Abstract
Tissue-specific gene expression using the UAS/GAL4 binary system has facilitated genetic dissection of many biological processes in Drosophila melanogaster. Refining GAL4 expression patterns or independently manipulating multiple cell populations using additional binary systems are common experimental goals. To simplify these processes, we have developed a convertible genetic platform, called the Integrase Swappable In vivo Targeting Element (InSITE) system. This approach allows GAL4 to be replaced with any other sequence, placing different genetic effectors under the control of the same regulatory elements. Using InSITE, GAL4 can be replaced with LexA or QF, allowing an expression pattern to be repurposed. GAL4 can also be replaced with GAL80 or split-GAL4 hemi-drivers, allowing intersectional approaches to refine expression patterns. The exchanges occur through efficient, in vivo manipulations, making it possible to generate many swaps in parallel. Furthermore, this system is entirely modular, allowing future genetic tools to be easily incorporated into the existing framework.
Introduction
Many in vivo manipulations rely on directing gene expression to a specific tissue, or to a particular developmental time. Two basic methods exist to do this. In one approach, transposable elements carrying genetic effectors with minimal promoters are inserted into the genome and expression is driven by local gene regulatory elements1–2. Alternatively, regulatory elements can be fused to genetic effectors in vitro and reinserted in the genome3–5. Such enhancer traps and enhancer fusions have been powerful tools in cell biology, development, physiology, and neuroscience6–9.
In Drosophila melanogaster, the UAS/GAL4 system allows for expression of UAS-linked genes in cells expressing the transcription factor GAL41. Two additional binary systems, using the LexA and QF transcriptional activators, allow independent manipulation of multiple populations of cells10–11. However, while many enhancer trap and enhancer fusion lines exist, particularly for the UAS/GAL4 system, the expression of such lines is seldom confined to a single tissue or cell type, limiting the resolution of these approaches4,12–13.
Several strategies exist for using the intersections of partially overlapping expression patterns to generate increased specificity. For example, FLP, GAL4, and QF have been used with FRT-flanked interruption or flip-out cassettes to target subsets of expression patterns11,13–15. The split-GAL4 system allows GAL4 activity to be reconstituted in cells that express both halves of the hemi-driver16. Finally, the GAL80 repressor can be used to subtract the overlap between two expression patterns12,17. While these intersectional methods are useful for generating lines with more specific gene expression patterns, a drawback of these approaches is that with each elaboration or extension of the GAL4 system, new combinations of these regulatory elements, or their reporters frequently need to be designed, and in the worst case, whole libraries need to be re-generated.
Recombinase-Mediated Cassette Exchange (RMCE) methods allow a sequence cassette to be replaced in vivo. Several strategies for RMCE, typically using a microinjected plasmid as the substrate for cassette exchange, have been developed18–24. Several of these approaches rely on FLP-mediated recombination using wild-type or mutant FRT sites to target an FRT-flanked cassette to a specific locus20–21,23. Another uses Cre recombinase and a pair of incompatible loxP sites22. The Streptomyces phage ΦC31 integrase, which catalyzes irreversible site-specific recombination between two sites (attP and attB)25–26, has had a profound impact on animal transgenesis and has made possible several novel RMCE strategies18–19.
We have combined these recombination systems into a versatile platform to facilitate the segmentation of complex expression patterns, and to allow GAL4 expression patterns to be repurposed. The Integrase Swappable In vivo Targeting Element (InSITE) system allows an enhancer trap or enhancer fusion to be rapidly converted from GAL4 to any other sequence (Fig. 1a). The InSITE system uses an RMCE strategy in which the substrate for RMCE can be genetically-derived, allowing replacement of GAL4 simply by crossing flies. We demonstrate that such swaps can be done either entirely genetically, using genomic donor lines, or with a microinjected donor plasmid. As this strategy is highly efficient, it is possible to perform high-throughput swapping of many enhancer trap lines to multiple different effector molecules in parallel. In addition, we describe an enhancer fusion vector that is compatible with this replacement strategy, allowing a single transgene to be diversified in vivo. Finally, because the InSITE system allows GAL4 to be converted into any other sequence, this platform is forward compatible with currently unanticipated future technologies.
Figure 1.
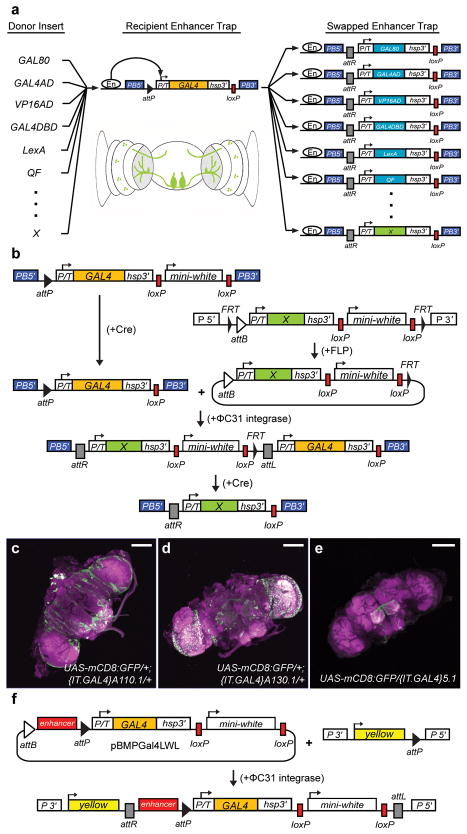
The InSITE system. (a) Schematic illustration of the InSITE system, which can convert a GAL4 enhancer trap to another sequence (“X”), which will then be expressed under the control of local enhancers (En). (b) Schematic illustration of the procedure for genetically swapping GAL4 with another sequence (“X”). (c-e) InSITE enhancer trap expression in the adult brain. Green: anti-mCD8. Magenta: anti-Bruchpilot (nc82). Scale bar: 100 μm. (c) P element line P{IT.GAL4}A110.1.(d) P element line P{IT.GAL4}A130.1.(e) PiggyBac line PBac{IT.GAL4}5.1. (f) The InSITE-compatible enhancer fusion vector, pBMPGal4LWL.
Results
A convertible enhancer trap platform
The InSITE enhancer trap contains a minimal (P transposase) promoter, the GAL4 transcriptional activator, and an attP recognition sequence for ΦC31 integrase (Fig. 1b)18–19,25–26. Swappable enhancer traps were constructed using two different transposons, the Drosophila melanogaster-specific P element and the piggyBac element, which has been used to transform a wide range of species, from insects to mammals27–30 (Supplementary Fig. 1). PiggyBac elements also have a different insertion spectrum from P elements, facilitating the generation of lines with distinct expression patterns31. Transformants were established, and mobile, X-linked insertions, which allow the isolation of additional lines for enhancer trap screens, were identified for both transposons. Both the P element and piggyBac transposons (referred to as P{IT.GAL4} or PBac{IT.GAL4}, respectively, where “IT” denotes InSITE Target) functioned as enhancer traps, and were expressed in diverse patterns in the adult brain (Fig. 1c–e, Supplementary Fig. 2).
The InSITE system uses a trio of site-specific recombinases to exchange GAL4 with any other sequence (Fig. 1b)19. First, the original enhancer trap is treated with Cre, which removes the loxP-flanked mini-white marker32. Next, an FRT-flanked genomic donor line is introduced, together with FLP and ΦC31 integrase. In a strategy similar to that used to generate knock-out alleles33, upon treatment with FLP, a circular, attB-containing molecule is excised from the donor chromosome (Fig. 1b). Targeting of a genomic FRT-flanked cassette to a second FRT-containing locus using FLP has been previously described20. We reasoned that the irreversible nature of ΦC31 integrase-mediated insertion would allow for high efficiency re-integration of the FLP-liberated donor molecule into the attP site of the InSITE enhancer trap (Fig. 1b). Integration events are detected by the re-appearance of the mini-white marker. Both constructs contain loxP sites oriented such that when treated with Cre, the original GAL4 and mini-white marker in the integrated donor are deleted, leaving just the donor sequence. Thus, GAL4 can be replaced with any other sequence, which should then be expressed under the control of the same regulatory elements.
In addition to the purely genetic swap, a microinjected plasmid analogous to the circular molecule that is liberated by FLP, can also be integrated into the attP site of the enhancer trap (Supplementary Fig. 3). A similar strategy using a microinjected LexA donor plasmid has recently been described24.
An InSITE-compatible enhancer fusion vector
Another approach to segmenting complex expression patterns is to use enhancer fusion constructs, where a DNA fragment corresponding to an endogenous regulatory region is cloned upstream of GAL4 or another effector. This approach has the advantage that lines containing enhancer subfragments are often expressed in fewer cells than enhancer traps, and enhancers can be further subdivided in vitro or the effector molecule can be switched by generating new constructs4. In order to take advantage of the enhancer fusion approach, but bypass the need to clone and microinject each new construct, we made an InSITE-compatible enhancer fusion vector (Fig. 1f). We cloned an enhancer fragment with a known expression pattern, ortc234, into this vector, and inserted this construct into the attP2 landing site25, and one of the Cre-reduced InSITE enhancer trap lines (PBac{IT.GAL4.w−}0096). While the presence of tandem attB and attP sites in this vector reduces the rate of transformation, likely through vector suicide via intramolecular recombination, integrants were obtained in both cases using standard injection procedures and verified by PCR. The insertion into attP2 drove the expected ortc2 expression pattern (data not shown)34. Such enhancer fusions are fully compatible with the InSITE system.
InSITE genetic donor lines and plasmids
A collection of attB-containing genetic donor lines and injectable donor plasmids have been made (Supplementary Fig. 1). All of the reagents described here are publicly available (see Methods section for details). Transgenic donor lines, referred to as P{ID.X} (“X” is the inserted effector gene and “ID” denotes InSITE Donor), were established for the GAL80 repressor17, the GAL4DBD, GAL4AD, and VP16AD split-GAL4 hemi-drivers16, as well as the LexA10 and QF transcriptional activators11 (Supplementary Fig. 4). Taken together, this collection of donor constructs represents a versatile toolkit for manipulating gene expression.
Enhancer trap lines are genetically swappable
To test the efficiency of the conversion procedure, four enhancer trap lines (PBac{IT.GAL4}1.1, PBac{IT.GAL4}3.1, PBac{IT.GAL4}5.1, and PBac{IT.GAL4}6.1) were crossed to Cre-expressing flies to remove the loxP-flanked mini-white marker32. Deletions were identified by loss of mini-white expression, and confirmed by PCR (Fig. 2a, b). This step of the conversion procedure was highly efficient, as no white+ flies were recovered after Cre treatment. Using eyFLP, we also confirmed that the donor molecules were readily excised by FLP, as followed by the loss of mini-white expression (Fig. 2c)35.
Figure 2.
Molecular and genetic validation of the enhancer trap exchange. (a) PCR confirmed each step of the genetic conversion of line PBac{IT.GAL4}6.1 to the VP16AD hemi-driver. Numbers refer to primer pairs shown in panel (b). (b) Schematics depicting the structure of the insertion being amplified in each set of PCR reactions. Locations of PCR primers are shown beneath each construct. (c) Genetic donor constructs lose mini-white expression when crossed to eyFLP2. Left: P{ID.VP16AD}D37.1/+, Right: y, w, eyFLP2; P{ID.VP16AD}D37.1/+. Scale bar: 100 μm. (de) When crossed to y, w, hs-FLP, vas-ΦC31 and heat shocked periodically throughout larval development, flies containing both recipient and genetic donor elements displayed either completely white eyes (not shown), or a small amount of variegated mini-white expression. Scale bar: 100 μm. (d) y, w, hs-FLP, vas-ΦC31; P{ID.VP16AD}D33.1/CyO; PBac{IT.GAL4.w−}3.1/+. (e) y, w, hs-FLP, vas-ΦC31; P{ID.VP16AD}D33.1/CyO; PBac{IT.GAL4.w−}6.1/+. (f) Correct integration events (genetic swaps) became resistant to eyFLP2 because one FRT site was lost during excision and re-integration. Top Right: PBac{IS.VP16AD.GAL4}6.1/+, Bottom: PBac{IS.VP16AD.w−}6.1/+, Top Left: y, w, eyFLP2; PBac{IS.VP16AD.GAL4}6.1/+. Scale bar: 100 μm. (g) Sequence of the PCR product of primer pair 5.
Next, eight donor lines, representing all six effectors, were crossed to one or more of three different recipient lines, PBac{IT.GAL4.w−}3.1, PBac{IT.GAL4.w−}5.1, and PBac{IT.GAL4.w−}6.1, in flies that also carried a heat-shock inducible FLP and expressed ΦC31 integrase in the germ-line19 (Supplementary Fig. 5). Larvae were heat shocked to liberate the circular donor molecule, and the resulting flies were crossed to flies carrying eyFLP (Fig. 2d, e)35. The presence of eyFLP in the following generation ensured that flies in which the FRT-flanked donor cassette had not been excised were not scored as false positives. For all thirteen recipient-donor pairs tested, many white+ putative genetic swap flies were recovered (ranging from 16.7% to 56.6% of crosses giving at least one white+ progeny, Table 1, Fig. 2f).
Table 1.
Efficiency of the InSITE genetic swap procedure. At least one line, chosen at random, for each of the six genetic donor elements was tested for the ability to excise and re-integrate into an attP-containing recipient site. Three different enhancer trap target lines were tested. Rates of eyFLP-resistant white+ flies and true integration events, as assayed by PCR, are shown.
| Recipient | Donor (Line #) | # of crosses | # of crosses with w+ flies | % of crosses with w+ flies | # tested by PCR | # of true integrations | % of true integrations | Recovered swap |
|---|---|---|---|---|---|---|---|---|
| PBac{IT.GAL4.w−}6.1 | P{ID.VP16AD}D33.1 | 26 | 9 | 34.6 | 9 | 7 | 77.8 | yes |
| PBac{IT.GAL4.w−}3.1 | P{ID.VP16AD}D33.1 | 39 | 9 | 23.1 | 8 | 1 | 12.5 | yes |
| PBac{IT.GAL4.w−}6.1 | P{ID.VP16AD}D37.1 | 34 | 8 | 23.5 | no data | no data | no data | yes |
| PBac{IT.GAL4.w−}3.1 | P{ID.VP16AD}D37.1 | 30 | 7 | 23.3 | 7 | 0 | 0.0 | no |
| PBac{IT.GAL4.w−}6.1 | P{ID.GAL4DBD}F32.1 | 30 | 5 | 16.7 | 5 | 3 | 60.0 | yes |
| PBac{IT.GAL4.w−}3.1 | P{ID.GAL4DBD}F32.1 | 30 | 10 | 33.3 | 9 | 4 | 44.4 | yes |
| PBac{IT.GAL4.w−}6.1 | P{ID.GAL80}E17.1 | 29 | 10 | 34.5 | no data | no data | no data | yes |
| PBac{IT.GAL4.w−}3.1 | P{ID.GAL80}E17.1 | 30 | 17 | 56.7 | no data | no data | no data | yes |
| PBac{IT.GAL4.w−}6.1 | P{ID.QF}Q10B | 20 | 4 | 20.0 | 4 | 3 | 75.0 | yes |
| PBac{IT.GAL4.w−}3.1 | P{ID.QF}Q12A | 18 | 5 | 27.8 | 5 | 3 | 60.0 | yes |
| PBac{IT.GAL4.w−}5.1 | P{ID.LexA}L34.2L | 19 | 6 | 31.6 | 6 | 6 | 100.0 | yes |
| PBac{IT.GAL4.w−}5.1 | P{ID.GAL4AD}G12.1 | 19 | 5 | 26.3 | 5 | 3 | 60.0 | yes |
| Total | - | 324 | 95 | 29.3 | 58 | 30 | 51.7 | 12 of 13 |
Several putative genetic swaps were selected for molecular and functional validation. For twelve out of thirteen recipient-donor pairs, successful genetic swaps were identified by PCR analysis (Fig. 2a), referred to as PBac{IS.X.GAL4} (“X” is the swapped effector gene, and “IS” denotes InSITE Swap). For one of the recipient-donor pairs, only aberrant events were recovered (see below). In addition to having the PCR products diagnostic for the novel junctions created by integration of the genetic donor constructs, the swaps lost PCR products specific to the intact donor transposon (Fig. 2a). The junction between the mini-white and GAL4 genes was sequenced and, as expected, a single FRT site from the genomic donor element was retained between the loxP and attL sites in the integrated constructs (Fig. 2g). To complete the swap, these lines were treated with Cre, and removal of GAL4 and mini-white was confirmed by PCR (Fig. 2a). The final, reduced constructs are referred to as PBac{IS.X.w−}.
In addition to verifying the genetic swap lines, a set of attB-containing donor plasmids was microinjected, along with a ΦC31 integrase helper plasmid, into one of the three InSITE enhancer traps (Supplementary Fig. 3). White+ integrants were obtained for all injections, with integration frequencies between 9.5% and 22.0%. Integration of all five donor plasmids was confirmed by PCR. Finally, the integrants were treated with Cre to remove GAL4 and mini-white, and the final conversion products were confirmed by PCR.
Avoiding genetic swap aberrant events
Typically, all integrants into a single attP landing site have similar eye colors36 (Supplementary Fig. 6). However, aberrant events with different eye colors were observed for many of the above genetic swap crosses (Table 1). Most of these events retained the original donor transposon, but one FRT site was inactivated (Supplementary Fig. 7). In addition, one reciprocal translocation was recovered, in which the recipient attP site and the donor attB site were directly fused (Supplementary Fig. 7). Such events have been described previously with ΦC31 integrase-mediated recombination in human cell lines37. However, by selecting appropriate recipient and donor pairs, and following specific markers in the crosses (see Methods), aberrant events can be essentially eliminated.
Functional validation of the enhancer trap swaps
To determine whether the swapped enhancer trap lines were functional, a number of Cre-reduced swaps were crossed to appropriate reporters. First, swaps to QF and LexA were tested. In PBac{IT.GAL4.w−}6.1 flies, expression was observed in the antennae and maxillary palps (Fig. 3a), and widely in the adult brain (Fig. 3b, c). The PBac{IT.GAL4}1.1 enhancer trap drove expression in a group of neurons in the subesophageal ganglion (Fig. 3d, e), as well as a small number of neurons in the central brain. In PBac{IS.QF.w−}6.1, expression of QUAS-mCD8:GFP recapitulated the pattern seen with the UAS-driven expression of PBac{IT.GAL4.w−}6.1 (Fig. 3f–h). In PBac{IS.LexA.w−}1.1 flies, expression from LexAOp-rCD2:GFP was detected in the subesophageal ganglion, but not in the central brain neurons (Fig. 3i, j). Thus the PBac{IS.LexA.w−}1.1 swap recapitulated some, but not all, features of the original GAL4 expression pattern.
Figure 3.
Functional validation of the QF and LexA enhancer trap swaps. (a-e) Expression of the PBac{IT.GAL4.w−}6.1 and PBac{IT.GAL4}1.1 enhancer traps. (a, f) Both PBac{IT.GAL4.w−}6.1 and PBac{IS.QF.w−}6.1 were expressed in the antennae (arrowheads) and maxillary palps (arrows) of adult flies. Scale bar: 100 μm. (b, g) Adult brains stained with anti-mCD8 (green) and anti-Bruchpilot (nc82, magenta). Scale bar: 50 μm. PBac{IT.GAL4.w−}6.1 and PBac{IS.QF.w−}6.1 were expressed in similar patterns in the adult brain. (c, h) mCD8 (green) channel only of images in panels (b) and (g). Scale bar: 50 μm. (d-e, i-j) Comparison of PBac{IT.GAL4}1.1 and PBac{IS.LexA.w−}1.1 lines. Scale bar: 50 μm. Asterisk denotes a group of cells where GFP expression was observed in PBac{IT.GAL4}1.1, but not in the PBac{IS.LexA.w−}1.1 swap. (d, i) Adult brains stained with anti-GFP (green) and anti-Bruchpilot (nc82, magenta). (e, j) GFP (green) channel only of images in (d) and (i).
Next, the conversion to split-GAL4 hemi-drivers and GAL80 was tested. Swaps of PBac{IT.GAL4.w−}3.1 to the GAL4DBD, VP16AD, and GAL4AD hemi-drivers and GAL80 were generated, and expression of these lines was assayed in the antennae (Fig. 4a–e) and antennal lobes (Fig. 4f–y). In PBac{IT.GAL4.w−}3.1, expression was observed in the antennae of adult flies (Fig. 4a), as well as in several olfactory glomeruli (Fig. 4f, k), and in a small number of central brain neurons. As expected, both PBac{IS.GAL4DBD.w−}3.1 and elav-VP16AD16 were incapable of driving UAS-mCD8:GFP expression on their own (Fig. 4b, c, g, h, l, m). Together, PBac{IS.GAL4DBD.w−}3.1/elav-VP16AD drove robust expression in a pattern similar to the original GAL4 line (Fig. 4d, i, n, Supplementary Table 1). As with PBac{IS.LexA.w−}1.1, a minor difference was detected between the two patterns, with no expression seen in the small number of central brain neurons in the PBac{IS.GAL4DBD.w−}3.1/elav-VP16AD brains (Fig. 4f, i, k, n).
Figure 4.
Functional validation of the split-GAL4 and GAL80 enhancer trap swaps. (a-e) GFP expression in the antennae (arrowheads) of adult flies. Scale bar: 100 μm. (f-j, p-t) Adult brains stained with anti-mCD8 (green) and anti-Bruchpilot (nc82, magenta). Scale bar: 50 μm. (k-o, u-y) mCD8 (green) channel only of images in panels (f-j) and (p-t). Scale bar: 50 μm. PBac{IT.GAL4.w−}3.1 was expressed in the antennae of adult flies (a), as well as in several olfactory glomeruli (f, k). Weak expression was also seen in PBac{IT.GAL4.w−}3.1 in a small number of central brain neurons (marked with an asterisk in panels (f) and (k)). No GFP or CD8 expression was seen in any of the samples carrying only one half of split-GAL4 (b, c, g, h, l, m, p-r, u-w). However, patterns similar to the original PBac{IT.GAL4.w−}3.1 were seen when both halves of split-GAL4 were present (d, i, n, s, t, x, y). PBac{IT.GAL4.w−}3.1 expression was repressed by PBac{IS.GAL80.w−}3.1 in both the antennae (e) and in the adult brain (j, o). In the adult brain, repression was strongest in the antennal lobe neurons and PBac{IS.GAL80.w−}3.1 failed to repress GAL4 in the small cluster of central brain neurons (marked with asterisk in panels (j) and (o)).
Neither of the activation domain hemi-driver lines drove UAS-mCD8:GFP expression on their own (Fig. 4p, q, u, v), nor did elav-GAL4DBD16 (Fig. 4r, w). PBac{IS.VP16AD.w−}3.1/elav-GAL4DBD drove expression in a pattern that was similar to, but somewhat broader than the original PBac{IT.GAL4}3.1 (Fig. 4s, x, Supplementary Table 1), while PBac{IS.GAL4AD.w− }3.1/elav-GAL4DBD drove weak expression in a subset of the glomeruli detected in the parent GAL4 line (Fig. 4t, y, Supplementary Table 1). Such differences are consistent with previous reports that the VP16AD hemi-driver is a stronger transcriptional activator than GAL4AD 16,38. Similarly, the PBac{IS.VP16AD.w−}3.1/PBac{IS.GAL4DBD.w−}3.1 pair drove expression in an essentially identical set of glomeruli as the parent GAL4 line, while PBac{IS.GAL4AD.w−}3.1/PBac{IS.GAL4DBD.w−}3.1 was expressed weakly in an overlapping but restricted subset of glomeruli (Supplementary Table 1). Likewise, PBac{IS.VP16AD.w−}6.1, in combination with elav-GAL4DBD, largely recapitulated the original PBac{IT.GAL4}6.1 pattern (Supplementary Fig. 8).
As expected, PBac{IS.GAL80.w−}3.1 eliminated most expression driven by the original PBac{IT.GAL4}3.1 line (Fig. 4e, j, o). Similar to the split-GAL4 lines, GAL80 failed to target the small group of central brain neurons (Fig. 4f, j, k, o), suggesting that this expression was influenced by sequences inside GAL4 itself.
For most of the swaps, the resultant expression was strongly predicted by the original GAL4 pattern. In three out of ten cases, the expression patterns overlapped with the original pattern, but also displayed substantial differences. Expression of PBac{IS.QF.w−}3.1 was similar to the original PBac{IT.GAL4.w−}3.1 line in the antennae and olfactory glomeruli, but new expression was observed in a single glial subtype (cortex glia)39 and in trachea (Supplementary Fig. 8, Supplementary Table 1). In a second case, PBac{IS.GAL4DBD.w−}6.1, the pattern of expression overlapped with PBac{IT.GAL4}6.1 in both the antennae and the adult brain, but was much sparser and was not detected in the maxillary palps. Finally, PBac{IS.GAL80.w−}6.1 repression of PBac{IT.GAL4}6.1 was incomplete (Supplementary Fig. 8). In this case, since GAL4 and GAL80 are presumably being expressed at similar levels, it is not clear that complete repression would be expected.
Discussion
Here we introduce a versatile platform designed to facilitate the functional diversification of expression patterns. The InSITE system uses ΦC31 integrase, FLP, and Cre-mediated recombination to convert a GAL4 effector in an enhancer trap or enhancer fusion line to another sequence. We have demonstrated that InSITE enhancer traps can be efficiently swapped and made to express a split-GAL4 hemi-driver, GAL80, LexA, or QF. This will allow lines with overlapping expression patterns to be used intersectionally to target restricted subsets of the original patterns. Using this system, it should be possible to generate highly specific driver lines targeting small populations of cells or tissues. This will be useful in a wide range of developmental and cell biological experiments, allowing the tissue-specific requirements of genes to be mapped with high precision, and the dissection of neural circuits at the level of single neuron types.
One advantage of the InSITE system is that the effector swap can be done genetically, through a series of crosses, bypassing the labor intensive step of DNA microinjection. While other approaches exist for repurposing enhancer expression patterns, such as constructed enhancer fusions, these strategies have previously required that new constructs must be cloned and transformed for each new desired fusion4. The InSITE-compatible enhancer fusion vector we describe allows one to make a single GAL4 construct, and then swap it for any of the available donor lines in vivo. In addition, the InSITE genetic swap approach is easily scalable, making it possible to simultaneously exchange large numbers of lines with multiple donor constructs.
The majority of the swaps captured most features of the original GAL4 expression patterns. However, in some cases, either prominent features of the GAL4 pattern were lost, or new expression patterns were observed. These changes may have resulted from differences in the strength or responsiveness of reporter lines. Alternately, the swap may have modified some combination of enhancer spacing and sequence composition flanking the promoter. Since the InSITE donors are essentially identical outside the effector sequence, these effects are likely intrinsic properties of the effector sequence itself. Therefore, any approach using these effectors would also likely encounter such context-specific cryptic regulatory activities. These occasional discrepancies between the original and swapped patterns underscore the importance of having a versatile, high-throughput system that allows many orthogonal intersectional strategies to be quickly tested in parallel.
The modular nature of the InSITE platform makes this system forward compatible. Recent work has described refinements to the existing binary systems 13,24,38. Such new reagents and many unanticipated future technologies can be readily incorporated into the InSITE system, and will be useful in designing additional intersectional strategies.
While we have focused primarily on binary system expression and the refinement of expression patterns, many additional elaborations of this system are possible due to the fact that GAL4 can be replaced with any other sequence. For instance, it is possible to design strategies to mutate or modify loci near the transposon insertion site. In particular, because the genetic swap leaves behind a single FRT site, it is possible to use genetic swap lines together with other FRT-containing transposons (like the Exelixis and Drosdel collections) to make deletions31. These deletions, which could disrupt nearby genes and regulatory elements, can be designed to leave behind either GAL4 or the swapped effector. This provides another approach to segmenting complex expression patterns, by altering the surrounding regulatory elements. Thus, the InSITE system is a versatile toolkit both for capturing and manipulating gene activity. As the methods described here use recombinases that work in other model organisms, this system should be easily adaptable.
Methods
Cloning enhancer trap transposons
pXL-BacII-attPGal4LWL: First, the attP site was excised from pUASTP2 (obtained from J. Bateman18) with EcoRI, and ligated into the EcoRI site of pBluescript, to make pBS-attP. Next a linker fragment, SPpolyF/R, was made by annealing and phosphorylating the SPpolyF and PSpolyR oligos (all oligos were obtained from Integrated DNA Technologies, see Supplemental Table 2 for oligo sequences) and this linker was ligated into PstI and SacI cut pBS-attP, destroying the SacI site, to make pBS-attPΔS3. A second linker fragment, HKpolyF/R, was made with the HKpolyF and KHpolyR oligos. pBS-attPΔS3 was cut with HindIII and KpnI, and the HKpolyF/R linker was inserted, to generate pBS-attPΔS+S1.
Next, a fragment containing the P promoter was amplified from pLAPVPRA (D. Gohl and M. Müller, unpub. data) using the pPromF and pPromR primers (see Supplemental Table 3 for sequences of PCR and sequencing primers). The P promoter fragment was cloned into pCRII-TOPO (Invitrogen), to generate pCRII-Pprom1–2. This vector and pBS-attPΔS+S1 were both digested sequentially with BamHI and HindIII, and the P promoter fragment was cloned into pBS-attPΔS+S1 to make pBS-attPΔS+SPprom1. This vector was cut with BamHI and SacI. A BamHI and SacI fragment containing GAL4 and the hsp70 3′ UTR was isolated from pGaTB (obtained from M. Wernet1) and ligated into pBS-attPΔS+SPprom1, to generate pBS-attPPpromGal4.
In parallel, the mini-white gene was obtained as an EcoRI fragment from pC4YM (from M. Müller40), and ligated between two loxP sites into the EcoRI site of pBLLΔ (from M. Müller, 40) to make pBLLΔ-miniwhite1. A NotI, BamHI fragment containing mini-white flanked by loxP sites was isolated and cloned into the NotI, BamHI sites of pXL-BacII-ECFP30 (obtained from L. Luo41) to make pXL-BacII-lox-miniwhite-lox. Finally, in order to make pXL-BacII-attPGal4LWL, a NotI fragment containing attP-Pprom-GAL4-hsp70 3′ UTR was isolated from pBS-attPPpromGal4 and ligated into the NotI site of pXL-BacII-lox-miniwhite-lox. The correct insert orientation was determined by diagnostic digests and by sequencing with primers: pPromF, pPromR, MW300Up, and 9-1.
pXN-attPGal4LWL: A KpnI, BamHI fragment from pXL-BacII-attPGal4LWL was ligated into the KpnI, BamHI sites of pXN (from Paul Schedl42), between the P element ends. This vector was re-cut with NotI and BamHI, and a NotI, BamHI fragment containing mini-white flanked by loxP sites (see above) was inserted, to make pXN-LWL. pXN-LWL was then cut with NotI, and was ligated to the NotI fragment containing attP-Pprom-GAL4-hsp70 3′UTR isolated from pBS-attPPpromGal4 (see above), to make pXN-attPGal4LWL. The correct orientation of the insert was confirmed by diagnostic digests and sequencing with the P5′inF and MW5′R primers.
Cloning injectable donor plasmids
pBPHLWL
Two pairs of oligos which encoded a multiple cloning site with the following restriction sites: KpnI-HindIII-BamHI-NotI-MluI-AscI-XbaI-ClaI-NheI-PacI-SphI-SpeI-BglII-SacI, were annealed and phosphorylated to make Oligo1F/R and Oligo2F/R. Next, pBluescriptKSII+ was cut with KpnI and XbaI and Oligo1F/R was inserted to generate pBS-Oligo1. pBS-Oligo1 was cut with XbaI and SacI, and Oligo2F/R was ligated into this backbone to make pBSO1O2. Next, a ClaI, SpeI fragment containing the hsp70 3′ UTR was isolated from pGaTB and cloned into the ClaI, SpeI sites of pBSO1O2. The resulting vector was cut with SpeI and BglII, and was ligated to an XbaI, BamHI fragment containing loxP-mini-white-loxP from pBLLΔ-miniwhite1, generating the plasmid pBSO1O2hsp70LWL.
In parallel, the attB site from piB-GFP (obtained from Jack Bateman18) was isolated as a KpnI, HindIII fragment and cloned into pBluescriptKSII+, to make pBS-attB. A HindIII, BamHI fragment containing the P minimal promoter (from pCRII-Pprom1–2, see above) was ligated into the HindIII, BamHI sites of pBS-attB, to make pBS-attBPprom.
Finally, a KpnI, BamHI fragment containing attB and the minimal P promoter was isolated from pBS-attBPprom, and ligated into the KpnI, BamHI sites of pBSO1O2hsp70LWL, to generate the pBPHLWL donor plasmid. pBPHLWL was sequenced with the following primers: M13For, M13Rev, hsp3′F, MW5′R, MW3′F, hsp3′R(seq), PpromF(seq), MW5′F(seq), MW2F(seq), MW2R(seq), MW3F(seq), MW3R(seq), MW4F(seq), MW4R(seq), MW6F(seq), and MW6R(seq).
pBPHLWL-Gal80: A NotI, XbaI fragment containing GAL80 from pCASPTubGAL80 (obtained from T. Schwabe17), was cloned into the NotI, XbaI sites of pBPHLWL. The insert was verified by sequencing using the following primers: PpromF(seq), MW5′R, Gal80F1(seq), and Gal80R1(seq).
pBPHLWL-Gal4AD: A NotI, AscI fragment containing the GAL4AD-leucine zipper fusion from pActPL-Gal4AD (obtained from Addgene16) was inserted in the MCS of pBPHLWL and verified by sequencing with the PpromF(seq) and MW5′R primers.
pBPHLWL-VP16AD: A NotI, AscI fragment containing the VP16AD-leucine zipper fusion from pActPL-VP16AD (obtained from Addgene16) was inserted in the MCS of pBPHLWL and verified by sequencing with the PpromF(seq) and MW5′R primers.
pBPHLWL-Gal4DBD: A NotI, AscI fragment containing the GAL4DBD-leucine zipper fusion from pActPL-Gal4DBD (obtained from Addgene16) was inserted in the MCS of pBPHLWL and verified by sequencing with the PpromF(seq) and MW5′R primers.
pBPHLWL-LexA: A NotI, XbaI fragment containing LexA from pGD319 (obtained from G. Dietzl) was cloned into the MCS of pBPHLWL and verified by sequencing with the following primers: PpromF(seq), MW5′R, LexA1F, and LexA1R.
pBPHLWL-QF: A BamHI and SpeI fragment containing QF11 from pXN-FBLWLF-QF (see below) was ligated into the BamHI, XbaI sites of pBPHLWL and verified by sequencing with the PpromF(seq) and MW5′R primers.
Cloning genetic donor transposons
pXN-FBLWLF
Two sets of FRT site-containing oligos were used to make 5′FRT(KpnI)F/R and 3′FRT(Not-Sac)F/R. Next, pBS-attBPprom was cut with KpnI and 5′FRT(KpnI)F/R was inserted to generate the plasmid pBSattBPprom+5′FRT5, which had a tandem insertion of the oligo. The orientation of the insert was verified by sequencing with M13For. To reduce this tandem insert, pBSattBPprom+5′FRT5 was cut with AscI, gel purified, and re-ligated to generate pBSFattBPprom. This plasmid was then cut with NotI and SacI, and 3′FRT(Not-Sac)F/R was inserted to make pBSFattBPpromF. Next, a KpnI, XhoI FattBPpromF fragment from pBSFattBPpromF was ligated into the KpnI, SalI sites of the pXN vector, to make pXN-FattBPpromF2-1. A linker containing specific restriction sites (NotI-EcoRV-PacI-SphI-AvrII-NheI-MluI-BglII) was made from a pair of oligos (GCNotI F (dXho) and GCNotI R (dXho)) and ligated into the NotI site of pXN-FattBPpromF2-1. This plasmid was cut with BglII and NheI, and an XbaI, BamHI loxP-mini-white-loxP from pBLLΔ-miniwhite1 was inserted to make pXN-FBLWLF. pXN-FBLWLF was confirmed by sequencing with the P5′inF and MW5′R primers.
pXN-FBLWLF-Gal80: A BamHI, SphI fragment containing GAL80 and the hsp70 3′ UTR from pBPHLWL-Gal80 was cloned into the MCS of pXN-FBLWLF and confirmed by diagnostic digests and sequencing with the P5′inF primer.
pXN-FBLWLF-GAL4AD: A BamHI, SphI fragment containing GAL4AD and the hsp70 3′ UTR from pBPHLWL-GAL4AD was cloned into the MCS of pXN-FBLWLF and confirmed by diagnostic digests and sequencing with the P5′inF primer.
pXN-FBLWLF-VP16AD: A BamHI, PacI fragment containing VP16AD and the hsp70 3′ UTR from pBPHLWL-VP16AD was cloned into the MCS of pXN-FBLWLF and confirmed by diagnostic digests and sequencing with the P5′inF primer.
pXN-FBLWLF-Gal4DBD: A BamHI, PacI fragment containing GAL4DBD and the hsp70 3′ UTR from pBPHLWL-Gal4DBD was cloned into the MCS of pXN-FBLWLF and confirmed by diagnostic digests and sequencing with the P5′inF and MW5′R primers.
pXN-FBLWLF-LexA: A NotI, PacI fragment containing LexA and the hsp70 3′ UTR from pBPHLWL-LexA was cloned into the MCS of pXN-FBLWLF and confirmed by diagnostic digests and sequencing with the P5′inF primer.
pXN-FBLWLF-QF: Using pBac-GH146-QF-hsp7011 as substrate, QF and hsp70 were separately PCR amplified with a shared sequence (TAAGCACTAGTGCAGATCTTATCGATAC) between the QF and hsp70 regions. For QF, the following primers were used: BHI-QF-FOR/QFREV-hsp70-LNKR. For hsp70, the following primers were used: pGaTn-hsp70REV-NotI-BH1/hsp70-FOR-QF-LNKR. The QF and hsp70 PCR products were annealed and the BHI-QF-FOR and pGaTn-hsp70REV-NotI-BH1 primers were used to PCR amplify a BamHI-QF-SpeI-BglII-hsp70-NotI-BamHI cassette, which was cloned into the BamHI site of pXN-FBLWLF. An insert in the correct orientation was verified by sequencing.
Cloning the enhancer fusion vector
pBMPGal4LWL
pBSO1O2 was cut with BamHI and BglII, and a BamHI fragment containing GAL4, the hsp70 3′ UTR and loxP-flanked mini-white from pXL-BacII-attPGal4LWL was ligated into these sites to make pBSO1O2-Gal4LWL. A correctly oriented insert was cut with NotI, treated with mung bean nuclease and re-ligated to destroy the NotI site. Next, to make a multi-cloning site, a pair of oligos (PstI-SacI EF linker For and PstI-SacI EF linker Rev) were phosphorylated, annealed, and ligated into the SacI and PstI sites of pBS-attP. Next, the multi-cloning site and attP site were liberated using HindIII, and ligated into the HindIII site of pBPHLWL. An insert with the correct orientation was identified and a fragment containing attB, the multi-cloning site, and attP was cut out of this plasmid with KpnI and BamHI. Finally, this fragment was ligated into the KpnI, BamHI sites of pBSO1O2-Gal4LWL to make pBMPGal4LWL.
pBMPGal4LWL-ortc2b
A fragment containing the ortc2b regulatory region was amplified from pChs-ATTB-OrtC2-Gal4 (obtained from C.-H. Lee)34 using the ortc2b For and ortc2b Rev primers. A NotI site was added to the 5′ end of the PCR product, which was then cloned into pCRII-TOPO (Invitrogen). The ortc2b fragment was cut out of pCRII-TOPO with NotI and BamHI and cloned into NotI, BglII cut pBMPGal4LWL.
Fly Methods
Flies were grown on molasses food at 22–25°C. Injections were performed as previously described by Rainbow Transgenic Flies43. P element constructs were transformed using a standard Δ2–3 helper plasmid. PiggyBac constructs were transformed using the pBSII-hs-orf30 helper plasmid (obtained from L. Luo). Injected attB donor constructs (pBPHLWL derivatives) were transformed using the ΦC31 integrase helper plasmid pBS130. Flies were obtained from the Bloomington Drosophila Stock Center (BDSC) (y, w; P{CaryP}attP2) to test the integration of pBMPGal4LWL.
Cre reductions
To prepare swappable enhancer trap lines for injection of the donor plasmid (or genetic conversion), females containing a convertible enhancer trap line were crossed to males carrying the Cre recombinase (y, w; MKRS, P{hsFLP}86E/TM6B, P{w[+mC]=Crew}DH2, Tb[1], or y, w; noc[Sco]/CyO, P{w[+mC]=Crew}DH1, from BDSC). Males containing both the enhancer trap and the Cre chromosome were crossed to the appropriate balancer line, and single male progeny were used to establish a balanced white minus enhancer trap stock (Supplementary Fig. 6). The clean excision of mini-white was confirmed by PCR (see below). The same procedure was used to remove the mini-white marker and original GAL4 gene from integrant flies. Excision using Cre was highly efficient, approaching 100%.
Genetic conversion crosses
To carry out the genetic swap procedure (see Fig. 1b, Supplementary Fig. 5), first a y, w, hs-FLP, vas-ΦC31 integrase recombinant X chromosome was established. Recombinants were scored for the presence of GFP (marking the J15, vas-ΦC31 integrase transgene19), and then tested by PCR for the presence of FLP (using the FLP Set1 Forward/FLP Set1 Reverse and FLP Set2 Forward/FLP Set2 Reverse primers). y, w, hs-FLP, vas-ΦC31; Recipient(enhancer trap)w−/Balancer stocks were made, and virgins were crossed to genetic donor males (Supplementary Fig. 5). These flies were allowed to lay eggs for 2–4 days, then flipped onto fresh food. The vials containing the progeny of this cross were then heat-shocked in a 37°C water bath for 1 hour, every day, until most of the progeny had pupated. The resulting progeny had eyes which were either completely white, or had small patches of orange or red variegation (Fig. 2d–e). Heat-shocked y, w, hs-FLP, vas-ΦC31; Donor/+; Recipient(w−)/+ males were crossed to y, w, eyFLP2; Balancer virgins, and white plus putative genetic swaps were balanced. Integration of the genetic donor construct in the enhancer trap was confirmed by PCR (see below). To complete the swap, the enhancer trap containing the integrated genetic donor construct was reduced using Cre (see above). Genetic donor constructs lose mini-white expression when crossed to eyFLP2, which excises the FRT-flanked donor cassette in the eye. At least eight independent lines were tested for the VP16AD, GAL80, GAL4AD, GAL4DBD, LexA, and QF constructs, and every line had white eyes in the presence of eyFLP2 (Fig. 2c), with the exception of one VP16AD line (P{ID.VP16AD}39.1D1), which was pupal lethal with eyFLP2.
Note: Maintaining the genetic donor lines together with the y, w, hs-FLP, vas-ΦC31 chromosome for many generations may lead to degradation of the donor construct.
Avoiding genetic swap aberrant events
Since all aberrant events were associated with the original donor site, crosses should be set up with recipient and donor lines on different chromosomes. This way, putative genetic swap lines that do not map to the recipient chromosome can be immediately discarded. In addition, by using recipient and donor lines with distinct eye colors, the level of mini-white expression can facilitate the identification of bona fide swaps. To avoid reciprocal translocations, recipient and donor lines can be molecularly mapped (Supplementary Fig. 4)44–45 and selected such that any translocations will be lethal (for instance, by generating a dicentric or acentric chromosome). Finally, by selecting white+ flies in the final step that also contain the balancer or dominant marker that was opposite the donor chromosome in the previous generation, it is possible to select against aberrant events. In our experience, selecting matched donors and recipients in this way eliminates all aberrant events. Even if no effort is made to select against aberrant events, the frequency of true events among w+ putative swaps is quite high (averaging 51.7%, Table 1).
Functional tests of enhancer trap swaps
The following lines were used to functionally test the split-GAL4 enhancer trap swaps: w; P{UAS:2xEGFP}; P{elav-GAL4.DBD}H4A1, w; P{UAS:2xEGFP}; P{elav-GAL4.AD}I1A1, w; P{UAS:2xEGFP}; P{elav-VP16.AD}G3A1 (from BDSC)16. For testing the enhancer trap swaps, the UAS:2xEGFP chromosome present in the elav split-GAL4 lines was replaced with UAS:mCD8-GFP17. w; LexAop:rCD2-GFP10 (obtained from Chi-Hon Lee) reporter lines was used to monitor LexA driven gene expression. w; P{QUAS-mCD8-GFP.P}5J was used to monitor QF expression11. The following injected enhancer trap swap lines were functionally tested: PBac{IS.VP16AD.w−}6.1(11.1), PBac{IS.GAL80.w−}6.1(39.1), PBac{IS.GAL4AD.w−}3.1(33.1), PBac{IS.GAL4DBD.w−}3.1(51.1), PBac{IS.LexA.w−}1.1 (44.1). The following genetic swap lines were functionally tested: PBac{IS.VP16AD.w−}6.1(D37-6), PBac{IS.GAL80.w−}6.1(E17-30), PBac{IS.GAL4DBD.w−}6.1(F32-2), PBac{IS.GAL80.w−}3.1 (E17-12), PBac{IS.VP16AD.w−}3.1(D33-16), PBac{IS.QF.w−}6.1(Q10B-7), and PBac{IS.QF.w−}3.1(Q12A-10). GFP expression in adult animals was visualized on a Zeiss M2Bio stereomicroscope and fluorescence images were acquired using a SPOT-RT digital camera (Diagnostic Instruments, Inc.).
Confirming Cre-mediated reductions and injected integration events by PCR
To confirm the deletion of mini-white by Cre, the subsequent integration of the microinjected attB-containing donor construct, and the Cre-mediated reduction of the integrant, a set of diagnostic PCR primers was used (see Fig. 2b and Supplementary Fig. 3 for primer locations and Fig. 2a and Supplementary Fig. 3 for examples). The following primer pairs were used on the piggyBac constructs: 1) MW3′F2/pBAC3′R4, 2) Gal4 3′F2/MW5′R2, 3) pBAC5′F1/attP R1, 4) Gal4 3′F2/pBAC3′R4, 5) pBSF1/attP R1, 6) pBAC5′F1/attB R1, 7) construct-specific primer/pBAC3′R4. Construct-specific primers: Gal80 3′F1, Gal4AD 3′F1 (note: also works on VP16AD), Gal4DBD 3′F1, LexA 3′F1, or QF 3′F1.
To confirm integration into the P element constructs (data not shown), the above primer pairs can be used, with two modifications. P5′inF can be used in place of pBAC5′F1, and P3′Rnew2R can be used in place of pBAC3′R4.
Confirming genetic swaps by PCR
The primers that were used to confirm the injection-based enhancer trap swaps were also used to validate the genetic swaps, with one exception. In the genetic swaps, the sequence corresponding to the vector backbone in the injected swaps does not exist, so primer MW3′F2 was substituted for pBSF1 in primer pair number 5. In addition, another set of PCRs using the primer pairs P5′inF/attBR1 (primer pair 8 in Fig. 2b) and MW3′F2/P3′Rnew2R (primer pair 9 in Fig. 2b) were done on the genetic swap lines, to confirm the loss of the donor element 5′ and 3′ junctions.
Confirming insertion of the pBMPGal4LWL-ortc2 enhancer fusion vector
To confirm the insertion of pBMPGal4LWL into P{CaryP}attP2, the following primers were used: 1) pBSF1/attP R1, 2) yellow 3′F2/attB R1, 3) yellow 3′F2/ortc2 5′R1.
Splinkerette mapping of transposon insertion sites
Fifteen of the piggyBac enhancer trap lines and twenty four genetic donor P element lines were mapped using Splinkerettes44, and localized using Flybase to BLAST search the Drosophila melanogaster genome46. Line PBac{IT.GAL4}1.1 was inserted in an intron of the sarah (sra) gene. Line PBac{IT.GAL4}3.1 was inserted in an intron of the desaturase1 (desat1) gene. Line PBac{IT.GAL4}5.1 was inserted within a microRNA cluster in mir-2498. Line PBac{IT.GAL4}6.1 was inserted in an intergenic region between the Gr93a-d cluster and the gliolectin (glec) gene. Insertion sites and orientations of the genetic donor lines are shown in Supplementary Fig. 4.
Immunohistochemistry and Imaging
Brains were dissected and fixed for one hour in 2% paraformaldehyde in phosphate buffered lysine (50 mM lysine, 100 mM Na2HPO4, pH 7.4), then washed 3×5 minutes in PBST (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 0.1% Triton X-100, pH 7.4). Following the washes, the brains were blocked for 30 minutes in 10% Normal Goat Serum in PBST, and incubated with primary antibody overnight at 4°C. Brains were then washed 3×5 minutes in PBST and incubated with secondary antibody for two hours at room temperature. After secondary incubation, the brains were washed 3×5 minutes in PBST, and transferred to 70% glycerol. When staining for mCD8, the following primary and secondary antibodies were used: mouse anti-Bruchpilot (nc82)47 (1:30, Developmental Studies Hybridoma Bank), rat anti-mCD817 (1:100, Invitrogen), goat anti-rat Alexa 488 (1:200, Molecular Probes), and goat anti-mouse Alexa 594 (1:200, Molecular Probes). When staining lines containing the LexAop:rCD2-GFP construct (and corresponding controls) chicken anti-GFP (1:2000, Abcam) primary, and goat anti-chicken Alexa 488 (1:200, Molecular Probes) secondary antibodies were used. Brains were mounted in Vectashield (Vector Laboratories) for imaging. Images were acquired on a Leica TCS SP2 AOBS confocal microscope with either a 20x (N.A. = 0.7) or 40x (N.A. = 1.25) lens. Confocal images were rendered in three dimensions using Imaris (Bitplane) and adjusted as necessary in Photoshop (Adobe) using cropping and thresholding tools, and assembled into figures using Illustrator (Adobe).
InSITE reagents
Drosophila stocks, including the mobile, X-linked P element and piggyBac enhancer trap lines (P{IT.GAL4}A134.3 and PBac{IT.GAL4}0315), the y, w, hs-FLP, vas-ΦC31 line, and all of the genetic donor lines shown in Supplementary Fig. 4 have been deposited with the Bloomington Drosophila Stock Center. A collection of 100 InSITE enhancer trap lines is available upon request. All plasmids have been deposited with Addgene.
Supplementary Material
Supplementary Figure 1. List of constructs used in the InSITE system
Supplementary Figure 2. Diversity of InSITE enhancer trap expression patterns in adult fly tissues and brains
Supplementary Figure 3. Validation of injectable InSITE plasmids
Supplementary Figure 4. Insertion sites and orientations of genetic donor lines
Supplementary Figure 5. Crossing schemes for performing swaps
Supplementary Figure 6. Eye colors are consistent for constructs integrated in the same enhancer trap landing site
Supplementary Figure 7. Analysis of the aberrant events seen in genetic swapping
Supplementary Figure 8. Additional functional tests of InSITE swaps
Supplementary Table 1. Antennal lobe glomerular innervation patterns of PBac{IT.GAL4}3.1 and associated swaps
Supplementary Table 2. Oligonucleotides used for cloning in this study
Supplementary Table 3. PCR and sequencing primers used in this study
Acknowledgments
We thank members of the Clandinin lab for helpful advice, and M. Müller (University of Basel), M. Wernet (Stanford University), T. Schwabe (Stanford University), G. Dietzl (Stanford University), J. Bateman (Bowdoin College), L. Luo (Stanford University), C-H. Lee (NIH), and P. Schedl (Princeton University) for reagents. Thanks also to S. Burns for assistance with experiments, and to A. Parks at the Bloomington Drosophila Stock Center. M. Klovstad, L. Luo, and T. Schwabe provided valuable comments on the manuscript. M. Spletter helped score antennal lobes. Plasmids were also obtained from the Drosophila Genomics Resource Center and from Addgene. Flies were obtained from the Bloomington Drosophila Stock Center. This work was funded by an National Institutes of Health Director’s Pioneer Award DP1 OD003530 (T.R.C.) and by National Institutes of Health R01 EY015231 (T.R.C.). D.M.G. and M.A.S. were supported by Stanford Dean’s Postdoctoral Fellowships.
Footnotes
Author Contributions
D.M.G. and T.R.C. designed the experiments; D.M.G., M.A.S., X.J.G., F.J.L., C-C. L., C.J.P., and T.R.C. performed the experiments; S.B. provided critical reagents; D.M.G. and T.R.C. wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 2.O’Kane CJ, Gehring WJ. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987;84:9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejsmont RK, Sarov M, Winkler S, Lipinski KA, Tomancak P. A toolkit for high-throughput, cross-species gene engineering in Drosophila. Nat Methods. 2009;6:435–437. doi: 10.1038/nmeth.1334. [DOI] [PubMed] [Google Scholar]
- 4.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venken KJ, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korzh V. Transposons as tools for enhancer trap screens in vertebrates. Genome Biol. 2007;8 (Suppl 1):S8. doi: 10.1186/gb-2007-8-s1-s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanford WL, Cohn JB, Cordes SP. Gene-trap mutagenesis: past, present and beyond. Nat Rev Genet. 2001;2:756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- 9.Bellen HJ. Ten years of enhancer detection: lessons from the fly. Plant Cell. 1999;11:2271–2281. doi: 10.1105/tpc.11.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 11.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- 13.Bohm RA, et al. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci U S A. 2010;107:16378–16383. doi: 10.1073/pnas.1004669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 18.Bateman JR, Lee AM, Wu CT. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics. 2006;173:769–777. doi: 10.1534/genetics.106.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golic MM, Rong YS, Petersen RB, Lindquist SL, Golic KG. FLP-mediated DNA mobilization to specific target sites in Drosophila chromosomes. Nucleic Acids Res. 1997;25:3665–3671. doi: 10.1093/nar/25.18.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn C, Handler AM. Site-specific genomic targeting in Drosophila. Proc Natl Acad Sci U S A. 2005;102:12483–12488. doi: 10.1073/pnas.0504305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberstein A, Pare A, Kaplan L, Small S. Site-specific transgenesis by Cre-mediated recombination in Drosophila. Nat Methods. 2005;2:583–585. doi: 10.1038/nmeth775. [DOI] [PubMed] [Google Scholar]
- 23.Schlake T, Bode J. Use of mutated FLP recognition target (FRT) sites for the exchange of expression cassettes at defined chromosomal loci. Biochemistry. 1994;33:12746–12751. doi: 10.1021/bi00209a003. [DOI] [PubMed] [Google Scholar]
- 24.Yagi R, Mayer F, Basler K. Refined LexA transactivators and their use in combination with the Drosophila Gal4 system. Proc Natl Acad Sci U S A. 2010;107:16166–16171. doi: 10.1073/pnas.1005957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorpe HM, Smith MC. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc Natl Acad Sci U S A. 1998;95:5505–5510. doi: 10.1073/pnas.95.10.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn C, Offen N, Nystedt S, Hacker U, Wimmer EA. piggyBac-based insertional mutagenesis and enhancer detection as a tool for functional insect genomics. Genetics. 2003;163:647–661. doi: 10.1093/genetics/163.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rad R, et al. PiggyBac Transposon Mutagenesis: A Tool for Cancer Gene Discovery in Mice. Science. 2010 doi: 10.1126/science.1193004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. piggyBac internal sequences are necessary for efficient transformation of target genomes. Insect Mol Biol. 2005;14:17–30. doi: 10.1111/j.1365-2583.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 31.Thibault ST, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 32.Siegal ML, Hartl DL. Transgene Coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics. 1996;144:715–726. doi: 10.1093/genetics/144.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggert KA, Gong WJ, Golic KG. Methods for homologous recombination in Drosophila. Methods Mol Biol. 2008;420:155–174. doi: 10.1007/978-1-59745-583-1_9. [DOI] [PubMed] [Google Scholar]
- 34.Gao S, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- 36.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 37.Malla S, Dafhnis-Calas F, Brookfield JF, Smith MC, Brown WR. Rearranging the centromere of the human Y chromosome with phiC31 integrase. Nucleic Acids Res. 2005;33:6101–6113. doi: 10.1093/nar/gki922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer BD, et al. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gohl D, Muller M, Pirrotta V, Affolter M, Schedl P. Enhancer blocking and transvection at the Drosophila apterous locus. Genetics. 2008;178:127–143. doi: 10.1534/genetics.107.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuldiner O, et al. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagstrom K, Muller M, Schedl P. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 1996;10:3202–3215. doi: 10.1101/gad.10.24.3202. [DOI] [PubMed] [Google Scholar]
- 43.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 44.Potter CJ, Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS One. 2010;5:e10168. doi: 10.1371/journal.pone.0010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochman H, Gerber AS, Hartl DL. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tweedie S, et al. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagh DA, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. List of constructs used in the InSITE system
Supplementary Figure 2. Diversity of InSITE enhancer trap expression patterns in adult fly tissues and brains
Supplementary Figure 3. Validation of injectable InSITE plasmids
Supplementary Figure 4. Insertion sites and orientations of genetic donor lines
Supplementary Figure 5. Crossing schemes for performing swaps
Supplementary Figure 6. Eye colors are consistent for constructs integrated in the same enhancer trap landing site
Supplementary Figure 7. Analysis of the aberrant events seen in genetic swapping
Supplementary Figure 8. Additional functional tests of InSITE swaps
Supplementary Table 1. Antennal lobe glomerular innervation patterns of PBac{IT.GAL4}3.1 and associated swaps
Supplementary Table 2. Oligonucleotides used for cloning in this study
Supplementary Table 3. PCR and sequencing primers used in this study