Abstract
The photochemistry of 1-methyl-4-phenyl-1H-tetrazole-5(4H)-thione (1a) and 1-(3-methoxyphenyl)-4-methyl-1H-tetrazole-5(4H)-thione (1b) was studied in acetonitrile at 254 and 300 nm which involves expulsion of dinitrogen and sulfur to form the respective carbodiimides 5a – b as sole photoproducts. Photolysis of the title compounds in the presence of 1,4-cyclohexadiene trap led to the formation of respective thioureas, providing strong evidence for the intermediacy of a 1,3-biradical formed by the loss of dinitrogen. In contrast, a trapping experiment with cyclohexene provided no evidence to support an alternative pathway of photodecomposition involving initial desulfurization followed by loss of dinitrogen via the intermediacy of a carbene. Triplet sensitization and triplet quenching studies argue against the involvement of a triplet excited state. While the quantum yields for the formation of the carbodiimides 5a – b were modest, and showed little change on going from a C6H5 (1a) to mOMeC6H4 (1b) substituent on the tetrazolethione ring, the highly clean photodecomposition of these compounds to a photostable end product makes them promising lead structures for industrial, agricultural and medicinal applications.
Introduction
Compounds containing a tetrazolethione scaffold have attracted attention as corrosion inhibitors,1–3 pesticides,4 stabilizers in photography,5, 6 UV resists in photolithography,7 capping agents in semiconductor photocatalytic reactions,8, 9 and as pharmaceutical agents.10 Despite their widespread applications, studies on their structure and reactivity are limited, and a better understanding of the photochemistry of these ring systems is critical for designing compounds with improved performance. For instance, photostability of the tetrazolethione ring is of prime concern in exploiting these scaffolds for the synthesis of novel pesticides or pharmaceutical agents. It is therefore, important to identify any unwanted phototoxic intermediates/products that may form upon UV exposure of these compounds, and thus enable strategies for preventing those decomposition pathways and hence, ensure greater photostability. On the other hand, in photolithography where tetrazolethione scaffolds have been proposed for the in situ photogeneration of carbodiimides that subsequently react with carboxylic acids to form deep UV resists,7 high photolability of the tetrazolethione rings is desirable, so that carbodiimides can be generated cleanly, quickly and efficiently. Again, conclusive information about the various photochemical processes is required as the undesirable pathways may interfere with the formation of carbodiimides, and therefore, would need to be suppressed in order to increase the overall efficiency of the process.
There are a few reports in the literature that discuss the photochemistry of these heterocycles. For instance, work by Dunkin and coworkers in 1989 revealed that the photolysis of tetrazolethiones in low-temperature matrix yields carbodiimides as a result of loss of dinitrogen and sulfur.11 Several years later, the photochemistry of this ring system was revisited by Fausto et al. who reported that carbodiimides are not the only products in gas phase and a variety of other photoproducts are formed.12 The photolysis of 1-methyl-1H-tetrazole-5(4H)-thione in low-temperature argon matrix was found to proceed via three decomposition pathways involving: (1) the expulsion of dinitrogen to form 1-methyl-1H-diazirene-3-thiol, (2) the cleavage of the tetrazolethione ring to form methylisothiocyanate and the azide, and (3) the concurrent expulsion of dinitrogen and sulfur to form N-methyl carbodiimide.12 The authors also reported that the primary photoproducts underwent further decomposition to form methyl diazene, carbon monosulfide and nitrogen hydride.12 However, in solution the photolysis of tetrazolethiones is known to form the corresponding carbodiimides or their tautomers as the sole products.13–15
To the best of our knowledge there are no reports on the mechanism of the photodecomposition of tetrazolethiones. A few mechanistic studies on the related tetrazolones are known.16, 17 For instance, Cristiano and coworkers studied the photochemistry of 1-allyl-4-phenyltetrazolones in solution that resulted in the formation of pyrimidinones as the major product which underwent decomposition in non-alcoholic solvents to form secondary photoproducts such as allyl amine, aniline, phenyl isocyanate and allyl isocyanate.17 This photochemistry is believed to involve an intermediacy of a 1,3-triplet biradical formed via the elimination of molecular nitrogen from the photoexcited 1-allyl-4-phenyltetrazolones.17 Cristiano also investigated the photochemistry of 5-allyloxy tetrazoles in solution and reported the formation of 1,3-oxazines as the sole primary photoproducts that undergo further reactions leading to the formation of hydrazines, enamines, aniline and phenyl isocyanate.16 Laser flash photolysis experiments further revealed that photodecomposition of 5-allyloxy tetrazoles involves the formation of triplet 1,3-biradicals.16 We were interested in exploring if the mechanism of photodecomposition of tetrazolethiones also proceed through a 1,3-biradical, analogous to the tetrazolones, or if another type of intermediate is involved. Therefore, we synthesized 1-methyl-4-phenyl-1H-tetrazole-5(4H)-thione (1a) and 1-(3-methoxyphenyl)-4-methyl-1H-tetrazole-5(4H)-thione (1b), investigated their photochemistry in acetonitrile and gained preliminary insights into the mechanism of their photodecomposition (Scheme 1).
SCHEME 1.

1-Methyl-4-phenyl-1H-tetrazole-5(4H)-thiones 1a – b.
Results and discussion
Synthesis of tetrazolethiones 1a – b and their absorption spectra
1,3-Dipolar cycloaddition of phenylisocyanate (2a) with trimethylsilylazide resulted in the formation of 1-phenyl-1H-tetrazol-5(4H)-one (3a).18 3a was methylated with dimethyl sulfate to obtain the tetrazolone 4a which upon treatment with phosphorous pentasulfide yielded the desired 1-methyl-4-phenyl-1H-tetrazole-5(4H)-thione (1a).19 Similarly, 3-methoxyphenylisocyanate (2b) was converted to 4b as reported earlier,20 which was subjected to thionation to obtain the desired 1-(3-methoxyphenyl)-4-methyl-1H-tetrazole-5(4H)-thione (1b) (Scheme 2).
SCHEME 2.
Synthesis of 1-methyl-4-phenyl-1H-tetrazole-5(4H)-thiones 1a – b.
The UV spectral data of 1a has been reported19 and the spectra of compound 1b in cyclohexane, tetrahydrofuran, and acetonitrile are shown in Figure 1. Analogous to 1a, four UV bands λ1 – λ4 were observed for 1b in cyclohexane and MeCN, however, λ4 could not be observed in THF due to solvent interference (Figure 1). The corresponding values of molar absorptivities for these bands are shown in Table 1. λ1 and λ2 underwent slight blue-shifts as the polarity of the solvent was increased from cyclohexane→THF→MeCN. λ3 of 1b remained unchanged while λ4 showed a blue shift from cyclohexane→MeCN. Time dependent density functional calculations revealed that all bands in 1a19 and 1b result from π→π* transitions with some degree of intramolecular charge transfer (ICT) within the molecules (See supporting information for 1b).
FIGURE 1.

Absorption spectra of 1b in cyclohexane (red), THF (green) and acetonitrile (blue).
TABLE 1.
Energies (λ (E)) and molar absorptivities (log ε) for the peaks observed in the absorption spectra of 1b in cyclohexane, tetrahydrofuran and acetonitrile.
| Cyclohexane | THF | MeCN | ||||
|---|---|---|---|---|---|---|
| λ (E)a | log εb | λ (E)a | log εb | λ (E)a | log εb | |
| λ1 | 290.0 (4.27) | 3.82 | 285.0 (4.35) | 3.97 | 281.0 (4.41) | 3.82 |
| λ2 | 266.0 (4.66) | 3.89 | 264.0 (4.69) | 4.03 | 260.0 (4.77) | 3.90 |
| λ3 | 222.0 (5.58) | 4.31 | 223.0 (5.55) | 4.40 | 221.0 (5.61) | 4.34 |
| λ4 | 207.0 (5.98) | 4.27 | 196.0 (6.32) | 4.15 | ||
in nm (eV);
in M−1 cm−1
Product analysis
The photolyses of argon-saturated solutions of 1a – b in acetonitrile was carried out at 254 and 313 nm in a quartz cuvette. The UV spectral changes as a function of wavelength for compounds 1a and 1b at different irradiation times (as reported in the experimental section) are shown in Figures 2 and 3, respectively. The observance of clean isosbestic points in each case suggested the formation of a single photoproduct.
FIGURE 2.
Changes in the UV spectrum of 1a induced by irradiation at 254 (left) and 313 nm (right) in acetonitrile (arrows indicate the direction of spectral change upon irradiation).
FIGURE 3.
Changes in the UV spectrum of 1b induced by irradiation at 254 (left) and 313 nm (right) in acetonitrile (arrows indicate the direction of spectral change upon irradiation).
In order to identify the product(s), the photochemistry of argon-saturated solutions of 1a – b in acetonitrile-d3 was studied in a quartz NMR tube at 254 and 300 nm. The NMR spectrum of tetrazolethiones taken at different irradiation times indicated a clean photochemical reaction resulting into the formation of carbodiimides 5a – b with the loss of dinitrogen and sulfur from the heterocyclic ring (Scheme 3, see Figures S4 and Figure S5 in supporting information). The NMR peaks corresponding to the respective carbodiimides were assigned by comparison of their chemical shift values to that of authentic samples. The formation of carbodiimides as the primary photoproduct was consistent with the result reported by Quast.13–15 The photolyses were only carried out for 15 and 60 min at 254 and 300 nm, respectively. The amount of 1a – b that remained unreacted after irradiation and yields of corresponding products 5a – b are listed in Table 2. There were trace amounts of other photoproducts (< 5%) that also formed during the photolysis of 1a – b at 254 nm (See Figures S4 and S5). Their chemical shift values suggest that these may correspond to methyl isothiocyanate, corresponding aryl azides or benzimidazolethiones formed from the photodecomposition of 1a – b. Note that these type of photoproducts have been previously detected in the photolysis of tetrazolethiones in the gas phase12 as well as in the photodecomposition of their 5-oxo derivatives.21 Only trace amounts of 5a – b were formed after 60 minutes of exposure to 300 nm UV light. This was attributed to the low molar absorptivities of 1a – b at that irradiation wavelength.
SCHEME 3.

Photochemical conversion of tetrazolethiones 1a – b to carbodiimides 5a – b.
TABLE 2.
Unreacted 1a – b (%), products 5a – b (%) and quantum yields (Φ) for the photodecomposition in MeCN.
| λ | Irradiation time | Unreacted 1a [%] | Yield of 5a [%] | Φ | ||
|---|---|---|---|---|---|---|
| 1a | 254 | 15 | Ar-purged | 57 | 44 | 0.045 |
| 15 | O2-satd | 55 | 47 | |||
| 300 | 60 | Ar-purged | >95 | trace | ||
| 60 | O2-satd | >95 | trace | |||
| 1b | 254 | 15 | Ar-purged | 60 | 42 | 0.031 |
| 15 | O2-satd | 57 | 45 | |||
| 300 | 60 | Ar-purged | >95 | trace | ||
| 60 | O2-satd | >95 | trace |
Amounts calculated by NMR spectroscopy with 1,4-dioxane as an internal standard (average of three irradiated samples). The standard deviation was in the range 0.4 – 2% and was calculated from three separate irradiated samples in each case.
The quantum yields for the formation of 5a – b were determined at 254 nm by using the azoxybenzene actinometer22 and are reported in Table 2. Overall, the quantum yields were found to be modest, which indicates that the compounds might be involved in radiationless decay or fluorescence.
Furthermore, we investigated the formation of secondary photoproducts by irradiating the authentic samples of 5a – b in acetonitrile-d3 at 254 nm. The NMR spectrum recorded at 120 minutes of irradiation showed no decomposition, thus indicating that carbodiimides were stable photoproducts. This end product photostability is a desirable feature from an applications standpoint.
Mechanism of photodecomposition
The formation of carbodiimides 5 from tetrazolethiones 1 involves the loss of dinitrogen and sulfur. Therefore, there are two possible photodecomposition pathways that must be considered: Path A, which involves desulfurization followed by elimination of dinitrogen, and path B, which involves the expulsion of dinitrogen followed by desulfurization (Scheme 4). The former pathway will involve the formation of a heterocyclic carbene 6 as intermediate where as the latter will lead to the formation of biradical 7 as the reactive species. In order to distinguish between these two mechanistic pathways, we carried out the experiments described below.
SCHEME 4.
Two possible mechanistic pathways for the photodecomposition of 1a – b.
Investigating the intermediacy of a carbene
In order to explore the intermediacy of a heterocyclic carbene 6 in the photodecomposition, we carried out the photolyses of argon-purged solutions of 1a and 1b in acetonitrile-d3 containing an excess of cyclohexene at 254 and 300 nm. The expectation was that if photochemistry took place through the intermediacy of a carbene, it could be trapped by cyclohexene to form the typical carbene addition product. However, analyses of the crude reaction mixture by NMR spectroscopy and ESI-MS/MS did not indicate the formation of product corresponding to the trapped carbene.
Investigating the intermediacy of a biradical
Next, we considered the possibility that the photochemistry could proceed through the intermediacy of a 1,3-biradical. The photolyses of argon-purged solutions 1a – b in acetonitrile-d3 was carried out in the presence of a hydrogen atom donor, 1,4-cyclohexadiene (1,4-CHD) at 300 nm. We predicted that if the photoreaction of 1a – b occurred through the intermediacy of a biradical, it could be trapped by 1,4-CHD prior to expulsion of sulfur, yielding a thiourea reduction product. Indeed, the NMR spectroscopic analyses of the reaction mixture indicated the formation of thioureas 8a – b as major product (Scheme 5). In addition, trace amounts of the respective carbodiimides 5a – b were also formed. Our studies demonstrated that as the concentration of 1,4-CHD was increased the yield of thiourea also increased (Figure 4). The formation of thioureas 8a – b as the major products during the photolysis of 1a – b in the presence of 1,4-CHD is suggestive of the intermediacy of a 1,3-biradical 7a – b in the photodecomposition of the title compounds. While this trapping experiment strongly implicates the intermediacy of a biradical, it does not provide definitive evidence for whether the biradical exists in its triplet or singlet spin multiplicity. However, since singlet biradicals are known to be extremely short-lived,23, 24 in all probability the trapped species is the triplet biradical since these have lifetimes long enough to react with externally added traps.25 In addition, desulfurization from a triplet biradical 7a – b to produce carbodiimide 5a – b will yield a ground state triplet sulfur atom (3P)26 which is energetically more favorable than desulfurization from a singlet biradical 7a – b which will lead to the formation of an excited state singlet sulfur atom (1D). We believe that the lost sulfur atoms become S8 as this is the most common allotrope of sulfur.27 This argument is further supported by the yellow coloration of the reaction mixture produced after photolysis which is suggestive of S8 formation.
SCHEME 5.
Formation of thioureas 8a – b during the photolysis of 1a – b in the presence of 1,4-CHD via the intermediacy of a 1,3-biradical 7a – b.
FIGURE 4.

Plot showing an increase in the amount of thioureas 8a (left) and 8b (right) with an increase in the concentration of 1,4-CHD during the photolysis of 1a and 1b in acetonitrile, respectively.
Investigating the involvement of a triplet excited state
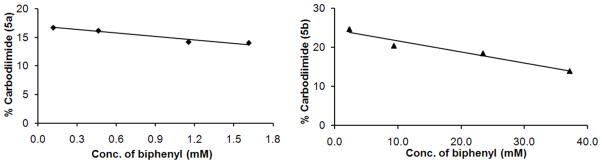
Since the 1,4-CHD trapping experiments were suggestive of a triplet biradical intermediate, a plausible precursor to such a species would be the triplet excited state of the photoprecursor 1a – b. In order to explore the possibility of photochemistry from a triplet excited state, we irradiated argon-purged solutions of 1a – b in acetonitrile-d3 at 300 nm in the presence of triplet sensitizers of varying energies (e.g. benzophenone, acetophenone and acetone). The analyses of the reaction mixture by NMR spectroscopy after 60 minutes of irradiation revealed that no carbodiimides 5a – b were formed and therefore, no photosensitization was observed. In order to rule out the possibility of high lying triplet state, we also performed the photolyses at 300 nm in the presence of a triplet quencher, e.g. biphenyl (Figure 5). Our results indicate that as the concentration of biphenyl was increased, the yields of carbodiimide were only slightly decreased. This minimal decrease in the yield of photoproduct in the presence of a quencher could be explained by a slight competitive absorption of the quencher at 300 nm. Photolyses of the oxygen-saturated solutions of 1a – b in acetonitrile-d3 were also carried out at 254 and 300 nm. The conversion of the tetrazolethiones and the product yields were found to be almost identical to that of the argon-saturated solutions indicating that the effect of the oxygen on the photochemistry of tetrazolethiones was negligible (Table 2).
FIGURE 5.
Modest quenching of carbodiimides 5a (right) and 5b (left) during the photolysis of 1a and 1b in the presence of biphenyl, respectively.
The inability to detect any photoproducts during triplet sensitization of 1a – b, as well as only a modest inhibition of carbodiimide formation observed in the triplet quenching experiments with biphenyl, does not provide a strong support for the intermediacy of a triplet excited state. Given that our computations indicate that the excited state of 1a – b is of π,π* nature19 (Supporting information), this may be due to a slow rate of intersystem crossing relative to singlet bond scission (1π,π*➔3π,π* intersystem crossings are frequently slowed due to this being a spin-orbit coupling ‘forbidden’ conversion28). While negative results do not provide conclusive evidence, dissociation of dinitrogen from the singlet excited state seems more plausible.
Conclusions
We have reported a clean photoconversion of 1-methyl-4-phenyl-1H-tetrazole-5(4H)-thione (1a) and 1-(3-methoxyphenyl)-4-methyl-1H-tetrazole-5(4H)-thione (1b) to the corresponding photostable carbodiimides 5a – b. Analogous to the 5-oxo derivatives of tetrazoles, the photodecomposition 5-thio derivatives occurs via the intermediacy of a 1,3-biradical 7a – b which is believed to be in its triplet spin multiplicity. We find no evidence for an alternative pathway involving a carbene, wherein desulfurization occurs prior to loss of dinitrogen. We were further interested in identifying the nature of precursor that leads to this biradical. The photosensitization and triplet quenching experiments argue against the involvement of a triplet excited state. The obvious alternative mechanistic pathway that could lead to the formation of a 1,3- triplet biradical is a diradicaloid species generated directly from the singlet excited state of 1a – b after the expulsion of dinitrogen. Once formed, this diradicaloid species could be envisioned to undergo intersystem crossing to generate the triplet biradical 7a – b which then would undergo desulfurization to form 5a – b.
Overall, these studies indicate that 1a and 1b are highly promising lead compounds for industrial, agricultural and medicinal applications. The search for derivatives with improved quantum yields that retain the clean photochemistry and end product photostability of 1a – b would be of considerable interest. Our mechanistic studies suggest that derivatives should be sought that favor dinitrogen dissociation in the excited state, since this appears to be the favored pathway in the photodecomposition of these compounds.
Experimental Section
General Procedures
Thin layer chromatography was carried out on 250 μm silica gel plates and UV-light was used as a visualizing agent. Standard column chromatography was performed using 63–200 μm silica gel. 1H and 13C NMR spectra for the structural characterization of the compounds were recorded on 400 MHz NMR spectrometer. The carrier frequencies were 399.75 MHz (1H) and 100.53 MHz (13C). Number of scans used was 64 for 1H NMR spectra and for 13C, it ranged from 3–5K depending on the sample concentration. Both the 1H and 13C spectra were recorded with longer relaxation time (10 s). Chemical shifts and the coupling constants are reported in parts per million and Hertz, respectively. All the quantitative analyses of the photolyzed reaction mixtures were performed by NMR spectroscopy with 1,4-dioxane as an internal standard.29 These experiments were carried out on a 500 MHz NMR spectrometer equipped with a 3 mm triple resonance inverse detection pulse field gradient probe operating at 499.848 MHz for 1H. The spectra were an accumulation of 64 individual scans. The photoproducts were assigned by comparison of their chemical shift values to that of authentic samples. The infrared frequencies are reported in cm–1. High resolution mass spectra were acquired on quadrupole/time-of-flight mass spectrometer. The samples were prepared in methanol/acetonitrile (containing 0.1% formic acid in some cases) and were introduced by continuous infusion into the electrospray ionization (ESI) source at a rate of 30 μL/min. TOF scans were carried out in positive ionization mode. In most cases, both [M+H]+ and [M+Na]+ ions were detectable for each species. All irradiations were carried out in a Rayonet reactor at 254 nm and broad band 300 nm UV light. Monochromatic 313 nm was obtained by using broad band 300 nm UV lamps and by filtering the radiation through a solution of 0.002M K2CrO4 in 5% Na2CO3.
Note that the syntheses of 1a – b require TMSN3 and Me2SO4 and extreme caution must be taken during the handling of these reagents because of their hazardous nature.
1-(3-Methoxyphenyl)-4-methyl-1H-tetrazole-5(4H)-thione (1b)
P2S5 (1.3 g, 5.8 mmol) was added to a solution of 4b (0.5 g, 2.4 mmol) in dry toluene (15 mL). The mixture was refluxed at 110°C until the starting material disappeared. The reaction mixture after filtration was concentrated under reduced pressure. Purification by column chromatography (SiO2, hexane:ethyl acetate, 93:7) gave 1b (0.42 g, 78% yield) as a white solid: Rf 0.64 (70:30 hexane:ethyl acetate); mp 52–54°C; FTIR (ATR) 1600, 1590, 1492, 1440, 1356, 1324, 1238, 1195, 1160, 1065, 1022, 865, 839, 782, 681. 1H NMR (400 MHz, CD3CN ): δ 7.53 (s, 1H), 7.51–7.49 (m, 1H), 7.46–7.43 (m, 1H), 7.13–7.10 (m, 1H), 3.89 (s, 3H), 3.85 (s, 3H) ; 13C NMR (400 MHz, CD3CN): δ 165.0, 161.0, 137.0, 131.2, 117.2, 116.2, 111.0, 56.4, 35.5. HRMS: exact mass calculated for (M+H+) C9H11N4OS+ = 223.0654, found 223.0648; (M+Na+) C9H10N4OSNa+ = 245.0473, found 245.0468.
Spectroscopic data
1-Phenyl-1H-tetrazol-5(4H)-one (3a)18, 19
White solid (1.67 g, 82%); 1H NMR (400 MHz, DMSO-d6): δ 7.85 (d, J = 7.68 Hz, 2H), 7.56 (t, J = 7.60 Hz, 2H), 7.42 (t, J = 7.51 Hz, 1H); 13C NMR (400 MHz, DMSO-d6): δ 150.2, 134.1, 129.4, 127.5, 119.5.
1-Methyl-4-phenyl-1H-tetrazol-5(4H)-one (4a)19
Crystalline solid (0.30 g, 91%); 1H NMR (400 MHz, DMSO-d6): δ 7.85 (d, J = 8.27 Hz, 2H), 7.58 (t, J = 7.03 Hz, 2H), 7.44 (t, J = 7.40 Hz, 1H), 3.62 (s, 3H, CH3); 13C NMR (400 MHz, DMSO-d6): δ 148.8, 134.2, 129.5, 127.7, 119.4, 31.2.
4-Methyl-1-phenyl-1H-tetrazole-5(4H)-thione (1a)19
Yellow solid (2.30 g, 53%); 1H NMR (400 MHz, CD3CN ): δ 7.89–7.85 (m, 2H), 7.63–7.55 (m, 3H), 3.89 (s, 3H); 13C NMR (400 MHz, CD3CN): δ 165.1, 136.2, 130.8, 130.3, 125.3, 35.5.
1-(3-Methoxyphenyl)-1H-tetrazol-5(4H)-one (3b)20
Pale white solid (0.52 g, 81% yield); 1H NMR (400 MHz, DMSO-d6): 7.47–7.41 (m, 3H), 6.98 (d, J = 4 Hz, 1H), 3.80 (s, 3H); 13C NMR (400 MHz, DMSO-d6): 159.8, 150.2, 135.3, 130.4, 113.0, 111.3, 105.0, 55.4.
1-Methyl-4-(3-methoxyphenyl)-1H-tetrazol-5(4H)-one (4b)20
Crystalline solid (0.50 g, 90%); 1H NMR (400 MHz, DMSO-d6): 7.49 – 7.43 (m, 3H), 7.15 (d, 1H), 3.81 (s, 3H), 3.61 (s, 3H); 13C NMR (400 MHz, DMSO-d6): 159.8, 148.7, 135.3, 130.6, 113.2, 111.3, 105.0, 55.5, 31.3.
The synthesis of the authentic samples is described below:
1-Methyl-3-phenylthiourea (8a)30
8a was prepared by a slight modification of the previously reported procedure. To a solution of aniline (1.5 g, 16.1 mmol) in methanol (75 mL) was added methylisocyanate (1.3 g, 17.7 mmol), and the reaction mixture was stirred at 65°C for 24 – 30 h. After the completion of the reaction, solvent was removed by distillation under reduced pressure to obtain crude product. Recrystallization in ethanol yielded 8a (2.46 g, 92% yield) as white solid: Rf 0.29 (60:40 hexane:ethyl acetate); mp 110–112°C; FTIR (ATR) 3259, 3155, 2989, 2937, 1515, 1490, 1287, 1246, 1210, 1026, 1001, 723, 689, 640, 602. 1H NMR (400 MHz, CD3CN ): δ 8.12 (s, 1H), 7.41–7.37 (m, 1H), 7.29–7.22 (m, 3H), 6.56 (s, 1H), 2.97 (d, J = 8 Hz, 3H); 13C NMR (400 MHz, CD3CN): δ 182.8, 138.7, 130.4, 127.0, 126.0, 32.1. HRMS: exact mass calculated for (M+H+) C8H11N2S+ = 167.0643, found 167.0637; (M+Na+) C8H10N2SNa+ = 189.0462, found 189.0457.
1-(3-Methoxyphenyl)-3-methylthiourea (8b)31
8b was prepared as previously reported.31 White solid (2.1 g, 87% yield); mp 98–100°C; 1H NMR (400 MHz, CD3CN ): δ 8.06 (s, 1H), 7.29 (t, 1H), 6.88–6.78 (m, 3H), 6.62 (s, 1H), 3.78 (s, 3H), 2.97 (d, J = 8 Hz, 3H); 13C NMR (400 MHz, CD3CN): δ 182.7, 161.5, 139.7, 131.3, 117.7, 112.5, 111.4, 56.1, 32.2. HRMS: exact mass calculated for (M+H+) C9H13N2OS+ = 197.0749, found 197.0743; (M+Na+) C9H12N2OSNa+ = 219.0568, found 219.0563.
N-((Methylimino)methylene)aniline (5a)
5a was prepared by a different method than previously reported.32 Mercuric oxide (3.9 g, 18.1 mmol) was added to a solution of 8a (1.0 g, 6.0 mmol) in CH2Cl2: H2O (4:1, 30 mL), and the mixture was stirred at room temperature for 30 min. The reaction mixture was filtered through celite and washed with ample amounts of methylene chloride. The filterate was concentrated under reduced pressure. Purification by column chromatography (SiO2, hexane:ethyl acetate, 95:5) gave 5a (0.1 g, 12% yield) as a yellow oil: Rf 0.83 (70:30 hexane:ethyl acetate); FTIR (ATR) 3059, 3024, 2935, 2879, 2121, 2026, 1592, 1499, 1406, 1282, 1156, 1070, 891. 1H NMR (400 MHz, CD3CN ): δ 7.33–7.29 (t, 2H), 7.14–7.09 (m, 3H), 3.14 (s, 3H); 13C NMR (400 MHz, CD3CN): δ 142.0, 137.0, 130.5, 125.6, 124.4, 32.8. HRMS: exact mass calculated for (M+H+) C8H9N2+ = 133.0766, found 133.0760.
3-Methyl-N-((methylimino)methylene)aniline (5b)
Similarly, compound 5b (0.35 g, 42% yield) was obtained from 8b (1.00 g, 5.1 mmol). Purification by column chromatography (SiO2, hexane:ethyl acetate, 95:5) gave 5b (0.35 g, 42% yield) as a yellow oil: Rf 0.71 (70:30 hexane:ethyl acetate); FTIR (ATR) 2937, 2834, 2123, 1594, 1581, 1493, 1464, 1421, 1281, 1243, 1127, 1038, 945, 840, 769, 684 589. 1H NMR (400 MHz, CD3CN ): δ 7.20 (t, 1H), 6.70 - 6.63 (m, 3H), 3.76 (s, 3H), 3.14 (s, 3H); 13C NMR (400 MHz, CD3CN): δ 161.6, 143.2, 136.7, 131.1, 116.7, 111.3, 109.9, 56.0, 32.8. HRMS: exact mass calculated for (M+H+) C9H11N2O+ = 163.0871, found 163.0866.
Photochemistry
UV spectral changes
An argon-purged solution of 1a (0.07 mM) and 1b (0.13 mM) in acetonitrile was irradiated in a quartz cuvette at 254 nm. In case of 1a, a UV spectrum was obtained after each irradiation at 0, 20, 40, 60, 120, 200, 300 and 420s, where as in case of 1b, a UV spectrum was obtained after each irradiation at 0, 20, 40, 60, 80, 120 and 150s, respectively. Similarly, the irradiation of an argon-purged solution of 1a (0.2 mM) and 1b (0.075 mM) in acetonitrile was carried out in a quartz cuvette at 313 nm and a UV spectrum was obtained after irradiation at different time intervals (at 0, 1, 5, 10, 15, 20, 30, 40 and 50 min for 1a and 0, 5, 10, 15, 20, 25, 25, 30, 35 and 40 min for 1b).
Product analysis
An argon-purged solution of 1a – b (0.45 mL) in acetonitrile-d3 (approximately in the range 4.63 mM) was irradiated in a quartz NMR tube at 254 and 300 nm and a NMR spectrum was obtained at every 5 min time interval for 15 min and 60 min, respectively.
Photolysis of carbodiimides 5a – b
An argon-purged solution of 5a – b (0.7 mL) in acetonitrile-d3 (approximately in the range 4.70 mM) was irradiated in a quartz NMR tube at 254 for 120 min and a NMR spectrum was obtained.
Photolysis in the presence of cyclohexene
An argon-purged solution of 1a – b (0.7mL) in acetonitrile-d3 (approximately in the range 4.55 mM) containing 10 equivalents of cyclohexene was irradiated at 254 and 300 nm in a quartz NMR tube for 30 and 60 minutes, respectively, and a NMR spectrum was obtained. For the ESI-MS/MS experiments, the concentration of 1a and 1b was in the range 1.5 mM. 8 mL of this solution was irradiated at 254 and 300 nm. The solvent was removed on a rotary evaporator and crude was analyzed.
Photolysis in the presence of 1,4-cyclohexadiene
Four separate solutions of 1a – b (approximately in the range 4.63 mM) in acetonitrile-d3 (0.7 mL) containing varying amounts of 1,4-CHD (between 10 – 50 equivalents) were taken in quartz NMR tubes. The mixture in each tube was purged with argon for 15 minutes and irradiated with broad band 300nm UV lamp for 60 minutes and a NMR spectrum was recorded.
Photosensitization and triplet quenching
The concentration of 1a and 1b for the following experiments was approximately 4.63 mM in acetonitrile-d3.
Three separate solutions of 1a – b (0.7 mL) in acetonitrile-d3 containing benzophenone, acetophenone and acetone (between 1 – 20 equivalents), respectively were taken in quartz NMR tubes, purged with argon for 15 minutes and irradiated with broad band 300 nm UV lamp for 60 minutes. Subsequently, a NMR spectrum was obtained.
Similarly, the irradiation of the oxygen-saturated solutions of 1a – b in acetonitrile-d3 was carried out at 254 nm and 300 nm to determine the effect of oxygen on product yields.
Four separate solutions of 1a – b in acetonitrile-d3 (0.7 mL) containing varying amounts of biphenyl (0 – 15 equivalents) were taken in different quartz NMR tubes. The mixture in each tube was purged with argon for 15 minutes and irradiated with broad band 300nm UV lamp for 60 minutes and NMR spectrum was recorded.
Effect of oxygen
An oxygen-purged solution of 1a – b (0.65 mL) in acetonitrile-d3 (approximately in the range 4.63 mM) was irradiated in a quartz NMR tube at 254 and 300 nm for 15 min and 60 min, respectively and NMR spectrum was recorded.
Actinometry
5.04 mM solution of azoxybenzene in 95% ethanol and 0.1 M solution of KOH in 95% ethanol were prepared. The azoxybenzene solution was irradiated for 30 minutes in a quartz cuvette at 254nm (no purging with Ar required) and 1 mL of the irradiated solution was mixed with 1mL of 0.1 M KOH solution followed by the addition of 8mL of 95% EtOH in a volumetric flask. A UV spectrum was subsequently recorded and the incident photons were calculated in moles/L/sec as described by Bunce et al.22 The quantum yields were calculated at less than 10% conversions.
Supplementary Material
Acknowledgments
Acknowledgment is made to the donors of the American Chemical Society Petroleum Research Fund for the partial support of the research described herein (ACS PRF # 48202-G4). This study was also supported in part by grants from Kansas State University (Startup funds and Innovative Research Award from the Terry C. Johnson Center for Basic Cancer Research). The authors acknowledge Professor Ruth Welti and Ms. Pamela Tamura at the Kansas Lipidomics Research Center (KLRC) for providing the mass spectra on our compounds and Professor Om Prakash and Mr. Alvaro Herrera for assistance with the 500MHz NMR experiments at the Biomolecular NMR facility which is supported by NIH (grants RR-025441 and RR-017686) and KSU Targeted Excellence Programs.
Footnotes
Supporting Information Available. Theoretical calculations on the electronic properties of 1b; computational methods; resonance structures of 1b (Scheme S1); ground state geometry of 1b (Figure S1); cartesian coordinates for the optimized 1b; experimental absorption spectra and TDDFT calculated vertical excitations as stick spectra for 1b in cyclohexane, THF and acetonitrile (Figure S2); molecular orbitals for the optimized geometries of 1b in acetonitrile (Figure S3); TDDFT calculated vertical excitation energies, oscillator strengths, MO character and transition type of 1b in cyclohexane, THF and acetonitrile (Tables S1); NMR spectra of 1a – b before and after irradiation at 254 nm (Figures S4 and S5); 1H NMR and 13C NMR spectra of 1b and 5b; 1H NMR spectra of 5a and 8a. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mihit M, Salghi R, El Issami S, Bazzi L, Hammouti B, Ait Addi E, Kertit S. Pigm Resin Technol. 2006;35:151. [Google Scholar]
- 2.Mihit M, El Issami S, Bouklah M, Bazzi L, Hammouti B, Ait Addi E, Salghi R, Kertit S. Appl Surf Sci. 2006;252:2389. [Google Scholar]
- 3.Essoufi H, Kertit S, Hammouti B, Benkaddour M. Bull Electrochem. 2000;16:205. [Google Scholar]
- 4.Foldenyi R. Monatsh Chem. 1995;126:1035–43. [Google Scholar]
- 5.Eachus RS, Muenter AA, Pawlik TD, Lenhard JR. J Phys Chem B. 2005;109:10126. doi: 10.1021/jp0504672. [DOI] [PubMed] [Google Scholar]
- 6.Tamura M, Hada H, Noguchi J, Hayashi S. J Phys Chem. 1962;66:559. [Google Scholar]
- 7.Taylor JW, Jiang Y, Bassett DR. Polym Mater Sci Eng. 1991;64:50. [Google Scholar]
- 8.Uchihara T, Kamiya T, Maedomari S, Ganeko M, Kinjo S, Tamaki Y, Kinjo A. J Photochem Photobiol, A. 2000;130:63. [Google Scholar]
- 9.Wang C-y, Liu C-y, Shen T. J Photochem Photobiol, A. 1997;109:65. [Google Scholar]
- 10.Nesmerak K, Pospisek M, Nemec I, Waisser K, Gabriel J. Folia Microbiol. 2000;45:138. doi: 10.1007/BF02817412. [DOI] [PubMed] [Google Scholar]
- 11.Dunkin IR, Shields CJ, Quast H. Tetrahedron. 1989;45:259. [Google Scholar]
- 12.Gomez-Zavaglia A, Reva ID, Frija L, Cristiano ML, Fausto R. J Mol Struct. 2006;786:182. [Google Scholar]
- 13.Quast H, Nahr U. Chem Ber. 1985;118:526. [Google Scholar]
- 14.Quast H, Bieber L. Chem Ber. 1981;114:3253. [Google Scholar]
- 15.Quast H, Nahr U. Chem Ber. 1983;116:3427. [Google Scholar]
- 16.Frija LMT, Khmelinskii IV, Serpa C, Reva ID, Fausto R, Cristiano MLS. Org Biomol Chem. 2008;6:1046. doi: 10.1039/b718104c. [DOI] [PubMed] [Google Scholar]
- 17.Frija LMT, Khmelinskii IV, Cristiano MLS. J Org Chem. 2006;71:3583. doi: 10.1021/jo060164j. [DOI] [PubMed] [Google Scholar]
- 18.Tsuge O, Urano S, Oe K. Reactions of trimethylsilyl azide with heterocumulenes. J Org Chem. 1980;45:5130. [Google Scholar]
- 19.Rayat S, Chhabra R, Alawode O, Gundugola AS. J Mol Struct. 2009;933:38–45. [Google Scholar]
- 20.Rayat S, Alawode O, Desper J. CrystEngComm. 2009:1892. [Google Scholar]
- 21.Frija LMT, Reva ID, Gomez-Zavaglia A, Cristiano MLS, Fausto R. Photochem Photobiol Sci. 2007;6:1170. doi: 10.1039/b703961a. [DOI] [PubMed] [Google Scholar]
- 22.Bunce NJ, LaMarre J, Vaish SP. Photochem Photobiol. 1984;39:531. [Google Scholar]
- 23.Hoyle CE, Clark SC, Viswanathan K, Jonsson S. Photochem Photobiol Sci. 2003;2:1074. doi: 10.1039/b307087e. [DOI] [PubMed] [Google Scholar]
- 24.Casey CP, Boggs RA. J Amer Chem Soc. 1972;94:6457. [Google Scholar]
- 25.Bucher G, Mahajan AA, Schmittel M. J Org Chem. 2008;73:8815. doi: 10.1021/jo801689w. [DOI] [PubMed] [Google Scholar]
- 26.Breckenridge WH, Taube H. J Chem Phys. 1970;53:1750. [Google Scholar]
- 27.Greenwood NN, Earnshaw A. Chemistry of the Elements. 2. 1997. [Google Scholar]
- 28.Turro NJ. Modern Molecular Photochemistry. 1978:628. [Google Scholar]
- 29.Peterson J. J Chem Educ. 1992;69:843. [Google Scholar]
- 30.Ambati NB, Anand V, Hanumanthu P. Synth Commun. 1997;27:1487. [Google Scholar]
- 31.Vassilev GN, Vassilev NG. Oxid Commun. 2002;25:608. [Google Scholar]
- 32.Fell JB, Coppola GM. Synth Commun. 1995;25:43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.