Abstract
Cardiac toxicity is an infrequent, but potentially serious side effect of 5-fluorouracil (5-FU). The reported incidence of 5-FU-induced cardiotoxicity is approximately 3%, although estimates vary from 1.2% to 18%. Cardiac death occurs in less than 1%. The prompt recognition of cardiac toxicity demands a thorough understanding of the myriad of potential cardiac manifestations and a high index of suspicion. The most common presentation is angina pectoris while other manifestations, namely myocardial infarction, left ventricular dysfunction, arrhythmias and sudden death have been recognised. The authors report an unusual case of myopericarditis masquerading as myocardial infarction.
Background
Although pericarditis-like changes after 5-fluorouracil (5-FU) administration have been noted in animal studies,1 it has not been well documented in humans. Physician awareness of this rare complication is required for prompt diagnosis and management, and to potentially avoid invasive testing with coronary angiography.
Case presentation
An 83-year-old gentleman with a medical history of coronary artery disease requiring coronary artery bypass grafting, hypertension and hyperlipidemia was diagnosed with non-operable oesophageal adenocarcinoma. Palliative chemotherapy was commenced with a modified FOLFOX regimen consisting of folinic acid, oxaliplatin and 5-FU infusion without a bolus. Forty hours after the initiation of 5-FU infusion, he experienced sudden-onset retrosternal chest pain and dyspnoea. Upon arrival to the emergency department, physical examination revealed tachycardia (heart rate 112 beats/min), tachypnoea (respiratory rate 24 breaths/min), normal blood pressure and an otherwise unremarkable cardiovascular examination.
Investigations
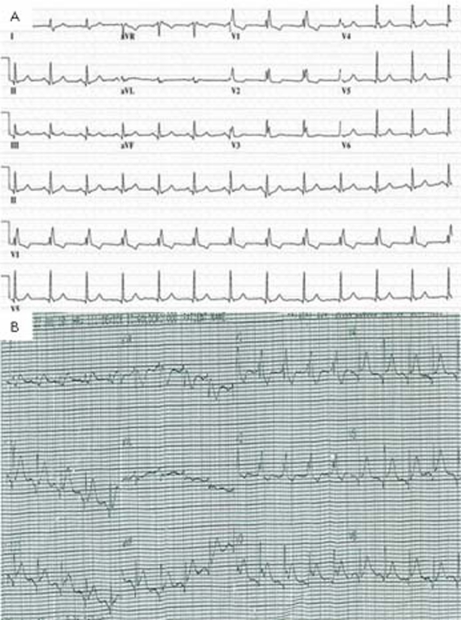
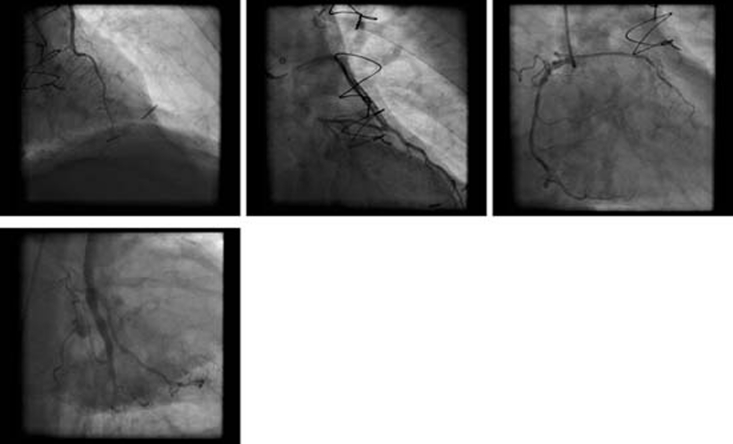
ECG showed sinus tachycardia, ST-segment elevation in multiple vascular territories, PR-segment depression and pre-existing right bundle branch block (figure 1). Laboratory testing was remarkable for normal blood counts, electrolytes and mildly elevated troponin-T of 0.05 ng/ml (reference range <0.01 ng/ml). He received aspirin, sublingual nitroglycerin, intravenous infusion of nitroglycerin and intravenous unfractionated heparin without relief of chest pain. Acute myocardial infarction was suspected and he proceeded to emergency coronary angiography which showed severe native coronary artery disease with patent grafts. No evidence of acute plaque rupture, thrombus, embolus or coronary artery spasm was identified (figure 2). Transthoracic echocardiogram was performed immediately following the procedure, and during persisting chest pain, revealed normal left ventricular ejection fraction and no regional wall motion abnormality, valvular or right ventricular dysfunction. Pericardial effusion was not detected. Serial troponin-T measurements showed a peak level of 0.11 ng/ml. Subsequent ECG showed persistent PR-segment depression and ST-segment elevation.
Figure 1.
(A) Pretreatment ECG shows right bundle branch block and inferior and lateral lead Q waves. (B) ECG obtained at the onset of chest pain during 5-fluorouracil infusion. This illustrates widespread ST-segment elevation and PR depression.
Figure 2.
Clockwise from top left: RAO cranial – LIMA to LAD; RAO caudal – SVG to diagonal; RAO – native RCA; RAO caudal – SVG to OM. No evidence of thrombus, embolism or vasospasm. LAD, left anterior descending artery; LIMA, left internal mammary artery; OM, obtuse marginal; RCA, right coronary artery; RAO, right anterior oblique; SVG, saphenous vein graft.
Differential diagnosis
-
▶
Acute coronary syndrome
-
▶
Pericarditis
-
▶
Myocarditis
-
▶
Drug-induced coronary vasospasm
-
▶
Oesophageal spasm.
Outcome and follow-up
The patient continued to report chest pain which became pleuritic and positional in nature. Based on the clinical presentation, absence of acute coronary lesions on angiography and preserved left ventricular function, a diagnosis of myopericarditis was made. Management consisted of discontinuation of 5-FU infusion and commencement of non-steroidal anti-inflammatory agents leading to complete resolution of chest pain within 24 h. At 3 months follow-up, the patient was doing well with platinum-based chemotherapy.
Discussion
Pre-existing ischaemic heart disease is a risk factor for 5-FU cardiotoxicity. Schöber et al reported an incidence of cardiac toxicity of 15.1% and 1.5% in those with and without a history of cardiac disease, respectively.2 Furthermore, asymptomatic ST-segment deviation is known to occur more frequently during 5-FU infusion in those with known coronary artery disease.3 Other risk factors for 5-FU cardiotoxicity include renal impairment and previous chest wall radiotherapy. Although cardiotoxicity can occur with all forms of 5-FU administration, the risk is higher with continuous infusion as compared to bolus therapy.4 The precise mechanism of 5-FU-induced cardiotoxicity remains undetermined. The most commonly suggested mechanism is coronary vasospasm and myocardial ischaemia. Although numerous reports of angiographic evidence of coronary vasospasm exist, this is not a universal phenomenon.5 The role of coronary vasospasm has been further brought into question by observed failure of ergonovine and 5-FU to induce vasospasm during catheterisation.5 Likewise, the role of coronary vasodilators such as calcium-channel blockers and nitrates in preventing and treating 5-FU-induced cardiotoxicity is controversial. As was illustrated in our patient, oral and intravenous nitroglycerin was ineffective in improving chest pain. Furthermore, his pain was more characteristic of pericarditis than vasospasm-induced ischaemia. The resolution of chest pain with non-steroidal anti-inflammatory agents further supports the diagnosis of myopericarditis in our patient. Hence, mechanisms other than vasospasm are likely, at least, in some reported cases of 5-FU cardiotoxicity. Animal studies have shown both pericarditis and myocarditis in rats following 5-FU infusion.1 6 Kumar et al postulated that 5-FU-induced endothelial damage leading to extravasation of 5-FU-containing blood into the myocardium could result in an inflammatory reaction.6 In addition, Matsubara et al administered 5-FU to open-chested guinea pigs and showed that ECG changes were not produced by ischaemia, rather a metabolic abnormality due to a direct toxic effect of 5-FU on the myocardium.7
5-FU-induced cardiotoxicity is usually reversible and management involves discontinuing the drug and instituting supportive measures. In cases of pericarditis, therapy with non-steroidal anti-inflammatory agents aimed at symptom relief is the mainstay. Complete discontinuation of the drug is required since relapse of symptoms can occur in up to 90% of patients upon re-challenge with 5-FU.
Cardiotoxicity is a serious consequence of 5-FU therapy and the decision to use this drug should be made carefully. While those with a history of coronary artery disease may represent a population at increased risk, it remains difficult to ascertain which patients will develop cardiotoxicity, warranting close monitoring of this high-risk group. 5-FU cardiotoxicity has a variety of presentations and therefore a high index of suspicion is required. If cardiotoxicity occurs, 5-FU should be discontinued and an alternative chemotherapy regimen initiated. As the number of patients treated with chemotherapy for solid organ malignancies rises, further studies to characterise the pathophysiology of 5-FU-induced cardiotoxicity are needed to optimise prevention and treatment.
Learning points.
-
▶
5-FU is associated with significant cardiac toxicity which includes acute pericarditis
-
▶
The mechanisms for the cardiac toxicity are unclear
-
▶
Discontinuation of the drug is required since relapse of symptoms occur in up to 90% of patients upon re-challenge with 5-FU.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Levillain R. Experimental myocarditis; anatomical study of 210 rat hearts after ingestion of 5-fluorouracil. C R Seances Soc Biol Fil 1972;166:340–2 [PubMed] [Google Scholar]
- 2.Schöber C, Papageorgiou E, Harstrick A, et al. Cardiotoxicity of 5-fluorouracil in combination with folinic acid in patients with gastrointestinal cancer. Cancer 1993;72:2242–7 [DOI] [PubMed] [Google Scholar]
- 3.Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol 1989;7:509–14 [DOI] [PubMed] [Google Scholar]
- 4.de Forni M, Malet-Martino MC, Jaillais P, et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol 1992;10:1795–801 [DOI] [PubMed] [Google Scholar]
- 5.Freeman NJ, Costanza ME. 5-Fluorouracil-associated cardiotoxicity. Cancer 1988;61:36–45 [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Gupta RK, Samal N. 5-fluorouracil induced cardiotoxicity in albino rats. Mater Med Pol 1995;27:63–6 [PubMed] [Google Scholar]
- 7.Matsubara I, Kamiya J, Imai S. Cardiotoxic effects of 5-fluorouracil in the guinea pig. Jpn J Pharmacol 1980;30:871–9 [DOI] [PubMed] [Google Scholar]