Abstract
The diagnosis of nonalcoholic steatohepatitis (NASH) is defined by the presence and pattern of specific histological abnormalities on liver biopsy. A separate system of scoring the features of nonalcoholic fatty liver disease (NA) called the NAFLD Activity Score (NAS) was developed as a tool to measure changes in NAFLD during therapeutic trials. However, some studies have used threshold values of the NAS, specifically NAS ≥ 5, as a surrogate for the histologic diagnosis of NASH. To evaluate whether this unintended use of the NAS is valid, biopsy and clinical data from the 976 adults in NASH CRN studies were reviewed. Biopsies were evaluated centrally by the NASH CRN Pathology Committee. Definite steatohepatitis (SH) was diagnosed in 58.1%, borderline SH in 19.5% and “not SH” in 22%. The NAS was ≥ 5 in 50% and ≤ 4 in 49%; in this cohort only 75% of biopsies with definite SH had a NAS ≥ 5, while 28% of borderline SH and 7% of "not SH" biopsies had NAS ≥ 5. Of biopsies with a NAS ≥ 5, 86% had SH and 3% "not SH". NAS ≤ 4 did not indicate benign histology; 29% had SH and only 42% had "not SH". Higher values of the NAS were associated with higher levels of ALT and AST, while the diagnosis of SH was associated with features of the metabolic syndrome.
Conclusions
The diagnosis of definite SH or the absence of SH based on evaluation of patterns as well as individual lesions on liver biopsies does not always correlate with threshold values of the semiquantitative NAS. Clinical trials and observational studies should take these different performance characteristics into account.
Keywords: NAS, NAFLD, NASH, histopathology, clinical correlations
Introduction
The diagnosis of NASH is established by the presence of a characteristic pattern of steatosis, inflammation and hepatocellular ballooning on liver biopsies in the absence of significant alcohol consumption. The value of establishing a diagnosis of NASH is that it identifies individuals who are at risk for progressive liver disease to the point of cirrhosis and death from chronic liver disease. However, the dichotomous assessment of liver biopsies as either having steatohepatitis or not is less helpful in treatment trials of therapeutic agents to improve NASH because it cannot identify patients in whom NASH significantly lessened in severity with treatment but continued to fulfill diagnostic criteria for NASH. For this reason, a scoring system was needed that included the full spectrum of nonalcoholic fatty liver disease and would be sensitive to changes in the underlying disease process independent of the diagnosis of NASH.
To meet this need, a scoring system for nonalcoholic fatty liver disease (NAFLD) was developed and validated by the NIDDK sponsored Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) Pathology Committee1. The methodology proposed for feature-based scoring of histologic lesions of NAFLD has been widely utilized, as evidenced by its application in numerous clinical and experimental settings in NAFLD-related studies. The recognized strengths of the method include the relative ease of understanding and therefore, application of the system; division of lesions of active and potentially reversible injury (“grade”) in the NAFLD Activity Score (NAS) and those potentially less reversible and characterized by collagen deposition and architectural alterations that may evolve toward more permanent parenchymal remodeling (“stage”). The proposed NAS also clearly separates the three lesions that comprise grade: steatosis, lobular inflammation, and ballooning. This allows detailed analysis of histologic changes for comparative and correlative studies in therapeutic intervention trials.
The histologically-based NAS was derived from 10 pathologists’ blinded and individual readings of biopsies from 32 adults and 18 children with clinically presumed NAFLD. The adult biopsies were read twice, and the pediatric biopsies once by each pathologist. It was noted in the publication of the validation study that the numeric scores correlated closely but not perfectly with separately derived diagnoses of “definite steatohepatitis (SH)”, “not SH” and “borderline SH”.
It is, however, increasingly apparent from ongoing and published studies that the numeric value of the composite NAS is considered by some investigators to be either “synonymous” with, or actually a replacement for, a microscopic diagnosis that is based on overall pattern of injury as well as the presence of additional lesions such as zonality of lesions, portal inflammation and fibrosis.2 The validity of this unintended use of the NAS has not been formally evaluated.
In order to objectively assess the relationships of the NAS, the diagnosis of SH, and important clinical characteristics of NAFLD, we availed ourselves of the large, well characterized dataset from the NASH CRN. We demonstrate that the NAS and the diagnostic category of definite SH are closely correlated, but also have distinct clinico-pathologic relationships. The study further highlights that not all biopsies with NAS ≥ 5 have findings that meet diagnostic criteria of definite SH, and that some cases of NAS ≤ 4 do, indicating that the a threshold value of a NAS > 5 cannot be used reliably to establish the presence or absence of NASH.
Materials and Methods
Biopsies from adult patients enrolled in either the Database study or pretreatment biopsies from the adult treatment trial (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with NASH) (PIVENS) were reviewed in a standardized blinded fashion by the Pathology Committee of the NASH CRN, composed of a pathologist from each of the 8 clinical centers, and one from the National Cancer Institute. Assignment of a diagnostic category was based on consensus recognition of the distinctive features of SH independent of the degree of NAFLD severity as indicated by the NAS. Biopsies that had been classified by the Pathology Committee during Central Review as “cirrhosis with or without features of NAFLD or NASH” were excluded from this analysis, as it is well recognized that the active lesions of steatohepatitis may not be retained in cirrhosis. Biopsies with the zone 1 borderline pattern were also excluded as this is a pattern that most commonly occurs in pediatric NAFLD and was rare among our adult cases. When more than one biopsy for a subject was available, only the first biopsy was used in the analysis. Histologic and clinical data were analyzed as described below.
Histologic Data
The following histologic data were analyzed: diagnosis rendered by the Pathology Committee (i.e. “not steatohepatitis”, “borderline, zone 3 pattern”, “definite steatohepatitis”); the aggregate NAS; the score of each component of the NAS (steatosis (0–3), lobular inflammation (0–3), ballooning (0–2)), and fibrosis scores (0,1a,1b,1c,2,3). In addition, portal chronic inflammation and steatosis location were included. The "borderline zone 3 pattern" was reserved for biopsies that have zone 3 accentuation of lesions, but not all the lesions for definite steatohepatitis were present. This definition is purposely left broad so as to neither preclude further evaluation nor include the biopsies in the "not SH" category.
Clinical Data
Clinical data obtained at baseline were used in the analyses, and laboratory measures were limited to those values within 6 months of the biopsy for each subject. From the dataset, the following were analyzed: demographic features (age at biopsy, gender, race, ethnicity), BMI, and laboratory values including ALT, AST, fasting serum glucose and insulin, ANA, and triglycerides. Calculations were performed to derive presence or absence of criteria for Metabolic Syndrome3, HOMA-IR, and QUICKI. QUICKI is an inverse log transformation of HOMA, and has been found to be linearly related to formal clamp measures of IR4.
Statistical analyses
Chi-square tests were used to compare univariate associations of categorical variables with NASH diagnosis (not SH, borderline SH, definite SH) and with NAS (≤ 4 vs. ≥ 5). Fisher’s Exact test was used for categorical variables with small expected numbers. Continuous variables were compared to NASH diagnosis (3 categories) using ANOVA for normally distributed variables (age at biopsy and BMI). The nonparametric Kruskal-Wallis test was used to compare laboratory measures with the three-category NASH diagnosis, and the Wilcoxon rank sum test was used for the binary NAS (≤ 4 vs. ≥ 5). Also examined were the associations between patient characteristics (demographics and laboratory measures) and diagnosis, within categories of NAS (≤ 4 vs. ≥ 5). Components of the NAS (steatosis, lobular inflammation, and ballooning) were excluded from statistical analysis when making comparisons to the binary NAS variable (≤ 4 vs. ≥ 5). Methods for evaluating a diagnostic test (sensitivity, specificity, percent agreement, and Cohen's kappa statistic) were used to evaluate the information loss in a NAS cutoff (≥5 vs. ≤4 ) as a surrogate for the histological diagnosis of steatohepatitis (definite SH vs. borderline/not SH).
Univariate regression analyses were performed to assess the individual associations between the NAS (≥ 5 vs. ≤ 4) and select patient characteristics. These results were compared to the univariate regression analyses of SH diagnosis and these same patient characteristics. Outcome measures included: ALT (U/L), AST (U/L), diabetes, metabolic syndrome, HOMA-IR, and QUICKI. Linear regression was used for continuous outcome measures (ALT, AST, HOMA-IR, and QUICKI) and logistic regression was used for binary outcome measures (diabetes and metabolic syndrome). Multivariable regression analyses were used to assess the independent association between diagnosis of definite SH and these same patient characteristics, controlling for the NAS. That is, both the binary NAS variable and SH diagnosis were included as covariates in the models. The β coefficients (for continuous outcomes), odds ratios (for binary outcomes), 95% confidence intervals, and p-values were compared for each model.
Nominal, two-sided P-values were used and were considered to be statistically significant if P<0.05; no adjustments for multiple comparisons were made. Analyses were performed using SAS statistical software (version 9; SAS Institute Inc., Carey, NC) and Stata (Release 10; Stata Corp., College Station, TX).
Results
Comparison of Demographic and Clinical Characteristics across NASH Diagnostic Categories
Data from a total of 934 adult liver biopsies without cirrhosis or the “zone 1 borderline” diagnosis were available for this analysis. The diagnoses were “not steatohepatitis” in 208 (22%), “borderline steatohepatitis” in 183 (20%) and “definite steatohepatitis” in 543 (58%). Table 1 highlights clinical correlates in these categories. Definite steatohepatitis (SH) was observed in a higher proportion of women (p=0.004) and tended to be seen in older individuals (p=0.05). There were no significant differences in the distribution of diagnostic categories amongst races, but Hispanics had proportionally fewer borderline cases (p=0.03). BMI did not differ among the diagnostic categories. Definite steatohepatitis was associated with higher serum ALT, triglycerides, insulin, and calculated HOMA-IR, lower QUICKI, as well as a higher frequency of diabetes. No association was found with presence or absence of serum ANA or serum fasting glucose. Thus, the most important clinical correlations conventionally associated with steatohepatitis, ie, older age, female gender, evidence of insulin resistance, and elevated ALT,5 were confirmed by blinded analysis of the biopsies for diagnostic category, regardless of the NAS.
Table 1.
Baseline characteristics by diagnosis*
| Not Steatohepatitis |
Borderline Steatohepatitis |
Definite Steatohepatitis |
Total | P† | |
|---|---|---|---|---|---|
| N | 208 | 183 | 543 | 934 | |
| Age at biopsy-yrs (range) | 47.0 (18.2–78.5) |
46.7 (19.1–68.6) |
48.7 (18.3–75.0) |
47.9 (18.2–78.5) |
0.05 |
| Female gender | 115 (55.3) | 102 (55.7) | 360 (66.3) | 577 (61.8) | 0.004 |
| Race | 0.14 | ||||
| White | 168 (83.6) | 147 (82.6) | 439 (84.4) | 754 (83.9) | |
| Black | 7 (3.5) | 7 (3.9) | 14 (2.7) | 28 (3.1) | |
| Asian or Pacific Islander | 11 (5.5) | 17 (9.6) | 25 (4.8) | 53 (5.9) | |
| American Indian or AK Native | 9 (4.5) | 1 (0.6) | 18 (3.5) | 28 (3.1) | |
| More than 1 race | 6 (3.0) | 6 (3.4) | 24 (4.6) | 36 (4.0) | |
| Hispanic/Latino | 28 (13.5) | 12 (6.6) | 75 (13.8) | 115 (12.3) | 0.03 |
| BMI (kg/m2) | 33.8 ± 6.2 | 33.8 ± 6.8 | 34.4 ± 6.3 | 34.2 ± 6.4 | 0.37 |
| Diabetes (Type 2) | 38 (18.3) | 37 (20.2) | 170 (31.3) | 245 (26.2) | <0.001 |
| ALT (U/L)‡ | 51 (33–73) | 67 (38–91) | 77 (51–116) | 67 (46–98) | <0.0001 |
| Triglycerides (mg/dL) | 144 (104–194) | 151 (104–208) | 159 (115–238) | 157 (110–219) | 0.02 |
| Glucose (mg/dL)‡ | 96 (87–107) | 93 (85–104) | 96 (85–110) | 95 (85–108) | 0.34 |
| Insulin (µU/mL)‡ | 15.4 (10.0–20.8) | 17.5 (11.8–24.0) | 20.5 (13.1–30.0) | 18.3 (12.1–28.2) | <0.0001 |
| HOMA-IR‡ | 3.5 (2.3–5.3) | 4.3 (2.6–6.2) | 4.9 (3.1–7.6) | 4.3 (2.8–7.0) | <0.0001 |
| QUICKI‡ | 0.319 ± 0.029 | 0.313 ± 0.029 | 0.307 ± 0.034 | 0.311 ± 0.032 | 0.001 |
| ANA positive‡ | 33 (25.4) | 20 (17.1) | 87 (23.8) | 140 (22.9) | 0.24 |
Values are N (%), means ± SD, or medians (IQR), unless otherwise specified.
P-values derived from chi-square tests for categorical variables (Fisher’s Exact test when expected numbers were small), from ANOVA for age at biopsy and BMI, and from the Kruskal-Wallis Test for laboratory measures.
Only laboratory values collected within 6 months of the liver biopsy were included. N=573 for ALT and glucose measurements; N=577 for triglycerides; N=563 for insulin and HOMA-IR measurements; N=612 for ANA. HOMA-IR is the homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (µU/mL)*fasting glucose (mmol/L))/22.5. QUICKI is calculated as: 1/log(insulin µU/mL*glucose mg/dL).
Comparison of Demographic and Clinical Characteristics by NAS Category
Table 2 shows the results of similar comparisons utilizing the subsets of NAS ≤ 4 (n=461) and NAS ≥ 5 (n=473). The biopsies in the higher NAS category were associated with female gender, as well as elevated ALT, triglycerides, insulin, and HOMA-IR, and lower QUICKI values. There was a trend of association of the higher NAS with diabetes (p=0.05), but this categorization of low versus high NAS was not associated with age, race, ethnicity, BMI, serum fasting glucose, or presence of positive ANA.
Table 2.
Baseline characteristics by NAS*
| NAS ≤4 | NAS ≥5 | Total | P† | |
|---|---|---|---|---|
| N | 461 | 473 | 934 | |
| Age at biopsy - yrs (range) | 47.7 (18.3–78.5) |
48.1 (18.2–72.5) |
47.9 (18.2–78.5) |
0.56 |
| Female gender | 257 (55.8) | 320 (67.7) | 577 (61.8) | 0.0002 |
| Race | 0.74 | |||
| White | 368 (82.7) | 386 (85.0) | 754 (83.9) | |
| Black | 16 (3.6) | 12 (2.6) | 28 (3.1) | |
| Asian or Pac. Islander | 30 (6.7) | 23 (5.1) | 53 (5.9) | |
| Am Indian or AK Native | 13 (2.9) | 15 (3.3) | 28 (3.1) | |
| More than 1 race | 18 (4.0) | 18 (4.0) | 36 (4.0) | |
| Hispanic/Latino | 52 (11.3) | 63 (13.3) | 115 (12.3) | 0.34 |
| BMI (kg/m2) | 34.0 ± 6.5 | 34.3 ± 6.3 | 34.2 ± 6.4 | 0.51 |
| Diabetes (Type 2) | 108 (23.4) | 137 (29.0) | 245 (26.2) | 0.05 |
| ALT (U/L)‡ | 58 (36–78) | 82 (53–121) | 67 (46–98) | <0.0001 |
| Triglycerides (mg/dL) | 149 (105–202) | 161 (115–239) | 157 (110–219) | 0.007 |
| Glucose (mg/dL)‡ | 95 (86–107) | 96 (84–110) | 95 (85–108) | 0.85 |
| Insulin (µU/mL)‡ | 17.0 (11.5–24.0) | 20.1 (13.0–30.0) | 18.3 (12.1–28.2) | 0.001 |
| HOMA-IR‡ | 3.9 (2.6–6.3) | 4.8 (3.1–7.5) | 4.3 (2.8–7.0) | 0.004 |
| QUICKI‡ | 0.314 ± 0.032 | 0.307 ± 0.033 | 0.311 ± 0.032 | 0.008 |
| ANA positive‡ | 74 (24.8) | 66 (21.0) | 140 (22.9) | 0.26 |
Values are N (%), means ± SD, or medians (IQR), unless otherwise specified.
P-values derived from chi-square tests for categorical variables (Fisher’s Exact test when expected numbers were small), from ANOVA for age at biopsy and BMI, and from the Kruskal-Wallis Test for laboratory measures.
Only laboratory values collected within 6 months of the liver biopsy were included. N=573 for ALT and glucose measurements; N=577 for triglycerides; N=563 for insulin and HOMA-IR measurements; N=612 for ANA. HOMA-IR is the homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (µU/mL)*fasting glucose (mmol/L))/22.5. QUICKI is calculated as: 1/log(insulin µU/mL*glucose mg/dL).
Comparison of Histology and Diagnostic Category. (Table 3)
Table 3.
Histology by Steatohepatitis
| Histology variable* | No steatohepatitis (n=208) |
Borderline (n=183) |
Definite steatohepatitis (n=543) |
Total (n=934) |
P |
|---|---|---|---|---|---|
| NAS | < 0.0001 | ||||
| ≤ 4 | 194 (93.3) | 131 (71.6) | 136 (25.1) | 461 (49.4) | |
| ≥ 5 | 14 (6.6) | 52 (28.4) | 407 (75.0) | 473 (50.6) | |
| Steatosis grade | <0.0001 | ||||
| 0 - <5% | 24 (11.5) | 4 (2.7) | 8 (1.5) | 37 (4.0) | |
| 1 - 5–33% | 106 (51.0) | 68 (37.2) | 175 (32.2) | 349 (37.4) | |
| 2 - 34–66% | 49 (23.6) | 56 (30.6) | 201 (37.0) | 306 (32.8) | |
| 3 – > 66% | 29 (13.9) | 54 (29.5) | 159 (29.3) | 242 (25.9) | |
| Steatosis location | <0.0001 | ||||
| 0 – Zone 3 | 105 (51.0) | 86 (47.0) | 200 (36.8) | 391 (42.0) | |
| 1 – Zone 1 | 5 (2.4) | 1 (0.6) | 2 (0.4) | 8 (0.9) | |
| 2 - Azonal | 51 (24.8) | 36 (19.7) | 140 (25.8) | 227 (24.4) | |
| 3 – Panancinar | 45 (21.8) | 60 (32.8) | 201 (37.0) | 306 (32.8) | |
| Lobular inflammation | <0.0001 | ||||
| 0 - none | 3 (1.4) | 0 (0.0) | 0 (0.0) | 3 (0.4) | |
| 1 - <2 | 167 (80.3) | 104 (56.8) | 190 (35.0) | 461 (49.4) | |
| 2 - 2–4 | 36 (17.3) | 70 (38.3) | 261 (48.1) | 367 (39.3) | |
| 3 - >4 | 2 (1.0) | 9 (4.9) | 92 (16.9) | 103 (11.0) | |
| Chronic portal inflammation | <0.0001 | ||||
| 0 - none | 60 (28.9) | 33 (18.0) | 58 (10.7) | 151 (16.2) | |
| 1 - mild | 125 (60.1) | 128 (70.0) | 354 (65.2) | 607 (65.0) | |
| 2 – > mild | 23 (11.1) | 22 (12.0) | 131 (24.1) | 176 (18.8) | |
| Ballooning | <0.0001 | ||||
| 0 - none | 199 (95.7) | 115 (62.8) | 2 (0.4) | 316 (33.8) | |
| 1 - few | 6 (2.9) | 64 (35.0) | 174 (32.0) | 244 (26.1) | |
| 2 - many | 3 (1.4) | 4 (2.2) | 367 (67.6) | 374 (40.0) | |
| Fibrosis | <0.0001 | ||||
| 0- none | 149 (73.0) | 59 (32.4) | 39 (7.3) | 247 (26.7) | |
| 1a | 17 (8.3) | 47 (25.8) | 75 (13.9) | 139 (15.0) | |
| 1b | 2 (1.0) | 12 (6.6) | 94 (17.5) | 108 (11.7) | |
| 1c | 18 (8.8) | 7 (3.9) | 2 (0.4) | 27 (2.9) | |
| 2 | 7 (3.4) | 36 (19.8) | 145 (27.0) | 188 (20.4) | |
| 3 - bridging | 11 (3.4) | 21 (11.5) | 183 (34.0) | 215 (23.3) |
Values are N (%). Biopsies with cirrhosis were excluded. P-values derived from chi-square tests or Fisher’s exact test (for categorical variables with small expected numbers).
All histologic components of the NAS, as well as fibrosis scores and amounts of portal chronic inflammation were highly correlated with the diagnostic categories (p<0.0001 for all). Steatosis scores of <5% or 5–33% were more often found in the “not” SH category while those of grades 2 (33–66%) and 3 (>66%) were evenly distributed between borderline and definite SH. Only 3 biopsies had no lobular inflammation; they were all in the not SH category; there was a clear association of increased lobular inflammation with definite SH. The majority of all biopsies had mild portal chronic inflammation; however, a greater percentage of the not SH biopsies had none, and more of the definite SH were classified as greater than mild portal chronic inflammation (p<0.0001). Ballooning was clearly absent in the majority of “not SH” (95.7%) and borderline (62.8%), and clearly present in the majority of definite SH (99.6%) (p<0.0001). Of note, 2 cases of definite SH did not have ballooning, and 7 cases with “many” ballooned hepatocytes had been categorized as either not SH (n=3) or as borderline (n=4).
Histologic Features and the NAS
Table 4 shows the sensitivity (0.75; 95% CI: 0.72 – 0.78), specificity (0.83; 95% CI: 0.80 – 0.85), percent agreement (78.4%, 95% CI: 75.6 – 81.0), and Cohen's kappa statistic (0.57; 95% CI: 0.51 – 0.62) when using a NAS cutoff (≥5 vs. ≤4) as a substitute for the histological diagnosis of steatohepatitis (definite SH vs. borderline/not SH). Taken together, these measures indicate a substantial loss in information if the NAS were used as a surrogate for the diagnosis of steatohepatitis.
Table 4.
Sensitivity, specificity, percent agreement, and Cohen’s kappa statistic using NAS cutpoint of 5 for classification of NASH diagnosis
| NAS | NASH Diagnosis | ||
|---|---|---|---|
| Definite | Borderline/not | ||
| ≥5 | 407 | 66 | 473 |
| ≤4 | 136 | 325 | 461 |
| 543 | 391 | 934 | |
Sensitivity (95% CI): 0.75 (0.72 – 0.78)
Specificity (95% CI): 0.83 (0.80 – 0.85)
Percent agreement (95% CI): 78.4% (0.76 – 0.81)
Kappa (95% CI): 0.57 (0.76 – 0.81)
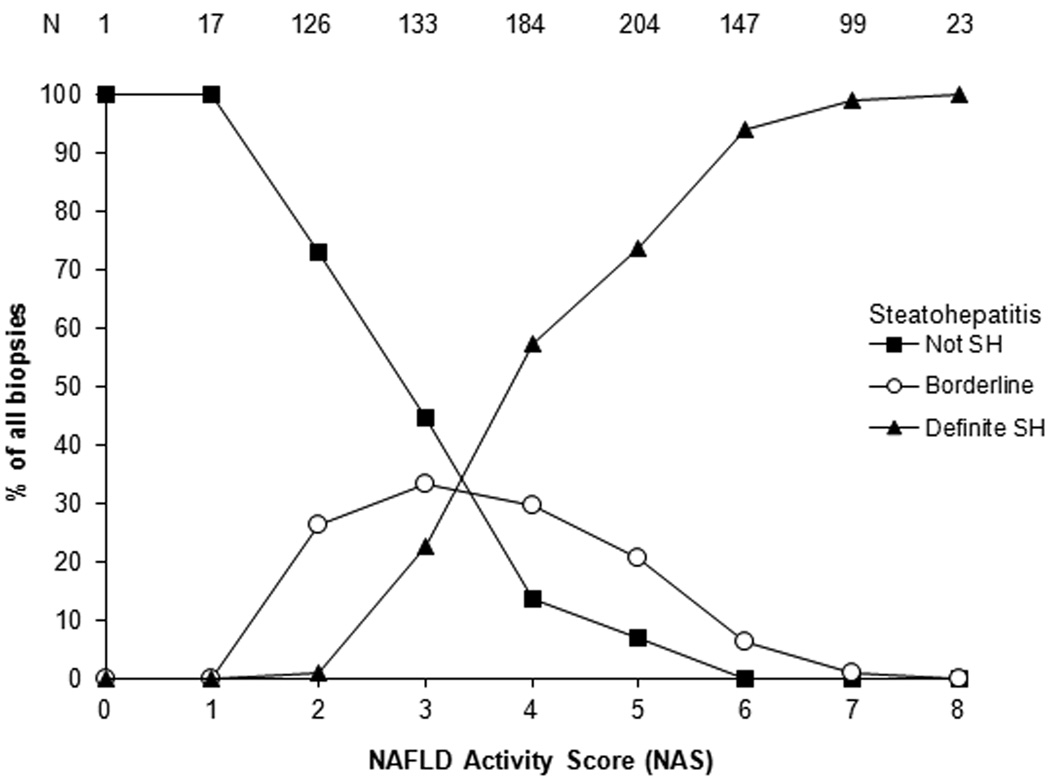
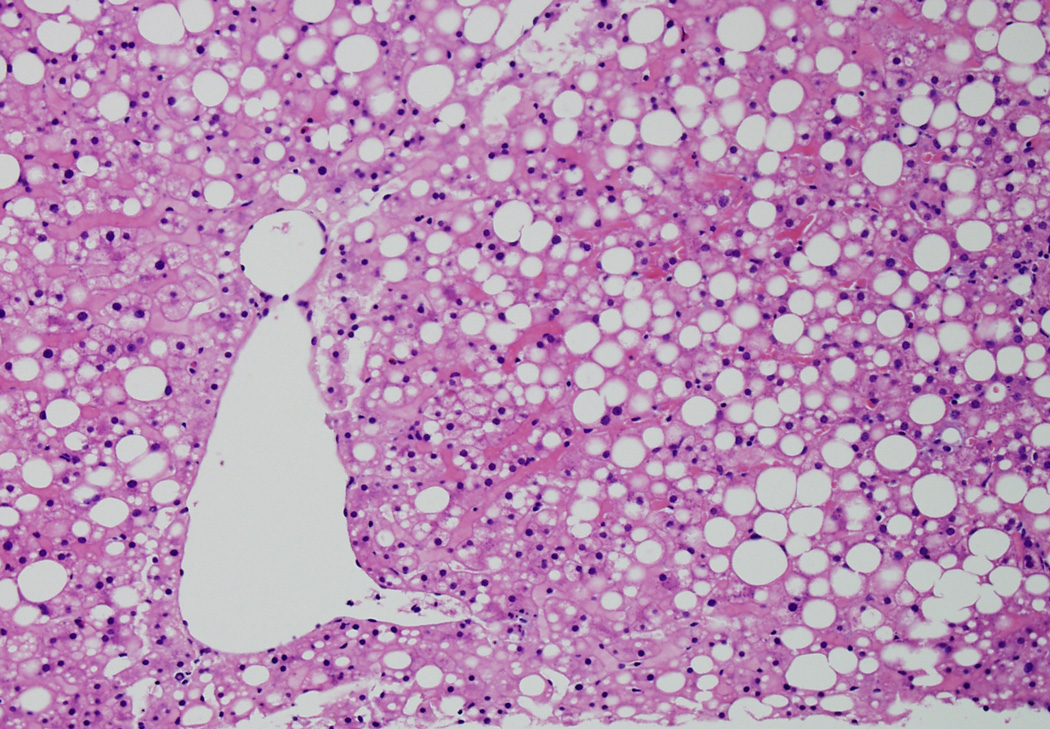
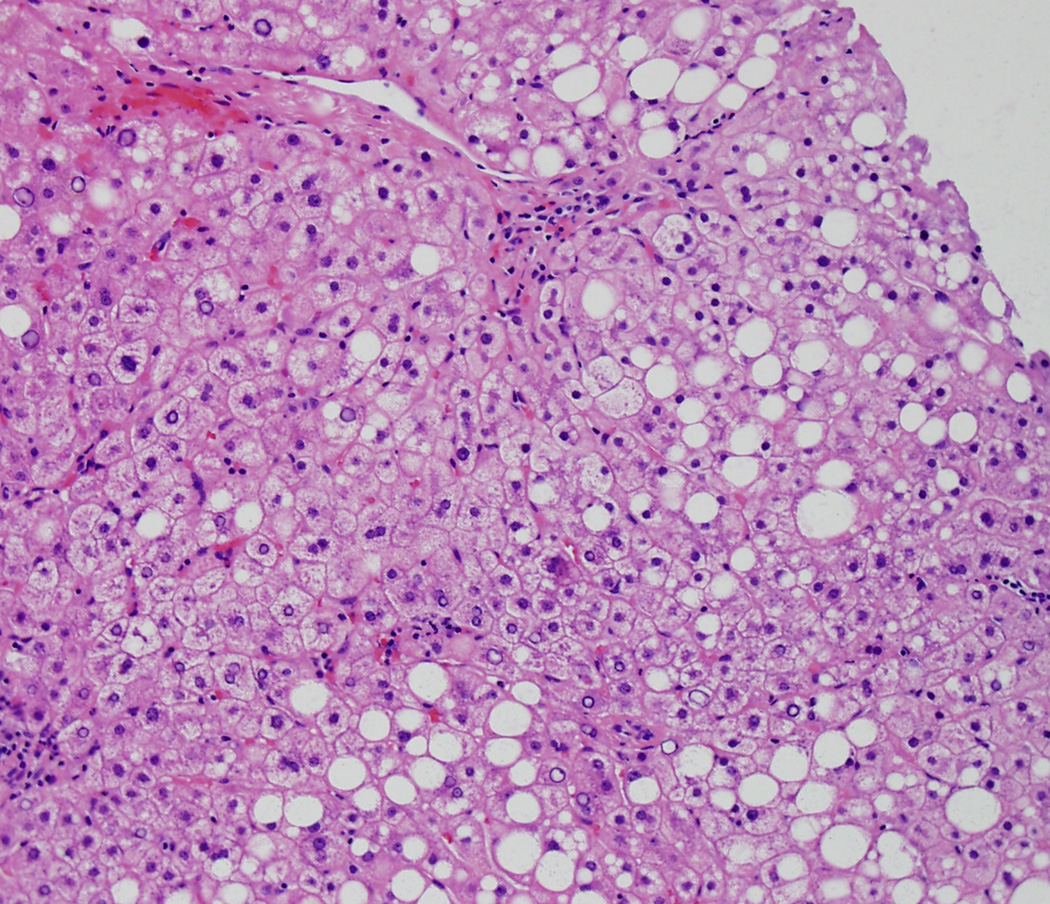
Figure 1 shows the relationship between the NAS and the diagnostic category, which is nearly identical to the relationship our group previously reported1. Table 5 shows a detailed breakdown of histologic features between the NAS ≥5 and the NAS ≤ 4 biopsies. Although NAS ≥ 5 biopsies were most commonly categorized as definite SH (86%), 66 biopsies were diagnosed as either not SH (3%) or borderline (11%). Less than half of NAS ≤ 4 biopsies were diagnosed as not SH (42%), while 28% were considered borderline, and nearly 30% had definite SH. Portal inflammation and fibrosis were more severe in NAS ≥ 5 biopsies compared to NAS ≤ 4. Of note, however, was the finding that ballooning, a central feature of the diagnosis of SH, was classified as none in 41/473 (9%) of NAS ≥ 5; on the other hand, ballooning was not only present, but was marked in 60/461 (13%) NAS ≤ 4. Figures 2a and 2b illustrate high NAS, "not SH" and low NAS, definite SH respectively.
Figure 1.
shows the percentages of biopsies with diagnoses of definite steatohepatitis (closed triangle), borderline (probable) steatohepatitis (open circle) and definitely not steatohepatitis (closed square). As can be noted, the majority of definite SH are > 5 and the majority of not SH are <3, however, the scores and diagnostic categories are not as easily separated in the NAS 3–5 ranges.
Table 5.
Histology by NAS
| Histology variable* | NAS ≤4 (n=461) | NAS ≥5 (n=473) | Total (n=934) | P |
|---|---|---|---|---|
| Diagnosis | < 0.0001 | |||
| Not steatohepatitis | 194 (42.1) | 14 (3.0) | 208 (22.3) | |
| Borderline | 131 (28.4) | 52 (11.0) | 183 (19.6) | |
| Definite steatohepatitis | 136 (29.5) | 407 (86.0) | 543 (58.1) | |
| Steatosis grade | n/a | |||
| 0 - <5% | 33 (7.2) | 4 (0.9) | 37 (4.0) | |
| 1 - 5–33% | 267 (57.9) | 82 (17.3) | 349 (37.4) | |
| 2 - 34–66% | 129 (28.0) | 177 (37.4) | 306 (32.8) | |
| 3 - > 66% | 32 (6.9) | 210 (44.4) | 242 (24.3) | |
| Steatosis location | <0.001 | |||
| 0 – Zone 3 | 231 (50.3) | 160 (33.8) | 391 (42.0) | |
| 1 – Zone 1 | 5 (1.1) | 3 (0.6) | 8 (0.9) | |
| 2 - Azonal | 132 (28.8) | 95 (20.1) | 227 (24.4) | |
| 3 – Panancinar | 91 (19.8) | 215 (45.5) | 306 (32.8) | |
| Lobular inflammation | n/a | |||
| 0 - none | 3 (0.7) | 0 (0.0) | 3 (0.3) | |
| 1 - <2 | 379 (82.2) | 82 (17.3) | 461 (49.4) | |
| 2 - 2–4 | 79 (17.1) | 288 (60.9) | 367 (39.3) | |
| 3 - >4 | 0 (0.0) | 103 (21.8) | 103 (11.0) | |
| Chronic portal inflammation | <0.0001 | |||
| 0 - none | 91 (19.7) | 60 (12.7) | 151 (16.2) | |
| 1 - mild | 306 (66.4) | 301 (63.6) | 607 (65.0) | |
| 2 - > mild | 64 (13.9) | 112 (23.7) | 176 (18.8) | |
| Ballooning | n/a | |||
| 0 - none | 275 (59.7) | 41 (8.7) | 316 (33.8) | |
| 1 - few | 126 (27.3) | 118 (25.0) | 244 (26.1) | |
| 2 - many | 60 (13.0) | 314 (66.4) | 374 (40.0) | |
| Fibrosis | <0.0001 | |||
| 0- none | 192 (42.3) | 55 (11.7) | 247 (26.7) | |
| 1a | 71 (15.6) | 68 (14.5) | 139 (15.0) | |
| 1b | 35 (7.7) | 73 (15.5) | 108 (11.7) | |
| 1c | 25 (5.5) | 2 (0.4) | 27 (2.9) | |
| 2 | 59 (13.0) | 129 (27.5) | 188 (20.4) | |
| 3 - bridging | 72 (15.9) | 143 (30.4) | 215 (23.3) |
Values are N (%). Biopsies with cirrhosis were excluded. P-values derived from chi-square tests or Fisher’s exact test (for categorical variables with small expected numbers). P-values for components of the NAS (steatosis amount, lobular inflammation, and ballooning) were not included.
Figure 2.
Figure 2a is an example of high NAS (steatosis, grade 3; lobular inflammation grade 2; ballooning grade 0, NAS = 5), but not SH by diagnosis. (20X, Hematoxylin and Eosin)
Figure 2b is an example of low NAS (steatosis, lobular inflammation and ballooning all grade 1, NAS = 3), but diagnosed as definite SH. (20X, Hematoxylin and Eosin)
NAS, Histological Diagnoses and Clinical Characteristics
To better understand the clinical characteristics of patients with low NAS but a definite SH diagnosis, or conversely, a high NAS but not a definite SH diagnosis, the biopsies with low and high NAS were analyzed separately. Table 6 shows the demographic and clinical characteristics by diagnostic category among NAS ≤ 4 biopsies and NAS ≥ 5 biopsies across all diagnostic categories, respectively. In the NAS ≤ 4 biopsies, elevated ALT correlated with the diagnosis of definite SH (p=0.003). No other clinical finding was discriminatory in that group. For biopsies with high NAS (≥ 5), several clinical features showed strong associations with the category of definite SH. The strongest was diabetes (p<0.0001); other factors were Hispanic ethnicity, higher fasting insulin levels and HOMA-IR, and lower QUICKI. A trend toward positive ANA was noted (p=0.06). Table 7 compares diagnostic categories of not SH with definite SH according to the NAS. Elevated serum ALT and triglycerides correlated with NAS ≥ 5 in those with definite SH (p=0.002, p=0.05, respectively), but not in those without SH (p=0.14, p=0.95, respectively). However, other clinical features were not significantly different among either the not SH category or definite SH category based on NAS ≤ 4 or ≥ 5.
Table 6.
Characteristics of patients by NAS and diagnosis*
| Not steatohepatitis | Borderline steatohepatitis | Definite steatohepatitis | P* | |
|---|---|---|---|---|
| N - NAS≤4 | 194 | 131 | 136 | |
| N - NAS≥5 | 14 | 52 | 407 | |
| Age at biopsy (yrs) | ||||
| NAS≤4 | 47.3 ± 11.6 | 46.5 ± 11.6 | 49.3 ± 11.9 | 0.13 |
| NAS≥5 | 41.9 ± 13.1 | 47.1 ± 10.6 | 48.5 ± 11.3 | 0.08 |
| Female gender | ||||
| NAS≤4 | 107 (55.2) | 72 (55.0) | 78 (57.4) | 0.90 |
| NAS≥5 | 8 (57.1) | 30 (57.7) | 282 (69.3) | 0.16 |
| Caucasian race | ||||
| NAS≤4 | 156 (83.0) | 103 (81.1) | 109 (83.9) | 0.84 |
| NAS≥5 | 12 (92.3) | 44 (86.3) | 330 (84.6) | 0.90 |
| Hispanic ethnicity | ||||
| NAS≤4 | 26 (13.4) | 11 (8.4) | 15 (11.0) | 0.39 |
| NAS≥5 | 2 (14.3) | 1 (1.9) | 60 (14.7) | 0.02 |
| BMI (kg/m2) | ||||
| NAS≤4 | 33.8 ± 5.9 | 34.5 ± 7.4 | 33.9 ± 6.3 | 0.63 |
| NAS≥5 | 34.8 ± 9.5 | 32.1 ± 4.7 | 34.6 ± 6.3 | 0.02 |
| Diabetes (Type 2) | ||||
| NAS≤4 | 38 (19.6) | 31 (23.7) | 39 (28.7) | 0.16 |
| NAS≥5 | 0 (0) | 6 (11.5) | 131 (32.2) | <0.0001 |
| ALT (U/L)‡ | ||||
| NAS≤4 | 51 (33–72) | 60 (33–78) | 65 (48–86) | 0.003 |
| NAS≥5 | 57 (50–120) | 89 (57–111) | 82 (52–122) | 0.76 |
| Triglycerides (mg/dL) | ||||
| NAS≤4 | 146 (104–193) | 151 (105–207) | 151 (106–209) | 0.87 |
| NAS≥5 | 130 (104–201) | 147 (99–209) | 164 (118–249) | 0.09 |
| Glucose (mg/dL)‡ | ||||
| NAS≤4 | 96 (87–107) | 92 (85–104) | 97 (86–108) | 0.24 |
| NAS≥5 | 97 (93–100) | 93 (87–108) | 96 (84–111) | 0.92 |
| Insulin (µU/mL)‡ | ||||
| NAS≤4 | 16 (10–21) | 18 (12–26) | 18 (12–28) | 0.06 |
| NAS≥5 | 14 (10–15) | 17 (9–24) | 22 (13–31) | 0.01 |
| HOMA-IR‡ | ||||
| NAS≤4 | 3.6 (2.3–5.4) | 4.3 (2.6–6.3) | 4.2 (3.0–7.3) | 0.07 |
| NAS≥5 | 3.3 (2.3–3.3) | 4.2 (2.1–6.2) | 5.0 (3.1–7.6) | 0.02 |
| QUICKI‡ | ||||
| NAS≤4 | 0.318 ± 0.030 | 0.312 ± 0.026 | 0.311 ± 0.038 | 0.21 |
| NAS≥5 | 0.328 ± 0.029 | 0.315 ± 0.035 | 0.305 ± 0.032 | 0.04 |
| ANA positive‡ | ||||
| NAS≤4 | 33 (27.3) | 17 (20.2) | 24 (25.8) | 0.51 |
| NAS≥5 | 0 (0) | 3 (9.1) | 63 (23.2) | 0.06 |
Values are N (%), means ± SD, or medians (IQR), unless otherwise specified.
P-values derived from chi-square tests for categorical variables (Fisher’s Exact test when expected numbers were small), from ANOVA for age at biopsy and BMI, and from the Kruskal-Wallis Test for laboratory measures.
Only laboratory values collected within 6 months of the liver biopsy were included. N=278 for ALT and glucose measurements; N=280 for triglycerides; N=272 for insulin and HOMA-IR measurements; N=298 for ANA. HOMA-IR is the homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (µU/mL)*fasting glucose (mmol/L))/22.5. QUICKI is calculated as: 1/log(insulin µU/mL) + log(glucose mg/dL).
Table 7.
Characteristics by diagnosis* and NAS
| No steatohepatitis | Definite steatohepatitis | |||||
|---|---|---|---|---|---|---|
| NAS≤4 (n=194) |
NAS≥5 (n=14) |
P† | NAS≤4 (n=136) |
NAS≥5 (n=407) |
P† | |
| Age at biopsy (mean) | 47.3 | 41.9 | 0.10 | 49.3 | 48.5 | 0.46 |
| Gender (% female) | 55.1 | 57.1 | 0.89 | 57.4 | 69.3 | 0.01 |
| Race (% Caucasian) | 83.0 | 92.3 | 0.83 | 83.9 | 84.6 | 0.57 |
| Hispanic ethnicity (%) | 13.4 | 14.3 | 1.00 | 11.0 | 14.7 | 0.28 |
| BMI (kg/m2) (mean) | 33.8 | 34.8 | 0.53 | 33.9 | 34.6 | 0.33 |
| Diabetes (% with) | 19.6 | 0.0 | 0.08 | 28.7 | 32.2 | 0.44 |
| ALT (U/L)‡ (median) | 51.0 | 57.0 | 0.14 | 65.0 | 82.0 | 0.002 |
| Triglycerides (mg/dL)‡ (median) | 146 | 130 | 0.95 | 151 | 164 | 0.05 |
| Glucose (mg/dL)‡ (median) | 95.5 | 97.0 | 0.82 | 97.0 | 96.0 | 0.55 |
| Insulin (µU/mL)‡ (median) | 15.8 | 13.8 | 0.26 | 18.0 | 22.0 | 0.13 |
| HOMA-IR‡ (median) | 3.6 | 3.3 | 0.25 | 4.2 | 5.0 | 0.29 |
| QUICKI‡ (mean) | 0.318 | 0.328 | 0.35 | 0.311 | 0.305 | 0.17 |
| ANA positive (%)‡ | 27.3 | 0.0 | 0.11 | 25.8 | 23.2 | 0.61 |
| Steatosis grade (%) | ||||||
| 0 - <5% | 11.9 | 7.1 | 3.7 | 0.7 | ||
| 1 - 5–33% | 54.6 | 0.0 | n/a | 69.1 | 19.9 | n/a |
| 2 - 34–66% | 24.2 | 14.3 | 27.2 | 40.3 | ||
| 3 - > 66% | 9.3 | 78.6 | 0.0 | 39.1 | ||
| Steatosis location (%) | ||||||
| 0 – Zone 3 | 51.6 | 35.7 | 46.3 | 33.7 | ||
| 1 – Zone 1 | 2.6 | 0.0 | 0.18 | 0.0 | 0.5 | <0.0001 |
| 2 - Azonal | 25.3 | 14.3 | 36.0 | 22.4 | ||
| 3 – Panancinar | 19.6 | 50.0 | 17.7 | 43.5 | ||
| Lobular inflammation (%) | n/a | n/a | ||||
| 0 - none | 1.6 | 0.0 | 0.0 | 0.0 | ||
| 1 - <2 | 86.1 | 0.0 | 86.8 | 17.7 | ||
| 2 - 2–4 | 12.4 | 85.7 | 13.2 | 59.7 | ||
| 3 - >4 | 0.0 | 14.3 | 0.0 | 22.6 | ||
| Chronic portal inflammation (%) | ||||||
| 0 - none | 28.9 | 26.6 | 0.85 | 12.5 | 10.1 | 0.26 |
| 1 - mild | 60.3 | 57.1 | 68.4 | 64.1 | ||
| 2 - >mild | 10.8 | 14.3 | 19.1 | 25.8 | ||
| Ballooning (%) | n/a | n/a | ||||
| 0 - none | 96.4 | 85.7 | 0.7 | 0.3 | ||
| 1 - few | 2.6 | 7.1 | 58.8 | 23.1 | ||
| 2 - many | 1.0 | 7.1 | 40.4 | 76.7 | ||
| Fibrosis (%) | 0.37 | 0.005 | ||||
| 0- none | 73.2 | 71.4 | 14.2 | 5.0 | ||
| 1a | 7.4 | 21.4 | 14.9 | 13.6 | ||
| 1b | 1.1 | 0.0 | 18.7 | 17.1 | ||
| 1c | 9.5 | 0.0 | 0.8 | 0.3 | ||
| 2 | 3.7 | 0.0 | 19.4 | 29.5 | ||
| 3 - bridging | 5.3 | 7.1 | 32.1 | 34.7 | ||
Diagnoses of borderline steatohepatitis and cirrhosis were excluded. Values are means for age, BMI, and QUICKI, medians for laboratory measures and HOMA-IR, and % for categorical variables.
P-values derived from chi-square tests for categorical variables (Fisher’s Exact test when expected numbers were small), from ANOVA for age at biopsy and BMI, and from the Kruskal-Wallis Test for laboratory measures. P-values for components of the NAS (steatosis amount, lobular inflammation, and ballooning) were not included.
Only laboratory values collected within 6 months of the liver biopsy were included. N=462 for ALT and glucose measurements; N=465 for triglycerides; N=454 for insulin and HOMA-IR measurements; N=495 for ANA. HOMA-IR is the homeostasis model assessment method for insulin resistance, calculated as (fasting insulin (µU/mL)*fasting glucose (mmol/L))/22.5. QUICKI is calculated as: 1/log(insulin µU/mL*glucose mg/dL).
Regression Analyses
Univariate and multivariable linear and logistic regression analyses were performed as described, where serum ALT and AST, the presence of diabetes, metabolic syndrome as defined by the NCEP3, calculated HOMA-IR and its inverse log transformation, the QUICKI, were the outcome measures and the NAS (≥ 5 vs. ≤ 4) and SH diagnosis were covariates. (The results are shown in Table 8.). Both the NAS ≥ 5 and definite SH were individually highly associated with serum ALT and AST. When both the NAS ≥ 5 and definite SH were included in the model, the significant association with ALT remained, but the NAS ≥ 5 showed a stronger association (β=24.5, p<0.0001) compared to definite SH (β=11.8, p=0.02). The association with AST was highly significant for both NAS ≥ 5 and definite SH in the multivariable model (p<0.0001).With respect to the clinical conditions and tests associated with insulin resistance, the SH diagnosis alone was strongly associated with diabetes, metabolic syndrome, HOMA-IR, and QUICKI (p<0.01 for all). In comparison, the NAS ≥ 5 alone showed no association with diabetes or metabolic syndrome (p=0.06 and p=0.16, respectively), but was associated with HOMA-IR and QUICKI (p=0.003, p=0.008, respectively). However, when the diagnosis of definite SH was included in the model with NAS ≥ 5, the association between definite SH and these measures remained statistically significant, but any contribution by the NAS was lost.
Table 8.
Regression analysis of liver enzymes and measures of metabolic syndrome and insulin resistance on livery histology (NAS≥5 and NASH diagnosis)
| ALT (U/L) | |||
|---|---|---|---|
| Models | β | 95% CI | P |
| One variable model | |||
| NAS≥5 | 30.9 | 23.0 – 38.8 | <0.0001 |
| NASH diagnosis | 25.5 | 17.3 – 33.7 | <0.0001 |
| Two variable models | |||
| NAS≥5 | 24.5 | 15.1 – 34.0 | <0.0001 |
| NASH Dx | 11.8 | 2.1 – 21.3 | 0.02 |
| AST (U/L) | |||
| Models | β | 95% CI | P |
| One variable model | |||
| NAS≥5 | 25.9 | 20.2 – 31.7 | <0.0001 |
| NASH diagnosis | 25.7 | 19.8 – 31.6 | <0.0001 |
| Two variable models | |||
| NAS≥5 | 17.3 | 10.4 – 24.1 | <0.0001 |
| NASH Dx | 16.1 | 9.1 – 23.0 | <0.0001 |
| Diabetes | |||
| Models | OR | 95% CI | P |
| One variable model | |||
| NAS≥5 | 1.33 | 0.99 – 1.79 | 0.06 |
| NASH diagnosis | 1.92 | 1.41 – 2.62 | <0.0001 |
| Two variable models | |||
| NAS≥5 | 0.90 | 0.63 – 1.29 | 0.58 |
| NASH Dx | 2.04 | 1.40 – 2.96 | <0.0001 |
| Metabolic Syndrome | |||
| Models | OR | 95% CI | P |
| One variable model | |||
| NAS≥5 | 1.21 | 0.93 – 1.58 | 0.16 |
| NASH diagnosis | 1.43 | 1.09 – 1.98 | 0.009 |
| Two variable models | |||
| NAS≥5 | 0.98 | 0.71 – 1.36 | 0.91 |
| NASH Dx | 1.45 | 1.04 – 2.01 | 0.03 |
| HOMA-IR | |||
| Models | β | 95% CI | P |
| One variable model | |||
| NAS≥5 | 1.39 | 0.48 – 2.31 | 0.003 |
| NASH diagnosis | 1.70 | 0.77 – 2.62 | <0.0001 |
| Two variable models | |||
| NAS≥5 | 0.68 | −0.41 – 1.77 | 0.22 |
| NASH Dx | 1.32 | 0.21 – 2.42 | <0.0001 |
| QUICKI (X 1,000 ) | |||
| Models | β | 95% CI | P |
| One variable model | |||
| NAS≥5 | −7.17 | −12.49 – −1.85 | 0.008 |
| NASH diagnosis | −9.24 | −14.63 – −3.86 | 0.001 |
| Two variable models | |||
| NAS≥5 | −3.12 | −9.45 – −3.86 | 0.33 |
| NASH Dx | −7.51 | −13.95 – −1.06 | 0.02 |
Discussion
This study was undertaken using a large dataset of prospectively obtained clinical data and results from liver biopsies blindly reviewed by a committee comprised of the pathologists from 10 different centers in the United States involved in the NASH CRN. The aim was to evaluate if the diagnosis of SH made by the pathologists correlated with a threshold value of feature-based scores that comprise the NAS of ≥ 5. Several interesting observations can be made. First, of 934 noncirrhotic entry liver biopsies from adult patients with phenotypic NAFLD enrolled in either the Database study or PIVENS treatment trial, 543 (58%) met histologic criteria for definite steatohepatitis. In this subset, the NAS (the sum of steatosis, lobular inflammation and ballooning scores) was ≥ 5 in 75%, but ≤ 4 in the remaining 25%. Since liver biopsy review for rigorously conducted treatment trials is done by pathologist(s) blinded to any clinical information, and therefore not influenced by knowledge of gender, ALT, or insulin resistance status, the discordance between the NAS and the diagnosis of NASH has serious implications if a discriminating criterion for trial entry is based on NAS alone. On the other hand, of the 208 biopsies from the noncirrhotic adult Database and PIVENS cohorts that were definitely “not steatohepatitis”, 14 had NAS ≥ 5. These results collectively highlight the fact that diagnostic criteria for steatohepatitis and scoring of particular lesions are related, but also provide different results.
A second major observation is clear from the regression analyses. When the diagnosis of definite steatohepatitis and the NAS were analyzed together in relation to clinical features known to be associated with NAFLD, the diagnosis of steatohepatitis was a stronger predictor of metabolic abnormalities than the score. This further emphasizes the point that the recognition of the histologic pattern of steatohepatitis cannot be replaced by a numerical score based on the presence and severity of certain features. On the other hand, the NAS is highly correlated with aminotransferase levels, commonly assumed to be markers of liver disease severity.
The NAS was created in the same way and for the same reasons that other systems for “scoring” histologic lesions in liver disease were: by an individual or a group of focused liver pathologists evaluating the lesions of significance for the specific disease process, (steatohepatitis or chronic hepatitis) and assigning relative, graduated values to represent severity. None of these systems was developed to replace a diagnostic determination of the disease; that process is the result of assessing a combination of the features (lesions) and their pattern(s). Scoring systems for chronic hepatitis, such as Knodell, METAVIR, Scheuer, and Ishak, were developed for semi-quantitative evaluation of liver histology for clinical trials (reviewed in 6). On the other hand, the histopathologic diagnosis of a disease process derives from several pieces of visual information ultimately integrated to formulate either a diagnosis or a differential diagnosis. This information includes the parenchymal location(s), alterations of surrounding cell compartments, and types of tissue responses (inflammatory cell types, presence and location of fibrosis, cell necrosis or apoptosis etc). Also included in those “pieces” of information in liver biopsy evaluation are the relationships of the vascular structures within the parenchyma to one another, the amount and types of inflammation, the location of each of the lesions being assessed, the presence and relative abnormalities of the parenchymal components’ cellular types. The lesions themselves are important individually, as they relate to each other and to other features of parenchymal alterations, and thus as part of the composite. Thus, pathologists utilize multiple inputs to formulate a final diagnosis, a process more complex than the simple addition of any one type or types of lesions to derive a “score”. Although the qualitative recognition of a pattern of injury may at first seem to be less precise and more subjective than a numerical score, our regression analysis indicated that it is a powerful result. Scores, on the other hand, are quite useful in comparative analyses, such as interventional studies, for objective measures of change of specific lesions. The exercises of diagnosis and scoring, while leading to inter-related results, are thus distinct and separate, and, when done properly, serve distinct, separate, and important purposes.
The histologic feature of the NAS that appears to be most significant in the determination of the diagnosis of steatohepatitis is ballooning. Regardless of the final NAS, >99% of 543 cases with a diagnosis of definite steatohepatitis had ballooning. Ballooning as an individual feature was significantly correlated with clinical features of insulin resistance in regression analyses. Ballooning remains a challenge for pathologists; as depicted in textbooks, ballooned cells are enlarged and have pale, flocculent cytoplasm and may contain Mallory-Denk bodies. However, in practice, they are not always enlarged, and many do not contain Mallory-Denk bodies. A recent study highlighted the loss of K8/18 detectable by immunohistochemistry as a more sensitive indicator of ballooning7. This technique is useful in detecting more subtle ballooning, and it is available in most diagnostic pathology laboratories. Other features of hepatocellular injury include acidophil bodies and immunohistochemical markers of apoptosis8,9; however, these markers have not been validated in terms of replacing ballooning as a key feature in the diagnosis.
Biopsies with definite SH but NAS ≤ 4 are clinically important because a low NAS could be interpreted as indicating absence of significant disease. These biopsies had milder steatosis (grades 0–1 in 73%) and inflammation (grades 0–1 in 87%) but ballooning in >99% and fibrosis ≥ 2 in 52%. Conversely, in the NAS ≥5 biopsies with a diagnosis of not SH, 86% had no ballooning, but 93% had > 33% steatosis (grades 2–3) and all had at least grade 2 lobular inflammation; 93% had either no fibrosis (71%) or delicate zone 3 perisinusoidal fibrosis only (21%).
On the other end of the spectrum, the cases with NAS ≤ 4, but with definite SH by diagnostic criteria were compared with both NAS ≥ 5, definite SH and NAS ≤ 4, not SH. There were higher ALT values and greater percentage of females with NAS ≥ 5 compared to NAS ≤ 4 in the definite SH group, but no other demographic or clinical data that were significantly different.
Major strengths of this study include the large amount of prospectively obtained clinical data from the NASH CRN, and biopsy results obtained over a period of several readings from a Central Review process from up to 10 liver pathologists. It is our hope that our work will allow others in the field to have the confidence to continue to “split” their diagnostic and scoring efforts, and not confuse diagnosis with “scoring” nor compromise diagnostic categories by using a summation numeric value.
Acknowledgments
Source of funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD).
Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359, UL1RR025741).
Appendix
Members of the Nonalcoholic Steatohepatitis Clinical Research Network:
Clinical Centers
Baylor College of Medicine, Houston, TX: Stephanie H. Abrams, MD, MS; Leanel Angeli Fairly, RN
Case Western Reserve University Clinical Centers:
MetroHealth Medical Center, Cleveland, OH: Arthur J. McCullough, MD; Patricia Brandt; Diane Bringman, RN (2004–2008); Srinivasan Dasarathy, MD; Jaividhya Dasarathy, MD; Carol Hawkins, RN; Yao-Chang Liu, MD (2004–2009); Nicholette Rogers, PhD, PA-C (2004–2008); Margaret Stager, MD (2004–2009)
Cleveland Clinic Foundation, Cleveland, OH: Arthur J. McCullough, MD; Srinivasan Dasarathy, MD; Mangesh Pagadala, MD; Ruth Sargent, LPN; Lisa Yerian, MD; Claudia Zein, MD
California Pacific Medical Center: Raphael Merriman, MD; Anthony Nguyen
Children’s National Medical Center, Washington DC: Parvathi Mohan, MD; Kavita Nair
Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Stephanie DeVore; Rohit Kohli, MD; Kathleen Lake; Stavra Xanthakos, MD
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD; Stephanie Buie; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Meryt Hanna; Paul Killenberg, MD (2004–2008); Samantha Kwan, MS (2006–2009); Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith
Indiana University School of Medicine, Indianapolis, IN: Elizabeth Byam, RN; Naga Chalasani, MD; Oscar W. Cummings, MD; Ann Klipsch, RN; Jean P. Molleston, MD; Linda Ragozzino, RN; Girish Subbarao, MD; Raj Vuppalanchi, MD
Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer, RN; Ann Scheimann, MD; Michael Torbenson, MD
Mount Sinai Kravis Children’s Hospital: Nanda Kerkar, MD; Sreevidya Narayanappa; Frederick Suchy, MD
Northwestern University Feinberg School of Medicine/Children’s Memorial Hospital: Mark H. Fishbein, MD; Katie Jacques; Ann Quinn, RD; Cindy Riazi, RN; Peter F. Whitington, MD
Seattle Children’s Hospital & Research Institute, WA: Melissa Coffey; Sarah Galdzicka, Karen Murray, MD; Melissa Young
Saint Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD; Joyce Hoffmann; Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Neuschwander-Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Janis Durelle; Tarek Hassanein, MD (2004–2009); Joel E. Lavine, MD, PhD; Rohit Loomba, MD; Anya Morgan; Steven Rose, MD (2007–2009); Heather Patton, MD; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, MD (2005–2009)
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD (2006–2010); Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, MD; Bo Gu (2009–2010); Raphael Merriman, MD (2002–2007); Mark Pabst; Monique Rosenthal (2005–2010); Philip Rosenthal, MD; Tessa Steel (2006–2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Puneet Puri, MD; Bimalijit Sandhu, MD (2007–2009); Arun J. Sanyal, MD; Carol Sargeant, RN, BSN, MPH; Kimberly Noble; Melanie White, RN, BSN (2006–2009)
Virginia Mason Medical Centeri, Seattle, WA: Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Ackermann; Cheryl Saunders, MPH; Vy Trinh; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (Project Scientist)
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS (2003–2009); Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson, BS; Wana Kim, BS; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH (2008–2009); Laura Miriel; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
i original grant with University of Washington
REFERENCES
- 1.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM. Nonalcoholic steatohepatitis: Definition and pathology. Seminars in Liver Disease. 2001;21(1):3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 3.Panel. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 5.Eeuschwander-Tetri BA. Fatty liver and the metabolic syndrome. Current Opinions in Gastroenterology. 2007;23:193–198. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM. Grading and staging the histopathologcial lesions of chronic hepatitis: The Knodell histology activity index and beyond. Hepatology. 2000;31:241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 7.Lackner C, Gogg-Kammerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: The value of keratin immunohistochemistry for diagnosis. Journal of Hepatology. 2008;48:821–828. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Yeh M, Belt P, Brunt EM, et al. Acidophil body index may help diagnosing nonalcoholic steatohepatitis. Modern Pathology. 2009;22:326A. [Google Scholar]
- 9.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]