Abstract
BACKGROUND
Whether ovarian follicular rupture involves contractile activity or not has been debated for decades. Recently, study in the rodents has indicated that an endogenously produced potent vasoconstrictive peptide, endothelin-2 (EDN2), may induce follicular constriction immediately prior to ovulation. This study was aimed to systematically characterize the human ovarian endothelin system and localize smooth muscle cells to assess the possible involvement of contractile activity in human ovulation.
METHODS
This is a prospective experimental study. Study subjects were 20 women aged 20–38 years who underwent IVF owing to tubal or male factors. Expression patterns of messenger RNAs (mRNAs) for EDN1, EDN2, EDN3, endothelin-converting enzyme-1 (ECE1 and ECE2), endothelin receptor A (ETA) and ETB in the granulosa cells (GCs) and cumulus cells and endothelin peptide concentration in the pre-ovulatory follicles were measured at 36 h after hCG injection. In addition, localization of ovarian smooth muscle cells and endothelin receptor expression were determined in normal (non-IVF patient) ovaries.
RESULTS
Pre-ovulatory follicles express mRNA for EDN1 and EDN2, ECE1, ECE2, ETA and ETB, but not EDN3, contain highly concentrated endothelin peptides (105.9 pg/ml) and are surrounded by theca externa that are made mostly of multicell layer non-vascular smooth muscle cells. ETA expression is localized in the smooth muscle cells of theca externa, theca interna and GC, whereas ETB expression is confined to theca interna.
CONCLUSIONS
Pre-ovulatory follicles contain highly concentrated endothelins and are surrounded by non-vascular smooth muscle cells that express endothelin receptor, suggesting involvement of endothelin-induced contractile action in ovulation in the human ovary.
Keywords: ovary, ovulation, endothelin, smooth muscle, contraction
Introduction
The program of events governing ovulation is activated by a surge of LH and FSH, the pituitary gonadotrophins, that initiates dramatic changes in molecular, biochemical and physical aspects of the ovary, eventually leading to follicle rupture and release of the oocyte (Richards et al., 2002). In regard to the mechanism that governs rupture of the follicle, the ‘follicular constriction’ theory has been a mainstream proposition, with an overwhelming amount of data provided by multiple laboratories in support of this theory. Ironically, however, the constriction theory has remained a century-long controversy (Espey, 1978) needing a critical piece of evidence to support the theory: identification of the trigger for constriction and therefore follicle rupture (Martin and Talbot, 1981a,b).
Since the identification of endothelin-1 (EDN1) in 1988 (Yanagisawa et al., 1988a,b), EDN2 and EDN3 were subsequently identified (Masaki, 2004; Nakas-Icindic et al., 2004). Endothelins are structurally similar and exhibit potent vasoconstrictive activity. Bioactive endothelins are produced from ∼200 amino acid-long precursors, preproendothelins, which are processed by endopeptidases to form ‘big’ endothelins (Karam et al., 1999; Barton and Yanagisawa, 2008). The big endothelins are then further digested into ‘bioactive’ 21 amino acid-long forms via the action of endothelin-converting enzymes (ECE1 or ECE2; Karam et al., 1999; Masaki, 2004). Although low levels of endothelins are universally present throughout the body, each isoform is abundant in a tissue-dependent manner. EDN1 is mostly expressed in vascular smooth muscles, the central nervous system and Sertoli cells of the testis. EDN2 is reported to be expressed in the kidneys, intestine and ovary, whereas EDN3 is mainly expressed in the brain (Masaki, 2004); however, new evidence suggests an additional role of the EDN3 isoform in the oviduct (Jeoung et al., 2010). Endothelins convey their function via at least two distinct G protein coupled receptors: ETA and ETB (Sitbon et al., 2003; Channick et al., 2004). These receptors are distinctive in location of expression, binding affinities and their physiological responses to endothelin binding. Endothelins and their receptors are co-localized in various tissues, signifying the presence of autocrine and paracrine functions (Murakoshi et al., 2002). It has been previously proposed that the action of endothelins is conveyed via their receptors causing an increase in intracellular Ca2+ via phospholipase C activation and the subsequent formation of 1,4,5-inositol triphosphate, eventually resulting in cellular response.
Recently, we and others carried out a genome-wide gene-expression analysis to identify such a trigger in the ovulating ovary in rodents. This approach led us to the identification of EDN2, a potent vasoconstrictive peptide (Ko et al., 2006; Palanisamy et al., 2006). EDN2 was exclusively and transiently expressed in the granulosa cells (GCs) of periovulatory follicles prior to ovulation. A subsequent study showed that EDN2 induced instant and sustained constriction in isolated ovarian tissues and that administration of tezosentan (an endothelin receptor antagonist) delayed or inhibited follicle rupture in vivo, indicating a potential critical role of follicular constriction in ovulation (Ko et al., 2006). In support of the constriction theory, we and others also demonstrated the presence of a well-organized ‘non-vascular’ smooth muscle layer in the theca externa of each follicle and the expression of endothelin receptors in neighboring ovarian tissues (Abberton et al., 1999; Ko et al., 2006). Therefore, the objective of this study was to determine whether ovulating human ovaries produce EDN2 and associated endothelin components. Concentrations of endothelins in follicular fluids, levels of proteins and/or messenger RNAs (mRNAs) for endothelins (EDN1, EDN2 and EDN3), endothelin-converting enzymes that are required for producing bioactive forms of endothelins and endothelin receptors (ETA and ETA) in GCs and cumulus cells (CCs) were measured. Finally, the distribution of smooth muscle cells and the localization of endothelin receptors were determined in the human ovary.
Materials and Methods
Study subjects for the measurements of mRNA levels and endothelin concentration
The study participants were female IVF patients, 20–38 years of age (mean 30.9 years), with 1–10 years of infertility duration (mean 3.6 years). Inclusion criteria for this study were a regular menstrual cycle (26–34 days), basal FSH level of ≤10 mIU/ml (mean 6.60 mIU/ml), no evidence of an endocrine anomaly and adequate response to controlled ovarian hyperstimulation (COH). The participants' infertility causes were either tubal or male factors without any sign of ovulatory defects. The data presented herein are from 20 patients who met the inclusion criteria. The participants were informed about this study and submitted written consent prior to the study. This study was approved by the Institutional Review Board of Bundang CHA Medical Center. Subjects used for localization of ovarian smooth muscle cells and endothelin receptor expression are described in the ‘Immunohistochemistry’ section.
COH protocol
For the ovulation induction, patients were stimulated with recombinant human FSH (Gonal-F, Merck Serono SA, Geneva, Switzerland) following a mid-luteal GnRH agonist (GnRH-a) long protocol (San Roman et al., 1992) or a GnRH antagonist (GnRH-anta) protocol (Olivennes et al., 1994; Tur-Kaspa and Ezcurra, 2009). For the GnRH-a long protocol, the patients were treated with GnRH-a (0.1 mg/day of Lucrin, Abbott, USA) from the mid-luteal phase of their previous cycle. One to two weeks later, ovarian suppression was confirmed using transvaginal ultrasound by the presence of a quiescent ovary and thin endometrium, and serum 17β-estradiol (E2) <50 pg/ml. On Day 3, the GnRH-a dose was reduced to half and follicular development was induced with recombinant human FSH. For the GnRH-anta protocol, recombinant human FSH treatment was started on the 3rd day of the menstrual cycle. The GnRH-anta (Cetrotide 0.25 mg; Merck Serono SA) was administered daily, starting when the leading follicle reached a mean diameter of 14 mm and until the day of hCG administration. A recombinant hCG (Ovidrel 250 µg; Merck Serono SA) was injected to induce final follicular maturation when one or more follicles reached a mean diameter ≥18 mm. For both protocols, the ovarian follicle diameter was assessed by transvaginal sonography. Cumulus–oocyte complexes (COCs) were retrieved 36 h after hCG administration by transvaginal ultrasound-guided aspiration.
Serum collection, retrieval of follicular fluid and retrieval of CC and GC
On the 2nd or 3rd menstrual-cycle day, blood samples were collected by venipuncture and the serum separated for the measurement of basal testosterone, E2, dehydroepiandrosterone sulfate (DHEA-S), FSH, LH, prolactin and thyroid-stimulating hormone (TSH). These hormones were measured by either radioimmunoassay (testosterone, E2 and DHEA-S; Beckman Coulter, Inc., USA) or enzyme immunoassay (EIA; FSH, LH, prolactin, TSH; Slemens, USA) following the manufacturer's instructions. Follicular fluids, GCs and CCs were collected from the largest follicle of each ovary at the time of oocyte retrieval. For follicular endothelin peptide measurement, the follicular fluids were centrifuged at 2000g for 20 min, and the supernatants were transferred to sterile tubes, snap frozen and kept at −80°C until assayed. For GC and CC isolation, COCs and GCs were manually separated from follicular fluid under a microscope. The CCs were then divided into two subgroups according to oocyte maturity: CCs from mature oocytes (metaphase II) and from immature oocytes (germinal vesicle stage). Both groups of CC and GC were washed twice in cold phosphate-buffered saline (Dulbelcco's medium; Gibco, Invitrogen, USA) before storage in liquid nitrogen.
Immunometric assay
Follicular fluid and serum concentrations of human endothelins were determined using a commercially available EIA (No. 583151, Cayman Chemical Company, MI, USA), as described previously (Bridges et al., 2010). Briefly, samples were acidified with 1.5 ml of 4% acetic acid in water then loaded on activated solid phase extraction (C-18) columns. After washing, the bound endothelins were eluted from each column, oven dried at 35°C and resuspended in the supplied EIA buffer. The immunometric assay was then performed on the samples following the manufacturer's directions and absorbance determined at a wavelength of 420 nm. The intra- and inter-assay coefficients of variance were 5.9 and 4.2%, respectively. Concentrations of endothelins are expressed in pg/ml.
RNA isolation and real-time quantitative PCR
mRNA was isolated from human CC and GC using the Dynabeads mRNA DIRECT kit (Invitrogen Dynal Asa, Oslo, Norway), according to the manufacturer's instructions. Briefly, human CCs or GCs were resuspended in lysis/binding buffer [100 mM Tris–HCl (pH 7.5), 500 mM LiCl, 10 mM EDTA, 1% LiDS, 5 mM dithiothreitol] for 5 min at room temperature. After vortexing, prewashed Dynabeads oligo dT25 were mixed with the lysates and annealed by rotating for 5 min at room temperature. The beads were separated with a Dynal MPC-S magnetic particle concentrator and poly-(A)+ RNAs were eluted by incubation with 10 µl of Tris–HCl (10 mM Tris–HCl, pH 7.5) at 65°C for 2 min. Complementary DNA (cDNA) was synthesized using oligo-dT primers according to the manufacturer's protocol (Promega, Madison, WI, USA). An equal amount of cDNAs was used as templates for semi-quantitative PCR analysis. PCR primers were designed using Primer3 software (Rozen and Skaletsky, 2000). The primer binding sites were on two different exons spanning an intron so that any non-specific amplification of genomic DNA or cDNA from primary transcripts should be prevented. The size of PCR products and primer sequences for the specific genes are listed in Table I. PCR products were separated by 1.5% agarose gel electrophoresis. To quantitatively measure the amount of target gene mRNA, real-time RT–PCR analysis was performed using the iCycler (Bio-Rad, Hercules, CA, USA). The iQ™ SYBR Green Supermix PCR reagents (Bio-Rad) and results were evaluated with the iCycler iQ real-time detection system software. Briefly, the reaction mixture contained cDNA, 20 pmol of each primer and SYBR Green Supermix 2 [100 mM KCl, 40 mM Tris–HCl (pH 8.4), 0.4 mM each dNTP, 50 U/ml iTaq DNA polymerase, 6 mM MgCl2, SYBR Green I, 20 nM fluorescein and stabilizers]. Template was amplified with 40 cycles of denaturation at 95°C for 40 s, annealing at 60°C for 40 s and extension at 72°C for 40 s. Upon completion of PCR, fluorescence was monitored continuously while slowly heating the samples from 60°C to 95°C at 0.5°C intervals. These melting curves were used to identify any non-specific amplification products. Quantification of gene amplification was performed by determining the cycle threshold (CT), based on the fluorescence detected within the geometric region of the semi-log amplification plot. Relative quantification of target gene expression was evaluated using the comparative CT method.
Table I.
Primer sequences for real-time PCR analyzes.
| Gene | GeneBank accession number | Oligonucleotide sequence | Exon | Size (bp) |
|---|---|---|---|---|
| EDN1 | BC009720 | F- TCGTCCCTGATGGATAAAGA | exon2 ∼ exon3 | 205 |
| R- GGCAAAAATTCCAGCACTTC | ||||
| EDN2 | BC034393 | F- TCCTGAACAGACAGCTCCTT | exon2 ∼ exon8 | 242 |
| R- GAAATGTCCCTCAGCCTTTG | ||||
| EDN3 | BC053866 | F- CTATTGCCACCTGGACATCA | exon2 ∼ exon4 | 231 |
| R- GCCGTCCTTGAATTACTGCT | ||||
| ETA | BC022511 | F-AAGGAATGGCAGCTTGAGAA | exon6 ∼ exon9 | 300 |
| R-CAGAGGCATGACTGGAAACA | ||||
| ETB | BC014472 | F-TTTGCCTGGTCCTTGTCTTT | exon6 ∼ exon8 | 286 |
| R-AAGCACGACTGCTTTTCCTC | ||||
| ECE1 | BC117256 | F-AGCTCTTCTTCCTGGGCTTT | exon20 ∼ exon21 | 203 |
| R-TTACCAGACTTCGCACTTGT | ||||
| ECE2 | BC069005 | F-CTTCCCCAGTGCTTCTTTTG | exon2 ∼ exon3 | 207 |
| R-GCATAGTGTCTGGTCCGAAA | ||||
| β-Actin | NM_001101 | F-AGGCCAACCGCGAGAAGATGACC | exon1 | 311 |
| R-GAAGTCCAGGGCGACGTAGCAC | ||||
| GAPDH | NM_002046 | F-ACCACAGTCCATGCCATCAC | exon5 ∼ exon6 | 451 |
| R-TCCACCACCCTGTTGCTGTA |
GADPH, glyceraldehyde-3-phosphate dehydrogenase; EDN, endothelin; ECE, endothelin-converting enzyme; ET, endothelin receptor.
Immunohistochemistry
We retrospectively reviewed the slides from formalin-fixed, paraffin-embedded tissues of ovaries that were surgically resected between 2007 and 2009. Ovaries (n = 7) that were intact and exhibited no pathologic defects were selected for the immunohistochemical analysis. The patients were diagnosed with leiomyomas (n = 4), adenomyosis (n = 2) and contralateral ovary tumor (n = 1), and their age ranged from 42 to 48 years, with a mean age of 46 years at the time of ovarian resection. The ovaries were presumed to be functional (ovulating) as they all had various stages of follicles and healthy corpora lutea. Briefly, immunohistochemical staining was performed on deparaffinized sections, serially sectioned at 4 µm on poly-l-lysine covered slides using an LSAB detection kit (DAKO, Carpinteria, CA, USA) according to the manufacturer's instructions. Antigen retrieval was performed using Target Retrieval Solution (pH 8.0, S2367, DAKO) in an autoclave for 20 min at 120°C, then the endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. Slides were then incubated without or with primary antibodies that recognized smooth muscle actin alpha (αSMA, mouse monoclonal αsma-1, 1:50 dilution; Novocastra, Newcastle, UK), ETA (rabbit polyclonal, 1:200 dilution; GeneTex, Irvine, CA, USA) and ETB (rabbit polyclonal, 1:200 dilution; Abcam, Cambridge, UK). The chromogen was aminoethylcarbazole (DAKO) and the slides were counterstained with 10% Mayer's hematoxylin (Sigma, St Louis, MO, USA). The staining was examined using a BX51 microscope (Olympus, Japan) provided with a digital camera and pictures acquired using a DP-70 imaging system (Olympus).
Statistics
Data were presented as the mean ± SEM. Statistical analysis of real-time PCR data was evaluated using a one-way analysis of variance and Student's t-test. A value of P < 0.05 was considered as significant.
Results
Endocrine profiles of the study participants
The study subjects that participated in this study were 20 IVF patients who received treatment owing to tubal factors or male factors at the Bundang CHA Medical Center, Korea (see the inclusion criteria in the ‘Study subjects’ in Materials and Methods). The patients' serum hormone levels were all within the normal ranges (Nilsson et al., 1985): TSH (1.97 ± 0.24 µIU/ml), FSH (6.60 ± 0.45 mIU/ml), LH (5.43 ± 0.81 mIU/ml), prolactin (7.74 ± 0.72 ng/ml), E2 (18.06 ± 2.69 pg/ml), testosterone (0.28 ± 0.12 ng/ml) and DHEA-S (162.46 ± 30.52 µg/ml) (±SEM).
All endothelin components except EDN3 were expressed in the ovary
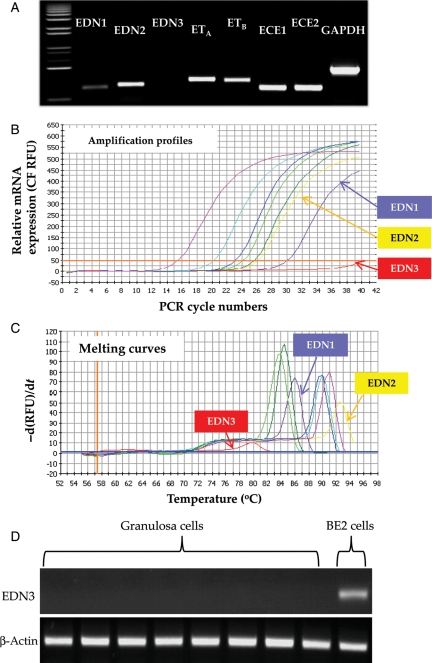
The relative mRNA level of each endothelin system component was first assessed using mRNA samples that were extracted from a patient's GC. A semi-quantitative RT–PCR analysis showed that transcripts of the entire endothelin system except EDN3 were present (Fig. 1A). Absence of the EDN3 transcript in GC was further verified by real-time PCR, in which no sign of EDN3 transcript was detected by either amplification or melting curve profiling analysis (Fig. 1B and C). Subsequent RT–PCR assay in eight randomly chosen patients' GC samples produced no EDN3 transcript, whereas amplification of EDN3 mRNA was apparent in the BE2 cell line (Moore and Johnson, 1998), an EDN3-expressing human neuronal cell line (Fig. 1D).
Figure 1.
Expression of endothelin system components in the GCs of a human pre-ovulatory follicle. (A) mRNA expression profiles of endothelin components in GCs obtained from a pre-ovulatory follicle. After 40 cycles of PCR amplifications, the PCR products were separated by electrophoresis on 1.2% agarose gel. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The real-time amplification profiles (B) and melting curves (C) of endothelin component mRNA. Purple, EDN1; yellow, EDN2; red, EDN3; yellowish green, ETA; green, ETB; sky blue, ECE1; blue, ECE2; pink, GAPDH. (D) EDN3 mRNA expression profiles in GC of eight different patients and BE2 cell line that constitutively expresses EDN3 mRNA. β-Actin was used as an internal control.
EDN2 is a predominant endothelin isoform in the GC
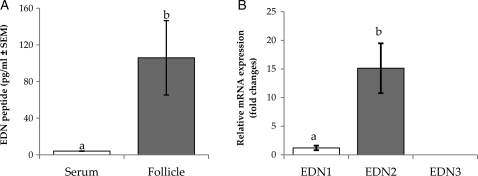
Follicular fluids from the largest follicle from each ovary were collected, free of blood contamination, at the time of oocyte retrieval and used to measure endothelin peptide levels by an immunometric method. The endothelin concentration in the follicular fluid (105.9 pg/ml) was significantly higher than in serum (4.1 pg/ml; Fig. 2A). Interestingly, the endothelin concentrations showed a wide range of variation among the follicular fluid samples (26.2–266.9 pg/ml), whereas a very narrow variation was seen in the serum samples (3.7–4.9 pg/ml). It should be noted that the immunometric method that was used for the assay could not separate the major endothelin peptide isoforms owing to cross-reactivity of the anti-endothelin antibody to both EDN1 and EDN2 peptides. The cross-reactivity of these antibodies is common, owing to the small sizes and high level of structural similarity of the endothelin isoforms (Janes and Wallace, 1994; Rubanyi and Polokoff, 1994). We, therefore, compared the mRNA levels of endothelin subtypes as an alternative way to determine a dominant isoform expressed by the GC. Real-time PCR followed by CT value analysis revealed an apparent dominant expression of EDN2 mRNA over EDN1 and EDN3 mRNA (Fig. 2B).
Figure 2.
Relative mRNA levels of endothelin subtypes in the GC and endothelin peptide levels of human pre-ovulatory follicles. (A) Endothelin peptide concentrations in the pre-ovulatory follicles (n = 6). The serum endothelin level was measured for reference. (B) Relative mRNA levels of endothelin subtypes calculated by using CT values from the real-time PCR (n = 8). The level of each mRNA was normalized with the corresponding β-actin transcript level. Data are the mean ± SEM, and different letters indicate significance at P < 0.05. The CT values of the EDN1 and EDN2 are 27.97 ± 0.58 and 24.38 ± 0.57, respectively.
GC expressed both forms of endothelin receptors and converting enzymes
Both forms of endothelin receptor (ETA and ETB) and endothelin-converting enzymes (ECE1 and ECE2) were readily detected in the GC (data not shown).
CC showed different expression patterns from GC
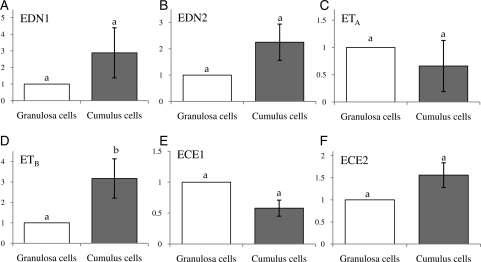
GC and CC are of the same origin and share a multitude of common characteristics. CCs however are unique in that they are physically associated with an oocyte in a follicle, regulate oocyte physiology and are released together with oocyte at the time of ovulation. We therefore compared the relative mRNA levels of the endothelin system components between GC and CC. Although not statistically significant, both EDN1 and EDN2 appeared to show higher levels in the CC than in the GC (Fig. 3A and B). Interestingly, although the ETA mRNA level was similar between the two cell types, the ETB mRNA level was significantly higher in the CC than the GC (Fig. 3C and D); no significant difference in the level of ECE1 and ECE2 mRNA was seen between these two cell types (Fig. 3E and F).
Figure 3.
Comparison of the endothelin component mRNA levels between human GCs and CCs. After real-time PCR, expression levels of each target gene in GCs and CCs were calculated from CT values, and the fold difference in the relative transcript level was determined against that of GC (n = 8). Data are the mean ± SEM, and different letters indicate significance at P < 0.05. Note a higher expression of ETB in the CC compared with GC.
No difference in expression of endothelin components between the CC of mature COC and immature COC
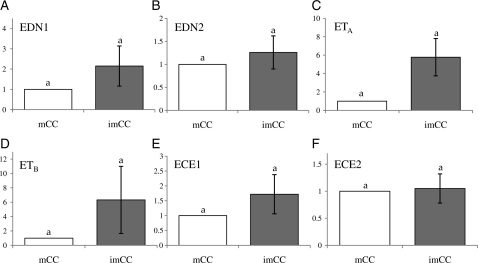
At the time of oocyte retrieval, COCs that had mature oocytes were separated from immature COCs and the CCs were isolated. Real-time PCR analysis showed no significant differences in any of the mRNA levels between the two different CCs (Fig. 4).
Figure 4.
Comparison of endothelin system component expression between human pre-ovulatory follicles with immature oocyte versus mature oocytes. Equal amounts of mRNAs isolated from human immature or mature CCs that were isolated from the same ovary were reverse transcribed and amplified using real-time PCR. The expression level was calculated from CT values, and the mRNA ratio was determined relative to that of mature CC (n = 7). Data are the mean ± SEM. Note that there was no difference in the mRNA level for any of the endothelin components.
Spatial localization of the smooth muscle network and endothelin receptor protein in the ovary
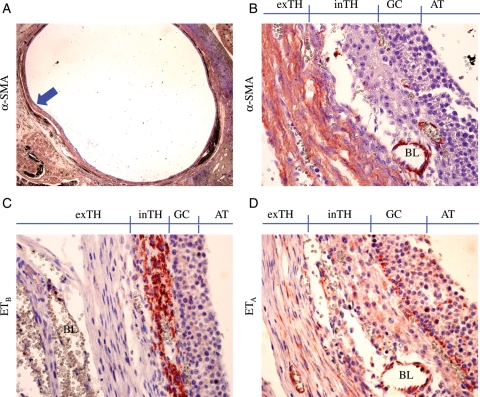
Localization of smooth muscle cells and ETA- and ETB-expressing cells were determined by immunohistochemistry using antibodies for αSMA, ETA and ETB, respectively. As was expected, αSMA antibody stained smooth muscle cells of the blood vessels localizing the interstitial as well as follicular vasculature of the human ovary (Fig. 5A). In addition, the same staining revealed multilayered ‘non-vascular’ αSMA-positive cells in the theca externa of antral follicles (Fig. 5B). No such ‘non-vascular’ αSMA-positive cell layer was seen in the pre-antral stage follicles (data not shown). Staining of adjacent sections of the ovary with endothelin receptor antibodies showed that the follicular smooth muscle cells expressed ETA, but not ETB (Fig. 5C and D). Interestingly, ETB protein expression was limited to the theca interna (Fig. 5C), whereas ETA expression was also apparent in the GC, theca interna and endothelia cells (Fig. 5D).
Figure 5.
Localization of αSMA, ETA and ETB protein in large antral follicle. Specific antibodies for αSMA (A and B), ETB (C) and ETA (D) proteins were used to stain adjacent sections of a normal human ovary followed by counter staining by Mayer's hematoxylin. The high magnification images (×400; B–D) were taken from the marked (arrow) area of (A) (×12.5). inTH, theca interna; exTH, theca externa; BL, blood vessel; AT, antrum. Note that ETB expression is specific to theca interna (C), whereas ETA expression is seen in the GC, theca interna and theca externa (D) where αSMA staining is most intense (B). No positive signal was seen in the negative controls (no primary antibody; not shown). These are representative images from one of the seven ovaries that had at least one healthy large follicle in each ovary.
Discussion
The ovulatory process, ending with rupture of the follicular wall and expulsion of the oocyte, is a central event in the reproductive cycle. Although a hypothesis was presented in the 1960s that increased intrafollicular pressure would be the driving force of follicle rupture, this concept was challenged by no success in recording an increase in intrafollicular pressure prior to follicle rupture and failure in inducing follicle rupture by artificially increasing intrafollicular pressure in rabbit follicles (Espey and Lipner, 1963; Rondell, 1964). These reports suggested a ‘decreasing tensile strength of the follicular wall’ as the main contributing factor in follicle rupture, which emphasized the importance of proteolytic enzymes and the plasminogen system (Curry and Osteen, 2003; Komar et al., 2001). In the early 1980s, Talbot and her colleagues revisited the concept of follicular contraction in follicle rupture (Martin and Talbot, 1981a,b), and the idea was further strengthened by the presence of smooth muscle layer around follicles (Schroeder and Talbot, 1982; Talbot and Chacon, 1982; this manuscript). In support of the constriction theory, we recently identified a potent vasoconstrictor, EDN2, that is produced by the pre-ovulatory follicle immediately prior to ovulation using a genome-wide ovarian gene expression profiling approach (Ko et al., 2006). This approach also showed the presence of endothelin receptors and converting enzymes in the ovary. As was expected, when applied to ovarian strips in vitro, EDN2 immediately induces a sustained constriction.
The presence of individual endothelin components in the human and primate ovary has been reported previously (Haq et al., 1996; Mancina et al., 1997; Apa et al., 1998; Karam et al., 1999; Korth et al., 1999), and their functionality determined in relation to regulating steroidogenesis in the follicle and corpus luteum (Apa et al., 1998; Usuki et al., 1998; Denkova et al., 2000; Meidan and Levy, 2007; Kawamura et al., 2009) and blood flow (Mancina et al., 1997; Usuki et al., 1998; Meidan and Levy, 2007). In the current study, we specifically aimed to assess the potential involvement of the endothelin system in controlling follicle rupture by characterizing the expression pattern of endothelin system components in the pre-ovulatory human ovary. The GCs, CCs and follicular fluids were collected from mature/dominant follicles 36 h post-hCG injection, a time point comparable with 11–12 h post-hCG injection in rodents when a pre-ovulatory follicle was about to rupture (Ko et al., 2006; Palanisamy et al., 2006; Na et al., 2008). Therefore, the data presented here represent the patterns of endothelin component expression when a pre-ovulatory follicle is temporally close to ovulation.
In general, the expression patterns of human endothelins, receptors and converting enzymes closely resembled those found in rodents. In complete accordance with the expression patterns of rodent counterparts, the human GC of pre-ovulatory follicles expressed mRNA for EDN1 and EDN2 but not EDN3 (Figs 1 and 2; Ko et al., 2006; Palanisamy et al., 2006; Na et al., 2008). As in rodents, EDN2 mRNA appeared dominant in comparison to EDN1 mRNA in these pre-ovulatory follicles (Fig. 2B) and is therefore believed to account for the majority of endothelin peptides detected in the follicular fluid. The human follicular fluidal concentration of endothelin peptides (105.9 pg/ml; Fig. 2) was significantly higher than that in serum (4.1 pg/ml) as was the ovarian level in the rat (68.0 pg/ml; Bridges et al., 2010). These high levels of follicular endothelin concentration in both rodents and human, and the similarity of the ovarian expression patterns for components of the endothelin system between rodents and human, may signify the importance of a conserved intra-ovarian role of the endothelin system across the species. Meanwhile, the wide range of variation of follicular endothelin peptide concentrations seen among the subjects of the present study (26.2–266.9 pg/ml; Fig. 2) indicates that follicular endothelin synthesis may be strictly restricted to the periovulatory period, as was seen in rodents (Ko et al., 2006; Palanisamy et al., 2006; Na et al., 2008): the low and high endothelin concentrations were likely from follicles temporally far from and close to the time point of maximal production. In line with this interpretation, Plonowski et al. (1999) also reported a wide range of endothelin concentrations in the follicular fluids obtained from hyperstimulated IVF patients (5–60 pmol/l). In regard to the effect of hCG on the endothelin peptide level, it was previously shown that that endothelin concentrations were higher in the follicular fluids of hCG-injected than non-injected subjects (1.29 pg/ml before hCG versus 4.85 pg/ml after hCG; Magini et al., 1996; Plonowski et al., 1999). In fact, the bioactive forms of endothelin peptides have a very short half-life and therefore are locally produced immediately prior to use (Meidan and Levy, 2007). Meanwhile, a high variance is seen in the absolute values of the follicular fluidal endothelin concentrations reported by different laboratories (Kamada et al., 1993; Schiff et al., 1993; Magini et al., 1996; Plonowski et al., 1999). Although the reason for this variation is not known, it is likely related to differences in the sensitivity or specificity of the individual assays or other procedural differences employed for the individual measurements.
Similar to GCs, CCs expressed all the components of the endothelin system except EDN3 (Fig. 3). Interestingly, although mRNA for each receptor subtype was expressed in both cell types, abundance of the receptor subtype transcripts differed in the two cell types: while the ETA mRNA levels were similar in GC and CC, the ETB mRNA level was higher in CC than GC (Fig. 3). The physiological significance of the difference is not known and awaits further investigation. Nonetheless, endothelin receptors expressed in the GC and CC would not mediate the constrictive action of endothelin but may regulate cell proliferation, steroidogenesis or oocyte maturation, as reported previously (Tedeschi et al., 1994; Kamada et al., 1995; Calogero et al., 1998; Denkova et al., 2002; Meidan and Levy, 2007; Kawamura et al., 2009). In this regard, we were interested in whether CCs that were retrieved with mature oocytes showed a different expression pattern of endothelin components compared with CCs that were with immature oocytes. Real-time PCR analyses, however, found no difference between the two groups (Fig. 4), indicating that the endothelin system was not a contributing factor for the oocyte immaturity in these cells. These data must be interpreted with caution, however, as no difference in the expression of components of the endothelin system in these two groups does not mean that endothelin action is not important for oocyte maturation or other known endothelin functions in the follicle.
The presence of smooth muscle-like theca externa cells was previously reported in the ovaries of diverse species and is believed to be responsible for the demonstrated constrictive responses to endothelin treatment (O'Shea, 1970; Amsterdam et al., 1977; Ko et al., 2006). In the present study, immunostaining of human ovarian sections with anti-αSMA and endothelin receptor antibodies localized the expression of ETA in these non-vascular αSMA-positive cells of the theca externa, whereas ETB expression was specifically localized to theca interna (Fig. 5). This tissue-type-dependent localization of endothelin receptor expression is suggestive of endothelin-mediated contractile regulation of follicles. Vasoconstriction is well documented in blood vessels, in which ETA is expressed in smooth muscle cells, whereas ETB is expressed in the endothelial cells and responsible for vasodilation (Magini et al., 1996; Karam et al., 1999; Boss et al., 2002; Masaki, 2004). In regard to the ETB expression pattern in the GC, a large discrepancy was seen between mRNA and protein expression. As was shown in Figs 1 and 3, ETB mRNA expression is apparent in the GC of pre-ovulatory follicles but ETB protein was not detectable in GC of the ovarian tissues that we examined (Fig. 5). The reason for this apparent discrepancy between mRNA and protein expression patterns is currently unknown. It would, however, be interesting to determine whether the translation of ETB protein is temporally controlled, in that ETB protein synthesis is limited to the periovulatory period.
In summary, the present study demonstrates the presence of the entire endothelin system (minus EDN3) and smooth muscle network in human periovulatory follicles, suggesting the involvement of endothelin-induced contractile action in regulating ovulation in the human ovary, as was proposed by recent animal studies (Ko et al., 2006; Al-Alem et al., 2007; Bridges et al., 2010).
Authors' roles
D-H.C.: experimental design, patient treatment, tissue collection and manuscript writing; E.K.K.: experimental design, patient selection, tissue handling and endothelin peptide measurement; K-H.K.: RNA extraction, RT–PCR, real-time PCR and manuscript writing; D-W.K.: immunostaining for localization of endothelin receptor and smooth muscle protein expression; H.Y.K.: reagent preparation and antibody testing; P.B.: experimental design and endothelin peptide measurement; C.K.: project leader, coordination of team works, experimental planning, data interpretation and manuscript writing; K-A.L.: experimental design and manuscript writing.
Funding
This work was supported by the NIH (R01HD052694 to C.K.) and the grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084923 to D-H.C. and K-A.L.).
References
- Abberton KM, Healy DL, Rogers PA. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod. 1999;14:3095–3100. doi: 10.1093/humrep/14.12.3095. [DOI] [PubMed] [Google Scholar]
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Lindner HR, Groschel-Stewart U. Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Rec. 1977;187:311–328. doi: 10.1002/ar.1091870304. [DOI] [PubMed] [Google Scholar]
- Apa R, Miceli F, de Feo D, Pierro E, Ayala G, Mancuso S, Napolitano M, Lanzone A. Endothelin-1: expression and role in human corpus luteum. Am J Reprod Immunol. 1998;40:370–376. doi: 10.1111/j.1600-0897.1998.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- Boss C, Bolli M, Weller T. Endothelin receptor antagonists: structures, synthesis, selectivity and therapeutic applications. Curr Med Chem. 2002;9:349–383. doi: 10.2174/0929867023371139. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al-Alem L, Na G, Su W, Gong MC, Jeoung M, Ko C. Production and binding of endothelin-2 (EDN2) in the rat ovary: Endothelin subtype A (EDNRA) mediated contraction. Reprod Fertil Dev. 2010;22:780–787. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Burrello N, Ossino AM. Endothelin (ET)-1 and ET-3 inhibit estrogen and cAMP production by rat granulosa cells in vitro. J Endocrinol. 1998;157:209–215. doi: 10.1677/joe.0.1570209. [DOI] [PubMed] [Google Scholar]
- Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:62S–67S. doi: 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- Denkova R, Bourneva V, Baleva K, Yaneva E, Nikolov B, Christov I, Ivanov I. Modulation of steroidogenesis in human ovarian granulosa cells during aging. Endocr Regul. 2000;34:157–160. [PubMed] [Google Scholar]
- Denkova R, Bourneva V, Yaneva E, Baleva K, Nikolov B, Ivanov I, Simeonov K, Timeva T. Potential role of nitric oxide in endothelin-1 provoked inhibition of progesterone secretion by isolated ovarian granulosa cells. Endocr Regul. 2002;36:19–22. [PubMed] [Google Scholar]
- Espey LL. Ovarian contractility and its relationship to ovulation: a review. Biol Reprod. 1978;19:540–551. doi: 10.1095/biolreprod19.3.540. [DOI] [PubMed] [Google Scholar]
- Espey LL, Lipner H. Measurements of intrafollicular pressures in the rabbit ovary. Am J Physiol. 1963;205:1067–1072. doi: 10.1152/ajplegacy.1963.205.6.1067. [DOI] [PubMed] [Google Scholar]
- Haq A, Kayali M, Hammami MM, Jaroudi K, al-Sedairy ST. Immunoreactive endothelin-1, endothelin-2 and big endothelin-1 in follicular fluids of women undergoing ovulation induction for in-vitro fertilization. Hum Reprod. 1996;11:269–273. doi: 10.1093/humrep/11.2.269. [DOI] [PubMed] [Google Scholar]
- Janes RW, Wallace BA. Modelling the structures of the isoforms of human endothelins based on the crystal structure of human endothelin-I. Biochem Soc Trans. 1994;22:1037–1043. doi: 10.1042/bst0221037. [DOI] [PubMed] [Google Scholar]
- Jeoung M, Lee S, Hawng HK, Cheon YP, Jeong YK, Gye MC, Iglarz M, Ko C, Bridges PJ. Identification of a novel role for endothelins within the oviduct. Endocrinology. 2010;151:2858–2867. doi: 10.1210/en.2009-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada S, Kubota T, Taguchi M, Aso T. High levels of immunoreactive endothelin-1 in human follicular fluids. Hum Reprod. 1993;8:674–677. doi: 10.1093/oxfordjournals.humrep.a138118. [DOI] [PubMed] [Google Scholar]
- Kamada S, Blackmore PF, Kubota T, Oehninger S, Asada Y, Gordon K, Hodgen GD, Aso T. The role of endothelin-1 in regulating human granulosa cell proliferation and steroidogenesis in vitro. J Clin Endocrinol Metab. 1995;80:3708–3714. doi: 10.1210/jcem.80.12.8530623. [DOI] [PubMed] [Google Scholar]
- Karam H, Valdenaire O, Belair MF, Prigent-Sassy C, Rakotosalama A, Clozel M, Itskovitz J, Bruneval P. The endothelin system in human and monkey ovaries: in situ gene expression of the different components. Cell Tissue Res. 1999;295:101–109. doi: 10.1007/s004410051216. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Ye Y, Liang CG, Kawamura N, Gelpke MS, Rauch R, Tanaka T, Hsueh AJ. Paracrine regulation of the resumption of oocyte meiosis by endothelin-1. Dev Biol. 2009;327:62–70. doi: 10.1016/j.ydbio.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Komar CM, Matousek M, Mitsube K, Mikuni M, Brannstrom M, Curry TE., Jr Effects of genistein on the periovulatory expression of messenger ribonucleic acid for matrix metalloproteinases and tissue inhibitors of metalloproteinases in the rat ovary. Reproduction. 2001;121:259–265. doi: 10.1530/rep.0.1210259. [DOI] [PubMed] [Google Scholar]
- Korth P, Bohle RM, Corvol P, Pinet F. Cellular distribution of endothelin-converting enzyme-1 in human tissues. J Histochem Cytochem. 1999;47:447–462. doi: 10.1177/002215549904700403. [DOI] [PubMed] [Google Scholar]
- Magini A, Granchi S, Orlando C, Vannelli GB, Pellegrini S, Milani S, Grappone C, De Franco R, Susini T, Forti G, et al. Expression of endothelin-1 gene and protein in human granulosa cells. J Clin Endocrinol Metab. 1996;81:1428–1433. doi: 10.1210/jcem.81.4.8636346. [DOI] [PubMed] [Google Scholar]
- Mancina R, Barni T, Calogero AE, Filippi S, Amerini S, Peri A, Susini T, Vannelli GB, Burrello N, Forti G, et al. Identification, characterization, and biological activity of endothelin receptors in human ovary. J Clin Endocrinol Metab. 1997;82:4122–4129. doi: 10.1210/jcem.82.12.4447. [DOI] [PubMed] [Google Scholar]
- Martin GG, Talbot P. The role of follicular smooth muscle cells in hamster ovulation. J Exp Zool. 1981a;216:469–482. doi: 10.1002/jez.1402160316. [DOI] [PubMed] [Google Scholar]
- Martin GG, Talbot P. Drugs that block smooth muscle contraction inhibit in vivo ovulation in hamsters. J Exp Zool. 1981b;216:483–491. doi: 10.1002/jez.1402160317. [DOI] [PubMed] [Google Scholar]
- Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–385. doi: 10.1016/j.tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Moore SW, Johnson AG. Hirschsprung's disease: genetic and functional associations of Down's and Waardenburg syndromes. Semin Pediatr Surg. 1998;7:156–161. doi: 10.1016/s1055-8586(98)70011-3. [DOI] [PubMed] [Google Scholar]
- Murakoshi N, Miyauchi T, Kakinuma Y, Ohuchi T, Goto K, Yanagisawa M, Yamaguchi I. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodeling after injury revealed by endothelin-B receptor-knockout mice. Circulation. 2002;106:1991–1998. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- Na G, Bridges PJ, Koo Y, Ko C. Role of hypoxia in the regulation of periovulatory EDN2 expression in the mouse. Can J Physiol Pharmacol. 2008;86:310–319. doi: 10.1139/y08-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakas-Icindic E, Zaciragic A, Hadzovic A, Avdagic N. Endothelin in health and disease. Bosn J Basic Med Sci. 2004;4:31–34. doi: 10.17305/bjbms.2004.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Wikland M, Hamberger L, Hillensjo T, Chari S, Sturm G, Daume E. Simplification of the method of in vitro fertilization: sonographic measurements of follicular diameter as the sole index of follicular maturity. J In Vitro Fert Embryo Transf. 1985;2:17–22. doi: 10.1007/BF01130827. [DOI] [PubMed] [Google Scholar]
- Olivennes F, Fanchin R, Bouchard P, de Ziegler D, Taieb J, Selva J, Frydman R. The single or dual administration of the gonadotropin-releasing hormone antagonist Cetrorelix in an in vitro fertilization-embryo transfer program. Fertil Steril. 1994;62:468–476. doi: 10.1016/s0015-0282(16)56933-7. [DOI] [PubMed] [Google Scholar]
- O'Shea JD. An ultrastructural study of smooth muscle-like cells in the theca externa of ovarian follicles in the rat. Anat Rec. 1970;167:127–131. doi: 10.1002/ar.1091670202. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Plonowski A, Kaplinski AP, Radzikowska M, Borowiec M, Baranowska B. Correlation between 21 amino acid endothelin, intrafollicular steroids and follicle size in stimulated cycles. Hum Reprod. 1999;14:2323–2327. doi: 10.1093/humrep/14.9.2323. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- Rondell P. Follicular pressure and distensibility in ovulation. Am J Physiol. 1964;207:590–594. doi: 10.1152/ajplegacy.1964.207.3.590. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- San Roman GA, Surrey ES, Judd HL, Kerin JF. A prospective randomized comparison of luteal phase versus concurrent follicular phase initiation of gonadotropin-releasing hormone agonist for in vitro fertilization. Fertil Steril. 1992;58:744–749. doi: 10.1016/s0015-0282(16)55322-9. [DOI] [PubMed] [Google Scholar]
- Schiff E, Alcalay M, Peleg E, Rosenthal T, Mashiach S, Dor J. Immunoreactive endothelins in human follicular fluid. Gynecol Obstet Invest. 1993;36:12–14. doi: 10.1159/000292585. [DOI] [PubMed] [Google Scholar]
- Schroeder PC, Talbot P. Intrafollicular pressure decreases in hamster preovulatory follicles during smooth muscle cell contraction in vitro. J Exp Zool. 1982;224:417–426. doi: 10.1002/jez.1402240315. [DOI] [PubMed] [Google Scholar]
- Sitbon O, Badesch DB, Channick RN, Frost A, Robbins IM, Simonneau G, Tapson VF, Rubin LJ. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary arterial hypertension: a 1-year follow-up study. Chest. 2003;124:247–254. doi: 10.1378/chest.124.1.247. [DOI] [PubMed] [Google Scholar]
- Talbot P, Chacon RS. In vitro ovulation of hamster oocytes depends on contraction of follicular smooth muscle cells. J Exp Zool. 1982;224:409–415. doi: 10.1002/jez.1402240314. [DOI] [PubMed] [Google Scholar]
- Tedeschi C, Lohman C, Hazum E, Ittoop O, Ben-Shlomo I, Resnick CE, Payne DW, Adashi EY. Rat ovarian granulosa cell as a site of endothelin reception and action: attenuation of gonadotropin-stimulated steroidogenesis via perturbation of the A-kinase signaling pathway. Biol Reprod. 1994;51:1058–1065. doi: 10.1095/biolreprod51.5.1058. [DOI] [PubMed] [Google Scholar]
- Tur-Kaspa I, Ezcurra D. GnRH antagonist, cetrorelix, for pituitary suppression in modern, patient-friendly assisted reproductive technology. Expert Opin Drug Metab Toxicol. 2009;5:1323–1336. doi: 10.1517/17425250903279969. [DOI] [PubMed] [Google Scholar]
- Usuki S, Kobayashi S, Sugimoto M, Kotani E, Otani S, Kubo T, Ishii T, Murakami K, Miyazaki H. Effects of follicle-stimulating hormone on endothelin receptors in cultured rat ovarian granulosa cells. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S225–S229. doi: 10.1097/00005344-199800001-00063. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci USA. 1988a;85:6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988b;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]