Abstract
Purpose of review
Plant pollens are one of the most common outdoor allergens. Pollen grains and subpollen particles can reach lower airways and induce symptoms of seasonal asthma and allergic rhinitis. Plants possess NAD(P)H oxidase activity that generates reactive oxygen species for physiological functions such as root-hair and pollen-tube growth, defense against microbial infections and cell signaling. The presence of NAD(P)H oxidases in pollens and their role in induction of airway inflammation have not been described until recently.
Recent findings
We discovered the presence of NAD(P)H oxidase in ragweed and other plant pollens. These oxidases induce reactive oxygen species in mucosal cells (signal 1) independent of adaptive immunity. This reactive oxygen species facilitates antigen (signal 2)-induced allergic inflammation. Inhibiting signal 1 by administration of antioxidants attenuated ragweed extract-induced allergic inflammation. Likewise, abrogating signal 2 by antigen challenge in mice lacking T cells failed to induce allergic inflammation.
Summary
Reactive oxygen species generated by pollen NAD(P)H oxidase play a major role in pathogenesis of allergic airway inflammation and airway hypersensitivity. Based on our findings, we propose a ‘two signal hypothesis of allergic inflammation’ in which both signal 1 (reactive oxygen species) and signal 2 (antigen presentation) are required in order to induce full-blown allergic inflammation.
Keywords: airway inflammation, NAD(P)H oxidase, reactive oxygen species
Introduction
The worldwide prevalence of allergic diseases is increasing. One of the prominent theories of this phenomenon is the ‘Hygiene Hypothesis of Asthma and Allergic Diseases’. According to this theory, improvement in hygiene in developed countries has contributed to an increase in allergic diseases [1]. Other studies, however, have failed to show the protection conferred by poor hygiene [2]. This has led to a scientific enigma: are there factors other than hygiene that are contributing to increased prevalence of allergic diseases?
Allergic asthma is characterized by airway eosinophilia, mucus hypersecretion and development of airway hyper-responsiveness (AHR). In patients with asthma markers of oxidative stress, such as 8-isoprostane, malondialdehyde, nitrotyrosine, protein carbonyls, H2O2 and nitric oxide are increased [3-7]. These reactive oxygen species (ROS) cause respiratory epithelial cell damage and induce hyperreactivity of human peripheral airways [8]. These and other studies indicate that oxidative stress is an important contributor to the pathogenesis of asthma.
Several studies indicate synergistic effects of allergens with environmental pro-oxidants like diesel exhaust particles (DEP) or ozone in increasing intensity of allergic symptoms. In urban areas rich in DEPs such as near busy roads, people are more likely to have severe allergic symptoms compared with people living in areas with high pollen loads but with less traffic [9]. Other studies have confirmed the augmenting effect of environmental pro-oxidants in allergic symptoms [10,11].
Does induction of allergic inflammation always need a combination of protein allergen from pollens and environmental pro-oxidants? While studying this question, we recently discovered the presence of NAD(P)H oxidase in pollens [12]. These oxidases are packaged with pollen antigens. In a series of papers, we demonstrated that these oxidases induce ROS in mucosal cells and perpetuate antigen-induced allergic inflammation [12]. Here we review the role of NAD(P)H oxidases in pollens and subpollen particles, their effect on allergic airway inflammation, and utility of antioxidants in prevention of allergic airway inflammation.
Reactive oxygen species and their effects on airway function
The term ‘reactive oxygen species’ denotes oxygen radicals that have unpaired electrons like superoxide (O2•–) and hydroxyl radical (OH−), or paired electrons like hydrogen peroxide (H2O2), hypochlorous acid (HOCl) and peroxynitrite (ONOO−). ROS are formed in biological systems by oxidases and as a byproduct of respiration. At the same time, a variety of antioxidant and ROS scavenging mechanisms counterbalance these deleterious effects on cells. The excess ROS unbalanced by antioxidants, termed ‘oxidative stress’, play an important role in the pathogenesis of allergic airway diseases.
The respiratory system, by virtue of its direct contact with the external environment, is continuously exposed to environmental pro-oxidants. ROS generated from this exposure induce inflammation, and airway smooth-muscle activation, contraction and proliferation [13-18]. Hospital admissions for asthma increase on days with high O3 concentrations [19]. H2O2 augments airway smooth-muscle tone by increasing Ca2+ influx from extracellular space and cellular sensitization to Ca2+ by Rho kinase [20•]. ONOO−, an ROS present in exhaled air, induces nitration of protein tyrosines to form 3-nitrotyrosine, and increases airway resistance in mice [4,21-23]. These reports indicate that ROS not only influence the extent of inflammation but also the tone of the airway smooth muscle.
Pollens contain intrinsic NAD(P)H oxidases
During the plant fertilization process, a pollen tube grows from the pollens that land on the stigma of the receptive flower. The process of pollen-tube growth demands high energy and requires rapid oxygen uptake, and is regulated by Rac homologs and Ca2+ signaling [24-26]. Foreman and colleagues [27] reported that development of root-hair growth involves NAD(P)H oxidase, which exerts control on cell expansion through ROS-induced Ca2+ channel signaling. These studies showed the importance of NAD(P)H oxidase in plant physiology.
In 2005, while studying the factors that induce pollen-mediated allergic inflammation, we discovered the presence of NAD(P)H oxidase in ragweed and 41 other pollens [12]. These pollen oxidases generate O2•– anions, which reduce redox-sensitive nitroblue tetrazolium into formazan and dihydro dichlorofluorescein diacetate into fluorescent dichlorofluorescein. The ROS-generating activity of these oxidases was inhibited by NAD(P)H oxidase inhibitors diphenylneiodonium (DPI), quinacrine and heat inactivation. The pollen NAD(P)H oxidase reduced cytochrome c, indicating that it generates O2− and this was inhibited by superoxide dismutase.
Although most pollens cannot reach the lower airway due to their large size, pollen hydration and hypotonic shock by rainwater can lead to release of subpollen particles (SPP) varying from 0.5 to 4.5 μm in size that can penetrate to the lower airways because they are in the respirable range [28••]. These SPP retain the Amb a 1 antigen, as well as NAD(P)H oxidase activity. In fact, the process of releasing SPP itself requires oxidase activity, as addition of NAD(P)H oxidase inhibitors quinacrine and DPI inhibited this phenomenon [28••].
Why do pollens have NAD(P)H oxidases? This question was answered recently by two groups. The first group demonstrated that NAD(P)H oscillates in the growing pollen tip [29]. The second group identified that pollentube growth requires ROS generated by NAD(P)H oxidase [30]. Transfection of pollen tubes with antisense oligonucleotides to tobacco NAD(P)H oxidase decreased mRNA levels, and NAD(P)H oxidase activity and inhibited pollen-tube growth [30]. Addition of exogenous H2O2 rescued pollen-tube growth inhibition caused by NADPH oxidase antisense oligos [30]. Similarly, NAD(P)H oxidase inhibitor, DPI, decreased ROS formation in tobacco pollen-tube cultures and thereby inhibited their growth [30]. These studies indicate that intrinsic pollen NAD(P)H oxidases are required to generate ROS and induce pollen-tube growth.
Pollen NAD(P)H oxidase induces reactive oxygen species and augments allergic airway inflammation
We reported that ragweed pollen extract (RWE) NAD(P)H oxidase increased intracellular ROS levels in airway epithelial cells and normal human bronchial epithelial (NHBE) cells cultured at air–liquid interphase to mimic physiologic conditions [12]. The NAD(P)H oxidase inhibitors DPI and quinacrine inhibited this increase in intracellular ROS levels. Administration of RWE into murine lungs ex vivo enhanced the ROS levels in airway epithelium. RWE challenge increased oxidative stress markers, oxidized glutathione (GSSG), 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) in the lungs in wild-type, as well as in mice lacking T cells, B cells or mast cells, suggesting that the ability of NAD(P)H oxidase to induce ROS in lungs is independent of adaptive immune response.
Next we investigated how this induction of ROS influences antigen-induced allergic airway inflammation. Challenge of sensitized mice with Amb a 1 (the most abundant antigenic component of RWE that lacks pro-oxidant activity) or RWE column chromatography fractions that lack NAD(P)H oxidase activity (pRWEOX−) induced minimal inflammatory cell recruitment and mucin production in the airways. Co-administration of a surrogate ROS generator with Amb a 1 induced full-blown inflammation observed with RWE challenge. Addition of NADPH oxidase high pro-oxidant containing activity fraction (pRWEOX+) to pRWEOX− resulted in increased eosinophilic inflammation in the airways. RWE-sensitized mice challenged with RWE showed increased airway hyperresponsiveness to increasing concentration of methacholine compared with pRWEOX− challenge (Fig. 1). pRWEOX− challenge induced lower pRWEOX− specific IgE levels in serum than RWE challenge. These findings indicate that pollen NAD(P)H oxidase-induced ROS are essential for generating robust allergic inflammatory response, airway hyperresponsiveness and IgE production.
Figure 1. Ragweed extract NAD(P)H oxidase-induced reactive oxygen species increase airway hyperresponsiveness.
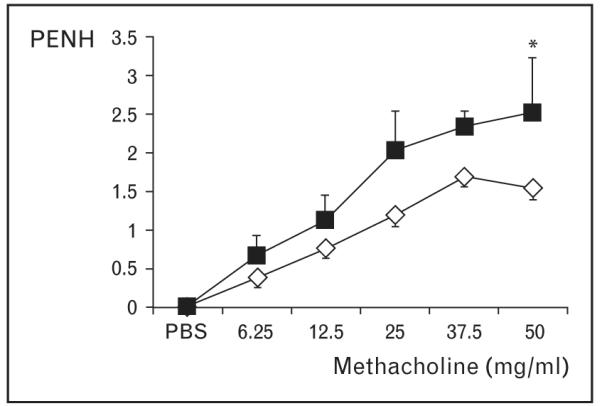
Ragweed extract-sensitized Balb/c mice were challenged with either ragweed extract (■) or pRWEOX+ (◇). Airway hyperresponsiveness to increasing dose of methacholine was measured using BUXCO instrument 48 h postchallenge. Results are means ± SEM (n = 3–6 mice per group). *P < 0.05.
Antioxidants inhibit pollen NAD(P)H oxidase-induced angmentation of allergic airway inflammation
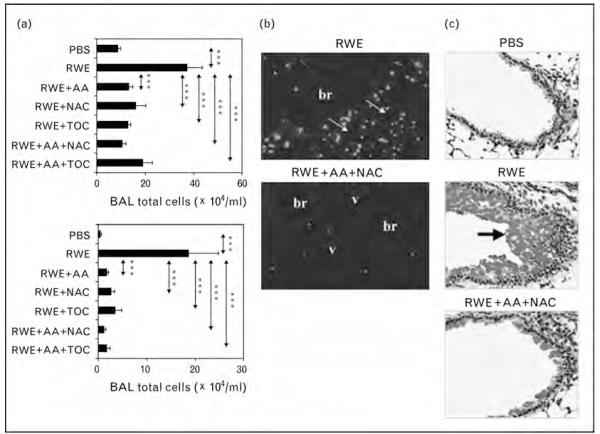
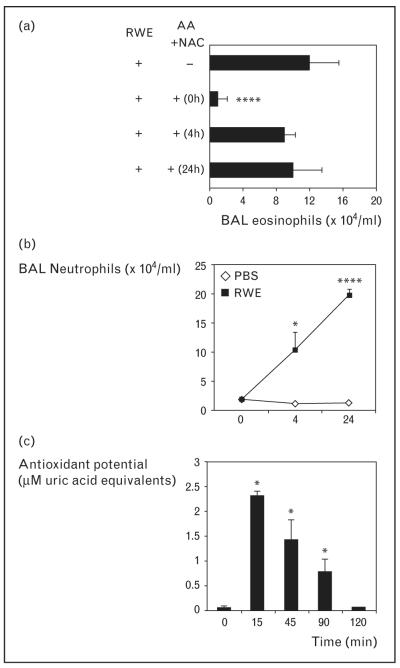
Early this year we reported that antioxidants N-acetyl cysteine (NAC), ascorbic acid, reduced glutathione or tocopherol or their combination significantly decreased ROS generated by RWE pollen NAD(P)H oxidase [31•]. Intranasal administration of ascorbic acid+NAC significantly decreased RWE-induced allergic airway inflammation in sensitized mice (Fig. 2). This change was associated with decreased IL-4 and Clca3 transcripts in the lungs. Of particular importance is our finding that ascorbic acid+NAC co-administered with RWE challenge prevented airway eosinophilia. When ascorbic acid+NAC were administered 4 or 24 h later, when neutrophilic infiltration in the airways was highest, there was no reduction in airway eosinophilia (Fig. 3a, b). This finding suggests that it is the decrease in pollen NAD(P)H oxidase-induced ROS and not ROS generated by neutrophils that prevents subsequent development of airway inflammation.
Figure 2. Scavenging pollen NAD(P)H oxidase-generated reactive oxygen species inhibit allergic lung inflammation.
Ragweed extract-sensitized Balb/c mice were challenged with PBS (phosphate-buffered saline), ragweed extract (RWE) or RWE+ascorbic acid+N-acetyl cysteine and sacrificed at 72 h. (a) Total inflammatory cells and eosinophils in bronchoalveolar lavage fluids of RWE-challenged mice. (b) Impact of antioxidants on recruitment of eosinophils in peribronchial (br) and perivascular (v) regions (arrows). (c) Periodic acid schiff-Stained lung sections showing mucin content of airway epithelial cells. Arrow shows PAS-positive mucin globules. Results are means ± SEM (n=7–9 mice per group). ***P < 0.001. Reproduced with permission from [31•].
Figure 3. Scavenging reactive oxygen species generated by ragweed extract pollen NAD(P)H oxidase and not by inflammatory cells inhibit allergic airway inflammation.
(a) Analysis of eosinophil recruitment in the airways after administration of antioxidants at various time points. (b) Kinetics of neutrophil recruitment in the bronchoalveolar lavage (BAL) fluid. Ragweed extract (RWE)-sensitized mice were challenged with either PBS or RWE and sacrificed kinetically and BAL fluid was examined for cellular content. (c) Ascorbic acid+N-acetyl cysteine increased total antioxidant potential in the airways for 90 min. X-axis represents time after administration of antioxidants. Results are means ± SEM (n=3–6 mice per group). *P < 0.05, ****P < 0.0001. Reproduced with permission from [31•].
To further validate these observations, we studied the role of the iron-binding protein natural antioxidant, lactoferrin, on allergic airway inflammation. Adding lactoferrin to cultured NHBE cells decreased RWE-induced intracellular ROS generation [32]. Co-administration of lactoferrin significantly decreased RWE-induced airway eosinophilia and mucin production in the lungs [32]. Similarly, Lee and colleagues [33] have shown that intraperitoneal administration of l-2-oxothiazolidine-4-carboxylic acid, a prodrug of cysteine, decreased OVA-induced airway inflammation and AHR.
Plant NAD(P)H oxidases are biochemically and functionally similar to mammalian NAD(P)H oxidase. Unlike their mammalian counterpart, the structure of plant NAD(P)H oxidases are not well characterized [34••]. Mammalian NAD(P)H oxidase is composed of membrane-bound gp91phox and p22phox and cytosolic p40phox, p47phox, p67phox and small G-proteins Rac2 and Cdc2. Rac2 is a critical component of NAD(P)H oxidase and is essential for its function and ROS production. Recently, Lo and colleagues personal communication used IL-5 transgenic (IL-5tg) mice and Rac2 knock-out mice crossed with IL-5tg mice to study the role of eosinophil NAD(P)H oxidase on airway inflammation. Even though eosinophils from IL-5tg-Rac2KO showed reduced superoxide production compared with IL-5tg mice, OVA-induced airway inflammation and AHR were similar in both mice. These observations further validated our hypothesis that ROS generated by pollen NAD(P)H oxidase and not those generated by inflammatory cells are critical for initiating allergic airway inflammation.
In randomized control trials, administration of ascorbic acid or vitamin E have failed to exert any beneficial effect on outcome measures of asthma [35,36]. This can be explained by a recent study that showed that even though administration of oral ascorbic acid and vitamin E increased its level in the serum, there was no significant difference in the total serum antioxidant capacity [37]. In our experiments, ascorbic acid+NAC increased the antioxidant potential in the airways only transiently, and it returned to basal levels by 2 h (Fig. 3c). This result, combined with our finding that administration of ascorbic acid+NAC 4 h after RWE challenge had no beneficial role, suggests that it is the local antioxidant potential that is likely to play a major role in circumventing airway inflammation.
Mechanisms by which antioxidant activity modulates allergic inflammation and airway hypersensitivity
We have shown that ragweed extract NAD(P)H oxidases increase GSSG and 4-HNE levels in the lungs [12]. These products of oxidative stress induced tyrosine phosphorylation of p38 mitogen-activated protein kinase and increased IL-8 promoter activity and IL-8 production in cultured airway epithelial cells. Challenging naïve mice with GSSG or pro-oxidant products induced three-fold to four-fold greater neutrophil and total inflammatory cell recruitment in the lungs at 24 h. Co-administration of NAC 4-HNE RWE challenge with RWE-sensitized mice of decreased 4-HNE adduct formation in the lungs [31•]. These observations indicate that pollen NAD(P)H oxidase-generated ROS induce a ‘danger signal’ and this can be inhibited by increasing the antioxidant potential of the airways [38]. This danger signal upregulated IL-4 transcripts in the lungs within 4 h and was inhibited by co-administration of ascorbic acid+NAC. IL-4 is a critical cytokine for generating Th2 response, airway inflammation and AHR [39-41]. Thus, decreasing transcripts of pro-inflammatory cytokines like IL-4 may be another mechanism by which antioxidants prevent RWE NAD(P)H oxidase-induced airway inflammation.
The mechanism of cellular response to oxidative stress is a field of increasing interest. Li and colleagues [42] have proposed a three-tier response to oxidative stress induced by particulate matter. In the first-tier response, cells exposed to oxidative stress upregulate their antioxidant capabilities by nuclear regulatory factor 2 (Nrf2)-dependent transcriptional activation of antioxidant response elements. If antioxidants fail to counterbalance the oxidative stress, tier-2 response sets in by upregulating pro-inflammatory genes and intracellular signaling cascades [43]. Eventually, oxidative stress induces cellular toxicity, mitochondrial perturbation and death by apoptosis or necrosis, and these effects compose the third-tier response. Our findings add a new dimension to these observations in which ROS are packaged with antigenic proteins as an intrinsic part of pollens. It needs to bedetermined whether the three-tier response is operative in pollen NAD(P)H oxidase-induced allergic inflammation.
Conclusion
We have shown that pollens contain intrinsic NAD(P)H oxidase. Blocking ROS generation by pollen NAD(P)H oxidase inhibited AHR, allergic airway inflammation and allergic conjunctivitis in mice [44]. These studies indicate that pollen NAD(P)H oxidases likely contribute substantially to induction of allergic diseases.
Could it be that pollen NAD(P)H oxidases contribute to the increasing prevalence of allergic diseases worldwide? As elegantly described in a movie by Vice-President Al Gore, ‘An Inconvenient Truth’, global warming is changing many environmental dynamics all across the globe. Rapid climatic changes in the last few decades have altered flowering behavior in many plants [45]. Ragweed plants grown in CO2-enriched environments produced more allergenic pollens [46]. Rising temperature leads to early spring and lengthening of the growing season in various plants. In climate-controlled experiments, ragweed plants generated greater biomass and had 54.8% greater pollen production when grown under simulated early-spring conditions [47•]. Also, at high CO2 levels, ragweed plants increased pollen production in simulated late-spring environments [47•]. Based on these findings, the authors suggested that ragweed-pollen production is likely to increase significantly under predicted future climatic conditions [47•]. These findings suggest that global warming may increase the total environmental pollen NAD(P)H oxidase load and contribute to the increasing prevalence of allergic disorders. Future studies should carefully test the validity of this ‘Global Warming Hypothesis of Allergic Disorders’ as an alternative to the hygiene hypothesis.
Acknowledgements
This work was supported by grants from National Institute of Health (1R01 HL071163, SS), NIAID Program Project Grant (1P01 AI062885, SS and IB) and National Heart, Lung and Blood Institute Proteomics Initiative (N01-HV28184, SS).
Footnotes
The authors do not have any conflict of interest in terms of relationships with commercial companies.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 89–90).
- 1.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 2.Ellertsen LK, Wiker HG, Egeberg NT, Hetland G. Allergic sensitisation in tuberculosis and leprosy patients. Int Arch Allergy Immunol. 2005;138:217–224. doi: 10.1159/000088722. [DOI] [PubMed] [Google Scholar]
- 3.Montuschi P, Corradi M, Ciabattoni G, et al. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 4.Kaminsky DA, Mitchell J, Carroll N, et al. Nitrotyrosine formation in the airways and lung parenchyma of patients with asthma. J Allergy Clin Immunol. 1999;104:747–754. doi: 10.1016/s0091-6749(99)70283-6. [DOI] [PubMed] [Google Scholar]
- 5.Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress in acute exacerbations of asthma. J Asthma. 2005;42:45–50. doi: 10.1081/jas-200044774. [DOI] [PubMed] [Google Scholar]
- 6.Emelyanov A, Fedoseev G, Abulimity A, et al. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- 7.Kharitonov SA, Yates D, Robbins RA, et al. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 8.Hulsmann AR, Raatgeep HR, den Hollander JC, et al. Oxidative epithelial damage produces hyperresponsiveness of human peripheral airways. Am J Respir Crit Care Med. 1994;149:519–525. doi: 10.1164/ajrccm.149.2.8306055. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaki T, Koizumi K, Ikemori R, et al. Studies of prevalence of Japanese cedar pollinosis among the residents in a densely cultivated area. Ann Allergy. 1987;58:265–270. [PubMed] [Google Scholar]
- 10.Knox RB, Suphioglu C, Taylor P, et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997;27:246–251. [PubMed] [Google Scholar]
- 11.Motta AC, Marliere M, Peltre G, et al. Traffic-related air pollutants induce the release of allergen-containing cytoplasmic granules from grass pollen. Int Arch Allergy Immunol. 2006;139:294–298. doi: 10.1159/000091600. [DOI] [PubMed] [Google Scholar]
- 12.Boldogh I, Bacsi A, Choudhury BK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szarek JL, Schmidt NL. Hydrogen peroxide-induced potentiation of contractile responses in isolated rat airways. Am J Physiol. 1990;258:L232–237. doi: 10.1152/ajplung.1990.258.4.L232. [DOI] [PubMed] [Google Scholar]
- 14.Gupta JB, Prasad K. Mechanism of H2O2-induced modulation of airway smooth muscle. Am J Physiol. 1992;263:L714–L722. doi: 10.1152/ajplung.1992.263.6.L714. [DOI] [PubMed] [Google Scholar]
- 15.Perkins WJ, Lorenz RR, Bogoger M, et al. A novel mechanism by which hydrogen peroxide decreases calcium sensitivity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2003;284:L324–332. doi: 10.1152/ajplung.00159.2002. [DOI] [PubMed] [Google Scholar]
- 16.Stewart RM, Weir EK, Montgomery MR, Niewoehner DE. Hydrogen peroxide contracts airway smooth muscle: a possible endogenous mechanism. Respir Physiol. 1981;45:333–342. doi: 10.1016/0034-5687(81)90016-5. [DOI] [PubMed] [Google Scholar]
- 17.Rabe KF, Dent G, Magnussen H. Hydrogen peroxide contracts human airways in vitro: role of epithelium. Am J Physiol. 1995;269:L332–338. doi: 10.1152/ajplung.1995.269.3.L332. [DOI] [PubMed] [Google Scholar]
- 18.Evans TW, Brokaw JJ, Chung KF, et al. Ozone-induced bronchial hyper-responsiveness in the rat is not accompanied by neutrophil influx or increased vascular permeability in the trachea. Am Rev Respir Dis. 1988;138:140–144. doi: 10.1164/ajrccm/138.1.140. [DOI] [PubMed] [Google Scholar]
- 19.Cody RP, Weisel CP, Birnbaum G, Lioy PJ. The effect of ozone associated with summertime photochemical smog on the frequency of asthma visits to hospital emergency departments. Environ Res. 1992;58:184–194. doi: 10.1016/s0013-9351(05)80214-2. [DOI] [PubMed] [Google Scholar]
- 20 •.Kojima K, Kume H, Ito S, et al. Direct effects of hydrogen peroxide on airway smooth muscle tone: roles of Ca2+ influx and Rho-kinase. Eur J Pharmacol. 2007;556:151–156. doi: 10.1016/j.ejphar.2006.11.007. This study showed that H2O2 induced airway smooth muscle contraction is mediated by Ca2+ release and is dependent on Rho kinase pathway.
- 21.Sadeghi-Hashjin G, Folkerts G, Henricks PA, et al. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. Am J Respir Crit Care Med. 1996;153:1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- 22.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;162:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 23.Saleh D, Ernst P, Lim S, et al. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 24.Tadege M, Kuhlemeier C. Aerobic fermentation during tobacco pollen development. Plant Mol Biol. 1997;35:343–354. doi: 10.1023/a:1005837112653. [DOI] [PubMed] [Google Scholar]
- 25.Kost B, Lemichez E, Spielhofer P, et al. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierson ES, Miller DD, Callaham DA, et al. Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 27.Foreman J, Demidchik V, Bothwell JH, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 28 ••.Bacsi A, Choudhury BK, Dharajiya N, et al. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. This study showed for the first time that subpollen particles possess NADPH oxidase activity and can induce ROS in the airways and allergic airway inflammation.
- 29.Cardenas L, McKenna ST, Kunkel JG, Hepler PK. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 2006;142:1460–1468. doi: 10.1104/pp.106.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potocky M, Jones MA, Bezvoda R, et al. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007;174:742–751. doi: 10.1111/j.1469-8137.2007.02042.x. [DOI] [PubMed] [Google Scholar]
- 31 •.Dharajiya N, Choudhury BK, Bacsi A, et al. Inhibiting pollen reduced nicotinamide adenine dinucleotide phosphate oxidase-induced signal by intrapulmonary administration of antioxidants blocks allergic airway inflammation. J Allergy Clin Immunol. 2007;119:646–653. doi: 10.1016/j.jaci.2006.11.634. This study shows role of antioxidant in preventing ragweed induced allergic airway inflammation.
- 32.Kruzel ML, Bacsi A, Choudhury B, et al. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006;119:159–166. doi: 10.1111/j.1365-2567.2006.02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee YC, Lee KS, Park SJ, et al. Blockade of airway hyperresponsiveness and inflammation in a murine model of asthma by a prodrug of cysteine, l-2-oxothiazolidine-4-carboxylic acid. FASEB J. 2004;18:1917–1919. doi: 10.1096/fj.04-2212fje. [DOI] [PubMed] [Google Scholar]
- 34 ••.Dharajiya NG, Bacsi A, Boldogh I, Sur S. Pollen NAD(P)H oxidases and their contribution to allergic inflammation. Immunol Allergy Clin North Am. 2007;27:45–63. doi: 10.1016/j.iac.2006.11.007. This review provides an in-depth overview of mammalian and plant NADPH oxidase and role of pollen NADPH oxidase in allergic airway inflammation.
- 35.Fogarty A, Lewis SA, Scrivener SL, et al. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003;33:1355–1359. doi: 10.1046/j.1365-2222.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- 36.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunstan JA, Breckler L, Hale J, et al. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on antioxidant status and immune responses in allergic adults: a randomized controlled trial. Clin Exp Allergy. 2007;37:180–187. doi: 10.1111/j.1365-2222.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 38.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 39.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of pre-cursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 40.Brusselle G, Kips J, Joos G, et al. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 41.Corry DB, Folkesson HG, Warnock ML, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Alam J, Venkatesan MI, et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 43.Xiao GG, Wang M, Li N, et al. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
- 44.Bacsi A, Dharajiya N, Choudhury BK, et al. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol. 2005;116:836–843. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 46.Wayne P, Foster S, Connolly J, et al. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88:279–282. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 47 •.Rogers CA, Wayne PM, Macklin EA, et al. Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ Health Perspect. 2006;114:865–869. doi: 10.1289/ehp.8549. This paper elegantly showed the effects of atmospheric CO2 on ragweed biomass and pollen production.