Abstract
Objective
Sexual dimorphism in the degree of high blood pressure has been observed in both animal and human hypertension. However, the mechanisms are still poorly understood. We tested the hypothesis that long term loss of sex steroids promotes changes in mesenteric vascular reactivity that impact the maintenance of hypertension in the SHR.
Methods
Male spontaneously hypertensive rats (SHR) were sham-operated (M-SHAM) or castrated (M-CX), and female SHR were sham-operated (F-SHAM) or ovariectomized (F-OVX) at 3 weeks of age. Seven months later, blood pressure (BP) was measured in anesthetized rats and vascular responsiveness was evaluated in the isolated perfused mesentery.
Results
Mean arterial BP (mmHg) was significantly greater in M-SHAM (186±6) compared to F-SHAM (159±5). Gonadectomy reduced BP in male SHR (M-CX, 160±4), but had no significant effect in female SHR (F-OVX, 153±7). Norepinephrine induced constriction was similar in all groups. Gonadectomy attenuated serotonin-induced vasoconstriction in the mesentery. Acetylcholine (ACh)- and isoproterenol (ISO)-induced vasodilation was greater in female than male SHR. Ovariectomy of female SHR blunted ACh and ISO dilatory responses. ISO dose response curves were shifted to the left in castrated male SHR.
Conclusions
Gonadectomy exerts long term effects on mesenteric vascular reactivity and hypertension in the SHR.
Keywords: Gender, Arterial blood pressure, Mesenteric Vascular Bed, Hypertension, Vascular Reactivity, sex
1. Introduction
Sexual dimorphism in the degree of high blood pressure has been observed in several forms of hypertension in both humans and animals. High blood pressure develops more rapidly and becomes more severe in males than in females in genetic and experimental models of hypertension (1-4). The relative importance of androgens and estrogens to these differences remains controversial (1, 4). For example, some studies reported that estrogen attenuated hypertension development in the spontaneously hypertensive rat (SHR) (5, 6). In contrast other data, including our own, showed that ovariectomy did not raise blood pressure in female SHR (3, 4, 7, 8) when blood pressure was measured relatively soon (3-8 weeks) after ovariectomy. On the other hand, natural estrogen loss induces a slow increase in arterial blood pressure that is only fully expressed in older rats (9). In contrast, castration of male SHR was effective at attenuating blood pressure elevation early in the hypertensive process (10-14) while testosterone supplementation augmented hypertension (12, 15) suggesting that androgens have an immediate effect on blood pressure control mechanisms. Whether these findings indicate fundamental differences in the interaction of male and female sex steroids on blood pressure control or simply a different time course of effect is not clear at this time. Since our previous studies showing a lack of effect of ovariectomy on blood pressure in female SHR were performed after a maximum duration of only 8 weeks (7), one goal of the current study was to determine the effect of endogenous sex steroids on arterial blood pressure at a later time (~ 7 months) after gonadectomy.
The mesenteric vascular bed contributes importantly to the total peripheral resistance (16). Both structural and functional alterations in mesenteric vascular function contribute to the hypertensive process (17-19). Mesenteric arteries expressed a greater density of calcium channels in SHR compared to WKY (20) and exhibited exaggerated constrictor responses to a variety of stimuli (17, 20, 21). Vasodilator function in mesenteric arteries may also be affected in hypertension. However, these data are less consistent. Endothelial dependent vasodilation has been reported to be increased (21), decreased (22) or unchanged (23) in mesenteric preparations obtained from hypertensive rats. Collectively, these data indicate that the mesenteric vascular bed is affected during the hypertensive process.
Mesenteric vascular function may also be modulated by sex steroids. A number of studies suggest that estrogens reduced constriction and enhanced dilation (1). However, in the SHR these data are also inconsistent. For example, it was reported that ovariectomy increased mesenteric vascular bed responses to norepinephrine and decreased responses to acetylcholine (24). On the other hand, Brandin reported that ovariectomy did not alter the mesenteric constrictor responses to norepinephrine in SHR (22). Previously we observed variable effects of estrogens on mesenteric vascular reactivity depending on the formulation of estrogen compounds administered to rats (25). Data concerning the effects of androgens on mesenteric vascular reactivity are relatively sparse. In the fructose-fed hypertensive rat, orchidectomy enhanced acetylcholine-mediated dilation but failed to affect phenylephrine-induced constriction in the mesenteric vascular bed (26). Similarly, testosterone treatment did not increase mesenteric vascular bed constrictor responses to norepinephrine (27). As a whole these data are consistent with the view that sex steroids may modulate mesenteric vascular reactivity; however, these actions require further clarification. The present study tested the hypothesis that long term loss of sex steroids promotes changes in mesenteric vascular reactivity that impact the maintenance of hypertension in the SHR.
2. Methods
All experiments and protocols were performed in accordance with the regulations set forth by the NIH Council on Animal Care and were approved by the Institutional Animal Care and Use Committee at the University of South Dakota. Blood pressure measurements and ex vivo vascular studies were carried out on adult (8 months old) male and female spontaneously hypertensive rats (SHR) (Harlan; Indianapolis, Indiana). Rats received low-phytoestrogen diet (Harlan Teklad) and tap water ad libitum, and were kept under standard conditions in the University of South Dakota animal house for ≥ 1 week before gonadectomy. Young (3-4 weeks old) male and female SHR were sham-operated or gonadectomized under isoflurane anesthesia to prevent exposure to the prepubertal surge in sex steroids. The rats were then assigned to four experimental groups of 24 animals each: Male sham-operated, male castrated, female sham-operated, and female ovariectomized.
2.1. Arterial blood pressure measurement
Seven months after gonadectomy, rats were fasted overnight and prepared for arterial blood pressure measurement (n=8/group). All surgical procedures were performed on anesthetized (3% isofluorane in 100% O2) rats while body temperature was maintained at 36-37°C using a heating lamp table controlled by a rectal thermistor probe. A polyethylene cannula (PE 50, Clay Adams, Parsippany, N.Y., USA) filled with saline (0.9% NaCl) was inserted into the left carotid artery. After completion of surgery, rats were allowed to equilibrate for 1 h before measurement of arterial blood pressure (~ 1 hour) using a low volume pressure transducer (Transpac Abott, Transonic, N.Y., USA). The pressure transducer was interfaced with a Gateway computer that acquired data for carotid blood pressure using a BIOPAC data acquisition software (model MP 100A, AcqKnowledge Software 3.1; BIOPAC Systems, Inc., Santa Barbara, CA, USA).
2.2 Mesenteric Vascular Reactivity
Another set of rats was used for in vitro studies in the isolated perfused mesenteric vascular bed. SHR were exsanguinated by aortic puncture under isoflurane anesthesia. The superior mesenteric artery was cannulated and the gut removed as previous described (25). Briefly, the isolated mesenteric vascular bed (MVB) was constantly perfused (5 ml/min) and superfused (0.2 ml/min) using two separate pumps with modified Krebs-Henseleit (in mM: 118 NaCl, 4.7 KCl, 1.2 MgCl2·6H2O, 1.0 NaH2PO4, 2.6 CaCl2·2H2O, 25 NaHCO3, 11.1 glucose; 37°C; pH 7.35-7.45), oxygenated with a 95% oxygen-5% carbon dioxide gas mixture. Arteriolar constriction or dilatation was determined by changes in perfusion pressure (mmHg) recorded from a pressure transducer placed in the perfusion circuit just before the MVB.
2.3. Experimental protocols
i.) Vasoconstrictor Responses
A set of MVBs (n=8/group) was used to study the vasoconstrictor responses to norepinephrine (NE), and serotonin (5-HT). After a 60-min stabilization period, dose response curves to NE (0.1-100 nmol) and 5-HT (0.1-30 nmol) were constructed by injecting increasing doses into the perfusion system at 5-min intervals. A 15-minute equilibration period was allowed between each drug. All drugs were given into the perfusate as bolus injections of 100 μl. The sequence of drug injection (NE, 5-HT) was randomized, but lower doses were always given before higher doses.
ii.) Vasodilator Responses
Another set of MVBs (n=8/group) was used to study the vasodilator responses to acetylcholine (ACh), isoproterenol (ISO), and papaverine (PPV). After a 30-min stabilization period, MVBs were preconstricted with high potassium chloride (75 mM). KCl-preconstricted MVBs were allowed to equilibrate for 30 minutes at the end of which time, ACh (0.3-3000 pmol)-, ISO (0.3-3000 nmol)-, or PPV (10 nmol)-induced relaxations were measured by injecting increasing doses into the perfusion system at 5-min intervals. A 15-minute equilibration period was allowed between each drug. All drugs were given into the perfusate as bolus injection of 100 μl. The sequence of drug injection (ACh, ISO, PPV) was randomized, but lower doses were always given before higher doses.
2.4. Drugs
All the chemicals were obtained from Sigma. Stock solutions of norepinephrine and serotonin were dissolved in 0.1 mM ascorbic acid in saline.
2.5. Statistical analysis
Results are expressed as means ± SEM. Values of n refer to the number of rats in each group. Significant differences between baseline means were evaluated with two-way ANOVA (factors: gender and gonadectomy). Dose response curves were analyzed by two-way ANOVA (factors: dose and surgery). Analyses were performed using GraphPad Prism 4.0 software. ANOVA was followed by Fisher post hoc test. Differences were considered significant at P<0.05. Baseline perfusion pressure values were not different amongst the groups prior to the constrictor responses elicited by NE and 5-HT. Accordingly these responses were expressed in absolute values of mm Hg. Since perfusion pressures were different amongst the groups prior to the injection of vasodilators (during KCl preconstriction) these responses were expressed as percentage of baseline. .
3. Results
3.1. Characteristics of the animals
Table 1 summarizes the general characteristics of the 4 experimental groups. Body weight was higher in male compared to female rats. The body weight was significantly increased in ovariectomized female SHR compared to sham operated female SHR. Castration of male rats reduced body weight significantly. Heart weight was higher in male than female SHR when expressed as absolute weight, but not when corrected to body weight. Castration of male SHR reduced the absolute and relative heart weight. Ovariectomy of female SHR induced a slight but significant attenuation of cardiac weight. As expected, ovariectomy significantly reduced uterine weight.
Table 1.
Physical Characteristics of Rats. Values of body weight (g), absolute heart weight (g), relative heart weight (g/kg of body weight), absolute uterine weight (g) and relative uterine weight (g/kg of body weight). N=8/group.
| Female |
Male |
|||
|---|---|---|---|---|
| SHAM | OVX | SHAM | CX | |
| Body Weight | 219±7¶ | 280±5*¶ | 402±5 | 324±9* |
| Absolute Heart Weight | 0.738±0.019 ¶ | 0.831±0.045¶ | 1.388±0.066 | 0.968±0.038* |
| Relative Heart Weight | 3.38±0.05 | 3.00±0.13* | 3.50±0.16 | 3.00±0.24* |
| Absolute Uterine Weight | 0.479±0.049 | 0.066±0.04* | -- | -- |
| Relative Uterine Weight | 2.19±0.20 | 0.24±0.01* | -- | -- |
SHAM= sham operated; OVX= ovariectomized; CX= castrated
Gonadectomy effect; ANOVA, followed by Fisher test.
Gender effect; ANOVA, followed by Fisher test.
3.2. Arterial pressure and heart rate
Baseline mean arterial pressure (panel A) and heart rate (panel B) are shown in figure 1. Mean arterial pressure (panel A) and heart rate (panel B) values were significantly higher in male SHAM compared to female SHAM SHR. Castration of male rats lowered the arterial blood pressure and heart rate to a level similar in females. Mean arterial blood pressure values (mmHg) values were ~ 30 mm Hg higher in male sham operated SHR compared to castrated male SHR, female SHR and ovariectomized female SHR respectively. Ovariectomy of female rats had no significant effect on arterial blood pressure or heart rate compared to female sham operated SHR. Pulse pressure was similar in all groups. Arterial pulse pressure values (mmHg) values were 47±2, 43±2, 44±2 and 41±2 in male sham operated, male castrated, female sham operated and female ovariectomized SHR respectively.
Figure 1.
This figure shows mean arterial blood Pressure (panel A) and heart rate (panel B) in anesthetized male sham operated (open bars), male castrated (solid bars), female sham operated (vertically hatched bars) and female ovariectomized rats (horizontally hatched bars). Bars represent means ± SEM. □ p<0.05 male sham operated vs male castrated, □ p<0.05 male sham operated vs female sham operated.
3.4. Vascular Reactivity to Norepinephrine and Serotonin in the MVB
Perfusion of the MVB at a constant flow rate (5ml/min) induced a steady basal perfusion pressure (BPP, mmHg) after an initial stabilization period of 60 min. BPP (mmHg) values were similar in all groups (23±1, 24±1, 24.±1 and 25±2 in male sham operated, male castrated, female sham operated and female ovariectomized SHR respectively).
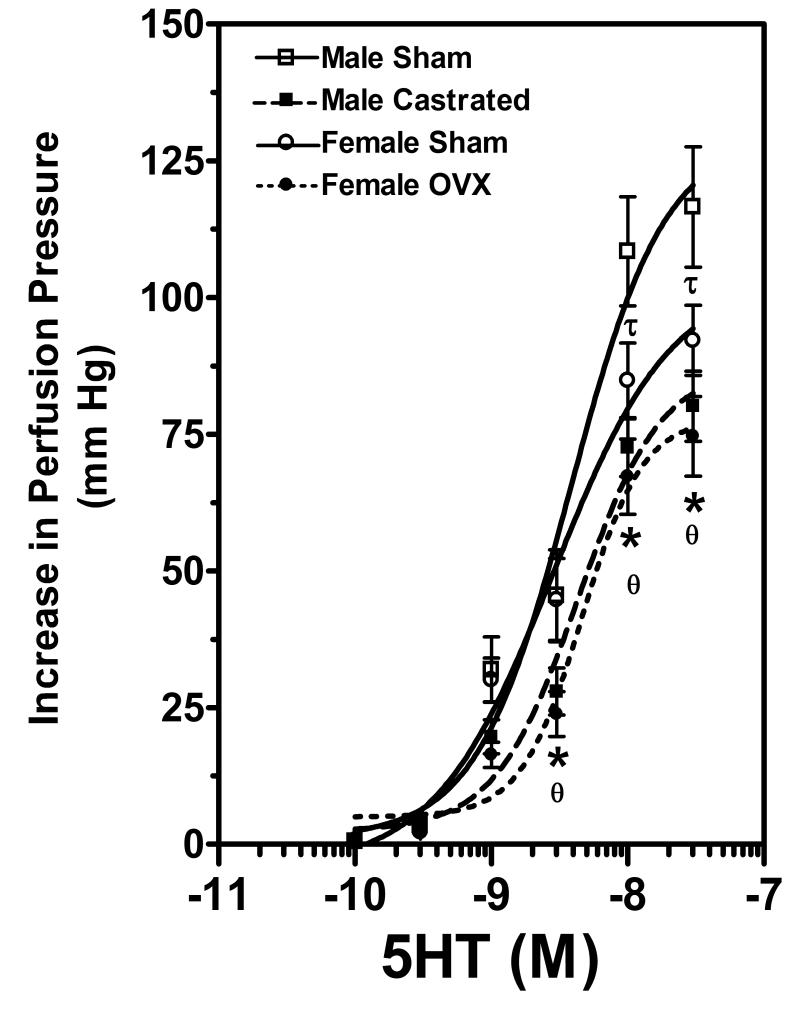
Bolus injection of norepinephrine (NE) dose-dependently increased PP (i.e. constrictor response) (Figure 2) in all groups. However, there were no differences in these responses amongst the four experimental groups. Bolus injection of serotonin (5-HT) increased in PP dose-dependently (i.e. constrictor response) (Figure 3). The constrictor responses to the two highest doses of 5-HT were exaggerated in male sham operated SHR in comparison to sham operated female SHR. Gonadectomy of male and female rats produced a rightward shift in the 5-HT dose response. The increases in perfusion pressure in response to 5HT were significantly attenuated in female ovariectomized rats compared to female sham operated SHR. Similarly orchidectomy of male SHR attenuated the perfusion pressure responses to 5HT.
Figure 2.
This figure illustrates the dose-dependent norepinephrine (NE)-induced changes in perfusion pressure (PP) in the MVB isolated from male sham operated (open squares), male castrated (solid squares), female sham operated (open circles) and female ovariectomized rats (solid circles). Data are shown as means ± SEM., n=8/group.
Figure 3.
This figure shows dose-dependent serotonin (5-HT)-induced changes in perfusion pressure (PP) in the MVB isolated from male sham operated (open squares), male castrated (solid squares), female sham operated (open circles) and female ovariectomized rats (solid circles). Data are shown as means ± SEM., n=8/group. * p<0.05 female sham operated vs female ovariectomized, □ p<0.05 male sham operated vs male castrated, □ p<0.05 male sham operated vs female sham operated.
3.5. Vascular Reactivity to Acetylcholine, Isoproterenol and Papaverine in the MVB
Baseline values were similar in all groups prior to perfusion with KCl (22±1 mm Hg, 24±1 mm Hg, 23.±2 mm Hg and 27±2 mm Hg in male sham operated, male castrated, female sham operated and female ovariectomized SHR respectively). Perfusion pressure increased in all groups after thirty minutes of perfusion with 75 mM KCl. However, there were significant differences in the perfusion pressures amongst the four groups (130±8 mm Hg, 178±8 mm Hg, 148±8 mm Hg and 179±8 mm Hg in male sham operated, male castrated, female sham operated and female ovariectomized SHR respectively). Accordingly, responses to the vasodilator agents were reported as percentage change from baseline.
Injection of the endothelium-dependent vasodilator, ACh, induced dose-dependent decreases in perfusion pressure in KCl-preconstricted MVBs (i.e. vasodilator response) (Figure 4). Dilatory responses to the three highest doses of Ach were blunted in MVBs removed from sham operated male SHR in comparison to sham operated female SHR. Ovariectomy similarly reduced ACh-induced dilatory responses. Castration of male SHR attenuated ACh responses as can be judged by the rightward shift of the ACh dose response curvewhich was most apparent at the lower doses of Ach.
Figure 4.
This figure shows acetylcholine (ACh)-induced relaxation (% preconstricted tone) in the MVB isolated from male sham operated (open squares), male castrated (solid squares), female sham operated (open circles) and female ovariectomized rats (solid circles). Data are shown as means ± SEM., n=8/group. * p<0.05 female sham operated vs female ovariectomized, □ p<0.05 male sham operated vs male castrated, □ p<0.05 male sham operated vs female sham operated.
Bolus injection of ISO reduced perfusion pressure in KCl-preconstricted MVBs (i.e. vasodilator response) in a dose-dependent manner (Figure 5). The dose response curve to ISO for sham operated SHR was shifted to the right of that for female sham operated SHR. Ovariectomy of female rats produced a slight non-significant rightward shift in the ISO dose response curve. Castration of male rats increased the vasodilator response to ISO significantly compared to sham operated male SHR. There were no significant differences in ISO responses between castrated male and ovariectomized female SHR. Papavarine was used to elicit maximal vasodilation to assess changes in resistance due to structural alterations. The endothelium-independent PPV-induced relaxations are represented in Figure 6. The responses to PPV were similar in all groups (Figure 6).
Figure 5.
This figure shows Isoproterenol (ISO)-induced dose-dependent relaxation (% preconstricted tone) in the MVB isolated from male sham operated (open squares), male castrated (solid squares), female sham operated (open circles) and female ovariectomized rats (solid circles). Data are shown as means ± SEM., n=8/group. *p<0.05 female sham operated vs female ovariectomized, □ p<0.05 male sham operated vs male castrated, □ p<0.05 male sham operated vs female sham operated.
Figure 6.
Papaverine (PPV)-induced vasodilator responses. PPV (10 nmol)-induced relaxation (% preconstricted tone) in the MVB isolated from male sham operated (open bar), male castrated (solid bar), female sham operated (vertically hatched bar) and female ovariectomized rats (horizontally hatched bar). Bars represent means ± SEM. Bars represent means ± SEM., n=8/group.
4. Discussion
The aim of this study was to investigate the long term effects of gonadectomy on blood pressure and vascular function ex vivo in the isolated perfused mesenteric vascular bed. Previous studies have addressed the issue of gender and arterial blood pressure; however, very few studies have attempted to evaluate the effect of the gonads on mesenteric vascular reactivity. The mesenteric arterial network which receives 25 % of cardiac output, contributes substantially to the total peripheral resistance, and therefore to arterial blood pressure (16). The present study indicates that endogenous sex steroids modulate both blood pressure and mesenteric vascular reactivity in middle aged SHR.
The development of hypertension is complex and is associated with alteration of a wide range of systems. Sexual dimorphism in the severity of high blood pressure has been observed in genetic and experimental models of hypertension in animals and in humans with females apparently protected at least until the time of menopause (1-4). The mechanisms by which females are protected against high blood pressure progression are not fully established. However, current evidence suggests that the sexually dimorphic pattern of hypertension in the spontaneously hypertensive rat is largely dependent on the sex steroids. The vasodilator and endothelial potentiating effects of estrogen have led to the proposal that estrogen or its metabolites are antihypertensive (1, 28). However, clinical trials have generally not supported an antihypertensive effect of estrogen (29, 30). On the other hand androgens may contribute to hypertension in males and postmenopausal females (4, 31). Our current findings support the view that androgens play an important role in amplifying hypertension development in male SHR. Ovariectomy did not affect blood pressure levels in female SHR. Our findings are consistent with those of others showing that hypertension was not exacerbated in SHR (3, 32) after ovariectomy. Conversely, orchidectomy of male SHR reduced the level of hypertension. These findings confirm earlier work from our laboratory (11, 12, 33) and those of others (34-36) and expand these observations to include longer term absence of these sex steroids. An assumption implicit in our approach is that gonadectomy results in the loss of sex steroids. In female rats we addressed this by measuring the weight of the uterus, an estrogen sensitive tissue. We observed that ovariectomy caused a marked reduction in uterine weight, consistent with loss of circulating estrogen. In separate male SHR we measured plasma testosterone levels 28 weeks following orchidectomy. Basal plasma testosterone averaged 1.7±0.04 ng/ml in sham operated rats and 0.4±0.3 ng/ml in castrated SHR (unpublished data). Thus, both ovariectomy and orchidectomy appear to have caused marked reductions in endogenous estrogen or testosterone respectively. In addition, it should be noted that blood pressures were measured under isoflurane anesthesia in the present study. Therefore, we cannot rule out the possibility that isoflurane may have affected the magnitude of blood pressure we have reported for each of the groups. The use of an anesthetic, in our case isoflurane, was necessary for harvest of the mesenteric vascular beds for the in vitro component of the study. Since all anesthetics, including isoflurane(37), may influence vascular reactivity, we decided to measure blood pressure under the same conditions as the vascular responses, namely under the influence of isoflurane. However, it is equally important to note that the pattern of blood pressure in the sham operated and gonadectomized SHR is similar to that which we (7, 8, 11, 12, 38) and others (e.g.,(36, 39, 40)) have reported previously. Thus, we are reasonably confident that the blood pressures reported in this study accurately reflect the blood pressure responses to gonadectomy.
Collectively, these findings suggest that whereas androgens amplify the development of hypertension in the SHR, ovariectomy does not have a profound effect on the hypertension development in this model. On the other hand ovariectomy has been shown to exacerbated the development of sodium dependent hypertension in the Dahl (41) or mRen(2).Lewis rat models (42). Thus the influence of sex steroids on hypertension development may depend on the etiology of the disease. Although these studies have implicated androgens in the progression and maintenance of hypertension, the mechanisms by which androgens affect blood pressure are not fully understood. Several hypotheses including enhanced renal norepinephrine release (43), renal dysfunction (14, 32), and vascular dysfunction (44, 45) have been proposed.
Since the mesenteric vascular bed contributes importantly to peripheral vascular resistance (16), we assessed potential differences in vascular reactivity in the isolated perfused mesenteric vascular bed. Our present data show a selective gender difference in the responsiveness of the mesenteric vasculature to vasoconstrictors. Since sex steroids were reported to modulate alpha adrenergic receptors in mesenteric arteries (46, 47) we predicted that ovariectomy or orchidectomy would have altered the MVB responses to NE. We observed that dose response curves to NE were not affected by gonadectomy in either male or female SHR. Our data are consistent with previous work in the normotensive rat (27) showing that testosterone did not affect vascular responses to NE and in the fructose-fed hypertensive rat (26) showing that testosterone did not affect MVB responses to phenylephrine. Zhang and Davidge reported that estradiol supplementation attenuated adrenergic vasoconstrictions (47) while ovariectomy was reported to amplify NE constrictor responses in the MVB (24). Conversely, others found that estradiol treatment increased adrenergic mesenteric vasoconstrictions (27, 48). In a study that most closely resembled the current work, Brandin reported that estradiol supplementation to SHR did not affect the EC50% for NE (22). The reasons for these discrepancies are unknown. Nevertheless, changes in perfusion pressure of the MVB are considered to represent vascular responses occurring in second and third degree branches of the entire mesenteric arterial cascade. Accordingly, our findings suggest that gonadectomy did not have a long term effect on NE-induced constriction of the MVB in SHR. Of course NE acts on multiple adrenergic receptor subtypes including alpha 1, alpha 2, beta 1 and beta 2 adrenergic receptors that may exert opposing effects on vascular tone. Since we did not block beta receptors in our preparations, we cannot exclude the possibility that testosterone had counterbalancing actions on alpha receptors and beta receptors activated by NE which resulted in no net change in the mesenteric constrictor responses. Future studies using selective adrenergic agonists and antagonists would address this issue.
In contrast to findings with NE, we observed interesting effects of gonadal steroids on the constrictor responses to 5-HT in the mesentery. Responses to higher doses of 5HT were greater in intact males compared to the other three groups. In addition, gonadectomy of both male and female SHR displaced the 5-HT dose response curves down and to the right. These data suggest that both male and female gonadal steroids augment the constrictor activity of 5-HT. Since NE and 5-HT may activate similar transduction pathways, the observation of differential effects of gonadectomy on NE and 5HT responses was unexpected. However, our data are consistent with previous work showing that testosterone enhanced MVB responses to 5-HT (49). Moreover, mesenteric vessels from hypertensive humans also show enhanced constrictor responses to 5HT (50) and 5HT release from sympathetic nerves appears to be augmented in the SHR (51). Lastly, aging selectively enhanced MVB responses to 5HT compared to NE (52), suggestive of differential modulation of these constrictor mechanisms. However, the precise role of 5HT in the hypertensive process remains to be fully delineated. Our data and those of others (53) suggest that 5-HT contractile mechanisms are enhanced in hypertension. However, other data indicate that, chronically, 5-HT may exert an antihypertensive effect (54, 55). Clearly there remain many unanswered questions regarding the role of 5HT in hypertension. Nevertheless, collectively, our data are consistent with the possibility that sex steroids modulate serotonin receptors and/or serotonin receptor coupling mechanisms in the mesenteric vascular bed of SHR.
The vasoconstrictor/vasodilator balance is generally acknowledged to be tipped toward vasoconstriction in hypertension. We also tested for potential changes in vasodilator function in the MVB. Vascular endothelial cells respond to physical and chemical stimuli via synthesis and release of several vasoactive factors that modulate vascular tone and systemic arterial blood (56). In general, endothelial-mediated dilation triggered by ACh administration is reported to be reduced in the mesenteric vasculature of the SHR (57). Moreover, gonadectomy altered endothelial dilation in mesenteric vessels. Ovariectomy reduced ACh induced dilation in mesenteric arteries (58, 59) while orchidectomy enhanced endothelial dilation in mesenteric arterial rings (26). In the present study, we observed that female sham operated SHR demonstrated the greatest responses to ACh compared to ovariectomized female rats and male rats. Since vasodilator responses were performed in the presence of high extracellular potassium, it is likely, as suggested by others (27, 60), that an increase in NO and/or vasodilator prostanoids, rather than endothelial derived hyperpolarizing factor which relies on potassium efflux, could account for the higher ACh-induced relaxation observed in female rats. Orchidectomy produced a slight rightward shift of the dose response curve to ACh but did not affect the maximal dilator responses. Our findings are similar to a report by Blanco-Rivero and co-workers (61). In contrast, other work showed that orchidectomy enhanced ACh induced dilation in the mesenteric vascular bed (26, 62). The reasons for these discrepancies are not immediately clear. In any case our data are consistent with the view that female sex steroids enhance endothelial-mediated dilation in the mesenteric vasculature.
We also assessed MVB bed responses to isoproterenol which is generally considered to be an endothelium-independent vasodilator that acts primarily by increasing cAMP synthesis in vascular smooth muscle of the MVB (63). The dose response curve for isoproterenol-induced vasodilation in sham operated male SHR was displaced to the right of those for the other three groups, indicating that isoproterenol-induced vasodilation was attenuated in sham operated male SHR. These findings differ from previous work in that a gender difference in isoproterenol ED50% was not detected in arterial rings but maximal dilation to isoproterenol was exaggerated in female SHR compare to male SHR (44). Nevertheless both studies show that isoproterenol-mediated dilation was impaired in male SHR. Orchidectomy shifted the isoproterenol dose response curve to the left while ovariectomy shifted the dose response curve to the right such that there were no differences in isoproterenol induced vasodilation between the male and female gonadectomized SHR. The effect of sex steroids on vasodilator function does not seem to be generalized since another endothelium-independent, smooth muscle relaxant, papaverine, provoked equal relaxation in all groups. As a whole these data support the view that both female and male sex steroids modulate isoproterenol-mediated vasodilation in the mesenteric arterial tree.
Conclusion
Collectively, our study supports a role for sex steroids in modulating hypertension in the SHR over the long term. Sex steroids also appear to influence contractile pathways and both endothelium-dependent and endothelium-independent vasodilator functions in the MVB. There appears to be selectivity in these effects since not all constrictors and dilators were affected equally by gonadectomy. This selectively of response suggests that further understanding of the sites and mechanisms of action of the sex steroids in these pathways may offer opportunities for future drug development.
Acknowledgments
This work was supported grant from the South Dakota EPSCoR 400049 (RTT) and NIH HLBI 63053 (DSM).
Funding: This work was supported grant from the South Dakota EPSCoR 400049 (RTT) and NIH HLBI 63053 (DSM).
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest associated with this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovascular Research. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 2.Khalil RA. Sex hormones as potential modulators of vascular function in hypertension. Hypertension. 2005;46:249–54. doi: 10.1161/01.HYP.0000172945.06681.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF. Sex and sex steroids in cardiovascular-renal physiology and pathophysiology. Gend Med. 2008;5(Suppl A):S1–2. doi: 10.1016/j.genm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl sensitive hypertension in female spontaneously hypertensive rats. Am. J. Physiol. 2001;281:R1934–1939. doi: 10.1152/ajpregu.2001.281.6.R1934. [DOI] [PubMed] [Google Scholar]
- 6.Iams SG, Wexler BC. Inhibition of the development of spontaneous hypertension in SH rats by gonadectomy or estradiol. J. Lab. Clin. Med. 1979;94:608–616. [PubMed] [Google Scholar]
- 7.Martin DS, Breitkopf NP, Eyster KM, Williams JL. Dietary soy exerts an antihypertensive effect in spontaneously hypertensive female rats. Am. J. Physiol. 2001;281:R553–R560. doi: 10.1152/ajpregu.2001.281.2.R553. [DOI] [PubMed] [Google Scholar]
- 8.Martin DS, Redetzke R, Vogel E, Mark C, Eyster KM. Effect of ovariectomy on blood pressure and venous tone in female spontaneously hypertensive rats. Am J Hypertens. 2008;21:983–8. doi: 10.1038/ajh.2008.237. [DOI] [PubMed] [Google Scholar]
- 9.Fortepiani LA, Zhang H, Racusen L, R. LJ, R. JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41:640–645. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 10.Ganten U, Witt GS, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effect of antiandrogen treatment. Journal of Hypertension. 1989;7:721–726. [PubMed] [Google Scholar]
- 11.Martin D, Biltoft S, Redetzke R, Vogel E. Castration reduces blood pressure and autonomic venous tone in spontaneously hypertensive rats. J. Hypertens. 2005;23:2229–36. doi: 10.1097/01.hjh.0000191903.19230.79. [DOI] [PubMed] [Google Scholar]
- 12.Song J, Eyster KM, Kost CK, Jr., Kjellsen B, Martin DS. Involvement of protein kinase C-CPI-17 in androgen modulation of angiotensin II-renal vasoconstriction. Cardiovasc Res. 2010;85:614–21. doi: 10.1093/cvr/cvp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Martin DS. Rho kinase contributes to androgen amplification of renal vasoconstrictor responses in the spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2006;48:103–9. doi: 10.1097/01.fjc.0000245403.45406.d8. [DOI] [PubMed] [Google Scholar]
- 14.Reckelhoff JF, Zhang J, Granger JP. Testosterone exacerbates hypertension and reduces pressure natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31:435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]
- 15.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289:F941–8. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 16.Christensen KL, Mulvany MJ. Mesenteric arcade arteries contribute substantially to vascular resistance in conscious rats. J Vasc Res. 1993;30:73–9. doi: 10.1159/000158978. [DOI] [PubMed] [Google Scholar]
- 17.Naito Y, Yoshida H, Konishi C, Ohara N. Differences in responses to norepinephrine and adenosine triphosphate in isolated perfused mesenteric vascular beds between normotensive and spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1998;32:807–818. doi: 10.1097/00005344-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Schiffrin EL. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture Hypertension. 1992;19:II1–9. doi: 10.1161/01.hyp.19.2_suppl.ii1-a. [DOI] [PubMed] [Google Scholar]
- 19.Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol. 2005;83:413–22. doi: 10.1139/y05-012. [DOI] [PubMed] [Google Scholar]
- 20.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–9. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 21.Chang HR, Lee RP, Wu CY, Chen HI. Nitric oxide in mesenteric vascular reactivity: a comparison between rats with normotension and hypertension. Clin Exp Pharmacol Physiol. 2002;29:275–80. doi: 10.1046/j.1440-1681.2002.03643.x. [DOI] [PubMed] [Google Scholar]
- 22.Brandin L, Bergstrom G, Manhem K, Gustafsson H. Oestrogen modulates vascular adrenergic reactivity of the spontaneously hypertensive rat. J. Hypertens. 2003;21:1695–1702. doi: 10.1097/00004872-200309000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Husken BC, Hendriks MG, Pfaffendorf M, Van Zwieten PA. Effects of aging and hypertension on the reactivity of isolated conduit and resistance vessels. Microvasc Res. 1994;48:303–15. doi: 10.1006/mvre.1994.1057. [DOI] [PubMed] [Google Scholar]
- 24.Dantas AP, Scivoletto R, Fortes ZB, Nigro D, Carvalho MH. Influence of female sex hormones on endothelium-derived vasoconstrictor prostanoid generation in microvessels of spontaneously hypertensive rats. Hypertension. 1999;34:914–9. doi: 10.1161/01.hyp.34.4.914. [DOI] [PubMed] [Google Scholar]
- 25.Mark CJ, Tatchum-Talom R, Martin DS, Eyster KM. Effects of estrogens and selective estrogen receptor modulators on vascular reactivity in the perfused mesenteric vascular bed. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1969–75. doi: 10.1152/ajpregu.00260.2007. [DOI] [PubMed] [Google Scholar]
- 26.Vasudevan H, Nagareddy PR, McNeill JH. Gonadectomy prevents endothelial dysfunction in fructose-fed male rats, a factor contributing to the development of hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H3058–64. doi: 10.1152/ajpheart.00598.2005. [DOI] [PubMed] [Google Scholar]
- 27.Vargas R, Delaney M, Farhat MY, Wolfe F, Rego A, Ramwell PW. Effect of estradiol 17ß on pressor responses of rat mesenteric bed to norepinephrine, K+ and U-46619. J. Cardiovasc. Pharmacol. 1995;25:200–206. doi: 10.1097/00005344-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Qiao X, McConnell KR, Khalil RA. Sex steroids and vascular responses in hypertension and aging. Gend Med. 2008;5(Suppl A):S46–64. doi: 10.1016/j.genm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Prelevic GM, Kwong P, Byrne DJ, Jagroop IA, Ginsburg J, Mikhailidis DP. A cross-sectional study of the effects of hormon replacement therapy on the cardiovascular disease risk profile in healthy postmenopausal women. Fertility and Sterility. 2002;77:945–951. doi: 10.1016/s0015-0282(02)03078-9. [DOI] [PubMed] [Google Scholar]
- 30.Preston RA. Comparative effects of conventional vs. novel hormone replacement therapy on blood pressure in postmenopausal women. Climacteric. 2009;12(Suppl 1):66–70. doi: 10.1080/13697130902775788. [DOI] [PubMed] [Google Scholar]
- 31.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43:918–923. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 32.Fortepiani LA, Yanes L, Zhang H, Racusen LC, Reckelhoff JF. Role of androgens in mediating renal injury in aging SHR. Hypertension. 2003;42:952–5. doi: 10.1161/01.HYP.0000099241.53121.7F. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Kost CK, Jr., Martin DS. Androgens augment renal vascular responses to ANG II in New Zealand genetically hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1608–15. doi: 10.1152/ajpregu.00364.2005. [DOI] [PubMed] [Google Scholar]
- 34.Reckelhoff JF, Granger JP. Role of androgens in mediating hypertension and renal injury. Clin Exp Pharmacol Physiol. 1999;26:127–31. doi: 10.1046/j.1440-1681.1999.02996.x. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JC, Sasser JM, Pollock JS. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R764–8. doi: 10.1152/ajpregu.00322.2006. [DOI] [PubMed] [Google Scholar]
- 36.Toot J, Jenkins C, Dunphy G, Boehme S, Hart M, Milsted A, Turner M, Ely D. Testosterone influences renal electrolyte excretion in SHR/y and WKY males. BMC Physiol. 2008;8:5. doi: 10.1186/1472-6793-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akata T, Kanna T, Yoshino J, Takahashi S. Mechanisms of direct inhibitory action of isoflurane on vascular smooth muscle of mesenteric resistance arteries. Anesthesiology. 2003;99:666–77. doi: 10.1097/00000542-200309000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Song J, Kost CK, Jr., Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res. 2006;72:456–63. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats. Role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1573–R1579. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 41.Laborde C. Hinojosa, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 42.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008;5(Suppl A):S65–75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones TJ, Dunphy G, Milsted A, Ely D. Testosterone effects on renal norepinephrine content and release in rats with different Y chromosomes. Hypertension. 1998;32:880–885. doi: 10.1161/01.hyp.32.5.880. [DOI] [PubMed] [Google Scholar]
- 44.Kahonen M, Tolvanen JP, Sallinen K, Wu X, Porsti I. Influence of gender on control of arterial tone in experimental hypertension. Am J Physiol. 1998;275:H15–22. doi: 10.1152/ajpheart.1998.275.1.H15. [DOI] [PubMed] [Google Scholar]
- 45.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 46.McConnaughey MM, Iams SG. Sex hormones change adrenoceptors in blood vessels of the spontaneously hypertensive rat. Clin Exp Hypertens. 1993;15:153–70. doi: 10.3109/10641969309041617. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Davidge ST. Effect of estrogen replacement on vasoconstrictor responses in rat mesenteric arteries. Hypertension. 1999;34:1117–22. doi: 10.1161/01.hyp.34.5.1117. [DOI] [PubMed] [Google Scholar]
- 48.Colucci WS, Gimbrone MA, McLaughlin MK, Halpern W, Alexander RW. Increased vascular catecholamine sensitivity and alpha adrenergic receptor affinity in female and estrogen treated male rats. Circ. Res. 1982;50:805–811. doi: 10.1161/01.res.50.6.805. [DOI] [PubMed] [Google Scholar]
- 49.Ammar EM, Said SA, Hassan MS. Enhanced vasoconstriction and reduced vasorelaxation induced by testosterone and nandrolone in hypercholesterolemic rabbits. Pharmacol Res. 2004;50:253–9. doi: 10.1016/j.phrs.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Tahvanainen A, Taurio J, Maki-Jouppi J, Koobi P, Mustonen J, Kahonen M, Sand J, Nordback I, Porsti I. Increased wall tension in response to vasoconstrictors in isolated mesenteric arterial rings from patients with high blood pressure. Basic Clin Pharmacol Toxicol. 2006;99:440–9. doi: 10.1111/j.1742-7843.2006.pto_572.x. [DOI] [PubMed] [Google Scholar]
- 51.Kawasaki H, Urabe M, Takasaki K. Enhanced 5-hydroxytryptamine release from vascular adrenergic nerves in spontaneously hypertensive rats. Hypertension. 1987;10:321–7. doi: 10.1161/01.hyp.10.3.321. [DOI] [PubMed] [Google Scholar]
- 52.Donoso MV, Fournier A, Peschke H, Faundez H, Domenech R, Huidobro-Toro JP. Aging differentially modifies arterial sensitivity to endothelin-1 and 5-hydroxytryptamine: studies in dog coronary arteries and rat arterial mesenteric bed. Peptides. 1994;15:1489–95. doi: 10.1016/0196-9781(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 53.Watts SW. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in deoxycorticosterone acetate-salt hypertension. Hypertension. 2002;39:825–9. doi: 10.1161/hy0302.104668. [DOI] [PubMed] [Google Scholar]
- 54.Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther. 2008;325:1031–8. doi: 10.1124/jpet.108.136226. [DOI] [PubMed] [Google Scholar]
- 55.Watts SW, Davis RP. 5-Hydroxtryptamine Receptors in Systemic Hypertension: An Arterial Focus. Cardiovasc Ther. 2011;29:54–67. doi: 10.1111/j.1755-5922.2010.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J. Cardiovasc. Pharmacol. 1993;22:S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 57.Bernatova I, Conde MV, Kopincova J, Gonzalez MC, Puzserova A, Arribas SM. Endothelial dysfunction in spontaneously hypertensive rats: focus on methodological aspects. J Hypertens. 2009;(Suppl 27):S27–31. doi: 10.1097/01.hjh.0000358834.18311.fc. [DOI] [PubMed] [Google Scholar]
- 58.Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+ Am J Physiol Endocrinol Metab. 2009;296:E503–12. doi: 10.1152/ajpendo.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, Miwa S, Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol. 2005;144:178–89. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000;292:375–80. [PubMed] [Google Scholar]
- 61.Blanco-Rivero J, Sagredo A, Balfagon G, Ferrer M. Protein kinase C activation increases endothelial nitric oxide release in mesenteric arteries from orchidectomized rats. J Endocrinol. 2007;192:189–97. doi: 10.1677/joe.1.07079. [DOI] [PubMed] [Google Scholar]
- 62.Ba ZF, Yokoyama Y, Toth B, Rue LW, 3rd, Bland KI, Chaudry IH. Gender differences in small intestinal endothelial function: inhibitory role of androgens. Am J Physiol Gastrointest Liver Physiol. 2004;286:G452–7. doi: 10.1152/ajpgi.00357.2003. [DOI] [PubMed] [Google Scholar]
- 63.Abiru T, Watanabe Y, Kamata K, Kasuya Y. Simultaneous measurement of vasodilation and changes in cyclic nucleotides in the perfused mesenteric arterial bed of the rat. Eur J Pharmacol. 1993;242:15–22. doi: 10.1016/0014-2999(93)90004-2. [DOI] [PubMed] [Google Scholar]