Abstract
BACKGROUND
An alginate-based matrix supports the three-dimensional (3D) architecture of non-human primate follicles and, in the presence of FSH, permits the in vitro development of pre-antral follicles to the small antral stage, including the production of ovarian steroids and paracrine factors. The current study investigated the ability of gonadotrophins, fetuin and oxygen (O2) to improve primate follicle growth and oocyte maturation in vitro.
METHODS
Macaque secondary follicles were isolated from the early follicular phase ovaries, encapsulated in a sodium alginate matrix and cultured individually for 40 days in supplemented medium. The effects of recombinant human (rh) FSH (15, 3 and 0.3 ng/ml for high, medium and low FSH, respectively), bovine fetuin (1 or 0 mg/ml) and O2 (5 or 20% v/v) were examined. Half of the follicles in each culture condition received rhLH on Day 30–40. Follicles that reached antral stage were treated with rh chorionic gonadotrophin for 34 h to initiate oocyte meiotic maturation. Media were analyzed for ovarian steroids and anti-müllerian hormone (AMH).
RESULTS
Improved culture conditions supported non-human primate, secondary follicle growth to the antral stage and, for the first time, promoted oocyte maturation to the MII stage. In the presence of fetuin at 5% O2, follicles had the highest survival rate if cultured with high or medium FSH, whereas follicles grew to larger diameters at Week 5 in low FSH. Oocyte health and maturation were promoted under 5% O2. High FSHstimulated steroid production by growing follicles, and steroidogenesis by follicles cultured with low FSH was promoted by LH. AMH biosynthesis was elevated with high compared with low FSH and for longer under 5% O2 than under 20% O2.
CONCLUSIONS
This encapsulated 3D culture model permits further studies on the endocrine and local factors that influence primate follicle growth and oocyte maturation, with relevance to enhancing fertility preservation options in women.
Keywords: follicle culture, follicle-stimulating hormone, luteinizing hormone, fetuin, oxygen
Introduction
Advances in ovarian tissue cryopreservation, followed by ovarian transplantation or in vitro follicle maturation (IFM), provide options for fertility preservation in female patients with cancer. Although ovarian tissue transplantation yielded viable offspring in monkeys (Lee et al., 2004) and women (Donnez et al., 2004; Silber et al., 2008, 2010), the IFM approach has the advantage of eliminating the possibility of reintroducing cancer cells into the patient following treatment and providing a way to harvest more mature oocytes (Woodruff, 2007). Advances in biomaterial engineering resulted in the development of an alginate-based, three-dimensional (3D) follicle culture system to maintain the cell–cell and cell–matrix connections important in regulating follicle development in vivo (West et al., 2007). This approach produced live offspring in mice (Xu et al., 2006) and studies demonstrated the potential application of IFM in human beings (Xu et al., 2009a; Smitz et al., 2010). Even though the meiotic competence and developmental capacity of human oocytes grown from pre-antral stages in vitro have not yet been reported, animal studies indicate that IFM is a valid prospect for clinical translation to humans to circumvent the destruction or damage to ovarian germline cells caused by radiotherapy and/or chemotherapy (Woodruff, 2007). Recently, efforts to grow non-human primate ovarian follicles from the early stages (secondary follicles) to the antral stage during 3D culture have been successful (Xu et al., 2010) and may be valuable in identifying the optimal conditions for primate follicle culture prior to human application. Besides the clinical relevance, IFM is a powerful instrument for monitoring follicular endocrine and paracrine function, which is essential to obtain knowledge of the factors implicated in the regulation of follicular development.
Evidence suggests that FSH receptors are expressed in pre-antral follicles during in vivo development of various species, including primates (Gougeon, 1996; Findlay and Drummond, 1999). Studies indicate that FSH is essential for in vitro survival of primate pre-antral follicles (Wright et al., 1999; Xu et al., 2010) and promotes the growth of rodent (Xu et al., 2006), non-human primate (Xu et al., 2010) and human (Xu et al., 2009a) follicles during 3D culture. On the basis of rodent data reported recently, follicles cultured with a physiological level (low) of FSH tend to have oocyte and cumulus cell gene expression levels that are comparable to those in vivo (Sánchez et al., 2010). In contrast, a supraphysiological level (high) of FSH alters the expression of oocyte and cumulus cell transcripts (Sánchez et al., 2010). Thus, low FSH may promote oocyte-regulated cumulus cell differentiation while supporting follicle growth, whereas high FSH may inappropriately stimulate granulosa cell proliferation or differentiation.
Follicles are cultured typically in the presence of atmospheric oxygen (O2) tension at 20% (v/v) (Xu et al., 2010), which is around 140 mmHg. However, the partial pressure of O2 in the peritoneal cavity where the ovaries are located is 40 mmHg (Tsai et al., 1998), which approximates 5% O2 (v/v). Low O2 tension at 5% increased the viability of canine cumulus cells (Silva et al., 2009) and promoted developmental competence of porcine oocytes during in vitro maturation (Iwamoto et al., 2005). Moreover, low O2 tension during the culture of rat pre-antral follicles benefited oocyte viability, maturation, parthenogenetic activation and fertilization (Heise et al., 2009). Therefore, it is hypothesized that a low O2 environment has a positive influence during follicular development in vitro.
During spontaneous maturation of mouse oocytes in serum-free medium, the zona pellucida (ZP) becomes ‘hardened’, i.e. resistant to chymotrypsin digestion (De Felici and Siracusa, 1982). The hardened ZP is resistant to sperm penetration, thus preventing fertilization. This phenomenon has also been characterized in non-human primate (VandeVoort et al., 2007) and human (Schiewe et al., 1995) oocytes. Serum, including fetal bovine serum (FBS), from various sources is effective in preventing ZP hardening in rodents (Eppig and Schroeder, 1986). Fetuin, a major glycoprotein in serum and follicular fluid, increased the solubility of the ZP during spontaneous oocyte maturation in mice and was able to provide a serum-free culture environment (Schroeder et al., 1990). Whether fetuin promotes or is essential for follicle or oocyte maturation during macaque IFM is unknown.
Therefore, using the encapsulated 3D culture system, the current study tested the dose–response of FSH on follicle survival and growth, and investigated O2 tension and fetuin effects on primate follicular development in vitro. Ovarian steroids (androstenedione, A4; estradiol, E2; and progesterone, P4) and anti-müllerian hormone (AMH) produced by individually cultured follicles, as well as oocyte maturation, were analyzed to evaluate follicular function.
Materials and Methods
Animals and ovary collection
The general care and housing of rhesus macaque monkeys were provided by the Division of Animal Resources at the Oregon National Primate Research Center (ONPRC). Animals were pair caged in a temperature-controlled (22°C) light-regulated 12 L:12 D room. Diet consisted of Purina monkey chow (Ralston-Purina, Richmond, IN, USA) provided twice a day, supplemented with fresh fruit or vegetables once a day and water ad libitum. Animals were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the ONPRC Institutional Animal Care and Use Committee.
Adult female rhesus macaques (n= 9; 5–10 years of age) exhibiting regular menstrual cycles of ∼28 days were evaluated daily for menstruation with the first day of menses termed Day 1 of the cycle. Ovariectomies were conducted on anesthetized monkeys by laparoscopy at early follicular phase, Day 1–4 of the cycle, as previously described (Duffy DM and Stouffer, 2002). Ovaries were immediately transferred into Hepes-buffered holding media (CooperSurgical, Inc., Trumbull, CT, USA) supplemented with 0.2% (v/v) human serum protein supplement (SPS, CooperSurgical, Inc.) and 10 µg/ml gentamicin (Sigma-Aldrich, St Louis, MO, USA).
Follicle isolation, encapsulation and culture
Follicle isolation and encapsulation were described previously (Xu et al., 2009b). Briefly, ovarian cortex was cut into 2 × 2 × 1 mm cortical strips and incubated in 6 ml holding media (as described above), containing 275 U/ml collagenase type I and 585 U/ml deoxyribonuclease I (Worthington Biochemical Corp., Lakewood, NJ, USA), at 37°C for 20 min. Follicles were mechanically isolated in the holding media using 31-gauge needles and the secondary follicles with diameters of 125–225 µm that displayed the following characteristics were selected for encapsulation: (i) an intact basement membrane, (ii) two to three layers of granulosa cells and (iii) a visible, healthy oocyte that was round and centrally located within the follicle, without vacuoles or dark cytoplasm. Follicles (128 ± 12/monkey from 9 monkeys) were divided among the treatment groups with 36–60 follicles per group.
Follicles were transferred individually into 5 µl 0.25% (w/v) sterile sodium alginate (FMC BioPolymers, Philadelphia, PA, USA)-phosphate-buffered saline (PBS) (137 mM NaCl, 10 M phosphate, 2.7 mM KCl, Invitrogen, Carlsbad, CA, USA) and the droplets were cross-linked in 50 mM CaCl2, 140 mM NaCl, 10 mM HEPES solution (pH = 7.2) for 1 min. Each alginate-encapsulated follicle was transferred into individual wells of 48-well plates containing 300 µl alpha minimum essential medium (αMEM, Invitrogen) supplemented with 0.3% (v/v) SPS, 5 µg/ml insulin, 5 µg/ml transferrin and 5 ng/ml sodium selenite (Sigma-Aldrich).
Encapsulated follicles were cultured at 37°C in a 5 or 20% (v/v) O2 environment (in 6% CO2/89% N2 or 5% CO2 in air atmosphere, respectively), with 0.3, 3 or 15 ng/ml recombinant human (rh) FSH (low, medium or high FSH; NV Organon, Oss, Netherlands), and 0 or 1 mg/ml purified bovine fetuin (Sigma-Aldrich) for 40 days. Half of the follicles in each culture condition received 0.4 ng/ml rhLH (EMD Serono, Inc., Randolph, MA, USA) in the media during Days 30–40. Follicles that reached the antral stage were treated with 100 ng/ml rh chorionic gonadotrophin (CG, Merck Serono, Geneva, Switzerland) for 34 h. Oocytes were retrieved to determine competence for meiotic maturation. Half of the culture media (150 µl) was collected and replaced every other day and stored at −20°C. The media samples from each culture week were assigned to ovarian steroids and AMH assays.
Follicle survival and growth
Follicle survival, diameter and antrum formation were assessed weekly using an Olympus CK40 inverted microscope and an Olympus DP11 digital camera (Olympus Imaging America Inc., Center Valley, PA, USA) as described previously (Xu et al., 2009b). Follicles were measured from the outer layer of cells which included a measurement at the widest diameter of the follicle and a second measurement perpendicular to the first. The mean of the values was calculated and reported as the follicle diameter. Follicles were considered to be undergoing atresia if the oocyte was dark or not surrounded by a layer of granulosa cells, the granulosa cells became dark and fragmented, or the diameter of the follicle decreased (Xu et al., 2009b). Follicle photographs were imported into ImageJ 1.42 software (National Institutes of Health, Bethesda, MD, USA) and the diameter of each follicle was measured.
Oocyte retrieval, maturation and fertilization
Retrieved oocytes were photographed and oocyte diameters (excluding the zona pellucida) were measured using the same camera and software as described earlier.
The germinal vesicle (GV)-intact and metaphase II (MII) oocytes were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for immunofluorescence imaging to identify nuclear maturation, spindle and polar body organization (Barrett and Albertini, 2007). Briefly, oocytes were incubated with primary antibody overnight at 4°C and secondary antibody for 1 h at room temperature. Spindle microtubules were labeled with α-tubulin clone DM1A (1:100; Sigma) followed by Alexa 633 goat anti-mouse IgG (1:500; Invitrogen). F-actin was probed with Alexa 488-phalloidin (1:50) and chromosomes were labeled with 5 μM ethidium homodimer (Invitrogen). Oocyte images were captured using a Leica SP5 confocal-equipped DM6000 CFS microscope (Leica Microsystems Inc., Bannockburn, IL, USA).
Semen collection and ICSI were performed for one of the MII oocytes by the Assisted Reproductive Technology (ART)/Embryonic Stem Cell Support Core at the ONPRC, as reported previously (Lanzendorf et al., 1990; Meng and Wolf, 1997). The resulting zygote was transferred to 500 μl hamster embryo culture medium-9 with 5% FBS and cultured at 37°C in 6% CO2, 5% O2 and 89% N2 (Schramm and Paprocki, 2000). The embryo was photographed daily to document development. Reagents and protocols for embryo culture were provided by the ART Core (Meng and Wolf, 1997).
Ovarian steroids and AMH assays
One media sample collected weekly during culture Weeks 1–5 was analyzed for E2 and P4 concentrations by the Endocrine Technology Support Core at the ONPRC using an Immulite 2000, a chemiluminescence-based automatic clinical platform (Siemens Healthcare Diagnostics, Deerfield, IL, USA) validated for macaque follicle culture media as reported previously (Xu et al., 2009b). The inter- and intra-assay variations for both E2 and P4 assays were <15%. Media A4 levels were measured by radioimmunoassay using a DSL-3800 kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA) which was validated previously for macaque follicle culture media (Xu et al., 2010) with 0.05 ng/ml in sensitivity and <10% in inter- and intra-assay variations.
Another media sample collected weekly was analyzed for AMH concentrations by ELISA using a DSL-10-14400 kit (Diagnostic Systems Laboratories, Inc.) based on the manufacturers’ instructions (Fréour et al., 2007), as previously validated for macaque follicle culture media (Xu et al., 2010). This assay has a sensitivity of 0.16 ng/ml and the inter- and intra-assay variations were <15 and 10%, respectively. Due to the cross-reaction of fetuin with the AMH antibody, levels assayed in media containing fetuin without cultured follicles were subtracted from AMH levels in media samples from follicle cultured with fetuin, as previously described (Xu et al., 2010).
Statistical analysis
Statistical significance was analyzed by SigmaPlot 11 software (SPSS, Inc., Chicago, IL, USA) using a two-way analysis of variance (ANOVA) with repeated measures or one-way ANOVA followed by the Student–Newman–Keuls post hoc test for single time points. Differences were considered significant at P < 0.05 and values are presented as mean ± SEM. Follicle survival and distribution represent the percent (mean ± SEM) of three individual animals in each treatment group. Follicle growth, steroid and AMH production, and oocyte maturation were analyzed for each individual follicle with total follicle numbers indicated in the figure legends, and represent follicles obtained from the three individual animals.
Results
There was no difference between high- and medium-dose FSH-treated groups in any parameters analyzed (data not shown). Only data from follicles cultured with high- and low-dose FSH, except follicle survival under medium-dose FSH in Fig. 1B, are described below.
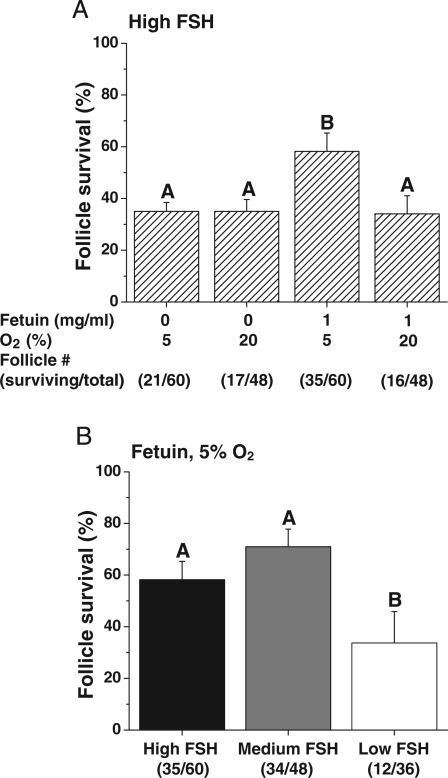
Figure 1.
The effects of FSH, fetuin and O2 on follicle survival in vitro. Follicle survival (percentage of those cultured) in media containing high FSH without LH supplementation at Day 30 (A). FSH dose–response for survival when follicles were cultured with fetuin at 5% O2 (B). Significant differences among culture conditions are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with three animals per treatment group. Parentheses indicate the total number of surviving/cultured follicles per treatment group.
Follicle survival and growth
In the presence of high FSH, the survival rate at Day 40 was higher (P < 0.05) when follicles were cultured with fetuin at 5% O2 (Fig. 1A). The survival rate was lower (P < 0.05) for follicles cultured with low compared with high FSH when in the presence of fetuin at 5% O2 (Fig. 1B). LH addition on Day 30 had no effect on follicle survival at Day 40 (data not shown).
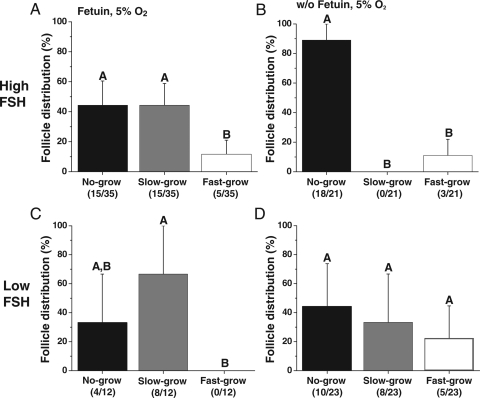
At the beginning of the culture, follicle diameters did not differ among treatment groups (data not shown). However, during culture, three distinct cohorts of surviving follicles were observed based on their growth rate as previously reported (Xu et al., 2010). The cohort that remained similar in size to the initial secondary follicles without significant change in diameter (<250 µm) through 5 weeks of culture was termed ‘no-grow’ follicles. Another cohort doubled their diameters (250–500 µm) and was termed ‘slow-grow’ follicles. Finally, another group of follicles increased their diameters by a minimum of 3-fold (>500 µm) and was termed ‘fast-grow’ follicles. An antral cavity was evident within 3–4 weeks of culture for all the slow- and fast-grow follicles. During culture with high FSH, fetuin and 5% O2, 57% of the surviving follicles fell into the growing (slow- and fast-grow) follicle category (Fig. 2A). In contrast, when fetuin was absent, the majority (86%) of the surviving follicles were no-grow follicles, despite the presence of high FSH (Fig. 2B). In the presence of low FSH, the proportions of growing follicles observed with or without fetuin were similar, 67 and 57%, respectively (Fig. 2C and D). However, fast-grow follicles were not obtained during culture with fetuin (Fig. 2C), while 22% were fast-grow follicles when cultured without fetuin (Fig. 2D). Similar patterns were observed for follicles cultured at 20% O2, except for lower (P < 0.05) percentages of growing follicles compared with those cultured at 5% O2 (data not shown). The addition of LH at Day 30 had no effect on the growth distribution of surviving follicles (data not shown).
Figure 2.
The effects of FSH and fetuin on growth patterns of surviving follicles. Distributions of no-, slow- and fast-grow follicles under culture conditions of high FSH with (A) or without (B) fetuin and low FSH with (C) or without (D) fetuin at 5% O2, without LH supplementation at Day 30. Significant differences among follicle categories are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with three animals per treatment group. Parentheses indicate the total number of follicles from each category/surviving follicles per treatment group.
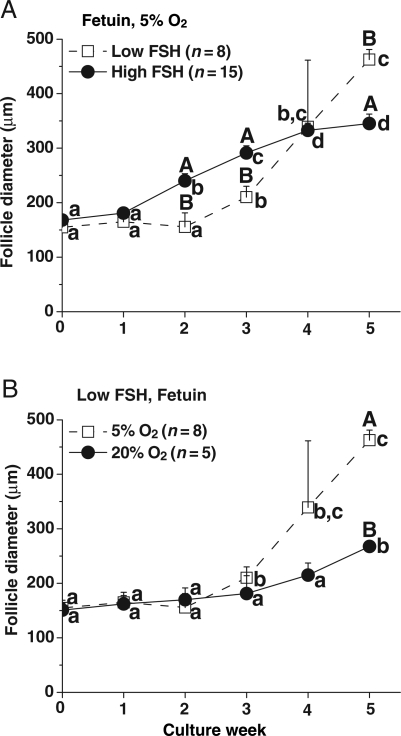
The dose of FSH and O2 tension influenced the growth rate of slow-grow (Fig. 3A and B), but not no- and fast-grow follicles (data not shown). In the presence of low FSH, slow-grow follicles cultured with fetuin at 5% O2 had lower (P < 0.05) growth rates at Weeks 2 and 3 compared with those in the high FSH group. In contrast, follicle diameters became larger (P < 0.05) in the presence of low FSH than in the presence of high FSH at Week 5 (Fig. 3A). The follicles maintained similar growth rates, when cultured with low FSH and fetuin, during the first 3 weeks in either 5% or 20% O2. However, diameters increased and became larger (P < 0.05) at Week 5 for follicles cultured at 5% O2 than those with 20% O2 (Fig. 3B). LH supplementation at culture Day 30 did not promote further growth of the no-, slow- or fast-grow follicles regardless of culture conditions (data not shown).
Figure 3.
The effects of FSH and O2 on follicle growth pattern. Growth patterns of slow-grow follicles during culture in media containing either low or high FSH and fetuin at 5% O2 (A), and in media containing low FSH and fetuin at either 20 or 5% O2 (B), without LH supplementation at Day 30. Significant differences over time (lowercase) or between culture conditions (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM. n, number of follicles.
Oocyte diameter, maturation and fertilization
Following exposure of antral (slow- and fast-grow) follicles to rhCG, healthy as well as degenerate (dark and condensed cytoplasm) oocytes were obtained at retrieval. Most of the healthy oocytes (22 oocytes) remained at the GV-intact stage. However, a few oocytes (three oocytes) from fast-grow follicles matured to the MII stage. Since LH had no effect on oocyte maturation, oocytes retrieved from follicles cultured with and without LH addition were combined for analysis of oocyte parameters among culture conditions. When cultured at 5% O2, more healthy oocytes (17 GV and 2 MII oocytes) were obtained from the follicles compared with those in 20% O2 culture (5 GV and 1 MII oocytes). Oocytes retrieved from follicles cultured at 5% O2 were analyzed among different culture conditions (Table I). Of the 20 oocytes retrieved from follicles cultured at high FSH with fetuin, 60% were healthy oocytes. In contrast, all the oocytes retrieved from the few slow- and fast-grow follicles that developed at high FSH without fetuin were degenerate. A few healthy oocytes were retrieved from follicles cultured at low FSH with or without fetuin. Furthermore, the diameters of GV-intact oocytes from follicles cultured at low FSH without fetuin were larger (P < 0.05) than those of the high FSH with fetuin group (Table I). MII oocytes were retrieved from follicles cultured with high FSH and fetuin at 5% O2 or with low FSH without fetuin at 5% O2 (Table I) or in 20% O2 (one oocyte).
Table I.
Characteristics of oocytes retrieved from antral follicles on Day 40 of encapsulated 3D culture with 5% O2 (34 h after addition of rhCG).
| Culture condition | Number (n) of |
Diameter (µm)* |
|||||
|---|---|---|---|---|---|---|---|
| Healthy oocytes |
|||||||
| Follicles harvested | Oocytes retrieved | Degenerate oocytes | GV-intact | MII stage | GV-intact oocytes | MII oocytes | |
| High FSH + fetuin | 25 | 20 | 8 | 11 | 1 | 94 ± 6a | 110 |
| High FSH | 5 | 3 | 3 | 0 | 0 | ||
| Low FSH + fetuin | 9 | 7 | 4 | 3 | 0 | 95 ± 8a,b | |
| Low FSH | 8 | 5 | 1 | 3 | 1 | 103 ± 2b | 112 |
*Values are the mean ± SEM with each oocyte diameter as an individual data point.
a,bDifferent letters indicate significant differences within the column (P < 0.05).
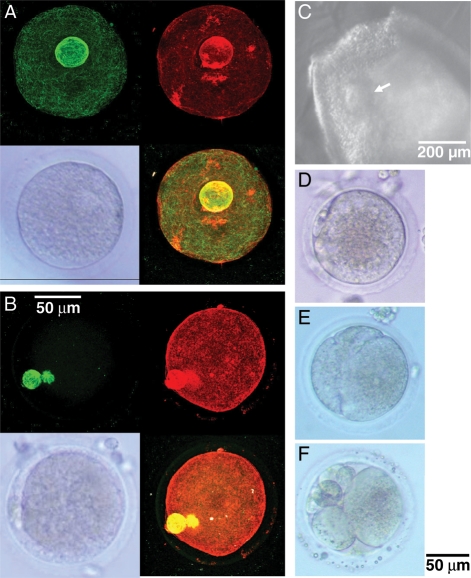
GV-intact oocytes and one MII-stage oocyte were analyzed using immunofluorescence imaging to visualize chromatin, spindle and actin elements (Fig. 4). In the GV-intact oocyte, the diffuse chromatin was evident as a perinucleolar ring (Fig. 4A). In the MII oocyte, the chromatin was condensed and reflected the chromosome organization for meiosis. A spindle and the first polar body were observed with normal sizes and positions in the MII oocyte (Fig. 4B).
Figure 4.
GV-intact (A, bottom left) and MII (B, bottom left) oocytes at retrieval with corresponding confocal microscopy images (A, B), and pictures of the cumulus–oocyte complex (arrow) in a fast-grow follicle (C) and retrieved MII oocyte prior to (D) and after (Day 1, E; Day 3, F) insemination using ICSI. The GV oocyte contains a perinucleolar ring (A) and the MII oocyte shows first polar body adjacent to spindle (B). Green tubulin (A and B, top left) and red F-actin (A and B, top right) staining were overlapped on bottom right of (A) and (B). The fertilized MII oocyte cleaved to two cells (E) and arrested with uneven cleavage (F). Scale bar = 50 µm for oocytes and 200 µm for follicle.
Figure 4D–F depicts fertilization and embryonic development of the other MII oocyte (Fig. 4D), retrieved from a fast-grow follicle (Fig. 4C), inseminated by ICSI. The fertilized oocyte cleaved to 2 cells within 24 h after ICSI (Fig. 4E). Subsequent cleavage was uneven and embryonic development arrested at Day 3 post-ICSI (Fig. 4F).
Follicular steroids
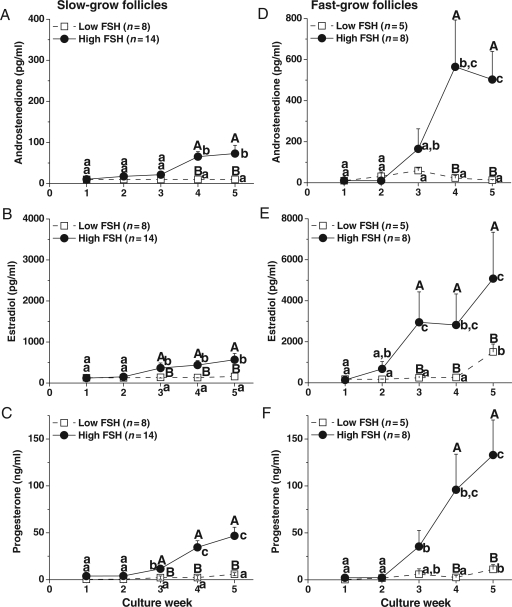
For no-grow follicles, media A4, E2 and P4 levels remained at baseline throughout culture and were not influenced by the experimental variables (data not shown). Since O2 tension and fetuin exposure did not alter steroid levels in culture media of slow- and fast-grow follicles (data not shown), data from 5% O2 with and without fetuin cultures were combined and presented to illustrate the dose-dependent effects of FSH (Fig. 5). For slow-grow follicles cultured with high FSH, A4 (Fig. 5A), E2 (Fig. 5B) and P4 (Fig. 5C) concentrations started rising at Week 3–4 and were higher (P < 0.05) at Week 3–5 compared with those observed in the beginning of culture. Similar patterns were observed for steroid concentrations of fast-grow follicles during the culture interval with higher (P < 0.05) levels at Week 3–5 compared with those of slow-grow follicles (Fig. 5D–F, note the differences in the y-axes scales of 5D and 5E compared with 5A and 5B). In contrast, A4 (Fig. 5A and D), E2 (Fig. 5B) and P4 (Fig. 5C) levels of slow- and fast-grow follicles cultured with low FSH stayed at baseline, except E2 (Fig. 5E) and P4 (Fig. 5F) produced by fast-grow follicles increased (P < 0.05) at Week 5. The steroid concentrations of both slow- and fast-grow follicles cultured with low FSH were lower (P < 0.05) than those of high FSH-treated follicles at Week 3–5 (Fig. 5A–F).
Figure 5.
Dose–response of FSH on steroid production by slow- (A–C) and fast- (D–F) grow follicles in vitro. Androstenedione (A and D), estradiol (B and E) and progesterone (C and F) levels in media containing either low- or high-dose FSH at 5% O2, without LH supplementation at Day 30. Significant differences over time (lowercase) or between the FSH dosage groups (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM. n, number of follicles.
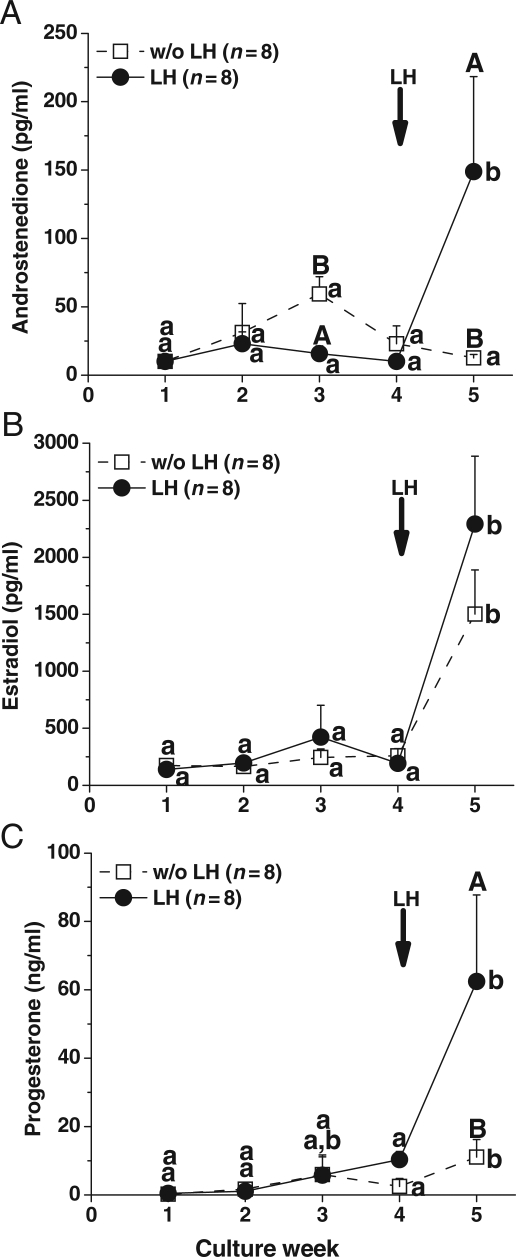
For slow-grow follicles cultured at 5% O2 with and without fetuin, media levels of A4 (Fig. 5A), E2 (Fig. 5B) and P4 (Fig. 5C) did not increase between Week 4 and 5 in the presence of high FSH. However, for these follicles, the addition of LH at Day 30 increased (P < 0.05) steroid production between pre-LH (Week 4) and post-LH (Week 5) exposure (data not shown). Moreover, LH treatment increased (P < 0.05) A4 (Fig. 6A) and P4 (Fig. 6C), but not E2 (Fig. 6B), levels in media for the slow-grow follicles cultured with low FSH and the levels were higher (P < 0.05) at Week 5 than those without LH administration. In contrast to the slow-grow follicles, LH supplementation at Day 30 had no effect on the patterns or levels of steroids during culture Week 5 for fast-grow follicles regardless of culture conditions (data not shown), as previously noted (Xu et al., 2010).
Figure 6.
LH effect on steroid production by slow-grow follicles. Androstenedione (A), estradiol (B) and progesterone (C) levels from slow-grow follicles during culture in media containing low FSH at 5% O2, without or with LH supplementation (arrow) at Day 30. Significant differences over time (lowercase) or between the LH treatment groups (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM. n, number of follicles.
Anti-müllerian hormone
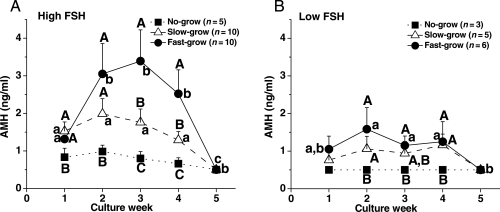
Since fetuin had no effect on AMH production by cultured follicles (data not shown), media AMH concentrations from follicles cultured with and without fetuin were combined for data analysis. When follicles were cultured with high FSH at 5% O2, AMH levels produced by no-grow follicles did not change throughout the culture (Fig. 7A). Although diameters of no-, slow- and fast-grow follicles were not different at the onset of culture, levels of AMH produced by slow- and fast-grow follicles at Week 1 were higher (P < 0.05) than those of no-grow follicles. Moreover, AMH levels of fast-grow follicles increased (P < 0.05) at Week 2 and remained at high levels until declining (P < 0.05) at Week 5 (Fig. 7A). AMH levels during Weeks 3 and 4 were distinct (P < 0.05) among all three follicle categories. By Week 5, all cultured follicles had basal levels of AMH (Fig. 7A). When cultured with low FSH at 5% O2, the slow- and fast-grow follicles produced higher (P < 0.05) levels of AMH during Week 2–4 than the no-grow follicles (Fig. 7B). However, the levels were lower (P < 0.05) than those of high FSH culture. Similar patterns were obtained for AMH produced by follicles cultured at 20% O2, except that AMH levels declined (P < 0.05) to basal levels earlier at Week 3 (data not shown) as previously reported (Xu et al., 2010). The addition of LH at Day 30 had no effect on AMH levels in any class of follicles or culture condition (data not shown).
Figure 7.
AMH concentrations from no-, slow- and fast-grow follicles during culture in media containing high (A) or low (B) FSH at 5% O2, without LH supplementation at Day 30. Significant differences over time (lowercase) or among the follicle categories (uppercase) are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM. n, number of follicles.
Discussion
Advances in the encapsulated 3D system allow primate secondary follicles to grow to the small antral stage and to produce local (AMH) and endocrine (steroids) factors. Compared with our previous study (Xu et al., 2010), the current culture conditions improved follicle growth (to >1000 µm in diameter) as well as the health and growth of the oocytes to reach the size of those that mature in vivo (>100 µm in macaques; Buse et al., 2008). For the first time, oocytes retrieved from in vitro developed primate follicles displayed the competence to reinitiate meiosis for fertilization, and hence early embryonic cleavage. Further indices of oocyte quality need to be examined, such as cumulus–oocyte communication (Kimura et al., 2007), to monitor the competence to undergo meiotic maturation, as well as maternal to zygotic transition.
In the present study, fetuin, in combination with high FSH and 5% O2, increased follicle survival and promoted growth of follicles. A variety of cell types in culture respond to fetuin in promoting cellular attachment, growth and differentiation (Nie, 1992; Demetriou et al., 1996). Previous studies found that fetuin is present in the ovarian follicular fluid of the mouse (Høyer et al., 2001), horse (Dell'Aquila et al., 1999) and human (Kalab et al., 1993). Our current data suggest that follicles cultured with low FSH without added fetuin grew faster after antrum formation. Whether cultured follicles require exogenous fetuin to promote further growth after antrum formation is unclear. Alternatively, endogenous fetuin production by cultured follicles could be inhibited by high FSH. Fetuin may also stimulate the action of macrophages (Wang et al., 1998), and healthy granulosa cells may behave in a macrophage-like manner during follicular atresia in the mouse (Inoue et al., 2000; Høyer et al., 2001), guinea pig (Kasuya, 1997) and cow (Van Wezel et al., 1999). The specific effects of fetuin on follicle cell proliferation and differentiation remain to be determined. It has been proposed that the protease inhibitory activity of fetuin plays an important role in preventing ZP hardening (Schroeder et al., 1990). Since one MII oocyte, which originated from a follicle grown in the absence of fetuin, was fertilized by ICSI, ZP ‘hardening’ that prevented sperm penetration was not evident. One unexpected observation was that alginate beads became non-transparent, brittle and fragmentary after 2 weeks of culture without fetuin. This phenomenon is not evident in murine follicle culture during the shorter culture period (<2 weeks; Xu et al., 2006). The mechanism whereby fetuin maintains alginate gel integrity is unknown. Since fetuin is currently not a recombinant protein, additional complexity caused by fetuin inpurity cannot be ruled out.
Compared with the typical culture condition of 20% O2, the lower O2 tension at 5% and fetuin supported higher follicle survival in the presence of high FSH, increased the rate of antrum formation and promoted the growth of slow-grow follicles after antrum formation when cultured with low FSH. Investigators using caprine pre-antral follicles cultured with 5% O2 reported an increased percentage of antrum formation relative to 20% (Silva et al., 2010). The current results are also consistent with observations from ovine pre-antral follicle culture, where low O2 concentration (5%) stimulated follicle growth with a high proportion of them developing an antral cavity and a healthy cumulus–oocyte complex (Cecconi et al., 1999). It is hypothesized that the ovarian follicle, in a low-oxygen environment, is often challenged by hypoxia (Redding et al., 2008), and that antrum formation provides a way to support further growth by avoiding hypoxia in the follicle wall (Redding et al., 2007). We have not tested the effects of lower O2 tensions (hypoxic) on cultured follicles in our system. It is noteworthy that the percentage of growing follicles in 20% O2 culture in the current study is lower than that of the same culture media in our previous report (Xu et al., 2010). This may be due to the animal variation from different reproductive seasons. The current study also indicates that more healthy oocytes are retrieved from the follicles cultured at 5% than at 20% O2. It is well established that high O2 tension is associated with higher levels of reactive oxygen species, and oxidative stress plays a role in cytotoxic activity (Evans et al., 2004). As such, low O2 tension (5%), compared with 20%, reduced cumulus cell apoptosis in canine cumulus–oocyte complexes during culture (Silva et al., 2009). Finally, the current results in macaques indicated that 5% O2 promoted AMH production after antrum formation. This is the first evidence that AMH, like other potential growth factors (e.g. vascular endothelial growth factor; Shweiki et al., 1992), is regulated by O2. On the basis of evidence from human (Weenen et al., 2004), non-human primate (Thomas et al., 2007) and rodent (Salmon et al., 2004) ovaries, AMH production by follicles cultured at 5% O2 after antrum formation may originate from mural or cumulus cells. Thus, a physiological level of O2 (5%) is beneficial for macaque follicle survival, growth and function, as well as oocyte quality, during encapsulated 3D culture.
The addition of FSH produced dose-dependent effects that are different for follicle survival and growth. In the presence of fetuin at 5% O2, follicles cultured with high FSH had a higher survival rate than those of low FSH. In contrast, most of the follicles cultured with low FSH died within 2 weeks, which extends our previous conclusion (Xu et al., 2010) that primate pre-antral follicle survival is FSH-dependent in vitro. However, after antrum formation, low FSH seems to further promote growth of slow-grow follicles compared with high FSH at 5% O2. Moreover, oocytes achieved larger diameters (>100 µm) when follicles were cultured with low FSH. High FSH negatively impacted pre-antral follicle development in mice (Kreeger et al., 2005). Prolonged high FSH exposure may disturb oocyte control of granulosa cell proliferation and differentiation, as well as cumulus cell function, during primate follicle growth in vitro. FSH increases steroid production of cultured follicles after antrum formation (Xu et al., 2010), presumably by acting on FSH receptors on granulosa cells. In the current study, high FSH markedly increased the levels of A4, E2 and P4, whereas E2 and P4 levels only modestly rose in the cultures with low FSH. The E2-to-P4 ratio at week 5 is higher in low, relative to high, FSH cultured follicles. Thus, high FSH may cause premature differentiation of granulosa cells which undergo luteinization to generate high levels of P4, which also serves as substrate for A4 and E2. The results provide the first evidence that high FSH also promoted AMH production by growing follicles. In rodents, AMH decreases the responsiveness of growing follicles to FSH stimulation (Durlinger et al., 2001). The factors that regulate AMH expression in primate follicles remain unclear, but oocyte-granulosa cell co-culture experiments determined that AMH mRNA expression within granulosa cells of mouse pre-antral follicles is regulated by signals from the oocyte (Salmon et al., 2004). Thus, high levels of AMH may be induced by oocyte-derived factors to reduce the negative effects caused by high FSH.
For the first time, MII-stage oocytes were retrieved from primate antral follicles, following growth from pre-antral follicles under chemically defined conditions. Nevertheless, most of the healthy oocytes obtained after the rhCG stimulus remained at the GV stage, and the MII oocyte that was fertilized only reached the 2-cell stage before embryonic development arrested. While insulin had deleterious effects on oocyte development in mouse follicle culture (Eppig et al., 1998), lowering insulin concentration in our 3D culture system did not improve oocyte quality in macaques (Xu et al., 2010). In rhesus monkeys, the transition from maternal to embryonic genome occurs at the 6- to 8-cell stage (Schramm and Bavister, 1999). Therefore, the oocyte must contain the appropriate instructions, involving the expression of new protein-coding genes (Kocabas et al., 2006), to drive the first few divisions and the awakening of the embryonic genome. Further studies are warranted to improve cytoplasmic and nuclear maturation, as well as the developmental competence of embryos produced from oocytes retrieved from primate follicles after encapsulated 3D culture.
For the first time, a significant LH effect was observed on A4 and P4, but not on E2, production in slow-grow follicles cultured with low FSH. A moderate increase in steroid production by slow-grow follicles following LH addition was reported previously during primate follicle culture with high FSH (Xu et al., 2010). According to the 2-cell, 2-gonadotrophin theory, LH-receptor signaling promotes A4 production from P4 by theca cells, which allows steroidogenic maturation of the follicles by providing substrate for E2 biosynthesis in granulosa cells (McNatty et al., 1980). This LH responsiveness suggests the presence of theca cells in in vitro developed primate follicles, although this has not yet been conclusively demonstrated in our system. In low FSH cultured follicles, well-developed theca cells may be stimulated by LH to produce A4 and P4, while granulosa cells undergoing appropriate proliferation may utilize A4 and P4 efficiently to synthesize high levels of E2. It is also possible that healthy theca cells produce insulin-like growth factors (Brogan et al., 2010) or other paracrine factors that can support steroidogenesis in granulosa cells independent of FSH stimulation. Steroid production by fast-grow follicles was not altered by LH addition, which extends our previous findings utilizing culture conditions with high FSH (Xu et al., 2010). High steroid production prior to the LH addition may prevent further stimulation. While there is a dose-dependent effect of FSH, LH addition does not appear to impact AMH production by in vitro developed primate follicles.
Three groups of surviving follicles with different growth rates were observed in the current, as well as our previous, studies in macaques (Xu et al., 2010). Also, AMH production in vitro by early pre-antral follicles correlated positively with growth rate as we reported previously (Xu et al., 2010). AMH acts as a paracrine factor to modulate folliculogenesis in the early stages of mouse follicular development (Durlinger et al., 2002). Thus, early AMH production may be a potential marker for predicting further development of pre-antral follicles with different growth rates during culture. Notably, all three MII oocytes were retrieved from fast-grow follicles. However, it is not known whether AMH production by early pre-antral follicles predicts the quality of the oocyte enclosed within a small antral follicle that develops during the encapsulated 3D culture.
In summary, under improved conditions, individual primate pre-antral follicles are able to grow in vitro and produce mature oocytes for fertilization. On the basis of the current data, the following conditions for primate follicle culture are being investigated: (i) medium (3 ng/ml) FSH for the first 3 weeks to support follicle survival, followed by low (0.3 ng/ml) FSH after antrum formation to promote follicle growth; (ii) low concentration (0.5 mg/ml) of fetuin to maintain alginate gel integrity without causing a negative effect on follicle development; and (iii) low O2 tension at 5%. AMH may be a marker of follicle growth potential to screen for no-grow follicles at the early stage of culture. This encapsulated 3D culture system provides a way to understand the process and regulation of folliculogenesis in primates, including gene and protein expression, as well as metabolic pathways during follicular development. This information can be used to discover biomarkers that predict or report follicle and/or oocyte condition during IFM. The ultimate goal is to translate the experimental results to patients, thereby facilitating diagnostic and therapeutic approaches for ovarian preservation, and hence female fertility.
Authors' roles
J.X. provided contributions to (i) experimental design, (ii) follicle collection, culture and oocyte retrieval, (iii) follicle growth recording and diameter measurement, (iv) data analysis and interpretation on follicle development, oocyte maturation, steroid and AMH production, (v) manuscript drafting and critical revising and (vi) final approval of the version to be submitted for publication. M.S.L. contributed to (i) experimental design, (ii) animal health and reproduction record, (iii) follicle collection and culture, (iv) critical revising on the manuscript and (v) final approval of the version to be submitted for publication. R.R.Y. contributed to (i) experimental design, (ii) follicle collection and culture, (iii) critical revising on the manuscript and (iv) final approval of the version to be submitted for publication. K.Y.P. contributed to (i) experimental design, (ii) steroids and AMH assays, (iii) critical revising on the manuscript and (iv) final approval of the version to be submitted for publication. S.L.B. contributed to (i) experimental design, (ii) immunofluorescence imaging and data interpretation, (iii) critical revising on the manuscript and (iv) final approval of the version to be submitted for publication. M.B.Z. provided contributions to (i) conception and design of the experiments, (ii) follicle collection and oocyte retrieval, (iii) data interpretation on follicle development, oocyte maturation, steroid and AMH production, (iv) critical manuscript revising for important intellectual content and (v) final approval of the version to be submitted for publication. R.L.S. provided contributions to (i) conception and design of the experiments, (ii) data interpretation on follicle development, oocyte maturation, steroid and AMH production, (iii) critical manuscript revising for important intellectual content and (iv) final approval of the version to be submitted for publication.
Funding
This work was supported by the National Institutes of Health [grant numbers NIH UL1 RR024926 (R01-HD058294, PL1-EB008542), U54-HD18185 (Specialized Cooperative Centers Program in Reproduction and Infertility Research), NCRR-RR000163, HD07133].
Acknowledgements
We are grateful to members of the Division of Animal Resources, the Endocrine Technology Support Core, the Assisted Reproductive Technology/Embryonic Stem Cell Support Core, and the Colony Demographics & Informatics Unit at the ONPRC, as well as Drs Min Xu, Lonnie Shea and Teresa Woodruff at the Northwestern University, for their valuable expertise and technical assistance.
References
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–957. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- Brogan RS, Mix S, Puttabyatappa M, Vandevoort CA, Chaffin CL. Expression of the insulin-like growth factor and insulin systems in the luteinizing macaque ovarian follicle. Fertil Steril. 2010;93:1421–1429. doi: 10.1016/j.fertnstert.2008.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse E, Zöller M, Van Esch E. The macaque ovary, with special reference to the cynomolgus macaque (Macaca fascicularis) Toxicol Pathol. 2008;36:24S–66S. [Google Scholar]
- Cecconi S, Barboni B, Coccia M, Mattioli M. In vitro development of sheep preantral follicles. Biol Reprod. 1999;60:594–601. doi: 10.1095/biolreprod60.3.594. [DOI] [PubMed] [Google Scholar]
- De Felici M, Siracusa G. Spontaneous hardening of the anna pellucida of mouse oocytes during in vitro culture. Gamete Res. 1982;6:107–113. [Google Scholar]
- Dell'Aquila ME, De Felici M, Massari S, Maritato F, Minoia P. Effects of fetuin on zona pellucida hardening and fertilizability of equine oocytes matured in vitro. Biol Reprod. 1999;61:533–540. doi: 10.1095/biolreprod61.2.533. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-b type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Anti-mullerian hormone attenuates the effects of fsh on follicle development in the mouse ovary. Endocrinology. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Culture systems for mammalian oocyte development: progress and prospects. Theriogenology. 1986;25:97–106. [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Findlay JK, Drummond AE. Regulation of the FSH Receptor in the Ovary. Trends Endocrinol Metab. 1999;10:183–188. doi: 10.1016/s1043-2760(98)00144-1. [DOI] [PubMed] [Google Scholar]
- Fréour T, Mirallié S, Bach-Ngohou K, Denis M, Barrière P, Masson D. Measurement of serum anti-Müllerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375:162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- Heise MK, Koepsel R, McGee EA, Russell AJ. Dynamic oxygen enhances oocyte maturation in long-term follicle culture. Tissue Eng Part C Methods. 2009;15:323–332. doi: 10.1089/ten.tec.2007.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer PE, Terkelsen OB, Grete Byskov A, Nielsen H. Fetuin and fetuin messenger RNA in granulosa cells of the rat ovary. Biol Reprod. 2001;65:1655–1662. doi: 10.1095/biolreprod65.6.1655. [DOI] [PubMed] [Google Scholar]
- Inoue S, Watanabe H, Saito H, Hiroi M, Tonosaki A. Elimination of atretic follicles from the mouse ovary: a TEM and immunohistochemical study in mice. J Anat. 2000;196:103–110. doi: 10.1046/j.1469-7580.2000.19610103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Onishi A, Fuchimoto D, Somfai T, Takeda K, Tagami T, Hanada H, Noguchi J, Kaneko H, Nagai T, et al. Low oxygen tension during in vitro maturation of porcine follicular oocytes improves parthenogenetic activation and subsequent development to the blastocyst stage. Theriogenology. 2005;63:1277–1289. doi: 10.1016/j.theriogenology.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Kalab P, Shultz RM, Kopf GS. Modifications of the mouse zona pellucida during oocyte maturation: inhibitory effects of follicular fluid, fetuin, and α2HS-glycoprotein. Biol Reprod. 1993;49:561–567. doi: 10.1095/biolreprod49.3.561. [DOI] [PubMed] [Google Scholar]
- Kasuya K. Elimination of apoptotic granulosa cells by intact granulosa cells and macrophages in atretic mature follicles of the guinea pig ovary. Arch Histol Cytol. 1997;60:175–184. doi: 10.1679/aohc.60.175. [DOI] [PubMed] [Google Scholar]
- Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signaling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–342. [PubMed] [Google Scholar]
- Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam WL, Rosa GJ, Halgren RG, Lim B, et al. The transcriptome of human oocytes. Proc Natl Acad Sci USA. 2006;103:14027–14032. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP. Collection and quality of rhesus monkey semen. Mol Reprod Dev. 1990;25:61–66. doi: 10.1002/mrd.1080250111. [DOI] [PubMed] [Google Scholar]
- Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, Wolf DP. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Makris A, Osathanondh R, Ryan KJ. Effects of luteinizing hormone on steroidogenesis by thecal tissue from human ovarian follicles in vitro. Steroids. 1980;36:53–63. doi: 10.1016/0039-128x(80)90067-7. [DOI] [PubMed] [Google Scholar]
- Meng L, Wolf D. Sperm-induced oocyte activation in the rhesus monkey: nuclear and cytoplasmic changes following intracytoplasmic sperm injection. Hum Reprod. 1997;12:1062–1068. doi: 10.1093/humrep/12.5.1062. [DOI] [PubMed] [Google Scholar]
- Nie Z. Fetuin: its enigmatic property of growth promotion. Am J Physiol. 1992;263:C551–C562. doi: 10.1152/ajpcell.1992.263.3.C551. [DOI] [PubMed] [Google Scholar]
- Redding GP, Bronlund JE, Hart AL. Mathematical modelling of oxygen transport-limited follicle growth. Reproduction. 2007;133:1095–1106. doi: 10.1530/REP-06-0171. [DOI] [PubMed] [Google Scholar]
- Redding GP, Bronlund JE, Hart AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reprod Fertil Dev. 2008;20:408–417. doi: 10.1071/rd07190. [DOI] [PubMed] [Google Scholar]
- Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–208. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus–oocyte complex in mice. Biol Reprod. 2010;83:514–524. doi: 10.1095/biolreprod.109.083311. [DOI] [PubMed] [Google Scholar]
- Schiewe MC, Araujo JR, Asch RH, Balmaceda JP. Enzymatic characterization of zona pellucida hardening in human eggs and embryos. J Asst Reprod Genet. 1995;12:2–7. doi: 10.1007/BF02214120. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod. 1999;60:721–728. doi: 10.1095/biolreprod60.3.721. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM. Birth of rhesus monkey infant after transfer of embryos derived from in-vitro matured oocytes: short communication. Hum Reprod. 2000;15:2411–2414. doi: 10.1093/humrep/15.11.2411. [DOI] [PubMed] [Google Scholar]
- Schroeder AC, Schultz RM, Kopf GS, Taylor FR, Becker RB, Eppig JJ. Fetuin inhibits zona pellucida hardening, conversion of ZP2 to ZP2f during spontaneous mouse oocyte maturation in vitro in the absence of serum. Biol Reprod. 1990;43:891–897. doi: 10.1095/biolreprod43.5.891. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;29:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- Silva AE, Rodriguez P, Cavalcante LF, Rodrigues BA, Rodrigues JL. The influence of oxygen tension on cumulus cell viability of canine COCs matured in high-glucose medium. Reprod Domest Anim. 2009;44(Suppl 2):259–262. doi: 10.1111/j.1439-0531.2009.01406.x. [DOI] [PubMed] [Google Scholar]
- Silva CM, Matos MH, Rodrigues GQ, Faustino LR, Pinto LC, Chaves RN, Araújo VR, Campello CC, Figueiredo JR. In vitro survival and development of goat preantral follicles in two different oxygen tensions. Anim Reprod Sci. 2010;117:83–89. doi: 10.1016/j.anireprosci.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas FH, Telfer EE, Fraser HM. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology. 2007;148:2273–2281. doi: 10.1210/en.2006-1501. [DOI] [PubMed] [Google Scholar]
- Tsai AG, Friesenecker B, Mazzoni MC, Kerger H, Buerk DG, Johnson PC, Intaglietta M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc Natl Acad Sci USA. 1998;95:6590–6595. doi: 10.1073/pnas.95.12.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wezel IL, Dharmarajan AM, Lavranos TC, Rodgers RJ. Evidence for alternative pathways of granulosa cell death in healthy and slightly atretic bovine antral follicles. Endocrinology. 1999;140:2602–2612. doi: 10.1210/endo.140.6.6758. [DOI] [PubMed] [Google Scholar]
- VandeVoort CA, Hung PH, Schramm RD. Prevention of zona hardening in non-human primate oocytes cultured in protein-free medium. J Med Primatol. 2007;36:10–16. doi: 10.1111/j.1600-0684.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ. Fetuin (α2-HS-glycoprotein) opsonizes cationic macrophage-deactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK. The emergence of a new interdiscipline: oncofertility. Cancer Treat Res. 2007;138:3–11. doi: 10.1007/978-0-387-72293-1_1. [DOI] [PubMed] [Google Scholar]
- Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009a;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009b;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]