Table 7.
Opening of vinyl aziridine.
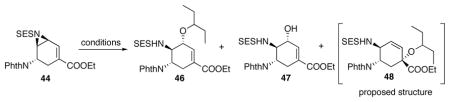 | ||
|---|---|---|
| Entry | Conditions | Yields |
| 1 | 1.5 equiv BF3•Et2O, 0.15 M in 3-pentanol, rt | 31% 46, 54% 47 |
| 2 | 1.2 equiv BF3•Et2O, 0.15 M in 3-pentanol, −20 °C | 20% 46, 41% 47 |
| 3 | 1.5 equiv BF3•Et2O, 0.15 M in 3-pentanol, 75 °C | 60% 46, 22% 47 |
| 4 | 1.5 equiv In(OTf)3, 0.15 M in 3-pentanol, 75 °C | 17% 46, 71% 47 |
| 5 | 10 mol% Cu(OTf)2, 0.1 M in 3-pentanol, 0-rt-100 °C | 87% 47 |
| 6 | 1.5 equiv BF3•Et2O, 0.15 M in 3-pentanol, 75 °C | 54% 46, 36% 47, 12% 48 |
| 7 | 1.5 equiv BF3•Et2O, 0.05 M in 3-pentanol, 70 °C | 54% 46, 34% 47, 12% 48 |
| 8 | 1.5 equiv BF3•Et2O, 0.05 M in 3-pentanol, 3Å MS, 70 °C | 50% 46, 34% 47, 16% 48 |
| 9 | 1.5 equiv B(OCHEt2)3, 0.15 M in 3-pentanol, 75 °C | No reaction |
| 10 | Azeotropically dired with toluene, then 1.5 equiv BF3•Et2O, 0.15 M in 3-pentanol, 75 °C | 60% 46, 26% 47, 13% 48 |
| 11 | 1.5 equiv BF3•Et2O, 1 equiv BSA in rxn, 0.15 M in 3-pentanol, rt | 25% 46, 57% 47, 7% 48, 11% starting material |
| 12 | Pre-mixed with BSA, volatile fully removed; then 1.5 equiv BF3•Et2O, 0.15 M in 3-pentanol, 75 °C | 65% 46, 20% 47, 15% 48 |
