, 2004). The finding of more extensive dendritic fields in the OFC of female rats may also help to explain the findings of larger volume (Goldstein et al., 2001) and thicker cortex (Sowell et al., 2007) in the OFC of women.
Conclusions
All of the lines of research reviewed herein – anatomical imaging, PANDAS, OCD as a consequence of brain injury, and neurosurgery for treatment-refractory OCD – suggest a role for OFC/ACC CBGTC loops in the pathogenesis of OCD, but each suffers from important limitations. Anatomical imaging findings point to abnormalities in the OFC/ACC CBGTC loops in both children and adults with OCD. These findings do not, however, per se license any conclusions regarding a causal role for those abnormalities in OCD. Studies of PANDAS and of OCD as a consequence of brain injury suggest that insults to OFC/ACC CBGTC loops may indeed cause OCD. Specifically, studies of PANDAS suggest that an autoimmune insult to the basal ganglia may cause OCD; however, controversy remains regarding this putative pathogenic mechanism and even the very existence of PANDAS. Studies of OCD as a consequence of brain injury suggest that lesions in key components of these loops may also cause OCD; however, much of this evidence is based on case reports. Finally, neurosurgical lesions used for treatment-refractory OCD suggest that lesions placed in key components of the OFC/ACC CBGTC loops can be effective in attenuating the symptoms of OCD; however, the improvement in symptoms is often gradual, raising the possibility that it may be due to degeneration or plasticity elsewhere in the brain, rather than an effect of the primary lesion itself. Despite the limitations when each of these lines of evidence is considered in isolation, the remarkable convergence of findings across all of them does suggest a causal role for the OFC/ACC CBGTC loops in OCD, in both children and adults.
Assuming that OCD is indeed caused by abnormalities in OFC/ACC CBGTC loops, do the findings reviewed above suggest a particular locus within those loops that may be responsible for OCD? In adults with OCD, anatomical imaging studies using ROI approaches point to volumetric or NAA abnormalities in all components of the OFC/ACC CBGTC loops that have been studied in detail: the OFC, ACC, striatum, and thalamus. Whole-brain approaches such as VBM have also found gray matter abnormalities in all of these areas. Abnormalities in other areas closely related to the OFC/ACC CBGTC loops, including the superior temporal gyrus, hippocampus, and amygdala, have also been found in both ROI and whole-brain anatomical studies. Several VBM studies have also highlighted abnormalities in the insula, another area closely related to the OFC/ACC CBGTC loops (Mesulam & Mufson, 1982; Mufson & Mesulam, 1982; Ongur & Price, 2000).
The implications for the pathogenesis of OCD of such widespread abnormalities within OFC/ACC CBGTC loops and potentially also in related areas in adults with OCD can be interpreted in at least four ways. First, the etiology of OCD may involve some mechanism that causes widespread abnormalities in all of these structures. Second, the etiology of OCD may involve some mechanism that causes an abnormality in just one or a small number of these structures, with the abnormalities in the remaining structures being a consequence of dynamic changes prompted by that primary etiologic abnormality. As we have seen, anatomical imaging studies after neurosurgery have shown that localized lesions in one structure or fiber bundle of the OFC/ACC CBGTC loops can cause volume changes in remote, albeit related structures (Rauch et al., 2000; Taren et al., 1994). Thus, abnormalities in one or a few structures in these loops could translate into structural abnormalities in all of the structures within the loops and related structures elsewhere. Third, the etiology of OCD may, as in the second hypothesis, consist of an initial abnormality in just one or a few structures, with the abnormalities in the remaining structures following from that, but with different cases of OCD possibly arising from initial abnormalities in different structures. Lesion studies suggest that damage to any one of several components of the OFC/ACC CBGTC loops is sufficient to cause OCD, raising the possibility that even non-lesion-related cases of OCD may be a consequence of different initial abnormalities. Fourth, the widespread abnormalities may actually be an artifact of averaging across subjects, with individual subjects having more localized abnormalities that vary across subjects.
One way of trying to disentangle these possibilities would be to conduct longitudinal anatomical imaging studies that followed patients with OCD over extended period of time, starting as close to their diagnosis as possible. Such studies could determine whether the abnormalities start in only one or a few structures (with the initial site of the abnormality possibly varying by case) and then spread to other structures. A cruder and less conclusive alternative is to compare the findings from anatomical imaging studies across children and adults with OCD to determine whether the findings in children suggest abnormalities in a smaller subset of regions than do the findings in adults. If so, this could indicate that abnormalities in OCD start in a specific subset of the regions that have been found abnormal in adults with OCD. Differences between findings in children and adults OCD could, however, be due to multiple factors, including a comparison across childhood- and adult-onset subtypes, differing types and rates of comorbidity, differing ascertainment biases, and differing durations of illness.
Unfortunately, the extant literature does not allow us to conclude with any certainty which areas are and are not affected in children with OCD. Existing studies clearly point to anatomical abnormalities in the ACC and metabolite abnormalities in the thalamus. No anatomical abnormalities have yet been reported in the other structures that are affected in adults with OCD, including the OFC and caudate, but that may be attributable either in whole or in part to the fact that those structures have not yet been properly studied in children with OCD. For example, even though the most consistently replicated volumetric finding in adults with OCD is a reduction in volume of the OFC, no ROI studies have analyzed the volume of the OFC in children with OCD. Similarly, even though the most consistent finding for the striatum of adults with OCD is a reduction in NAA levels, no studies have analyzed NAA levels in the striatum of children with OCD. In addition, of the two VBM studies of pediatric OCD, one had fairly small samples (Gilbert et al., 2008), and in the other the majority of children were on medication (Carmona et al., 2007).
A large-scale longitudinal study that followed children or adults with high familial risk for OCD from before their diagnosis through several years later would clearly provide a much richer and more dynamic view of the unfolding of pathology in the brain affected by OCD. Such a study should ideally be multi-modal, including not only anatomical MRI, fMRI, and 1H-MRS, but also, for example, investigation of white matter fiber tracts using diffusion tensor imaging and investigation of neurotransmitter and receptor levels using PET. The results of such a study could then inform a more detailed search for the ultimate cause or causes of OCD: clearly, the focus of such a search would be vastly different if OCD was found to be a consequence of, say, an abnormality localized initially in the striatum, than if it was found to be a consequence of abnormalities affecting, from the very beginning, several structures of the OFC/ACC CBGTC loops and related structures elsewhere.
Acknowledgments
This work was supported in part by NIMH grants K02 74677, 2T32 MH16434, and MH068318.
Footnotes
A more recent study using the same paradigm confirmed abnormal recruitment of the hippocampus in patients with OCD, even though it failed to detect differences in activation of the striatum between patients with OCD and healthy controls (Rauch et al., 2007).
References
- Ackerman DL, Greenland S, Bystritsky A, Morgenstern H, Katz RJ. Predictors of treatment response in obsessive-compulsive disorder: Multivariate analyses from a multicenter trial of clomipramine. Journal of Clinical Psychopharmacology. 1994;14:247–254. [PubMed] [Google Scholar]
- Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. Journal of Psychiatric Research. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen AJ, Leonard HL, Swedo SE. Case study: A new infection-triggered, autoimmune subtype of pediatric OCD and Tourette’s syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:307–311. doi: 10.1097/00004583-199503000-00015. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Anderson KE, Louis ED, Stern Y, Marder KS. Cognitive correlates of obsessive and compulsive symptoms in Huntington’s disease. American Journal of Psychiatry. 2001;158:799–801. doi: 10.1176/appi.ajp.158.5.799. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim BH, Ozdemir BH, Aydin BA, Tezcan AE, Ozler AS. Volumetric MRI assessment of brain regions in patients with refractory obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:1051–1057. doi: 10.1016/j.pnpbp.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Harris GJ, Hoehn-Saric R, Barta PE, Machlin SR, Pearlson GD. Normal caudate nucleus in obsessive-compulsive disorder assessed by quantitative neuroimaging. Archives of General Psychiatry. 1996;53:577–584. doi: 10.1001/archpsyc.1996.01830070021006. [DOI] [PubMed] [Google Scholar]
- Barker PB. N-acetyl aspartate - a neuronal marker? Annals of Neurology. 2001;49:423–424. [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. American Journal of Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Clark EC, Iqbal M, Ackermann RF. Cortical-subcortical systems in the mediation of obsessive-compulsive disorder: Modeling the brain’s mediation of a classic “Neurosis”. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: Guilford Press; 2001. pp. 207–230. [Google Scholar]
- Beglinger LJ, Langbehn DR, Duff K, Stierman L, Black DW, Nehl C, et al. Probability of obsessive and compulsive symptoms in Huntington’s disease. Biological Psychiatry. 2007;61:415–418. doi: 10.1016/j.biopsych.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Bellodi L, Sciuto G, Diaferia G, Ronchi P, Smeraldi E. Psychiatric disorders in the families of patients with obsessive-compulsive disorder. Psychiatry Research. 1992;42:111–120. doi: 10.1016/0165-1781(92)90075-e. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Kulisevsky JJ, Gironell A, Lopez OL. Obsessive-compulsive disorder and traumatic brain injury: Behavioral, cognitive, and neuroimaging findings. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2001;14:23–31. [PubMed] [Google Scholar]
- Bingley T, Leksell L, Meyerson BA, Rylander G. Long-term results of stereotactic capsulotomy in chronic obsessive-compulsive neurosis. In: Sweet WH, Obrador S, Martín-Rodríguez JG, editors. Neurosurgical treatment in psychiatry, pain and epilepsy. Baltimore, MD: University Park Press; 1977. pp. 287–299. [Google Scholar]
- Bland RC, Newman SC, Orn H. Age of onset of psychiatric disorders. Acta Psychiatrica Scandinavica. Supplementum. 1988;338:43–49. doi: 10.1111/j.1600-0447.1988.tb08546.x. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel-based morphometry” Should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Brain imaging in anxiety disorders. Expert Review of Neurotherapeutics. 2004;4:275–284. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Buchpiguel CA, Zamignani DR, Garrido GE, Glabus MF, Rosario-Campos MC, et al. Regional cerebral blood flow abnormalities in early-onset obsessive-compulsive disorder: An exploratory SPECT study. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:347–354. doi: 10.1097/00004583-200103000-00015. [DOI] [PubMed] [Google Scholar]
- Cardoner N, Soriano-Mas C, Pujol J, Alonso P, Harrison BJ, Deus J, et al. Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage. 2007;38:413–421. doi: 10.1016/j.neuroimage.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmin CN, Wiegartz PS, Yunus U, Gillock KL. Treatment of late-onset OCD following basal ganglia infarct. Depression and Anxiety. 2002;15:87–90. doi: 10.1002/da.10024. [DOI] [PubMed] [Google Scholar]
- Carmona S, Bassas N, Rovira M, Gispert JD, Soliva JC, Prado M, et al. Pediatric OCD structural brain deficits in conflict monitoring circuits: A voxel-based morphometry study. Neuroscience Letters. 2007;421:218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30:61–69. [PubMed] [Google Scholar]
- Castillo AR, Buchpiguel CA, de Araujo LA, Castillo JC, Asbahr FR, Maia AK, et al. Brain SPECT imaging in children and adolescents with obsessive-compulsive disorder. Journal of Neural Transmission. 2005;112:1115–1129. doi: 10.1007/s00702-004-0240-x. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex: A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: A volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Annals of Neurology. 1994;35:211–216. doi: 10.1002/ana.410350213. [DOI] [PubMed] [Google Scholar]
- Chabane N, Delorme R, Millet B, Mouren MC, Leboyer M, Pauls D. Early-onset obsessive-compulsive disorder: A subgroup with a specific clinical and familial pattern? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2005;46:881–887. doi: 10.1111/j.1469-7610.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Chacko RC, Corbin MA, Harper RG. Acquired obsessive-compulsive disorder associated with basal ganglia lesions. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:269–272. doi: 10.1176/jnp.12.2.269. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: The importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kang DH, Kim JJ, Ha TH, Lee JM, Youn T, et al. Left anterior subregion of orbitofrontal cortex volume reduction and impaired organizational strategies in obsessive-compulsive disorder. Journal of Psychiatric Research. 2004;38:193–199. doi: 10.1016/j.jpsychires.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim HS, Yoo SY, Ha TH, Chang JH, Kim YY, et al. Morphometric alterations of anterior superior temporal cortex in obsessive-compulsive disorder. Depression and Anxiety. 2006;23:290–296. doi: 10.1002/da.20171. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim SH, Yoo SY, Kang DH, Kim CW, Lee JM, et al. Shape deformity of the corpus striatum in obsessive-compulsive disorder. Psychiatry Research. 2007;155:257–264. doi: 10.1016/j.pscychresns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Clark AS, Goldman-Rakic PS. Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behavioral Neuroscience. 1989;103:1287–1295. doi: 10.1037//0735-7044.103.6.1287. [DOI] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurgery Clinics of North America. 2003;14:225–235. doi: 10.1016/s1042-3680(02)00115-8. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Bennett D, Lekwuwa G, Shaunak S, Deakin JF. Cognition and the inhibitory control of saccades in schizophrenia and parkinson’s disease. Progress in Brain Research. 2002;140:449–466. doi: 10.1016/S0079-6123(02)40068-4. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Cunningham K. Obsessive-compulsive disorder in Huntington’s disease. Biological Psychiatry. 1992;31:263–270. doi: 10.1016/0006-3223(92)90049-6. [DOI] [PubMed] [Google Scholar]
- Dager SR, Steen RG. Applications of magnetic resonance spectroscopy to the investigation of neuropsychiatric disorders. Neuropsychopharmacology. 1992;6:249–266. [PubMed] [Google Scholar]
- Dale RC, Heyman I, Giovannoni G, Church AW. Incidence of anti-brain antibodies in children with obsessive-compulsive disorder. British Journal of Psychiatry. 2005;187:314–319. doi: 10.1192/bjp.187.4.314. [DOI] [PubMed] [Google Scholar]
- De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harvard Review of Psychiatry. 2000;7:278–289. doi: 10.1093/hrp/7.5.278. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Curran T, Bohne A, Wilhelm S, Baer L, et al. A study of parallel implicit and explicit information processing in patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2002;159:1780–1782. doi: 10.1176/appi.ajp.159.10.1780. [DOI] [PubMed] [Google Scholar]
- Dekaban AS, Sadowsky D. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Diler RS, Kibar M, Avci A. Pharmacotherapy and regional cerebral blood flow in children with obsessive compulsive disorder. Yonsei Medical Journal. 2004;45:90–99. doi: 10.3349/ymj.2004.45.1.90. [DOI] [PubMed] [Google Scholar]
- do Rosario-Campos MC, Leckman JF, Curi M, Quatrano S, Katsovitch L, Miguel EC, et al. A family study of early-onset obsessive-compulsive disorder. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2005;136:92–97. doi: 10.1002/ajmg.b.30149. [DOI] [PubMed] [Google Scholar]
- DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Earp JD. Psychosurgery: The position of the Canadian psychiatric association. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 1979;24:353–364. [PubMed] [Google Scholar]
- Ebert D, Speck O, Konig A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: Evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Research. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Ebisu T, Rooney WD, Graham SH, Weiner MW, Maudsley AA. N-acetylaspartate as an in vivo marker of neuronal viability in kainate-induced status epilepticus: 1H magnetic resonance spectroscopic imaging. Journal of Cerebral Blood Flow and Metabolism. 1994;14:373–382. doi: 10.1038/jcbfm.1994.48. [DOI] [PubMed] [Google Scholar]
- Eichstedt JA, Arnold SL. Childhood-onset obsessive-compulsive disorder: A tic-related subtype of OCD? Clinical Psychology Review. 2001;21:137–157. doi: 10.1016/s0272-7358(99)00044-6. [DOI] [PubMed] [Google Scholar]
- Elliott R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Feldman RP, Alterman RL, Goodrich JT. Contemporary psychosurgery and a look to the future. Journal of Neurosurgery. 2001;95:944–956. doi: 10.3171/jns.2001.95.6.0944. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Saxena S, Zohar J, Craig KJ. Obsessive-compulsive disorder: Boundary issues. CNS Spectrums. 2007;12:359–364. 367–375. doi: 10.1017/s1092852900021167. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Moore GJ, Paulson LA, Stewart CM, Rosenberg DR. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biological Psychiatry. 2000;47:174–182. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Flament MF, Whitaker A, Rapoport JL, Davies M, Berg CZ, Kalikow K, et al. Obsessive compulsive disorder in adolescence: An epidemiological study. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27:764–771. doi: 10.1097/00004583-198811000-00018. [DOI] [PubMed] [Google Scholar]
- Foa EB, Steketee GS, Ozarow BJ. Behavior therapy with obsessive-compulsives: From theory to treatment. In: Mavissakalian M, Turner SM, Michelson L, editors. Obsessive-compulsive disorder: Psychological and pharmacological treatment. New York: Plenum Press; 1985. pp. 49–129. [Google Scholar]
- Fujii T, Otsuka Y, Suzuki K, Endo K, Yamadori A. Improvement of obsessive-compulsive disorder following left putaminal hemorrhage. European Neurology. 2005;54:166–170. doi: 10.1159/000090109. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. 3. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- Gamazo-Garran P, Soutullo CA, Ortuno F. Obsessive-compulsive disorder secondary to brain dysgerminoma in an adolescent boy: A positron emission tomography case report. Journal of Child and Adolescent Psychopharmacology. 2002;12:259–263. doi: 10.1089/104454602760386950. [DOI] [PubMed] [Google Scholar]
- Geller DA. Obsessive-compulsive and spectrum disorders in children and adolescents. Psychiatric Clinics of North America. 2006;29:353–370. doi: 10.1016/j.psc.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Jones J, Park K, Schwartz S, Shapiro S, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:420–427. doi: 10.1097/00004583-199804000-00020. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Jones J, Shapiro S, Schwartz S, Park KS. Obsessive-compulsive disorder in children and adolescents: A review. Harvard Review of Psychiatry. 1998;5:260–273. doi: 10.3109/10673229809000309. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. American Journal of Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Kruesi MJ, Parker C, Schapiro MB, Allen AJ, et al. Sydenham’s chorea: Magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199–2202. doi: 10.1212/wnl.45.12.2199. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Leonard HL, Richter D, Swedo SE. Case study: Acute basal ganglia enlargement and obsessive-compulsive symptoms in an adolescent boy. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:913–915. doi: 10.1097/00004583-199607000-00017. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Keshavan MS, Diwadkar V, Nutche J, Macmaster F, Easter PC, et al. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: A preliminary voxel-based morphometry study. Neuroscience Letters. 2008;435:45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, et al. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000;57:449–456. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman PS, Crawford HT, Stokes LP, Galkin TW, Rosvold HE. Sex-dependent behavioral effects of cerebral cortical lesions in the developing rhesus monkey. Science. 1974;186:540–542. doi: 10.1126/science.186.4163.540. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Breiter HC, Rauch SL, Savage CR, Baer L, Shera DM, et al. Structural abnormalities of frontal neocortex in obsessive-compulsive disorder. Archives of General Psychiatry. 1998;55:181–182. doi: 10.1001/archpsyc.55.2.181. [DOI] [PubMed] [Google Scholar]
- Grados MA, Riddle MA, Samuels JF, Liang KY, Hoehn-Saric R, Bienvenu OJ, et al. The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: The hopkins OCD family study. Biological Psychiatry. 2001;50:559–565. doi: 10.1016/s0006-3223(01)01074-5. [DOI] [PubMed] [Google Scholar]
- Grados MA, Vasa RA, Riddle MA, Slomine BS, Salorio C, Christensen J, et al. New onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depression and Anxiety. 2008;25:398–407. doi: 10.1002/da.20398. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Murphy DL, Rasmussen SA. Neuroanatomically based approaches to obsessive-compulsive disorder. Neurosurgery and transcranial magnetic stimulation. Psychiatric Clinics of North America. 2000;23:671–686. xii. doi: 10.1016/s0193-953x(05)70188-x. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Price LH, Rauch SL, Friehs G, Noren G, Malone D, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: Critical issues. Neurosurgery Clinics of North America. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: Parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. Journal of Neuroimmunology. 2000;111:195–202. doi: 10.1016/s0165-5728(00)00320-9. [DOI] [PubMed] [Google Scholar]
- Harris K, Singer HS. Tic disorders: Neural circuits, neurochemistry, and neuroimmunology. Journal of Child Neurology. 2006;21:678–689. doi: 10.1177/08830738060210080901. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Yucel M, Shaw M, Kyrios M, Maruff P, Brewer WJ, et al. Evaluating brain activity in obsessive-compulsive disorder: Preliminary insights from a multivariate analysis. Psychiatry Research. 2006;147:227–231. doi: 10.1016/j.pscychresns.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hoekstra PJ, Minderaa RB. Tic disorders and obsessive-compulsive disorder: Is autoimmunity involved? International Review of Psychiatry. 2005;17:497–502. doi: 10.1080/02646830500382003. [DOI] [PubMed] [Google Scholar]
- Hong SB, Shin YW, Kim SH, Yoo SY, Lee JM, Kim IY, et al. Hippocampal shape deformity analysis in obsessive-compulsive disorder. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:185–190. doi: 10.1007/s00406-006-0655-5. [DOI] [PubMed] [Google Scholar]
- Horwath E, Weissman MM. The epidemiology and cross-national presentation of obsessive-compulsive disorder. Psychiatric Clinics of North America. 2000;23:493–507. doi: 10.1016/s0193-953x(05)70176-3. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kwon JS, Jang DP, Moon WJ, Lee JM, Ha TH, et al. A proton MRSI study of brain n-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. British Journal of Psychiatry. 1998;(Supplement):79–90. [PubMed] [Google Scholar]
- Jenike MA, Breiter HC, Baer L, Kennedy DN, Savage CR, Olivares MJ, et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Archives of General Psychiatry. 1996;53:625–632. doi: 10.1001/archpsyc.1996.01830070073011. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114 (Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Joel D. Current animal models of obsessive compulsive disorder: A critical review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:374–388. doi: 10.1016/j.pnpbp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kanemura H, Aihara M, Aoki S, Araki T, Nakazawa S. Development of the prefrontal lobe in infants and children: A three-dimensional magnetic resonance volumetric study. Brain and Development. 2003;25:195–199. doi: 10.1016/s0387-7604(02)00214-0. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, et al. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:342–349. doi: 10.1176/jnp.16.3.342. [DOI] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, Burnam MA. The epidemiology of obsessive-compulsive disorder in five us communities. Archives of General Psychiatry. 1988;45:1094–1099. doi: 10.1001/archpsyc.1988.01800360042006. [DOI] [PubMed] [Google Scholar]
- Kellner CH, Jolley RR, Holgate RC, Austin L, Lydiard RB, Laraia M, et al. Brain MRI in obsessive-compulsive disorder. Psychiatry Research. 1991;36:45–49. doi: 10.1016/0165-1781(91)90116-7. [DOI] [PubMed] [Google Scholar]
- Kelly D, Richardson A, Mitchell-Heggs N, Greenup J, Chen C, Hafner RJ. Stereotactic limbic leucotomy: A preliminary report on forty patients. British Journal of Psychiatry. 1973;123:141–148. doi: 10.1192/bjp.123.2.141. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies: Tics and obsessive-compulsive symptoms. Journal of Developmental and Behavioral Pediatrics. 1994;15:421–425. [PubMed] [Google Scholar]
- Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, et al. Grey matter abnormalities in obsessive-compulsive disorder: Statistical parametric mapping of segmented magnetic resonance images. British Journal of Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- Kim KW, Lee DY. Obsessive-compulsive disorder associated with a left orbitofrontal infarct. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:88–89. doi: 10.1176/jnp.14.1.88. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Knight GC. The orbital cortex as an objective in the surgical treatment of mental illness: The development of a stereotactic approach. British Journal of Surgery. 1964;51:114–124. doi: 10.1002/bjs.1800510207. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB, Mrzljak L. Postnatal maturation of the layer III pyramidal neurons in the human prefrontal cortex: A quantitative Golgi analysis. Brain Research. 1994;653:173–182. doi: 10.1016/0006-8993(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain and Cognition. 2004;55:104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Koran LM. Quality of life in obsessive-compulsive disorder. Psychiatric Clinics of North America. 2000;23:509–517. doi: 10.1016/s0193-953x(05)70177-5. [DOI] [PubMed] [Google Scholar]
- Koran LM, Thienemann ML, Davenport R. Quality of life for patients with obsessive-compulsive disorder. American Journal of Psychiatry. 1996;153:783–788. doi: 10.1176/ajp.153.6.783. [DOI] [PubMed] [Google Scholar]
- Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: Rationale to understanding psychobiology and pharmacology. Psychiatric Clinics of North America. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. In: Kretschmann HJ, editor. Brain growth. Basel: Karger; 1986. pp. 1–26. [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kroll L, Drummond LM. Temporal lobe epilepsy and obsessive-compulsive symptoms. Journal of Nervous and Mental Disease. 1993;181:457–458. doi: 10.1097/00005053-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Kurlan R. The PANDAS hypothesis: Losing its bite? Movement Disorders. 2004;19:371–374. doi: 10.1002/mds.20107. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: Hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883–886. doi: 10.1542/peds.113.4.883. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Shin YW, Kim CW, Kim YI, Youn T, Han MH, et al. Similarity and disparity of obsessive-compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:962–964. doi: 10.1136/jnnp.74.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112 (Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Larson ER, Shear PK, Krikorian R, Welge J, Strakowski SM. Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. Journal of the International Neuropsychological Society. 2005;11:163–172. doi: 10.1017/s1355617705050228. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro L, Caldu X, Junque C, Bargallo N, Andres S, Morer A, et al. Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: A functional MRI study. Journal of Psychiatric Research. 2008 doi: 10.1016/j.jpsychires.2007.12.007. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The amygdala. Current Biology. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard HL, Swedo SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) International Journal of Neuropsychopharmacology. 2001;4:191–198. doi: 10.1017/S1461145701002371. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and Biobehavioral Reviews. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: Failure to induce behavioral changes. Movement Disorders. 2004;19:390–396. doi: 10.1002/mds.10522. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Gunay I, Glaser N. Obsessive compulsive disorder in a woman with left basal ganglia infarct: A case report. Behavioural Neurology. 1997;10:101–103. doi: 10.3233/BEN-1997-102-308. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Woods RP, Rex DE, Jancke L, et al. Mapping cortical gray matter in the young adult brain: Effects of gender. Neuroimage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luxenberg JS, Swedo SE, Flament MF, Friedland RP, Rapoport J, Rapoport SI. Neuroanatomical abnormalities in obsessive-compulsive disorder detected with quantitative x-ray computed tomography. American Journal of Psychiatry. 1988;145:1089–1093. doi: 10.1176/ajp.145.9.1089. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: Possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Banerjee SP, Bhandari R, et al. Pituitary volume in pediatric obsessive-compulsive disorder. Biological Psychiatry. 2006;59:252–257. doi: 10.1016/j.biopsych.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Maier M. In vivo magnetic resonance spectroscopy: Applications in psychiatry. British Journal of Psychiatry. 1995;167:299–306. doi: 10.1192/bjp.167.3.299. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Bartlett JR. A new lesion for the psychosurgical operation of stereotactic subcaudate tractotomy (sst) British Journal of Neurosurgery. 1998;12:335–339. doi: 10.1080/02688699844844. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Bartlett JR. Depression: A role for neurosurgery? British Journal of Neurosurgery. 2000;14:415–422. doi: 10.1080/02688690050175193. discussion 423. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: An event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Marquardt RK, Levitt JG, Blanton RE, Caplan R, Asarnow R, Siddarth P, et al. Abnormal development of the anterior cingulate in childhood-onset schizophrenia: A preliminary quantitative MRI study. Psychiatry Research. 2005;138:221–233. doi: 10.1016/j.pscychresns.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Marsh R, Leckman JF, Bloch MH, Yazgan Y, Peterson BS. Tics and compulsions: Disturbances of self-regulatory control in the development of habitual behaviors. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- Mataix-Cols D, Cullen S, Lange K, Zelaya F, Andrew C, Amaro E, et al. Neural correlates of anxiety associated with obsessive-compulsive symptom dimensions in normal volunteers. Biological Psychiatry. 2003;53:482–493. doi: 10.1016/s0006-3223(02)01504-4. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, et al. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex. 2001;11:335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- Max JE, Smith WL, Jr, Lindgren SD, Robin DA, Mattheis P, Stierwalt J, et al. Case study: Obsessive-compulsive disorder after severe traumatic brain injury in an adolescent. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:45–49. doi: 10.1097/00004583-199501000-00012. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, et al. Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal subcortical circuits. In: Salloway S, Malloy P, Duffy JD, editors. The frontal lobes and neuropsychiatric illness. 1. Washington, DC: American Psychiatric Pub; 2001. pp. 15–32. [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Dentate output channels: Motor and cognitive components. Progress in Brain Research. 1997;114:553–566. doi: 10.1016/s0079-6123(08)63386-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research. Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of Neuroscience. 2001a;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. A revised neuroanatomy of frontal-subcortical circuits. In: Lichter DG, Cummings JL, editors. Frontal-subcortical circuits in psychiatric and neurological disorders. New York: Guilford Press; 2001b. [Google Scholar]
- Miller LA, Taber KH, Gabbard GO, Hurley RA. Neural underpinnings of fear and its modulation: Implications for anxiety disorders. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:1–6. doi: 10.1176/jnp.17.1.1. [DOI] [PubMed] [Google Scholar]
- Mindus P, Rasmussen SA, Lindquist C, Noren G. Neurosurgical treatment for refractory obsessive-compulsive disorder: Implications for understanding frontal lobe function. In: Salloway S, Malloy P, Duffy JD, editors. The frontal lobes and neuropsychiatric illness. Washington, DC: American Psychiatric Publishing; 2001. pp. 233–245. [Google Scholar]
- Mirza Y, O’Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. Journal of Child Neurology. 2006;21:106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Monaco F, Cavanna A, Magli E, Barbagli D, Collimedaglia L, Cantello R, et al. Obsessionality, obsessive-compulsive disorder, and temporal lobe epilepsy. Epilepsy and Behavior. 2005;7:491–496. doi: 10.1016/j.yebeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. Journal of Comparative Neurology. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group a streptococcal infection (PANDAS) Archives of Pediatrics and Adolescent Medicine. 2002;156:356–361. doi: 10.1001/archpedi.156.4.356. [DOI] [PubMed] [Google Scholar]
- Murphy TK, Husted DS, Edge PJ. Preclinical/clinical evidence of central nervous system infectious etiology in PANDAS. Advances in Neurology. 2006;99:148–158. [PubMed] [Google Scholar]
- Murphy TK, Sajid MW, Goodman WK. Immunology of obsessive-compulsive disorder. Psychiatric Clinics of North America. 2006;29:445–469. doi: 10.1016/j.psc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a chinese character Stroop task. Psychiatry Research. 2005a;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biological Psychiatry. 2005b;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, 3rd, Liang KY, et al. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. American Journal of Human Genetics. 2000;67:1611–1616. doi: 10.1086/316898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe R. The lesion in stereotactic subcaudate tractotomy. British Journal of Psychiatry. 1975;126:478–481. doi: 10.1192/bjp.126.5.478. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Annals of the New York Academy of Sciences. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ogai M, Iyo M, Mori N, Takei N. A right orbitofrontal region and OCD symptoms: A case report. Acta Psychiatrica Scandinavica. 2005;111:74–76. doi: 10.1111/j.1600-0447.2004.00395.x. discussion 76–77. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Reviews Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A. The brain that plays music and is changed by it. Annals of the New York Academy of Sciences. 2001;930:315–329. doi: 10.1111/j.1749-6632.2001.tb05741.x. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. American Journal of Psychiatry. 1995;152:76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cerebral Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Development and Psychopathology. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Archives of General Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Pujol J, Torres L, Deus J, Cardoner N, Pifarre J, Capdevila A, et al. Functional magnetic resonance imaging study of frontal lobe activation during word generation in obsessive-compulsive disorder. Biological Psychiatry. 1999;45:891–897. doi: 10.1016/s0006-3223(98)00099-7. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Frangou S. Neuropsychology of bipolar disorder: A review. Journal of Affective Disorders. 2002;72:209–226. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: More neurons in males; more processes in females. Journal of Child Neurology. 1999;14:98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- Rachman S, Hodgson RJ. Obsessions and compulsions. Englewood Cliffs, N.J: Prentice-Hall; 1980. [Google Scholar]
- Rasmussen SA, Eisen JL. The course and clinical features of obsessive-compulsive disorder. In: Davis KL, Charney DS, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. American College of Neuropsychopharmacology; 2002. pp. 1593–1608. [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Archives of General Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, et al. Volume reduction in the caudate nucleus following stereotactic placement of lesions in the anterior cingulate cortex in humans: A morphometric magnetic resonance imaging study. Journal of Neurosurgery. 2000;93:1019–1025. doi: 10.3171/jns.2000.93.6.1019. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, et al. Probing striatal function in obsessive-compulsive disorder: A PET study of implicit sequence learning. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:568–573. doi: 10.1176/jnp.9.4.568. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals of the New York Academy of Sciences. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biological Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Curran T, Shin LM, Coffey BJ, Savage CR, et al. Probing striato-thalamic function in obsessive-compulsive disorder and Tourette syndrome using neuroimaging methods. Advances in Neurology. 2001;85:207–224. [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119 (Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Richardson A. Stereotactic limbic leucotomy: Surgical technique. Postgraduate Medical Journal. 1973;49:860–864. doi: 10.1136/pgmj.49.578.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffkin J, Yucel M, Maruff P, Wood SJ, Soulsby B, Olver J, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: Comparison with healthy controls and patients with schizophrenia. Psychiatry Research. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Gulisano M, Pavone P, Fogliani F, Robertson MM. Increased antistreptococcal antibody titers and anti-basal ganglia antibodies in patients with Tourette syndrome: Controlled cross-sectional study. Journal of Child Neurology. 2006;21:747–753. doi: 10.1177/08830738060210091001. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Jr, et al. Lifetime prevalence of specific psychiatric disorders in three sites. Archives of General Psychiatry. 1984;41:949–958. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Archives of General Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rodrigo Escalona P, Adair JC, Roberts BB, Graeber DA. Obsessive-compulsive disorder following bilateral globus pallidus infarction. Biological Psychiatry. 1997;42:410–412. doi: 10.1016/s0006-3223(97)00262-x. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1996;351:1433–1444. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The brain and emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Amponsah A, Sullivan A, MacMillan S, Moore GJ. Increased medial thalamic choline in pediatric obsessive-compulsive disorder as detected by quantitative in vivo spectroscopic imaging. Journal of Child Neurology. 2001;16:636–641. doi: 10.1177/088307380101600902. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Benazon NR, Gilbert A, Sullivan A, Moore GJ. Thalamic volume in pediatric obsessive-compulsive disorder patients before and after cognitive behavioral therapy. Biological Psychiatry. 2000;48:294–300. doi: 10.1016/s0006-3223(00)00902-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. A.E. Bennett research award. Toward a neurodevelopmental model of of obsessive-compulsive disorder. Biological Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS, O’Hearn KM, Dick EL, Bagwell WW, Seymour AB, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Archives of General Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biological Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Russell A, Cortese B, Lorch E, Ivey J, Banerjee SP, Moore GJ, et al. Localized functional neurochemical marker abnormalities in dorsolateral prefrontal cortex in pediatric obsessive-compulsive disorder. Journal of Child and Adolescent Psychopharmacology. 2003;13(Suppl 1):S31–38. doi: 10.1089/104454603322126322. [DOI] [PubMed] [Google Scholar]
- Sahni D, Jit I, Sodhi L. Brain weight of northwest Indian children and adolescents. American Journal of Human Biology. 1998;10:505–509. doi: 10.1002/(SICI)1520-6300(1998)10:4<505::AID-AJHB10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Sasson Y, Zohar J, Chopra M, Lustig M, Iancu I, Hendler T. Epidemiology of obsessive-compulsive disorder: A world view. Journal of Clinical Psychiatry. 1997;58(Suppl 12):7–10. [PubMed] [Google Scholar]
- Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Seminars in Clinical Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry. 1998;(Supplement):26–37. [PubMed] [Google Scholar]
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatric Clinics of North America. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: Detection with magnetic resonance imaging. Psychiatry Research. 1992;45:115–121. doi: 10.1016/0925-4927(92)90005-o. [DOI] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians. A model for functional and structural adaptation. Annals of the New York Academy of Sciences. 2001;930:281–299. [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, Winner E. Effects of music training on the child’s brain and cognitive development. Annals of the New York Academy of Sciences. 2005;1060:219–230. doi: 10.1196/annals.1360.015. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Schwartz JM. Neuroanatomical aspects of cognitive-behavioural therapy response in obsessive-compulsive disorder. An evolving perspective on brain and behaviour. British Journal of Psychiatry. 1998;(Supplement):38–44. [PubMed] [Google Scholar]
- Scicutella A. Late-life obsessive-compulsive disorder and Huntington’s disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:288–289. doi: 10.1176/jnp.12.2.288. [DOI] [PubMed] [Google Scholar]
- Shafran R, Speckens A. Reply to Rosenberg et al. Biological versus psychological approaches to OCD: War or peace? In: Abramowitz JS, Houts AC, editors. Concepts and controversies in obsessive-compulsive disorder. New York, NY: Springer; 2005. pp. 255–260. [Google Scholar]
- Shin YW, Yoo SY, Lee JK, Ha TH, Lee KJ, Lee JM, et al. Cortical thinning in obsessive compulsive disorder. Human Brain Mapping. 2007;28:1128–1135. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS. PANDAS and immunomodulatory therapy. Lancet. 1999;354:1137–1138. doi: 10.1016/S0140-6736(99)00204-4. [DOI] [PubMed] [Google Scholar]
- Singer HS, Giuliano JD, Hansen BH, Hallett JJ, Laurino JP, Benson M, et al. Antibodies against human putamen in children with Tourette syndrome. Neurology. 1998;50:1618–1624. doi: 10.1212/wnl.50.6.1618. [DOI] [PubMed] [Google Scholar]
- Singer HS, Loiselle C. PANDAS: A commentary. Journal of Psychosomatic Research. 2003;55:31–39. doi: 10.1016/s0022-3999(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Singer HS, Loiselle CR, Lee O, Minzer K, Swedo S, Grus FH. Anti-basal ganglia antibodies in PANDAS. Movement Disorders. 2004;19:406–415. doi: 10.1002/mds.20052. [DOI] [PubMed] [Google Scholar]
- Singer HS, Mink JW, Loiselle CR, Burke KA, Ruchkina I, Morshed S, et al. Microinfusion of antineuronal antibodies into rodent striatum: Failure to differentiate between elevated and low titers. Journal of Neuroimmunology. 2005;163:8–14. doi: 10.1016/j.jneuroim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, et al. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: A magnetic resonance spectroscopy study. Biological Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biological Psychiatry. 2005;57:788–792. doi: 10.1016/j.biopsych.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Snider LA, Swedo SE. PANDAS: Current status and directions for research. Molecular Psychiatry. 2004 doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B, et al. Long-term outcome of pediatric obsessive-compulsive disorder: A meta-analysis and qualitative review of the literature. Acta Psychiatrica Scandinavica. 2004;110:4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Research. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. American Journal of Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Schapiro MB, Casey BJ, Mannheim GB, Lenane MC, et al. Sydenham’s chorea: Physical and psychological symptoms of st vitus dance. Pediatrics. 1993;91:706–713. [PubMed] [Google Scholar]
- Swedo SE, Rapoport JL, Cheslow DL, Leonard HL, Ayoub EM, Hosier DM, et al. High prevalence of obsessive-compulsive symptoms in patients with sydenham’s chorea. American Journal of Psychiatry. 1989;146:246–249. doi: 10.1176/ajp.146.2.246. [DOI] [PubMed] [Google Scholar]
- Swoboda KJ, Jenike MA. Frontal abnormalities in a patient with obsessive-compulsive disorder: The role of structural lesions in obsessive-compulsive behavior. Neurology. 1995;45:2130–2134. doi: 10.1212/wnl.45.12.2130. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2004;161:1049–1056. doi: 10.1176/appi.ajp.161.6.1049. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Lorch E, Madden R, Ivey J, et al. Amygdala volume reductions in pediatric patients with obsessive-compulsive disorder treated with paroxetine: Preliminary findings. Neuropsychopharmacology. 2004;29:826–832. doi: 10.1038/sj.npp.1300399. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Talairach J, Hecaen H, David M, Monnier J, Ajuriaguerra J. Recherches sur la coagulation thérapeutique des structures sous-corticales chez l’homme. Revue Neurologique. 1949;81:4–24. [Google Scholar]
- Taren JA, Curtis GC, Gebarski SS. Late local and remote structural changes after capsulotomy for obsessive compulsive disorder. Stereotactic and Functional Neurosurgery. 1994;63:1–6. [PubMed] [Google Scholar]
- Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, et al. An animal model of Tourette’s syndrome. American Journal of Psychiatry. 2002;159:657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- Taylor S. Dimensional and subtype models of OCD. In: Abramowitz JS, Houts AC, editors. Concepts and controversies in obsessive-compulsive disorder. New York, NY: Springer; 2005. pp. 27–41. [Google Scholar]
- Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Valente AA, Jr, Miguel EC, Castro CC, Amaro E, Jr, Duran FL, Buchpiguel CA, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: A voxel-based morphometry study. Biological Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Valleni-Basile LA, Garrison CZ, Jackson KL, Waller JL, McKeown RE, Addy CL, et al. Frequency of obsessive-compulsive disorder in a community sample of young adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:782–791. doi: 10.1097/00004583-199407000-00002. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Archives of General Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- van der Wee NJ, Ramsey NF, Jansma JM, Denys DA, van Megen HJ, Westenberg HM, et al. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. Neuroimage. 2003;20:2271–2280. doi: 10.1016/j.neuroimage.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D, et al. Cognitive control in childhood-onset obsessive-compulsive disorder: A functional MRI study. Psychological Medicine. 2005;35:1007–1017. doi: 10.1017/s0033291704004295. [DOI] [PubMed] [Google Scholar]
- Ward CD. Transient feelings of compulsion caused by hemispheric lesions: Three cases. Journal of Neurology, Neurosurgery and Psychiatry. 1988;51:266–268. doi: 10.1136/jnnp.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilburg JB, Mesulam MM, Weintraub S, Buonanno F, Jenike MA, Stakes JW. Focal striatal abnormalities in a patient with obsessive-compulsive disorder. Archives of Neurology. 1989;46:233–235. [Google Scholar]
- Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: Evidence from event-related potentials in a go/nogo task. Biological Psychiatry. 2000;47:51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Jenike MA. Late-onset obsessive-compulsive disorder: A case series. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:265–268. doi: 10.1176/jnp.12.2.265. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Lee CK, et al. The cross national epidemiology of obsessive compulsive disorder. The cross national collaborative group. Journal of Clinical Psychiatry. 1994;55(Suppl):5–10. [PubMed] [Google Scholar]
- Wendlandt JT, Grus FH, Hansen BH, Singer HS. Striatal antibodies in children with Tourette’s syndrome: Multivariate discriminant analysis of IgG repertoires. Journal of Neuroimmunology. 2001;119:106–113. doi: 10.1016/s0165-5728(01)00370-8. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Research. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Whitty CW, Duffield JE, Tov PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;1:475–481. doi: 10.1016/s0140-6736(52)90051-2. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Current Opinion in Neurobiology. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson HA, Davidson KM, Davidson RI. Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery. 1999;45:1129–1134. doi: 10.1097/00006123-199911000-00023. discussion 1134–1126. [DOI] [PubMed] [Google Scholar]
- Woolley J, Heyman I, Brammer M, Frampton I, McGuire PK, Rubia K. Brain activation in paediatric obsessive compulsive disorder during tasks of inhibitory control. British Journal of Psychiatry. 2008;192:25–31. doi: 10.1192/bjp.bp.107.036558. [DOI] [PubMed] [Google Scholar]
- Yakovlev PA, Lecours IR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Yaryura-Tobias JA, Neziroglu F. Basal ganglia hemorrhagic ablation associated with temporary suppression of obsessive-compulsive symptoms. Revista Brasileira de Psiquiatria. 2003;25:40–42. doi: 10.1590/s1516-44462003000100008. [DOI] [PubMed] [Google Scholar]
- Yoo SY, Roh MS, Choi JS, Kang do H, Ha TH, Lee JM, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. Journal of Korean Medical Science. 2008;23:24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Archives of General Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: Implications for diagnosis, treatment and prevention. Drug and Alcohol Review. 2007;26:33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- Zald DH, Kim SW. The orbitofrontal cortex. In: Salloway SP, Malloy PF, Duffy JD, editors. The frontal lobes and neuropsychiatric illness. 1. Washington, DC: American Psychiatric Publishing, Inc; 2001. pp. 33–69. [Google Scholar]
- Zohar AH, Ratzoni G, Pauls DL, Apter A, Bleich A, Kron S, et al. An epidemiological study of obsessive-compulsive disorder and related disorders in Israeli adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:1057–1061. doi: 10.1097/00004583-199211000-00010. [DOI] [PubMed] [Google Scholar]