Abstract
Aims
To determine independent associations of diabetes mellitus with outcomes in a propensity-matched cohort of patients with acute myocardial infarction (AMI) and systolic heart failure (HF).
Methods and results
In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial, hospitalized AMI patients complicated by left ventricular ejection fraction ≤40% and symptoms of HF receiving standard therapy were randomized 3–14 days post-AMI to receive eplerenone 25–50 mg/day (n = 3319) or placebo (n = 3313). Of the 6632 patients, 2142 (32%) had a history of diabetes, who were older and sicker. Using propensity scores for diabetes, we assembled a cohort of 1119 pairs of patients with and without diabetes who were balanced on 64 baseline characteristics. Incident fatal or nonfatal recurrent AMI occurred in 136 (12%) and 87 (8%) of matched patients with and without diabetes, respectively, during 2.5 years of follow-up [hazard ratio (HR) when diabetes was compared with no-diabetes, 1.61; 95% confidence interval (CI), 1.23–2.10; P = 0.001]. Diabetes was associated with nonfatal AMI (HR, 1.68; 95% CI, 1.23–2.31; P = 0.001) but not with fatal AMI (HR, 1.42; 95% CI, 0.88–2.28; P = 0.146). Hazard ratios (95% CIs) for the association of diabetes with all-cause mortality, cardiovascular mortality, all-cause hospitalization, and cardiovascular hospitalization were 1.12 (0.93–1.37; P = 0.224), 1.11 (0.90–1.37; P = 0.318), 1.13 (1.00–1.27; P = 0.054), and 1.20 (1.01–1.44; P = 0.042), respectively.
Conclusion
In post-AMI patients with systolic HF, diabetes mellitus is a significant independent risk factor for recurrent short-term nonfatal AMI, but had no association with fatal AMI.
Keywords: Diabetes, Recurrent myocardial infarction
Introduction
Diabetes mellitus is a risk factor for acute myocardial infarction (AMI).1–3 Although the associations between diabetes and outcomes after AMI have been studied previously, the association of diabetes on recurrent AMI in a large cohort of post-AMI patients with systolic heart failure (HF) has not been well studied.4,5 Further, associations between diabetes and poor outcomes have often been attributed to older age and higher prevalence of cardiovascular risk factors among those with diabetes. To what extent diabetes has an independent association with poor outcomes in post-AMI patients, however, is unclear. Traditional regression-based multivariable risk adjustment models may be limited by lack of procedural transparency, concerns for residual bias, and strong and often untenable model assumptions.6 Propensity score matching, on the other hand, can be used to assemble cohorts in which patients are balanced on all measured baseline covariates and investigators are blinded to study outcomes.7–13 Therefore, the objective of the current study was to examine the association of diabetes with outcomes in a propensity-matched cohort of post-AMI patients with systolic HF in which those with and without diabetes would be well-balanced on all measured baseline covariates.
Methods
Source of study data
The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) was a multicentre, international (37 countries), randomized, double-blind, placebo-controlled clinical trial that randomized 6632 patients with AMI complicated by symptoms of HF and left ventricular ejection fraction ≤40% between 27 December 1999, and 31 December 2001 to receive eplerenone 25–50 mg daily or placebo.14 These patients were receiving standard medical therapy, including angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers, and coronary reperfusion therapies. The details of the design and results of the EPHESUS trial have been previously reported.14 Patients with a serum creatinine >2.5 mg/dL or a potassium >5.0 mEq/L were excluded. An original copy of the EPHESUS data was obtained from Pfizer Inc., which was also the sponsor of the trial. However, Pfizer played no role in the design, analysis, and interpretation of the study data.
History of diabetes mellitus
Data on history of diabetes mellitus was collected by study investigators at baseline based on a physician diagnosis of diabetes. Although baseline data on random plasma glucose was collected, there was no data on baseline fasting plasma glucose. Of the 6632 EPHESUS participants, 2142 (32%) patients had a history of diabetes.
Study outcomes
For the current analysis, our main outcomes of interest were three AMI related endpoints: incident recurrent fatal or nonfatal AMI, mortality due to AMI, and hospitalization due to AMI during 2.5 years of follow-up (median, 16 months and range, 0.3–30.42 months). Incident AMI was confirmed using two of three usual criteria: (i) electrocardiographic changes including ST-segment elevation, new Q waves, or both for transmural AMI, and T-wave inversion, ST-segment depression, or both for subendocardial AMI, (ii) typical symptoms, or (iii) elevation of cardiac biochemical markers. Definite AMI (including silent AMI) was diagnosed by unequivocal electrocardiographic evidence of a new MI with or without a typical history, or a typical increase and decrease in biochemical markers of myocardial damage in which the maximal value reached was greater than twice the upper limit of the hospital range for creatine kinase, or in which the creatine kinase-MB fraction value was ≥10% of the creatine kinase value with either a typical history, new equivocal changes on electrocardiogram, or both, indicating the presence of ischaemia; or elevation of troponin to over three times the upper limit of the normal range of laboratory values. Acute myocardial infarction related to a cardiac procedure was diagnosed by a typical increase and decrease in biochemical markers of myocardial damage in which the maximal value reached within 1 week is more than three times the upper limit of the hospital range for creatine kinase after catheterization or percutaneous transluminal coronary angioplasty or greater than five times the upper limit of the hospital range for CK after cardiac surgery. A death was considered to be due to AMI if it occurred within 28 days after AMI, or when the occurrence of AMI and/or the date of occurrence of AMI were uncertain, the primary cause of death was confirmed by autopsy to be AMI.
Among the 2238 matched patients, a total of 223 (10%) patients had fatal or nonfatal incident AMI, 163 (7%) patients had hospitalization due to nonfatal AMI, and 70 (3%) patients had fatal AMI. None of the 163 patients died during hospitalization for AMI. However, of the 163 patients with nonfatal AMI, 40 (25%) patients subsequently died from all causes, of which 10 were due to recurrent AMI. We also examined other major natural history endpoints including mortality and hospitalization due to all causes and cardiovascular causes. The cause of death or the primary diagnosis leading to hospitalization was adjudicated by an EPHESUS critical-events committee, members of which were blinded to the patient's study drug assignment.
Assembly of a balanced cohort
Because of the imbalances in baseline covariates between patients with and without diabetes, we used propensity score matching to assemble a cohort, in which those with and without diabetes would be well-balanced on all measured baseline covariates.7–13 The propensity score for diabetes for a patient is that patient's probability of having diabetes given his/her measured baseline characteristics. We estimated propensity scores for diabetes for each patient using a non-parsimonious multivariable logistic regression model. In the model, diabetes was the dependent variable and 64 clinically relevant baseline characteristics (Figure 1) were used as covariates.7–11 Using a greedy matching protocol, we attempted to match each patient with diabetes with another patient without diabetes who had a similar propensity score. In five repeated steps, patients were matched by propensity scores to five, four, three, two, and one decimal places, the details of which have been described elsewhere.7–11 In all, we were able to match 1119 pairs of patients with and without a history of diabetes with similar propensity for diabetes.
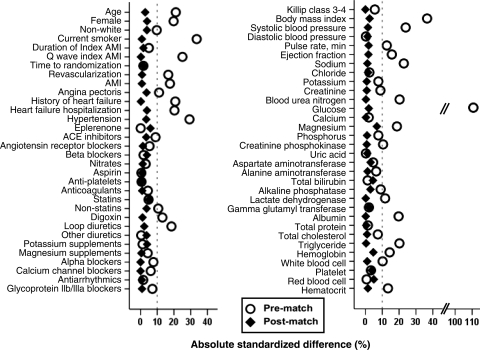
Figure 1.
Love plots for absolute standardized differences for covariates between patients with and without a history of diabetes mellitus, before and after propensity score matching. AMI, acute myocardial infarction; ACE, angiotensin-converting enzyme.
Because propensity score models are sample-specific adjusters and are not intended to be used for out-of-sample prediction or estimation of coefficients, measures of fitness and discrimination are not important for assessment of the model's effectiveness.7–11,15,16 Instead, the reduction in baseline covariate imbalance is a better marker of the efficacy of a propensity score model, which is best assessed by estimating absolute standardized differences. Absolute standardized differences directly quantify the bias in the means (or proportions) of baseline covariates across the groups and are expressed as a percentage of the pooled standard deviations. Therefore, to quantify pre-match imbalances and post-match balances, we calculated pre- and post-match absolute standardized differences and presented those findings as Love plots.8,11 An absolute standardized difference of 0% indicate no residual bias and <10% is considered of inconsequential bias.
Assembly of a pre-match cohort of similar sample size
To ensure that the post-match comparisions between patients with and without diabetes were not affected by the smaller sample size of the matched cohort, we assembled a pre-match cohort of the same sample size as that of the matched cohort. This was done by first identifying the 1119 patients with diabetes in the matched cohort. Then, we identified a random sample of 1119 patients without diabetes from the entire pre-match sample of 4490 patients without diabetes. Finally, we linked these two data sets, thus assembling a cohort of 1119 pairs of patients with and without diabetes.
Statistical analysis
For descriptive analyses, we used Pearson Chi-square and Wilcoxon rank-sum tests for the pre-match, and McNemar's test and paired sample t-test for the post-match comparisons of baseline covariates between patients with and without diabetes, as appropriate. We used Kaplan–Meier plots and matched Cox-regression analysis to estimate associations of a history of diabetes with outcomes during 2.5 years of follow-up. Log-minus-log scale survival plots were used to check proportional hazards assumptions. We then repeated our analysis in the pre-match cohort of 2238 patients. We performed subgroup analyses to determine whether the association between diabetes and recurrent fatal or nonfatal AMI was homogenous across various subgroups of matched patients. All statistical analyses were completed using SPSS for Windows, Rel. 15, 2006 (SPSS Inc., Chicago, IL, USA) and two-sided tests with a P-value < 0.05 were considered significant.
Sensitivity analysis
Despite excellent balance between matched patients with and without diabetes on all measured baseline covariates, confounding due to unmeasured covariates is still a possibility, as in all observational studies. A formal sensitivity analysis using Rosenbaum's formulation was conducted to quantify the degree a hidden bias that would need to be present to invalidate any conclusions based on significant associations between diabetes and primary outcomes among our matched patients.17
Results
Baseline characteristics
Matched patients had a mean age of 66 (±11) years, 31% were women and 10% were nonwhites. Before matching, patients with diabetes were older, had a higher prevalence of women, and a higher burden of cardiovascular comorbidities than those without diabetes. These and other imbalances in baseline characteristics between patients with and without diabetes were well-balanced after matching so that no significant differences remained (Table 1). Post-match absolute standardized differences for all measured covariates were <10% (most were <5%), suggesting substantial covariate balance across the groups (Figure 1).
Table 1.
Baseline patient characteristics by a history of diabetes mellitus before and after propensity matching
| N (%) or mean (±SD) | Before propensity matching |
After propensity matching |
||||
|---|---|---|---|---|---|---|
| No history of diabetes mellitus (n=1119) | History of diabetes mellitus (n=1119) | P-value | No history of diabetes mellitus (n=1119) | History of diabetes mellitus (n=1119) | P-value | |
| Age, years | 63 (±12) | 66 (±10) | <0.001 | 66 (±12) | 66 (±10) | 0.505 |
| Women | 300 (27) | 339 (30) | 0.068 | 359 (32) | 339 (30) | 0.375 |
| Non-white race | 110 (10) | 110 (12) | 1.000 | 123 (11) | 110 (10) | 0.413 |
| Smoking status | ||||||
| Current | 405 (36) | 270 (24) | <0.001 | 266 (24) | 270 (24) | 0.988 |
| Never | 411 (37) | 495 (44) | 502 (45) | 495 (44) | ||
| Former | 303 (27) | 354 (32) | 351 (31) | 354 (32) | ||
| Duration of index AMI hospitalization, days | 15.2 (±8.3) | 15.3 (±8.7) | 0.795 | 15.2 (±8.6) | 15.3 (±8.7) | 0.686 |
| Index AMI with ST elevation | 833 (74) | 736 (66) | <0.001 | 735 (66) | 736 (66) | 1.00 |
| Time from index AMI to randomization, days | 7 (±3) | 7 (±3) | 0.774 | 7 (±3) | 7 (±3) | 0.697 |
| Reperfusion or revascularization therapy within 14 days of index AMI | ||||||
| CABG | 11 (1) | 10 (1) | 0.826 | 11 (1) | 10 (1) | 1.00 |
| PCI | 257 (23) | 256 (23) | 0.960 | 255 (23) | 256 (23) | 1.00 |
| Thrombolysis | 332 (30) | 258 (23) | <0.001 | 251 (22) | 258 (23) | 0.759 |
| Past medical history | ||||||
| AMI | 280 (25) | 338 (30) | 0.006 | 342 (31) | 338 (30) | 0.890 |
| Angina pectoris | 433 (39) | 497 (44) | 0.006 | 517 (46) | 497 (44) | 0.415 |
| Heart failure | 140 (13) | 211 (19) | <0.001 | 210 (19) | 211 (19) | 1.00 |
| Heart failure hospitalization | 71 (6) | 107 (10) | 0.005 | 120 (11) | 107 (10) | 0.393 |
| Hypertension | 635 (57) | 746 (67) | <0.001 | 764 (68) | 746 (67) | 0.440 |
| Medications | ||||||
| Eplerenone | 569 (51) | 561 (50) | 0.735 | 594 (53) | 561 (50) | 0.163 |
| ACE-inhibitors | 943 (84) | 961 (86) | 0.286 | 974 (87) | 961 (86) | 0.463 |
| Angiotensin-receptor blocker | 40 (4) | 41 (4) | 0.910 | 44 (4) | 41 (4) | 0.828 |
| Beta-blockers | 826 (74) | 830 (74) | 0.847 | 811 (73) | 830 (74) | 0.388 |
| Nitrates | 687 (61) | 699 (63) | 0.601 | 706 (63) | 699 (63) | 0.795 |
| Aspirin | 993 (89) | 978 (87) | 0.328 | 984 (88) | 978 (87) | 0.750 |
| Anti-platelet drugs | 314 (28) | 312 (28) | 0.925 | 309 (28) | 312 (28) | 0.926 |
| Anticoagulants | 187 (17) | 177 (16) | 0.567 | 173 (16) | 177 (16) | 0.861 |
| Statins | 506 (45) | 535 (48) | 0.219 | 507 (45) | 535 (48) | 0.240 |
| Digoxin | 160 (14) | 204 (18) | 0.012 | 199 (18) | 204 (18) | 0.824 |
| Loop diuretics | 592 (53) | 672 (60) | 0.001 | 684 (61) | 672 (60) | 0.628 |
| Calcium channel blockers | 167 (15) | 189 (17) | 0.204 | 189 (17) | 189 (17) | 1.00 |
| Anti-arrhythmic drugs | 135 (12) | 147 (13) | 0.445 | 150 (13) | 147 (13) | 0.900 |
| Killip status | ||||||
| I | 105 (9) | 218 (20) | <0.001 | 236 (21) | 218 (20) | 0.857 |
| II | 813 (73) | 662 (59) | 644 (58) | 662 (59) | ||
| III | 163 (15) | 200 (18) | 196 (18) | 200 (18) | ||
| IV | 38 (3) | 39 (4) | 43 (4) | 39 (4) | ||
| Body mass index, kg/m2 | 26.9 (±4.2) | 27.9 (±4.2) | <0.001 | 28.1 (±4.8) | 27.9 (±4.2) | 0.544 |
| Systolic blood pressure, mmHg | 118 (±16) | 121 (±17) | <0.001 | 121 (±17) | 121 (±17) | 0.727 |
| Diastolic blood pressure, mmHg | 72 (±10) | 72 (±11) | 0.112 | 72 (±11) | 72 (±11) | 0.758 |
| Pulse, bpm | 74 (±11) | 75 (±12) | 0.053 | 75 (±12) | 75 (±12) | 0.653 |
| Laboratory values | ||||||
| Sodium, mEq/L | 140 (±4) | 140 (±4) | 0.059 | 139 (±4) | 140 (±4) | 0.765 |
| Potassium, mEq/L | 4.27 (±0.44) | 4.25 (±0.45) | 0.288 | 4.25 (±0.45) | 4.25 (±0.45) | 0.849 |
| Creatinine, mg/dL | 1.14 (±0.31) | 1.16 (±0.35) | 0.184 | 1.16 (±0.35) | 1.16 (±0.35) | 0.854 |
| Glucose, mg/dL | 111 (±33) | 135 (±52) | <0.001 | 134 (±71) | 135 (±52) | 0.382 |
| Albumin, g/dL | 3.73 (±0.62) | 3.67 (±0.57) | 0.016 | 3.7 (±0.6) | 3.7 (±0.6) | 0.964 |
| Haemoglobin, g/dL | 13.3 (±1.7) | 13.3 (±1.8) | 0.505 | 13 (±2) | 13 (±2) | 0.277 |
| Left ventricular ejection fraction, % | 33.1 (± 5.9) | 32.3 (± 6.2) | 0.152 | 32.7 (±6.1) | 32.8 (±6.2) | 0.669 |
ACE, angiotensin converting enzyme; AMI, acute myocardial infarction, CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention.
Diabetes and recurrent fatal and nonfatal acute myocardial infarction
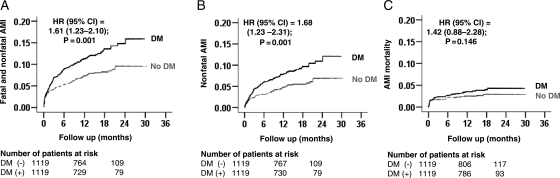
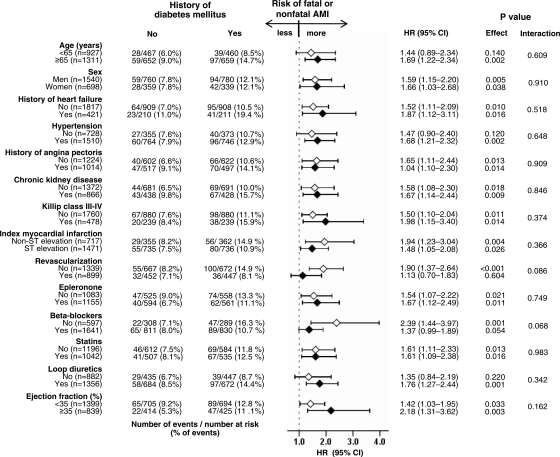
Fatal and nonfatal AMI occurred in 136 (12%) and 87 (8%) patients, respectively, with and without a history of diabetes [hazard ratio (HR) when diabetes was compared with no diabetes, 1.61; 95% confidence interval (CI), 1.23–2.10; P = 0.001; Figure 2A and Table 2]. In the absence of hidden bias, a sign-score test for matched data with censoring provides evidence (P = 0.003) that patients with a history of diabetes clearly had more incident fatal and nonfatal AMI than those without a history of diabetes. Our sensitivity analysis suggests that a hidden binary covariate, which is a near-perfect predictor of incident fatal and nonfatal AMI, would need to increase the odds of diabetes by 17% to potentially explain away this association. The results of our subgroup analyses demonstrated that a history of diabetes had, in general, a homogenous association with fatal and nonfatal AMI across a wide spectrum of patients (Figure 3).
Figure 2.
Kaplan–Meier plots for recurrent acute myocardial infarction (AMI) by a history of diabetes mellitus (DM): (A) fatal or nonfatal, (B) nonfatal, and (C) fatal. CI, confidence interval; HR, hazard ratio.
Table 2.
Association of a history of diabetes mellitus and recurrent acute myocardial infarction (AMI)
| Outcomes | Events (%) |
Absolute risk difference (%) | Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|---|---|---|
| No history of diabetes | History of diabetes | ||||
| Before matching | (n=1119) | (n=1119) | |||
| Fatal or nonfatal AMI | 83 (7) | 136 (12) | +5 | 1.73 (1.31–2.27) | <0.001 |
| Nonfatal AMI | 62 (6) | 101 (9) | +3 | 1.73 (1.26–2.37) | 0.001 |
| Fatal AMI | 21 (2) | 41 (4) | +2 | 2.00 (1.18–3.38) | 0.010 |
| After matching | (n=1119) | (n=1119) | |||
| Fatal or nonfatal AMI | 87 (8) | 136 (12) | +4 | 1.61 (1.23–2.10) | 0.001 |
| Nonfatal AMI | 62 (6) | 101 (9) | +3 | 1.68 (1.23–2.31) | 0.001 |
| Fatal AMI | 29 (3) | 41 (4) | +1 | 1.42 (0.88–2.28) | 0.146 |
Figure 3.
Association of a history of diabetes mellitus with subsequent fatal or nonfatal myocardial infarction in subgroups of patients. CI, confidence interval; HR, hazard ratio.
Nonfatal AMI occurred in 101 (9%) and 62 (3%) patients, respectively, with and without a history of diabetes (HR, 1.68; 95% CI, 1.23–2.31; P = 0.001; Figure 2B and Table 2). A history of diabetes was not associated with fatal AMI (HR, 1.42; 95% CI, 0.88–2.28; P = 0.146; Figure 2C and Table 2). Among the 2238 random-pair patients, diabetes was significantly associated with fatal and nonfatal AMI (HR, 1.73; 95% CI, 1.31–2.27; P < 0.001), nonfatal AMI (HR, 1.73; 95% CI, 1.26–2.37; P < 0.001), and fatal AMI (HR, 2.00; 95% CI, 1.18–3.38; P = 0.010).
Diabetes and other outcomes
The presence of a history of diabetes had significant unadjusted associations with nearly all outcomes among the 2238 random-pair pre-match cohort (Table 3). However, among the 2238 balanced matched cohort, diabetes was only associated with increased risk of cardiovascular hospitalizations and held a borderline association with all-cause hospitalization. Pre- and post-match associations between diabetes and other outcomes are displayed in Table 3.
Table 3.
Effects of a history of diabetes on all other outcomes in EPHESUS
| Events (%) |
Absolute risk difference (%) | Hazard ratio (95% confidence interval) | P-value | ||
|---|---|---|---|---|---|
| No history of diabetes | History of diabetes | ||||
| Before matching | (n=1119) | (n=1119) | |||
| All-cause death | 148 (13) | 212 (19) | +6 | 1.50 (1.21–1.85) | 0.001 |
| Cardiovascular death | 129 (12) | 182 (16) | +4 | 1.47 (1.17–1.84) | 0.001 |
| Heart failure death | 34 (3) | 50 (5) | +2 | 1.54 (1.00–2.38) | 0.052 |
| Sudden cardiac death | 52 (5) | 68 (6) | +1 | 1.37 (0.95–1.96) | 0.089 |
| All-cause hospitalization | 494 (44) | 554 (50) | +6 | 1.19 (1.06–1.35) | 0.004 |
| Cardiovascular hospitalization | 190 (17) | 263 (24) | +7 | 1.49 (1.23–1.79) | <0.001 |
| Heart failure hospitalization | 104 (9) | 164 (15) | +6 | 1.67 (1.31–2.13) | <0.001 |
| Cardiovascular hospitalization or cardiovascular death | 283 (25) | 374 (33) | +8 | 1.42 (1.21–1.65) | <0.001 |
| All-cause hospitalization or all-cause death | 578 (52) | 650 (58) | +6 | 1.20 (1.07–1.34) | 0.002 |
| After matching | (n=1119) | (n=1119) | |||
| All-cause death | 191 (17) | 212 (19) | +2 | 1.12 (0.93–1.37) | 0.224 |
| Cardiovascular death | 166 (15) | 182 (16) | +1 | 1.11 (0.90–1.37) | 0.318 |
| Heart failure death | 47 (4) | 50 (5) | +1 | 1.09 (0.73–1.62) | 0.678 |
| Sudden cardiac death | 71 (6) | 68 (6) | +0 | 0.97 (0.70–1.36) | 0.867 |
| All-cause hospitalization | 506 (45) | 554 (50) | +5 | 1.13 (1.00–1.27) | 0.054 |
| Cardiovascular hospitalization | 225 (20) | 263 (24) | +4 | 1.20 (1.01–1.44) | 0.042 |
| Heart failure hospitalization | 150 (13) | 164 (15) | +2 | 1.10 (0.89–1.38) | 0.382 |
| Cardiovascular hospitalization or cardiovascular death | 349 (31) | 374 (33) | +2 | 1.10 (0.95–1.27) | 0.198 |
| All-cause hospitalization or all-cause death | 618 (55) | 650 (58) | +3 | 1.08 (0.97–1.21) | 0.169 |
Diabetes and the effect of eplerenone
Among the pre-match 6632 EPHESUS participants, fatal or nonfatal AMI occurred in 293 (9%) and 313 (9%) patients in the eplerenone and placebo groups, respectively (HR when eplerenone was compared with placebo, 0.92; 95% CI, 0.79–1.08; P = 0.312; data not shown). As previously reported by the EPHESUS investigators, the effect of eplerenone on mortality did not vary by the presence or absence of diabetes at baseline.14,18
Discussion
Findings of the current analysis demonstrate that in post-AMI patients with systolic HF, a history of diabetes was associated with increased risk of recurrent fatal or nonfatal AMI, which was primarily driven by an increase in nonfatal AMI. Diabetes was also associated with cardiovascular hospitalization, but had no independent association with all-cause or cardiovascular mortality. To the best of our knowledge, this is the first report of an association between diabetes and recurrent AMI in a propensity-matched cohort of post-AMI patients with systolic HF. These findings provide important insights into the early effects of diabetes after AMI suggesting that nonfatal AMI may be the first major clinical cardiovascular manifestation after an index AMI in patients with diabetes. While there was no increase in fatal AMI or cardiovascular mortality in the diabetes group, it is likely that an excess of nonfatal AMI would over time result in progressive adverse left ventricular remodelling with ensuing worsening of left ventricular dysfunction leading to progressive chronic HF and associated morbidity and mortality.
There are two potential explanations of the significant associations of diabetes with recurrent AMI in our balanced cohort of matched patients: residual confounding by measured covariates and cofounding due to unmeasured covariates. Because our matched patients were balanced on 64 baseline characteristics, they are unlikely to explain the observed associations. However, it is possible that these characteristics may have changed during follow-up thus increasing the risk of subsequent AMI in those with diabetes.19–22 Findings from our sensitivity analysis suggest that the association between diabetes and recurrent AMI was rather insensitive to an unmeasured binary confounder. The lack of an intrinsic association between diabetes and fatal AMI is likely due to a relatively small number of events and/or short follow-up of the EPHESUS trial. However, diabetes also had no intrinsic association with all-cause or cardiovascular mortality, despite much higher event rates for those outcomes, suggesting that the early effect of diabetes may not be fatal in nature.
The association between diabetes and recurrent nonfatal AMI may be a direct effect of diabetes. The metabolic effect of diabetes on cardiovascular morbidity and mortality is complex.23–26 Diabetes is associated with activation of the renin–angiotensin–aldosterone system.23,24 Collagen cross-linking is a major mechanism by which vascular and cardiac compliance is diminished in diabetes and may also contribute to diabetic cardiomyopathy.25,26 Other potential underlying mechanisms may include accelerated atherosclerosis associated with diabetes. Hyperglycaemia, insulin resistance, and advanced glycation end-products have been implicated in vascular inflammation and endothelial dysfunction in patients with diabetes.27 Further important contributing factors may include increased platelet activation, presence of a chronic hypercoagulable state, and impaired fibrinolysis.28–30 Serum levels of insulin-like growth factor-binding protein-1 are elevated in patients with diabetes, which in turn has been shown to be associated with increased risk for cardiovascular mortality and morbidity in these patients.19
Studies of associations between a history of diabetes and outcomes after AMI are limited by relatively small sample size and failure to account for various important cardiovascular comorbidities and risk factors, including hyperglycaemia and hyperlipidaemia.2–5,31–34 Further, only a few of these studies examined the association between diabetes and recurrent AMI.4,5 In the Framingham Heart Study, of the 609 patients who survived an initial AMI, 92 had a history of diabetes and the age-adjusted incidence rates of recurrent AMI for participants with and without diabetes were approximately 650 and 400 per 10 000 person-years, respectively.4 Findings from the Finnish Social Insurance Institution registry data suggest that of the 238 post-AMI patients, 169 had a history of diabetes and the unadjusted incidence rates of recurrent fatal and nonfatal AMI for participants with and without diabetes were 780 and 300 per 10 000 person-years, respectively.5 In contrast to these studies, our study is distinguished by its contemporary nature, the presence of baseline systolic HF, larger sample size, the use of propensity-matched design to assemble a cohort that was balanced on 64 baseline covariates.
The findings of the current analysis are important as they help identify a subset of post-AMI patients who are at increased risk of subsequent nonfatal AMI. We observed that diabetes-associated increased risk of recurrent AMI was higher in those not receiving beta-blockers and without coronary revascularization. The use of these therapies may explain the relatively low incidence of fatal AMI in our study. In the Framingham Heart Study, of the 609 patients who survived an initial AMI, 92 had a history of diabetes and the age-adjusted incidence rates of recurrent AMI for participants with and without diabetes were 724 and 326 per 10 000 person-years, respectively.4 In contrast, the incidence rates for recurrent fatal AMI for matched patients with and without diabetes in our study were 291 and 200 per 10 000 person-years, respectively. Eplerenone has been shown to reduce adverse cardiovascular events in diabetes,18 and may help improve long-term prognosis in these patients.
Our study has several limitations. We used a history of diabetes and the diagnosis of diabetes was not centrally adjudicated. Similarly, patients without diabetes at baseline may have developed diabetes during follow up, which may have led to regression dilution and underestimation of true association.35 We also had no data on the duration, type, or control status of diabetes. The findings of the current study based on post-AMI patients with systolic HF may not be generalized to post-AMI patients without systolic HF.
In conclusion, in post-AMI patients with systolic HF receiving standard therapy, diabetes is a marker of poor outcomes. Although diabetes was independently associated with increased risk of recurrent nonfatal AMI during over 2 years of follow-up, there was no independent association with AMI mortality. Whether a more aggressive control of diabetes may reduce the risk of recurrent AMI in these patients is unknown and needs to be prospectively determined by future studies.
Funding
The EPHESUS study was funded by Pfizer Inc. However, Pfizer played no role in data analysis or manuscript preparation. A.A. is supported by the National Institutes of Health through grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms Jean B. Morris of Birmingham, Alabama.
Conflict of interest: P.D. has received grants and research support from Pfizer and AstraZeneca Pharmaceuticals LP and has also served as a consultant and participated in Speakers Bureau activities for Pfizer and AstraZeneca Pharmaceuticals LP. B.P. is a consultant to Pfizer, Merck, Novartis, Astra-Zenenca, Takeda, and Bayer.
References
- 1.Melchior T, Kober L, Madsen CR, Seibaek M, Jensen GV, Hildebrandt P, Torp-Pedersen C. Accelerating impact of diabetes mellitus on mortality in the years following an acute myocardial infarction. TRACE Study Group. Trandolapril Cardiac Evaluation. Eur Heart J. 1999;20:973–978. doi: 10.1053/euhj.1999.1530. [DOI] [PubMed] [Google Scholar]
- 2.Zuanetti G, Latini R, Maggioni AP, Santoro L, Franzosi MG. Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993;22:1788–1794. doi: 10.1016/0735-1097(93)90758-s. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572–1578. doi: 10.1161/01.CIR.0000142047.28024.F2. [DOI] [PubMed] [Google Scholar]
- 4.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260:3456–3460. [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 6.Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–583. doi: 10.1016/j.nut.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Aban IB, Vaccarino V, Lloyd-Jones DM, Goff DC, Jr., Zhao J, Love TE, Ritchie C, Ovalle F, Gambassi G, Dell'Italia LJ. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, Pitt B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–1343. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed MI, White M, Ekundayo OJ, Love TE, Aban I, Liu B, Aronow WS, Ahmed A. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 13.Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 14.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 15.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. 2nd edn. New York: Springer-Verlag; 2002. pp. 110–124. [Google Scholar]
- 18.O'Keefe JH, Abuissa H, Pitt B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: results from EPHESUS. Diabetes Obes Metab. 2008;10:492–497. doi: 10.1111/j.1463-1326.2007.00730.x. [DOI] [PubMed] [Google Scholar]
- 19.Wallander M, Norhammar A, Malmberg K, Ohrvik J, Ryden L, Brismar K. IGF binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes. Diabetes Care. 2007;30:2343–2348. doi: 10.2337/dc07-0825. [DOI] [PubMed] [Google Scholar]
- 20.Tenenbaum A, Fisman EZ, Boyko V, Goldbourt U, Auerbach I, Shemesh J, Shotan A, Reicher-Reiss H, Behar S, Motro M. Prevalence and prognostic significance of unrecognized systemic hypertension in patients with diabetes mellitus and healed myocardial infarction and/or stable angina pectoris. Am J Cardiol. 1999;84:294–298. doi: 10.1016/s0002-9149(99)00279-9. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez-Navarro M, Gomez-Doblas JJ, Hernandez Garcia JM, Alonso-Briales J, Garcia Alcantara A, Gomez G, Rodriguez-Bailon I, de Teresa E. Does angina pectoris the week before protect against first acute myocardial infarction in patients with diabetes mellitus? Am J Cardiol. 2002;90:160–162. doi: 10.1016/s0002-9149(02)02442-6. [DOI] [PubMed] [Google Scholar]
- 22.Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 23.Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–1748. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- 24.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10:1778–1785. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- 25.Spiro MJ, Kumar BR, Crowley TJ. Myocardial glycoproteins in diabetes: type VI collagen is a major PAS-reactive extracellular matrix protein. J Mol Cell Cardiol. 1992;24:397–410. doi: 10.1016/0022-2828(92)93194-o. [DOI] [PubMed] [Google Scholar]
- 26.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Lopez Y, Paloma MJ, Rifon J, Cuesta B, Paramo JA. Measurement of prethrombotic markers in the assessment of acquired hypercoagulable states. Thromb Res. 1999;93:71–78. doi: 10.1016/s0049-3848(98)00165-0. [DOI] [PubMed] [Google Scholar]
- 29.Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 30.Collier A, Rumley A, Rumley AG, Paterson JR, Leach JP, Lowe GD, Small M. Free radical activity and hemostatic factors in NIDDM patients with and without microalbuminuria. Diabetes. 1992;41:909–913. doi: 10.2337/diab.41.8.909. [DOI] [PubMed] [Google Scholar]
- 31.Barbash GI, White HD, Modan M, Van de Werf F. Significance of diabetes mellitus in patients with acute myocardial infarction receiving thrombolytic therapy. Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. J Am Coll Cardiol. 1993;22:707–713. doi: 10.1016/0735-1097(93)90180-9. [DOI] [PubMed] [Google Scholar]
- 32.Abbud ZA, Shindler DM, Wilson AC, Kostis JB. Effect of diabetes mellitus on short- and long-term mortality rates of patients with acute myocardial infarction: a statewide study. Myocardial Infarction Data Acquisition System Study Group. Am Heart J. 1995;130:51–58. doi: 10.1016/0002-8703(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 33.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Impact of history of diabetes mellitus on hospital mortality in men and women with first acute myocardial infarction. The National Registry of Myocardial Infarction 2 Participants. Am J Cardiol. 2000;85:1486–1489. doi: 10.1016/s0002-9149(00)00800-6. A1487. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara M, Sato H, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Kouno Y, Umemura T, Nakamura S. Impact of diabetes mellitus on long term survival after acute myocardial infarction in patients with single vessel disease. Heart. 2001;86:133–138. doi: 10.1136/heart.86.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]