Abstract
The endoderm gives rise to the lining of the esophagus, stomach and intestines, as well as associated organs. To generate a functional intestine, a series of highly orchestrated developmental processes must occur. In this review, we attempt to cover major events during intestinal development from gastrulation to birth, including endoderm formation, gut tube growth and patterning, intestinal morphogenesis, epithelial reorganization, villus emergence as well as proliferation and cytodifferentiation. Our discussion includes morphological and anatomical changes during intestinal development as well as molecular mechanisms regulating these processes.
Keywords: intestine, development, differentiation, epithelium, endoderm, mesoderm, villus, crypt
Introduction
The intestine is a highly organized, complex organ that serves many important functions, including digestion and nutrient absorption, endocrine function, and immunity. The mature (endoderm-derived) intestinal epithelium is comprised of the stereotypic crypt-villus unit and contains absorptive (enterocytes) and secretory (goblet, enteroendocrine, Paneth, tuft) cell types, as well as resident intestinal stem cells and rapidly dividing progenitors. Adjacent to the intestinal epithelium are a complex assortment of (non-endoderm derived) cells comprising the lamina propria, submucosa, and muscular layers. In order to reach its mature form, the intestine must go through several complex developmental stages.
In this review, we focus on vertebrate intestine development with an emphasis on mammalian development. The authors recognize the importance of invertebrate model organisms and their contribution to the overall understanding of gut development, however, in an attempt to cover intestine development from gastrulation to the mature organ, invertebrate development is beyond the scope of this review. Excellent reviews on invertebrate gut development may be found elsewhere (Wessel and Wikramanayake, 1999; Lengyel and Iwaki, 2002; Maduro and Rothman, 2002; McGhee, 2007; Hou).
We have organized this review to cover intestine development along a developmental timeline starting with gastrulation and endoderm specification and ending with the mature organ shortly after birth. Along the way, we will cover the topics of gut tube formation, hindgut patterning, intestinal epithelial reorganization and villus emergence as well as proliferation and cytodifferentiation of the embryonic intestine.
Endoderm Specification during Gastrulation
This section is meant only to be a brief review of endoderm specification during gastrulation. Many excellent reviews exist which are dedicated to gastrulation and endoderm specification (For review, see: (Wells and Melton, 1999; Zernicka-Goetz, 2002; Tam et al., 2003; Technau and Scholz, 2003; Rossant, 2004; Tam et al., 2006; Spence and Wells, 2007; Tam and Loebel, 2007; Zorn and Wells, 2007; Rossant and Tam, 2009; Zorn and Wells, 2009).
The process of gastrulation gives rise to three primary germ layers including ectoderm, mesoderm and endoderm. Experimental evidence from fish, frogs and mice indicate that mesendoderm progenitor cells give rise to either endoderm or mesoderm as gastrulation proceeds (Lawson and Pedersen, 1987; Lawson et al., 1991; Rodaway et al., 1999; Kimelman and Griffin, 2000; Rodaway and Patient, 2001; Spence and Wells, 2007; Zorn and Wells, 2007; Zorn and Wells, 2009). First demonstrated in Xenopus, The TGF-β superfamily member Nodal is required for mesoderm and endoderm specification in all vertebrates (Green and Smith, 1990; Clements et al., 1999; Aoki et al., 2002; Ben-Haim et al., 2006; Hagos and Dougan, 2007). Exposure to Nodal signaling as the mesendoderm progenitor cells move through the streak determines if a progenitor cell becomes endoderm or mesoderm, with high levels of Nodal specifying endoderm and lower levels promoting mesoderm (Tremblay et al., 2000; Lowe et al., 2001; Vincent et al., 2003; Weng and Stemple, 2003; Tada et al., 2005; Shen, 2007). Activin, another TGF-β superfamily member, is able to mimic Nodal activity by binding to the same receptors (Smith et al., 1990; Thomsen et al., 1990; Gamer and Wright, 1995; Henry et al., 1996; Gray et al., 2003).This universal requirement for Activin/Nodal signaling across species has been utilized to direct differentiation of human and mouse embryonic stem cells (hESCs and mESCs, respectively) into endoderm (Kubo et al., 2004; D'Amour et al., 2005; Tada et al., 2005; Spence and Wells, 2007).
Nodal signaling promotes a complex transcriptional network, which acts to separate the endodermal and mesodermal lineages as well as give regional identity to the newly formed endodermal layer (Lewis and Tam, 2006; Zorn and Wells, 2009).
Morphogenetic movements of the endoderm during gastrulation
In mouse, birds and humans gastrulation starts in the pluripotent epiblast layer with the formation of the primitive streak. In the mouse, cells migrate through the primitive streak displacing and intercalating with the underlying visceral endoderm (VE) (which gives rise to mainly extraembryonic structures) and forms the endodermal layer of the embryo (Lawson and Pedersen, 1987; Tam and Beddington, 1992) The first cells to emerge through the primitive streak migrate anteriorly and give rise to the anterior definitive endoderm (ADE) (Lawson and Pedersen, 1987; Lawson and Schoenwolf, 2003; Kimura et al., 2006; Tam et al., 2007; Franklin et al., 2008). The mid- and hindgut endoderm emerges at the mid-streak stage of gastrulation, with all definitive endoderm (DE) formation completed prior to somite formation (Tam et al., 2007; Franklin et al., 2008; Zorn and Wells, 2009). Recently, it has been demonstrated that definitive endoderm that migrates through the primitive streak does not entirely displace visceral endoderm as previously thought (Kwon et al., 2008). Instead, the newly specified DE intercalates and mixes with existing underlying VE such that in the gut tube at E8.75, the VE accounts for ~10% of foregut endoderm, ~15% of midgut and ~35% of hindgut endoderm (Kwon et al., 2008). These genetic studies are consistent with cell labeling studies, which suggested that the VE might contribute to the gut tube (Tam and Beddington, 1992). This recent evidence for intercalation confounds genetic loss-of function studies for endoderm development because a complete failure DE formation via gastrulation may be masked by the contribution of VE to the DE layer after gastrulation. This problem may be especially true in the hindgut, where up to 35% of the DE after gastrulation consists of VE.
Gut tubulogenesis and elongation
Once the primary germ layers are established, the endoderm undergoes a complex series of changes to give rise to a gut tube. Gut tube morphogenesis differs dramatically between organisms. For example, in zebrafish and Xenopus, the endoderm forms from a “rod” of cells which will give rise to the gut tube (Warga and Nusslein-Volhard, 1999; Chalmers and Slack, 2000; Horne-Badovinac et al., 2001), whereas in chick, mouse, and human, the endoderm exists as a sheet of simple epithelium covering the mesoderm. The endodermal sheet undergoes a series of morphogenetic movements, which will ultimately give rise to the gut tube (Figure 1). Fate-mapping studies have shown that specific regions of the naïve endoderm contribute to specific domains of the gut tube, and in general, anterior endoderm cells contribute to the anterior intestinal portal (AIP) whereas posterior endoderm cells contribute to the caudal (posterior) intestinal portal (CIP) (Lawson et al., 1986) (Lawson and Pedersen, 1987; Tam et al., 2004; Franklin et al., 2008). Furthermore, fate-mapping studies have shown that individual organs, such as the liver, can arise from multiple early endoderm domains (Rosenquist, 1971; Warga and Nusslein-Volhard, 1999; Chalmers and Slack, 2000; Tremblay and Zaret, 2005).
Figure 1. Gut tube formation.
A. Schematic of the morphogenetic movements giving rise to the gut tube from post-gastrulation (E7.25) through gut tube formation (E9.5) in the mouse embryo. At E7.25, after gastrulation, the embryo is organized in a cup shape with the endoderm on the outer-most surface (yellow) and the mesoderm and ectoderm on the inside of the cup (black). At E7.75, pits that will give rise to the AIP and CIP are evident (black arrows). Note that the AIP forms several hours prior to the CIP, but for ease of illustration they are shown together. By E8.0 the AIP and CIP are clearly visible. Between E8.0 and E9.0, the lip of both the portals moves toward the center of the embryo, and the lateral endoderm continues to fold ventrally. Between E9.0 and E9.5 the endoderm has finished folding and exists as a tube surrounded by mesenchyme.
B. Ventral view of the endoderm folding. At E7.5, shortly after gastrulation has ended, the endoderm is schematically represented as a relatively naive flat sheet. By E7.75, the endoderm begins to fold along the longitudinal axis, much like a sheet of paper being rolled into a tube. At the same time, the intestinal portals begin to form (black arrows point to invaginations where the portals will form). Between E8.0 and E8.5, the ends of the endodermal “tube” are formed and represent the anterior and caudal intestinal portals. Simultaneously, the endoderm continues to fold ventrally along the longitudinal axis. Since the endoderm is covered by mesoderm, as the endoderm forms a tube, mesoderm is visible. By E9.0–E9.5 the endodermal tube is closed such that the endoderm is entirely surrounded by mesoderm. The dashed line denotes a cross section through the closed tube, where the endoderm is surrounded by the mesoderm.
C. FoxA2creER;R26R embryos treated with Tamoxifen via maternal oral gavage (0.12mg/g) at E6.5. Tamoxifen activation of FoxA2creER causes extensive recombination of the R26R allele, leading to β-galactosidase expression in the endoderm and notochord. Embryos were collected and stained for β-galactosidase activity with X-Gal (shown in blue) at E8.5 and E9.5. Anterior is denoted by the red arrow and posterior is denoted by the blue arrow. The middle panel demonstrates the CIP at E8.5, with the arrow pointing to the lip of the CIP.
In chick and mouse, one of the first steps in gut tube closure is the formation of anterior and posterior pits in the endoderm, which are the beginning of the AIP and CIP respectively (Figure 1A,B; 2A). As the pits invaginate further, they form pockets. The intestinal portals expand rostrally (AIP) and caudally (CIP) while the openings of both the AIP and CIP move towards each other. The lateral endoderm of the midgut folds ventrally and meets together to form a closed gut tube as the embryo is turning at e9.0 (Figure 1; 2B,C) (Lewis and Tam, 2006).
Figure 2. Gut tube histology.
A–B. At left, a schematic drawing of the embryo shows the level of the histological sections shown at right. The endoderm in colored yellow in the schematic and pseudocolored yellow in the histological sections.
A. Transverse sections through the caudal region of an E7.75 embryo demonstrating the open endoderm mid-embryo (section 1) which is starting to form a CIP (section 2 and 3).
B. Transverse sections through the caudal region of an E8.5 embryo showing the open gut tube toward the middle of the embryo, which closes forming the CIP at the posterior of the embryo.
C. Changes in the gut tube epithelium between E9.0 and E11.5. The gut tube endoderm condenses between E9.0 and E9.5 to give rise to a pseudostratified epithelium. Between E9.5 and E10.5 the gut tube epithelium circumference increases giving rise to a bigger lumen and an increased epithelial surface area.
All images are the same magnification (40X). Endoderm is pseudocolored yellow in all histological sections.
Little is known about how intestinal portal formation and gut tubulogenesis is controlled. Extensive cross talk and inductive cues between the mesoderm and endoderm have been described (Schultheiss et al., 1995; Wells and Melton, 1999; Cleaver and Krieg, 2001; Deutsch et al., 2001; Rossi et al., 2001; Withington et al., 2001; Couly et al., 2002; David et al., 2002; Zaret, 2002; Sugi and Markwald, 2003; Serls et al., 2005; Wandzioch and Zaret, 2009). Despite this extensive body of work, it does not appear that mesodermal movements directly guide gut tube morphogenesis. That is, while the mesoderm is undoubtedly providing information to the endodermal layer, it does not appear as though the cellular movements of the mesoderm are directly tethered to endodermal movements since cell labeling experiments show that labeled adjacent mesoderm and endoderm can end up at different locations (Tremblay and Zaret, 2005).
Transcription factors and signaling pathways that disrupt gut tube formation have been identified. In most cases, however, disruption of gut tubulogenesis is a secondary consequence of disrupting endoderm specification or maintenance. For example, disruption of Fox factors (FoxA2, FoxH1), Gata factors, Sox17, Mixl1 or Smad signaling all lead to defects in gut tube morphogenesis which are secondary to endoderm development and specification defects (Soudais et al., 1995; Hudson et al., 1997; Narita et al., 1997; Bossard and Zaret, 1998; Dufort et al., 1998; Clements and Woodland, 2000; Tremblay et al., 2000; Hart et al., 2002; Kanai-Azuma et al., 2002; Liu et al., 2004; Tam et al., 2007; Hoodless et al., 2001). New evidence suggests that endoderm which will give rise to anterior or posterior endoderm may be specified differently during gastrulation. Analysis of FoxA2 and FoxH1 null embryos with novel markers for foregut, midgut and hindgut showed that mid and posterior endoderm is specified in these embryos while anterior endoderm fails to form (McKnight et al., 2010). As mentioned above, however, this study did not perform lineage-tracing experiments to rule out the possibility that the mid- and posterior endoderm seen in these mutant embryos was contributed by the VE.
Recently, it has been demonstrated that regulation of convergent extension movements control gut tube formation and elongation in mouse (Garcia-Garcia et al., 2008; Wen et al., 2010). Chato, a KRAB zinc-finger protein, regulates body axis elongation in all three germ layers through a process independent of non-canonical Wnt signaling. Chato was demonstrated to control convergent extension in the endoderm, and in embryos lacking Chato the endoderm failed to elongate and undergo gut tube closure (Garcia-Garcia et al., 2008). In a second study, Dact1 is involved in gut tube morphogenesis by regulating non-canonical Wnt-dependent planar cell polarity (PCP) signaling. Dact1 null mice had severely impaired posterior development and hindgut defects, including a failure of the hindgut endoderm to form a CIP and failure of the endoderm to fold ventrally at e8.25. By e10.5 Dact1 null mice fail to form a cloaca and have no obvious hindgut (Wen et al., 2010). While Dact1 regulates non-canonical Wnt signaling, canonical Wnt signaling is also important for caudal development as a lack of Wnt3a, Wnt5a, LRP6 or Tcf1/Lef1 all lead to posterior developmental defects that are mainly mesodermal in origin (Takada et al., 1994; Yamaguchi et al., 1999; Pinson et al., 2000). TCF1/4 double null mice have a lack of caudal structures, however, unlike other disruptions in canonical Wnt signaling, the primary defect in these mice is in the endoderm. At E8.5 TCF1/4 double null mice fail to form a CIP and lose expression of the hindgut endoderm markers such as FoxA1 and Sox17. By E9.5 embryos have an open midgut and completely lack hindgut structures concomitant with a loss of posterior endodermal Shh expression (Gregorieff et al., 2004). Lastly, studies in Xenopus have shown that generation of a lumen in the primitive gut tube, as well as gut tube elongation require Rho, ROCK and Myosin II for proper cellular movement and tissue rearrangement (Reed et al., 2009). Taken together, these studies demonstrate that both Wnt-dependant and Wnt-independent mechanisms control gut tube morphogenesis, and both canonical and non-canonical Wnt signaling are required for development of the posterior gut tube. Overall, our understanding of the dynamics and molecular regulation of gut tubulogenesis is still in its infancy and a more in-depth understanding of this process will require additional work.
Gut tube patterning
Nodal signaling, which is required for mesendoderm specification during gastrulation, plays a second role in specifying anterior identity during anterior-posterior (A-P) patterning (Osada and Wright, 1999; Schier and Shen, 2000; Brennan et al., 2001; Hoodless et al., 2001; Yamamoto et al., 2001; Robertson et al., 2003; Vincent et al., 2003; Duboc et al., 2004; Lu and Robertson, 2004; Yamamoto et al., 2004), such that by the end of gastrulation, the endoderm is patterned into molecularly distinct A-P domains as demonstrated by anterior Hhex, FoxA2 and Sox2 expression and posterior Cdx expression (Cdx1, 2, 4) (For review, see (Dufort et al., 1998; Martinez Barbera et al., 2000; Chawengsaksophak et al., 2004; Kinkel et al., 2008; Sherwood et al., 2009; Zorn and Wells, 2009).
After the initial A-P patterning during gastrulation, the endoderm continues to receive patterning and inductive signals, mostly from the mesoderm, such that by e8.5 distinct organ-specific molecular domains are already in place (Sherwood et al., 2009). These inductive and patterning steps occur simultaneously with tissue rearrangements and gut tube formation, making this time during embryogenesis extremely dynamic. The anterior gut tube will give rise to the esophagus, lungs, thyroid, liver, pancreas and biliary system, where as the midgut will give rise to the stomach and the small intestine and the hindgut will give rise to the large intestines as well as the lining of the genito-urinary system (Wells and Melton, 1999; Zorn and Wells, 2007; Seifert et al., 2008; McLin et al., 2009; Spence et al., 2009; Zorn and Wells, 2009).
Signaling pathways that play a role in A-P patterning of the endoderm include FGF, Wnt, BMP and retinoic acid signaling, and are reviewed elsewhere (Summarized in Figure 4A). (Wells and Melton, 1999; Wells and Melton, 2000; Tiso et al., 2002; Kumar et al., 2003; Grapin-Botton, 2005; Dessimoz et al., 2006; Lewis and Tam, 2006; Marikawa, 2006; Tam et al., 2006; McLin et al., 2007; Pan et al., 2007; Zorn and Wells, 2007; Goessling et al., 2008; Wills et al., 2008; Bayha et al., 2009; McLin et al., 2009; Zorn and Wells, 2009). An understanding of developmental signaling pathways that pattern the endoderm in the embryo has been used to inform embryonic stem cell (ESCs) differentiation into specific endodermal lineages (D'Amour et al., 2006; Cai et al., 2007; Kroon et al., 2008; Basma et al., 2009; Maehr et al., 2009; Brolen et al., 2010; Mfopou et al., 2010). Additionally, how multiple signaling inputs are integrated to determine positional A-P identity in the embryo is also starting to become clearer through the use of ESCs because of fine control over growth factor timing and dosage that in vitro studies allow. It has recently been demonstrated that a specific dose of FGF4 likely patterns hESC derived endoderm such that it is able to increase Pdx1 expression induced by RA signaling. In addition, RA and FGF4 appear to work synergistically to induce posterior Cdx2 expression (Johannesson et al., 2009). Moreover, it has recently been reported that varying concentrations of FGF2 can pattern hESC-derived endoderm from anterior (low doses) to posterior (high doses)(Ameri et al., 2010). Our work has recently shown that FGF and Wnt signaling synergize to efficiently push hESC derived endoderm into Cdx2+ hindgut epithelium, which gives rise to hindgut spheroids that are equivalent to early embryonic (E8.5) mouse hindgut. These spheroids can then be grown in intestine specific growth conditions in vitro and go through a series of developmental steps to give rise first to fetal gut-like tissue and then to tissue reminiscent of adult intestine (Spence JR, 2010). It is becoming evident that in vitro differentiation of different tissue lineages is able to greatly benefit from closely mimicing embryonic developmental cues, and that including very early embryonic patterning steps in differentiation protocols is an important first step in this process.
Figure 4. Schematic of mouse intestinal development from gastrulation to birth.
A. The grey box contains a schematic of an E8.0 mouse embryo with the endoderm in yellow. Signals involved in anterior-posterior patterning are sent from the mesoderm (Meso, white cells) to the endoderm (Endo, yellow cells). Signaling pathways important for A-P patterning are summarized, and include fine regulation of Bmp, Wnt, Fgf and RA signaling along the axis.
B–F. The grey boxes contain a schematic of a transverse section through the proximal intestine (duodenum) at different developmental time points. The endoderm-derived intestinal epithelium is shown in yellow. A close up of the intestinal epithelium and underlying mesoderm-derived mesenchyme is shown under the grey box. Key molecular events regulating intestine development are shown based on the developmental time during which evidence has been presented for each event.
B. Pseudostratified intestinal endoderm at E10.5.
C. At E14.5, intestinal morphogenesis is underway. The intestinal epithelium becomes stratified and the formation of Ezrin+ secondary lumina is apparent (Ezrin expression is shown in red).
D. By E15, secondary lumina have fused with the luminal surface and villus emergence is evident. In addition, condensed mesenchyme expressing higher levels of BMPs and PDGFR-α underlying nascent villi are present. Major signaling pathways (described in detail in the text) involved in epithelial-mesenchymal crosstalk responsible for modulating villus emergence are depicted.
E. At E16.5 villi and intervillus regions are evident. Sox9 expression is restricted to the proliferative intervillus region (green cells). Recent evidence has shown that β-catenin activity is present in the villus epithelium, but is excluded from the proliferative intervillus region.
F. By late E18.5, β-catenin activity has transitioned and is present in the proliferative Sox9+ intervillus villus, and is absent from the villi. A schematic of the pathways involved in mesenchymal-epithelial crosstalk at E18.5 that regulate both mesenchymal and epithelial proliferation is shown.
While it is clear that secreted factors signaling from the mesoderm to the endoderm are crucial in A-P patterning, these signaling events must ultimately induce an endodermal epithelium intrinsic program that will specify hindgut and intestinal identity. For example, xenografting studies show that the hindgut endoderm is only partially able to be reprogrammed along the A-P axis by the mesoderm just prior to cytodifferentiation (Duluc et al., 1994; Grapin-Botton and Melton, 2000) indicating that the intrinsic identity of the epithelium becomes fixed at a certain point during development. Hox factors play an important role in patterning the mesoderm and neurectoderm (McGinnis and Krumlauf, 1992; Krumlauf, 1994; Deschamps et al., 1999), however, mutations in Hox factors have only minor effects on the gut endoderm. (Manley and Capecchi, 1995; Boulet and Capecchi, 1996; Aubin et al., 1997; Warot et al., 1997; Zacchetti et al., 2007). It has recently been shown that a member of the ParaHox gene cluster, Cdx2, is perhaps the most critical intrinsic factor for hindgut and intestinal specification and patterning (Gao et al., 2009; Grainger et al., 2010), whereas a loss of Cdx1 or Cdx4 does not have an intestinal phenotype (Subramanian et al., 1995; van Nes et al., 2006). Cdx2 null mice die at E3.5, and therefore assessment of Cdx2 function at later stages requires a conditional loss-of-function approach. Conditional loss of Cdx2 in the endoderm around E9.5 results in the loss of intestinal identity. It becomes apparent by E12.5 that the gut epithelium is replaced by esophageal epithelium. In these mice, the posterior Hox code was only transiently affected such that only 5/13 Hox genes examined at e12.5 had reduced expression levels, which eventually recovered by e14.5. Furthermore other region-specific markers, such as Pdx1 (pancreas and duodenum) and Barx1 (stomach) remained unchanged. This indicates that while the endoderm has a posterior-to-anterior transformation in Cdx2 loss-of-function mice, other aspects of the A-P pattern remain intact (Gao et al., 2009). Conditional loss of Cdx2 at later developmental time points, around e13.5, led to a transformation of the small intestine to a stomach-like identity (Grainger et al., 2010). It is clear from these studies that Cdx2 is initially required for establishing and maintaining posterior identity and at later stages, it is required for maintaining A-P identity.
It is likely that a combination of signaling molecules responsible for A-P patterning integrate to drive endoderm specific Cdx2 activity, since the Cdx family is regulated in many different contexts by Wnt, Fgf, Bmp and RA signaling (Houle et al., 2000; Allan et al., 2001; Ikeya and Takada, 2001; Beland et al., 2004; Beland and Lohnes, 2005; Keenan et al., 2006; Pilon et al., 2006; Joo et al., 2010). It is also important to note that different signaling pathways will likely have different effects on Cdx2 expression and activity during different developmental time-points. For example, double-null mutations in the Wnt downstream effectors TCF1 and TCF4 have early posterior defects, including a loss of the caudal-most hindgut at E8.5. At E13.5 these TCF1/4 -mutant mice had a homeotic transformation such that the duodenum had reduced Cdx2 expression and expressed the stomach marker, Sox2 (Gregorieff et al., 2004). This phenotype is strikingly similar to loss of Cdx2 at E13.5 (Grainger et al., 2010) and indicates that Wnt signaling may positively regulate Cdx2 expression during early development. However, a second recent study shows that de-repression of Wnt signaling by loss of the Wnt antagonist Pinin around e13.5 also leads to downregulation of Cdx2 (Joo et al., 2010). Collectively, these studies show that there will be complex temporal, spatial and context specific roles for patterning factors in specifying hindgut identity.
Early intestinal development
Little is known about changes occurring in the intestinal epithelium after the hindgut epithelium is fully formed, but before villus emergence begins. In mouse, after the gut tube is fully formed around e9.0, the simple epithelium condenses and becomes a pseudostratified epithelium by e9.5 (Figure 2C). Between e9.5 and e13.5, the intestine lengthens with the growing embryo and the circumference of the gut mesenchyme and epithelium increases along with the size of the lumen (Figure 2C and 3) (Lepourcelet et al., 2005; Cervantes et al., 2009). Wnt5a, working through non-canonical Wnt signaling, has been implicated in the control of gut elongation and regulation of epithelial architecture. Wnt5a null mice have an 80% reduction in small intestine length and a 63% reduction in large intestine length by E18.5, but differences were apparent by E14.5. Wnt5a mutant mice also had disrupted apical-basal polarity of the intestinal epithelium (Cervantes et al., 2009). Similarly, mice lacking proteins of the Secreted Frizzled Related Protein (Sfrp) family (Sfrp1−/−;Sfrp2−/− or Sfrp1−/−;Sfrp2−/−;Sfrp5+/−), which physically interact with and inhibit Wnt5a, also had shortened intestines by E13.5. Furthermore, apical-basal polarity of the epithelium and core components of the PCP pathway were disrupted in these mice, consistent with a defect in non-canonical Wnt signaling (Matsuyama et al., 2009).
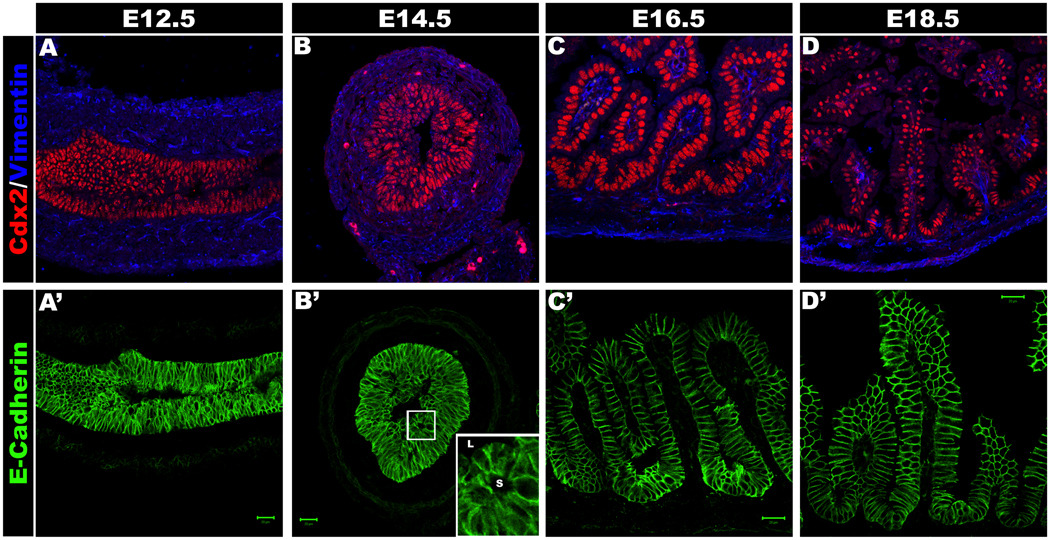
Figure 3. Intestinal epithelial reorganization.
Intestinal development at E12.5 (A, A’), E14.5 (B, B’), E16.5 (C, C’) and E18.5 (D, D’). A–D shows the Cdx2 positive epithelium (Red) and Vimentin positive mesenchyme. A’–D’ shows the E-cadherin positive epithelium. Secondary lumina can be seen at E14.5 (Boxed region is magnified in the inset; L indicates primary lumen, s indicates secondary lumen) (B’) as epithelial reorganization begins. By E16.5 there are clear intervillus regions and villi (C, C’).
In addition to Wnt signaling, Fgf signaling is also important during early intestinal development. Fgf9, Fgf10 and FgfR2b are all required for proper cecum development. Fgf9 is expressed in the intestinal epithelium and signals to the mesenchyme, driving mesenchymal proliferation and lengthening of the intestine (Geske et al., 2008). Furthermore, Fgf9 signals from the epithelium are required for mesenchymal Fgf10 expression. Fgf10 then signals back to the epithelium through FgfR2b, driving proliferation, which is required for cecal budding and growth (Burns et al., 2004; Fairbanks et al., 2004; Sala et al., 2006; Zhang et al., 2006).
During this time in intestinal development, in addition to an increase in length and circumference, there are dramatic changes in the intestine at the transcriptional level. Data mining approaches have been employed and proven useful to look at the entire transcriptome, as well as transcription factor specific regulation across developmental timepoints (Lepourcelet et al., 2005; Choi et al., 2006). Based on the limited understanding of early intestinal development, and the lack of literature available for intestinal development at this stage, it is clear that additional research is needed in this area.
Epithelial reorganization and villus emergence
Starting at about E14 in the mouse (E17 in the rat, 9 weeks in humans), the stratified epithelium of the midgut and hindgut endoderm begins to undergo extensive reorganization to form the simple columnar epithelium which covers the lumenal surface of the intestines (Figures 3 and 4). This reorganization has been carefully examined by light and electron microscopic analysis of the developing intestine in animal models and human fetuses. (Johnson, 1910; Patten, 1948; Grand et al., 1976; Trier and Moxey, 1979). Prior to these morphological changes, the endoderm transitions from a tightly packed simple epithelium with nuclei at several levels within the apicobasal axis, called pseudostratified epithelium, to a stratified epithelium in which the apical (lumenal) cells are connected by junctional complexes, but the basally located cells are more loosely associated (Figure 3A,B; 4B,C) (Dunn, 1967). By E14 in the mouse, a wave of rostral-to-caudal (proximal-to-distal) epithelial reorganization initiates formation of the columnar intestinal epithelium. This reorganization begins with secondary lumina (also called intra-epithelial cavities) forming in the deeper basal layers of the stratified epithelium, with nascent junctional complexes in cells surrounding these secondary lumina (Figure 3B; 4C) (Mathan et al., 1976; Matsumoto et al., 2002). Over the next day, extension of these junctional complexes to adjacent cells enlarge these secondary lumens, and they begin to fuse with the primary lumen (Figure 4D). Mitosis of cells lining the secondary lumina may also contribute to lumina expansion (Toyota et al., 1989). At the same time that the intestinal endoderm begins to reorganize into a simple columnar epithelium, the mesenchyme begins to invaginate into the epithelium to form nascent villi (Figure 3C; 4D,E). Like epithelial reorganization, villus emergence progresses in a rostral-to-caudal wave and is evident by E15 in the mouse, by E19 in the rat, and 9 weeks in human (Figure 4D,E) (Mathan et al., 1976; Madara et al., 1981).
Development of the large intestine (colon) follows a similar pattern as the small intestine, with some important distinctions. As in the small intestine, the early single-layer hindgut endoderm develops into a multilayered epithelium surrounded by mesenchyme and an outer mesothelium (mouse E13–E15, rat E16–18, human 9 weeks) (Polak-Charcon et al., 1980; Colony et al., 1989). Interestingly, the fetal colon develops villus-like structures before adopting the deeper crypts and flat intercrypt table that constitutes the mature colonic epithelium (Patten, 1948; Helander, 1973). These villus-like structures first appear as longitudinal ridges in the multilayered epithelium, which begin to develop secondary lumina in a process of epithelial reorganization similar to the small intestine (E16 in mouse, E19 in rat, 10 weeks in humans) (Helander, 1973; Bell and Williams, 1982). At the same time, the mesenchyme begins to invaginate into the epithelial ridges, forming longitudinal epithelial folds. These folds resolve into villi which are covered with stratified epithelium that has initiated cytodifferentiation, evident with the emergence of goblet cells (11–12 weeks in humans) (Lev and Orlic, 1974). These larger primary villi subsequently split in a process mediated by extensive epithelial and mesenchymal rearrangements. Crypt-shaped structures initially form as secondary lumina within the basal layers of the stratified intervillus epithelium, and extend as a single layered epithelium to become continuous with the lumen. At the same time, mesenchymal cells extend into the epithelial layer adjacent to the nascent single layered epithelium, thus dividing the primary villi into smaller secondary villi, each with a core of mesenchymal lamina propria covered by a simple columnar epithelium. (E17–18 in mice, 12–13 weeks in humans) (Bell and Williams, 1982).
Although the morphological changes in the fetal gut have been well described in several animal models, less is known about the mechanisms that initiate epithelial reorganization and villus emergence. Apicobasal polarity is required for epithelial reorganization, likely supported by the planar cell polarity pathway (Matsuyama et al., 2009). For example, the apical membrane organizing protein Ezrin is required for normal polarization of the epithelium from E14–E15 (Figure 4C,D). At E14, Ezrin is concentrated at both the lumenal surface as well as secondary lumina undergoing epithelial reorganization. At this stage, the stratified intestinal endoderm of Ezrin deficient mice appears grossly normal, but by E15 some cells fail to properly polarize, ultimately resulting in villus fusion and gross disorganization of the intestinal mucosa (Saotome et al., 2004).
Epithelial-Mesenchymal crosstalk during villus emergence
Extensive crosstalk between the developing endoderm and underlying mesenchyme are critical for normal development of the intestines. This concept was first established by tissue reassortment experiments in which the endoderm and mesenchyme from different regions, stages, and/or species of the developing gastrointestinal tract were recombined and allowed to develop (Lebenthal, 1989). These studies demonstrate that both permissive and instructive reciprocal interactions between the intestinal endoderm and mesoderm are required for normal morphogenesis and differentiation. For example, in a more recent study, xenografts of stratified endoderm from proximal jejunum combined with mesenchyme from proximal colon (ePJ/mPC) gave villi, jejunal-specific absorptive enterocytes expressing the digestive enzyme sucrase-isomaltase, and endocrine cells expressing jejunal-type hormones (CCK), but did not support production of small-intestine specific Paneth cells. The converse xenograft (ePC/mPJ) also gave villi, sucrase-isomaltase expressing enterocytes, and in this case the xenografts contained Paneth cells, but endocrine cells expressed distal-type hormones (PYY, GLP-1) (Ratineau et al., 2003). This example demonstrates the complex nature of the reciprocal epithelial-mesenchymal interactions in the developing intestines.
Further evidence supporting the role of epithelial-mesenchymal crosstalk comes from mice bearing mutations in Epimorphin, a syntaxin protein involved in targeting secretory vesicles to the plasma membrane of mesenchymal cells (Hirai et al., 1992). Loss of epimorphin inhibits epithelial morphogenesis and enhances epithelial proliferation, likely by interfering with multiple intercellular communication pathways including BMP and Hedgehog (Fritsch et al., 2002; Shaker et al., 2010). Several other intercellular signaling pathways, including TGF-β, PDGF, FGF, WNT, and EGF, have been implicated in epithelial-mesenchymal crosstalk during intestinal epithelial reorganization and villus formation.
Hedgehog signals from the developing epithelium regulate maturation of the underlying mesenchyme (reviewed in (van den Brink, 2007). Both Shh and Ihh are expressed throughout the pseudostratified epithelium of the early intestinal endoderm. Shh and Ihh continue to be expressed in the single-layer columnar epithelium with Shh becoming restricted to the villus base during villus emergence and eventually becoming extinguished, whereas Ihh expression is retained by the differentiated epithelium (Kolterud et al., 2009). Shh mutant mice display villus overgrowth, whereas Ihh mutant mice show reduced epithelial proliferation and have smaller, fewer villi, suggesting opposing effects of Shh and Ihh (Ramalho-Santos et al., 2000)). Interestingly, inhibition of all intestinal Hedgehog signals, by deletion of both Shh and Ihh in the early endoderm, antibody injection, or expression of the Hedgehog antagonist Hhip, causes defective intestinal mesenchymal development, leading to severely disrupted villus formation and epithelial maturation; moderate Hedgehog inhibition leads to impaired crypt-villus axis formation with ectopic and branched crypts proliferating on the villi (Wang et al., 2002; Madison et al., 2005; Mao et al., 2010). These results suggest that Hedgehogs may be one of the primary and most important signals during the phase of villus emergence (Figure 4D,F).
In the subepithelial mesenchyme, Hedgehog signals are interpreted by Gli transcription factors Gli2 and Gli3, which directly activate transcription factors of the Forkhead Box winged-helix superfamily (Madison et al., 2009). Mutation of the Gli target gene Foxl1/Fkh6 causes a delay in epithelial reorganization and villus emergence, leading to hyperproliferation and abnormal crypt branching in the adult (Kaestner et al., 1997). Additional Hedgehog targets FoxF1 and FoxF2 cooperate to support mesenchymal expansion, differentiation, and maintenance. Loss of these genes causes defects in the mesenchyme leading to disintegration of the tissue shortly after villus formation (Figure 4F) (Ormestad et al., 2006). In addition to cell-autonomous mesenchymal effects of Gli and Fox proteins, these transcription factors regulate secreted morphogens, such as Wnts and BMPs, that signal back to the developing endoderm.
BMPs, in particular BMP2, 4, and 7, play important roles in reciprocal epithelial-mesenchymal signaling downstream of the Hedgehog pathway in the developing intestine (Figure 4D,F) (Roberts et al., 1995; Madison et al., 2005). BMPs are primarily expressed in the subepithelial mesenchyme, especially in the mesenchymal condensations that underlie nascent villi (Karlsson et al., 2000). Perturbation of BMP signaling in the developing chick hindgut caused abnormal morphogenesis and differentiation of endoderm, mesoderm, and ectoderm derivatives in the gut (De Santa Barbara et al., 2005). Mutation of the BMP receptor BMPR1a causes aberrant hyperproliferation and ectopic crypt formation on the villi, leading to polyp formation in a disease termed Juvenile Polyposis (Howe et al., 2001; He et al., 2004). However, it is clear that loss of BMP signaling in nonepithelial cells is critical for this process, as epithelial-specific deletion of BMPR1a causes only hyperplasia but not polyp formation (Auclair et al., 2007; Shroyer and Wong, 2007). The hypothesis that BMP signaling is critical for development of the crypt-villus axis was further supported by experiments in which epithelial expression of the BMP2/4/7 antagonist Noggin caused abnormal villus formation in transgenic lines (Batts et al., 2006). In these mice, epithelial reorganization occurs but subepithelial mesenchymal condensation is less robust, leading to fewer but larger villi formed by birth. Later, BMP inhibition consistently causes extensive disorganization with ectopic proliferating crypts on the villi, leading to polyp formation in the small and large intestines (Haramis et al., 2004; Madison et al., 2005; Batts et al., 2006). These defects are associated with increased Wnt, PDGF and Hedgehog activities, suggesting multiple levels of feedback regulating epithelial-mesenchymal interactions (Haramis et al., 2004; Madison et al., 2005; Batts et al., 2006).
PDGF epithelial to mesenchymal signaling functions in parallel with the Hedgehog pathway. PDGF-A and its receptor, PDGFR-α, are expressed in the endoderm and mesenchyme respectively, prior to villus emergence (Figure 4D). PDGF-A becomes progressively more restricted to the intervillus epithelium and subsequently to the crypts, whereas PDGFR-α expression remains scattered throughout the subepithelial mesenchyme, with particularly strong expression in mesenchymal clusters beneath nascent villi which retain strong expression and remain at the growing tip of the villus core as the villi elongate. Genetic ablation of this epithelial-to-mesenchymal signaling system causes abnormally folded small intestinal villi and disrupted colonic mucosal architecture, likely due to early differentiation of mesenchymal smooth muscle cells. (Karlsson et al., 2000). However, unlike Hedgehog and BMP pathway manipulations, in PDGF mutants proliferation remains appropriately restricted to the intervillus epithelium and crypts.
EGF is another potent morphogen that acts on the developing intestine. Deletion of the EGF receptor, EGFR, caused delayed epithelial reorganization and villus formation, leading to reduced proliferation, villus blunting and tissue disintegration (Miettinen et al., 1995). Interestingly, this phenotype was highly variable in different inbred strains of mice (Sibilia and Wagner, 1995; Threadgill et al., 1995). Postnatally, EGF is considered a potent mitogen for the intestine. (Barnard et al., 1995) However, EGF has been reported to have varying effects on the intestine of different species at various developmental stages. For example, EGF delivered in utero or in organ culture enhances intestinal epithelial maturation in mice (Calvert et al., 1982; Beaulieu et al., 1985). In contrast, EGF decreased proliferation and maturation of human fetal intestinal organ cultures (Menard et al., 1988; Menard et al., 1990). EGF treatment of intestine explanted prior to villus emergence enhanced villus formation and expression of cytodifferentiation markers for enterocytes and goblet cells, along with enhanced epithelial proliferation. Conversely, inhibition of EGFR blocked mesenchymal growth and development, as well as reducing epithelial proliferation and goblet cell formation (Duh et al., 2000). Thus, EGF is important in the early development of the intestine, but has varying effects depending on the developmental stage, and proximodistal region, and species examined.
Various additional growth factors, including HGF, IGFs, KGF/FGF7, and TGF-β have poorly defined roles in intestinal development, but are expressed in the intestinal mucosa along with their receptors and have protective or reparative effects in various contexts (Goke and Podolsky, 1996; MacDonald, 1999; Howarth, 2003; Xian, 2003; Ido et al., 2005). Much work remains to be done to identify all of the important intercellular communication pathways involved in epithelial reorganization and villus formation.
Transcriptional control of villus emergence
Several transcription factors have been implicated in intestinal villus formation. Elf3 is an ETS transcription factor that cooperates with the Crif1 transcriptional coactivator to regulate morphogenesis and epithelial differentiation of the intestine (Ng et al., 2002; Kwon et al., 2009). Both Elf3- and Crif1-deficient mice develop fewer, abnormal villi with disorganized lamina propria mesenchyme and defective epithelial cells, likely arising at the time of villus formation. Both mutant lines also show reduced expression of the Tgf-β type II receptor, Tgf-βRII. In an elegant study, re-expression of Tgf-βRII in the epithelium rescued all intestinal phenotypes associated with Elf3 deficiency (Flentjar et al., 2007). These results suggest that Elf3/Crif1 plays an important role during villus emergence that is mediated by Tgf-β signaling. However, the specific ligands and cells of origin of this morphogenetic signal remain to be identified.
HNF4α is another transcription factor expressed throughout the intestinal epithelium. Deletion of HNF4α in the early embryonic endoderm causes a severe colonic phenotype including loss of crypt-villus architecture, defects in epithelial and mesenchymal maturation, and reduced epithelial proliferation (Garrison et al., 2006). In contrast, deletion after villus formation does not perturb intestinal development but instead leads to abnormal homeostasis in the adult crypt progenitors (Cattin et al., 2009; Darsigny et al., 2009).
Mutation of Nkx2.3, a mesenchymal transcription factor, causes a significant reduction in mesenchymal cells, reduced epithelial proliferation, and delayed villus formation, with about half of the mice dying prior to weaning. Mice that survive recover from the neonatal paucity of intestinal villi and instead show abnormal architecture of the small and large intestines including branched villi, thickened mucosa, and epithelial hyperproliferation (Pabst et al., 1999).
In addition to the specific transcription factors discussed here, global chromatin remodeling has also been implicated at this stage of intestinal development. A dominant negative mutation of the p300 histone acetyltransferase (HAT) causes delayed villus emergence associated with a failure of subepithelial mesenchymal condensation and weak BMP4 expression at the sight of presumptive villus formation (Shikama et al., 2003). Villi eventually emerge in these p300 mutant animals, along with differentiated epithelium, but continued proliferation of the villus core mesenchyme suggests a continual requirement for histone acetylation in the developing intestine, especially in the mesenchymal compartment. Interestingly, a similar mutation in the HAT protein CBP does not affect prenatal development of the intestine.
Histone deacetylases (HDACs) catalyze the reverse modification as HATs. Examination of HDAC 1 and 2 showed strong expression throughout the pseudostratified intestinal endoderm which became restricted to weaker, villus-specific expression once villi had formed (Tou et al., 2004). In intestinal explants, overexpression of HDACs blocked epithelial differentiation, whereas treatment with HDAC inhibitors caused premature villus formation and epithelial differentiation, associated with increased histone acetylation on genes expressed in differentiated epithelial cells (Tou et al., 2004). Thus, dynamic modification of chromatin is likely to be an important component regulating development of the intestine.
Proliferation and cytodifferentiation of the embryonic intestinal epithelium
Proliferation occurs throughout the stratified midgut and hindgut endoderm prior to villus emergence. No evidence of functional cytodifferentiation is present at this time, although the epithelial cells at the apical surface form a functional barrier to passive diffusion of macromolecules (reviewed in (Grand et al., 1976; Trier and Moxey, 1979)). As the pseudostratified endodermal epithelium reorganizes to form a simple columnar epithelium and villi begin to emerge, proliferation increases in the epithelium at the bases of emerging villi (Figure 4E). Later, proliferation becomes restricted exclusively to the intervillus epithelium, and then to the crypts of Lieberkühn.
Distinct epithelial cell types can be identified by morphological and molecular markers during villus emergence. Each lineage in the intestinal epithelium has a distinct function. In the embryo, four main cell types can be identified: columnar cells that bear apical microvilli, termed enterocytes in the small intestine and colonocytes in the large intestine; mucous-producing goblet cells; caveolated or tuft cells; and a diversity of hormone-producing enteroendocrine cells. Antimicrobial peptide secreting Paneth cells appear in the small intestine once crypts develop, which occurs after villus emergence in human fetuses and postnatally in rodents. Enterocytes and colonocytes are collectively termed absorptive cells to denote their primary role in absorbing nutrients and electrolytes. Goblet, enteroendocrine, and Paneth cells are collectively termed secretory cells to reflect the abundant secretory granules which typify these cells. Tuft cells, a relatively rare and understudied component of the intestinal epithelium, is also classified as a secretory cell based on recent lineage studies (Gerbe and Jay, personal communication). In addition, highly specialized enterocytes termed M cells develop to overlie the lymphoid follicles of maturing Peyer’s patches. All of these epithelial cell types are derived from multipotent stem cells in the adult intestine, but whether distinct stem cells exist in the embryo is unclear. Although it is beyond the scope of this review to detail all of the factors involved in proliferation and cytodifferentiation in the intestine, two major pathways that influence this process, Wnt/β-catenin and Notch, will be discussed here. For further information on intestine epithelial stem cells and cytodifferentiation see recent reviews by (Barker et al., 2008; Garrison et al., 2009; van der Flier and Clevers, 2009)
The Wnt/β-catenin signaling pathway is essential for supporting intestinal epithelial stem cell maintenance, proliferation, and differentiation in the adult intestine, but its role in the prenatal intestine is less clear. Canonical Wnt signaling blocks APC-mediated degradation of cytoplasmic β-catenin, leading to β-catenin accumulation and translocation to the nucleus (reviewed in (Gregorieff et al., 2005)). In the nucleus, β-catenin associates with DNA binding proteins of the Tcf/Lef family, and recruits coactivators to initiate transcription of target genes. Components of the Wnt/β-catenin signaling pathway are dynamically expressed in endodermal and mesodermal layers of the developing intestines, but β-catenin transcriptional activity is first detected in the intestinal epithelium after villus emergence (Kim et al., 2007; Garcia et al., 2009; Joo et al., 2010). Kim et al report that β-catenin activity is restricted to the postmitotic cells of the villus epithelium, where it continues to be expressed until birth, at which point β-catenin activity redistributes to the proliferative intervillus epithelium (Figure 4E,F). Conflicting reports on the localization of β-catenin activity in embryonic intestine suggest some controversy in the field.
Evidence supporting a role for the Wnt/β-catenin pathway in fetal intestines comes from mice in which Tcf4 is disrupted: in these mutant animals, villus emergence occurs but epithelial proliferation in the small intestine is subsequently halted, and some aspects of cytodifferentiation are abnormal (Korinek et al., 1998). This result was confirmed in Tcf4 mutant zebrafish, which also show loss of proliferation in the middle and distal intestines (Muncan et al., 2007). Similarly, in the mature intestine inhibition of Wnt/β-catenin causes acute loss of proliferation, depletion of the progenitor compartment, and a block in differentiation of goblet, enteroendocrine, and Paneth cells (Pinto et al., 2003; Ireland et al., 2004; Kuhnert et al., 2004). The similarity in these phenotypes led to the conclusion that β-catenin transcriptional activity drives proliferation in both the embryonic and adult intestine. Tcf4 is expressed in the intervillus epithelium of the embryonic intestine, whereas Tcf3 is expressed in the embryonic villus epithelium (Kim et al., 2007). In the adult, Tcf4 is the main Tcf/Lef factor and is expressed throughout the intestinal epithelium, and β-catenin is active in crypt progenitors (Gregorieff et al., 2005; Davies et al., 2008). Thus, based on the report that β-catenin is activated only in the embryonic villus epithelium, β-catenin may utilize different Tcf binding partners to activate distinct fetal or postnatal transcriptional programs, (Figure 4E,F) (Kim et al., 2007). These results may also suggest β-catenin independent functions for Tcf4 in the embryonic intestine. Conversely, β-catenin may partner with other nuclear proteins such as Sox factors to execute Tcf-independent transcriptional programs (Sinner et al., 2004; Sinner et al., 2007).
Premature activation of β-catenin throughout the intestinal endoderm causes disruption of villus emergence (Kim et al., 2007). However, this result may arise from a homeotic transformation of mutant tissue to non-intestinal endoderm, since Cdx2 was lost in cells with active β-catenin. A previous study in which hyperactive β-catenin was expressed in intestinal epithelium that was already appropriately patterned showed no defects in morphogenesis but instead caused apoptosis of the mutant cells (Wong et al., 2002). The concept that β-catenin is normally repressed in the developing intestinal endoderm is further supported by recent studies of Pinin, a nuclear speckle associated protein that physically binds to and represses transcriptional activity of nuclear β-catenin. Deletion of Pinin in the intestinal endoderm impaired villus formation and cytodifferentiation, associated with premature activation of β-catenin in the intervillus epithelium and misregualtion of Cdx1 and 2 (Joo et al., 2010). Together, these studies demonstrate the key role of β-catenin activity in intestinal development, but emphasize that clarifying the role of Wnt/β-catenin in the perinatal intestinal epithelium is an important area of future investigation.
The Notch signaling pathway is essential for regulating proliferation and cytodifferentiation in the developing intestine. Notch proteins are transmembrane receptors that are bound by ligands of the Delta/Serrate/Lag-2 family, which are expressed by adjacent cells. Once bound, Notch receptors are cleaved to release a cytoplasmic transcriptional activation domain (Notch Intracellular Domain; NICD). Overexpression of NICD in the emerging duodenum blocked proliferation and inhibited villus growth (Stanger et al., 2005). In contrast, after villus emergence in the intestine NICD blocked formation of secretory cells (goblet and enteroendocrine cells) and expanded proliferation (Fre et al., 2005). Conversely, inhibition of Notch activity enhanced secretory cell production and reduced proliferation (Milano et al., 2004; van Es et al., 2005b). Notch1 and Notch2 constitute the essential Notch receptors in the mature intestine (Riccio et al., 2008). The specific ligands essential for signaling in the intestine have yet to be identified.
In the nucleus, NICD binds to CSL/RBP-J proteins to activate transcription of target genes, the best known being Hes1. Studies of Hes1-deficient mice demonstrated that Hes1 promotes absorptive enterocyte differentiation and represses formation of secretory cells (Jensen et al., 2000). Subsequent studies showed that Hes1 represses Atoh1/Math1, which is required for secretory cell formation (Yang et al., 2001; Shroyer et al., 2007). A model emerged in which a balance between Hes1 and Atoh1 expression, controlled by Notch activity, determines absorptive vs. secretory cell differentiation. This model is further supported by studies in other model organisms: inhibition of Notch in zebrafish and Drosophila gut caused a change in epithelial cell fate and dysregulated cell turnover (Crosnier et al., 2005; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). Recent evidence suggests that Atoh1 is both necessary (Kazanjian et al., 2010; van Es et al., 2010) and sufficient (Vandussen and Samuelson, 2010) for epithelial progenitors to adopt a secretory cell fate. Thus, Notch and Atoh1 are critical gatekeepers of intestinal epithelial cell fate.
The Notch and Wnt/β-catenin pathways interact at several levels to regulate intestinal epithelial cell fate (reviewed in (Nakamura et al., 2007)). Crosstalk between these pathways was first suggested by studies in mice with global inhibition of intestinal Wnt activity (Pinto et al., 2003), which blocked epithelial proliferation and also phenocopied the loss of secretory lineages observed in Atoh1-mutant mice. Recent evidence from adult intestine suggests Wnt/β-catenin can enhance Notch signaling by upregulating receptor and ligand expression, and can also directly activate key Notch targets including Hes1 (Peignon G, 2010). In addition, Atoh1 can be targeted for degradation by the APC/axin complex when Wnt signals are present, and Atoh1 has also been reported to be a direct target of β-catenin transcriptional activation (Tsuchiya et al., 2007). On the other hand, active Notch signaling can enhance GSK3β-mediated β-catenin degradation (Koo et al., 2009). Thus, the Notch and Wnt/β-catenin pathways crossregulate one another via multiple complex mechanisms. Furthermore, both Notch and Wnt/β-catenin can be coordinately regulated, for example by the reactive oxygen species (ROS)-producing oxidase Nox1(Coant et al., 2010). Additional work is needed to define the precise mechanisms that mediate context-dependent crosstalk between these two important pathways.
Within the secretory lineage, several genes are known to function downstream of Atoh1. Gfi1 is a zinc-finger transcriptional repressor that is an important regulator of the fate of Atoh1-expressing secretory progenitors. Gfi1 mutants divert goblet and Paneth cell progenitors towards the enteroendocrine fate (Shroyer et al., 2005). Neurogenin3 is a transcription factor in the same basic helix-loop-helix family as Atoh1, which it is dependent upon for expression in the intestine. Neurogenin3 is required for formation of all enteroendocrine cells (Jenny et al., 2002). Conversely, overexpression of Neurogenin3 in the developing intestine can divert secretory progenitors towards the enteroendocrine fate at the expense of goblet cells (Lopez-Diaz et al., 2007). Recent evidence suggests that Gfi1 exerts its effects by repressing Neurogenin3 (Bjerknes and Cheng, 2010). Thus, a balance between Gfi1 and Neurogenin3 may control subtype allocation within the secretory lineage. After commitment to the enteroendocrine lineage, several transcription factors (e.g., NeuroD1, Pax6, Nkx2.2, Insm1) regulate differentiation and maintenance of specific hormone-producing cells (reviewed in (Schonhoff et al., 2004).
Several additional transcription factors function downstream of Wnt/β-catenin and Notch/Atoh1 to control intestinal epithelial cytodifferentiation and proliferation. SPDEF is a target of both the Wnt/β-catenin and Notch/Atoh1 pathways, and directs terminal differentiation and maturation of goblet cells (Gregorieff et al., 2009; Noah et al., 2010). Sox9 is a transcriptional target of β-catenin which can feed back to inhibit its activity, and regulates epithelial proliferation and formation of Paneth and goblet cells (Bastide et al., 2007; Mori-Akiyama et al., 2007). Klf5 and Klf4 are respectively expressed in the proliferating and differentiated cells of the intestinal epithelium. Klf5 is thought to stimulate whereas Klf4 inhibits proliferation by interactions with the Wnt/β-catenin pathway (McConnell et al., 2007; Flandez et al., 2008; McConnell et al., 2009). Klf4 is also a target of the Notch pathway where it regulates colonic goblet cell differentiation (Katz et al., 2002; Zheng et al., 2009).
Crypt development and postnatal changes to the intestinal epithelium
Crypt development initiates by anchorage of precursor stem cells to the intervillus epithelium, and subsequent remodeling of the tissue surrounding these cells to form flask-shaped crypts by upward migration of the crypt-villus junction, rather than downward penetration of the developing crypt (Calvert and Pothier, 1990). Crypts contain multiple stem cells but are monoclonal, that is, derived from a single stem cell, in adult intestines (Ponder et al., 1985; Griffiths et al., 1988). In mice, crypts become monoclonal during the first 2 weeks of postnatal development. In utero, progenitor cells mix extensively, with no clonal grouping of cells (Shiojiri and Mori, 2003). One week postnatally, mixed crypts are observed, but at 2 weeks most crypts are monoclonal (Schmidt et al., 1988). The mechanisms that produce this clonality have not been elucidated, but likely derive both from the rapid proliferation of the intestinal epithelium, and from the mechanism of crypt expansion by fission (see below). In the perinatal intestinal epithelium, proliferation of putative stem cells occurs at a rapid rate to maintain growth of the organ, resulting in patches of cells that are derived from a single earlier progenitor. As crypts develop, they are clonally derived within the patch, but are polyclonal at the border between two patches. Subsequently, new crypts arise by fission of existing crypts, with stem cells from the parent crypt partitioning randomly between the daughter crypts. As new crypts arise from rapid expansion of resident stem cells and subsequent fission, these polyclonal crypts become monoclonal. This clonality is maintained throughout adulthood, and can serve to fix genetic and epigenetic changes geographically within the intestines (Fuller et al., 1990; Endo et al., 1995; Novelli et al., 1996) For example, mutations can arise in crypts and these mutant crypts can expand by fission to create patches of clonally-derived cells with identical mutations (Greaves et al., 2006; Gutierrez-Gonzalez et al., 2009). Epigenetic marks are similarly stable and can be used to study stem cell dynamics in the intestines (Yatabe et al., 2001).
Less is known about pathways that control crypt development. As discussed above, the Hedgehog and BMP pathways regulate the location of developing crypts in the intervillus epithelium. BMP signals likely restrict stem cells to the emerging crypts, as phospho-SMAD 1/5/8 is observed primarily on the villus epithelium (Haramis et al., 2004). This restriction may be related to inhibition of the Wnt/β-catenin pathway via the PTEN/PI3K/AKT pathway (Tian et al., 2005). Wnt/β-catenin activity is likely to be required for emergence and maintenance intestinal stem cells, but direct evidence that it is required for crypt development is lacking. Wnt/β-catenin is required for expression of EphB2 and EphB3 in the intervillus epithelium, and for restriction of the EphB ligand Ephrin-B1 to the postmitotic villus epithelial cells. These EphB/EphrinB proteins contribute to crypt formation by restricting proliferating cells to the intervillus epithelium, and later for localization of Paneth cells to the crypt base (Batlle et al., 2002). The canonical β-catenin transcriptional target c-Myc also likely contributes to crypt emergence (Bettess et al., 2005), although conflicting data suggests that c-Myc may be required for stem cell maintenance rather than crypt formation (Muncan et al., 2006). Finally, several β-catenin target genes have been identified as molecular markers of crypt base columnar (CBC) stem cells. One such β-catenin target, Ascl2, is of special interest because regulation of its activity controls stem cell numbers: increased Ascl2 expression expanded proliferation and caused formation of crypt-like structures on the villi, whereas deletion of Ascl2 blocked renewal of CBCs and loss of mutant crypts (van der Flier and Clevers, 2009). Determining the mechanisms that control crypt development, and distinguishing these from those that regulate intestinal stem cells, is an important goal for the future.
Paneth cells begin to differentiate in the small intestinal epithelium coincident with the development of crypts. As with crypt development, the Wnt/β-catenin pathway is essential for maturation of Paneth cells. Mutation of the Wnt receptor Frizzled5 causes mislocalization of Paneth cells similar to EphB mutation, and blocks their final maturation (van Es et al., 2005a). Conversely, restriction of β-catenin activity in the late prenatal intestine via Lgr5-mediated feedback inhibition prevents premature emergence of Paneth cells (Garcia et al., 2009). Furthermore, β-catenin targets genes such as Sox9 and SPDEF are known to regulate Paneth cell differentiation and maturation.
Thus, Wnt/β-catenin activity appears to be centrally important to coordinate formation of crypts, stem cells, and Paneth cells. An excellent example of this coordinate regulation is seen in mice bearing mutations in FGFR3, which have defects in crypt formation, stem cell maintenance, Paneth cell maturation, and reduced expression and activity of β-catenin (Vidrich et al., 2009). However, these three properties of β-catenin can be separated, for example other genes that regulate Paneth cell maturation, such as PPAR-β, are not regulated by Wnt/β-catenin and do not show defects in crypt formation or stem cell maintenance (Varnat et al., 2006). Likewise, β-catenin is critical to regulate active CBC stem cells, but evidence is emerging for a slowly-cycling quiescent/reserve stem cell population that may not be directly regulated by β-catenin (reviewed in (Montgomery and Breault, 2008; Li and Clevers, 2010). Much work remains to fully understand how the simultaneous formation of crypts, stem cells, and Paneth cells is accomplished.
A final major step during development of the mammalian intestine occurs during the suckling to weaning transition (Pacha, 2000). During this period, the animal must change its nutritional absorptive capacity from a primarily lipid-based diet (milk) to a more complex carbohydrate-based mix (solid food). This transition is regulated by both cell-intrinsic and external cues, such as a surge in glucocorticoid levels. Precocious development of the adult digestive enzyme repertoire can be induced by systemic administration of steroids (Moog, 1953; Henning, 1981). However, the regional expression of appropriate transporters (i.e., jejunal expression of lactase; ileal expression of ASBT) appears to be primarily encoded by the intestine. Transcription factors of the GATA family are important for regulating this regional identity: mutation of Gata4 causes expression of ileal-specific digestive enzymes in the jejunum (Bosse et al., 2006; Battle et al., 2008). However, not all aspects of jejunal identity are lost in Gata4 mutant mice. Thus, much remains to be learned about the mechanisms that control regional identity in the intestine, and what controls intestinal digestive and absorptive capacity.
Perspectives
A daunting number of key developmental steps must be properly coordinated in order for the mature intestine to develop. Headway has been made over many decades in understanding how critical processes at each stage of development are regulated and our understanding of the cellular and molecular aspects of intestinal development has expanded significantly in recent years. However, several areas of intestinal development are understudied and unclear. Gut tubulogenesis, the process of making a gut tube from a sheet of endoderm, is only recently starting to be understood at the molecular level. It is currently unknown what controls the initial formation of the anterior and caudal pits which give rise to the AIP and CIP. Furthermore, endoderm specific deletion of genes controlling cellular movements leading to gut tube defects has not been performed. Thus, whether reported gut tube morphogenic defects are a consequence of endoderm-specific gene perturbations is unknown. While a lot of work has focused on mesenchymal-epithelial interactions in the foregut at this early stage of development, less work has been done studying crosstalk in the hindgut.
Another major area of intestine development that remains unclear is that of villus emergence. Many signaling pathways are clearly involved in this process, but it is unknown how they are all intimately tied together to drive villus formation. Additionally, transcriptional regulators that control the signaling pathways and drive villus emergence are lacking. During this process, it is also unclear how cells are designated to become an intervillus progenitor cell or a cell in the villus.
While there is some understanding of how the intervillus progenitor cells are regulated, existing literature and dogma surrounding regulation of adult intestinal stem cells (ISCs) make this an area for further investigation. For example, it is well established in the adult that Wnt/β-catenin signaling is required for regulation and maintenance of ISCs. Furthermore, the downstream effector of Wnt signaling, TCF4, is required for maintenance of the intervillus progenitor region in the embryo. In contrast to this, recent studies suggest that active canonical Wnt/β-catenin signaling is absent from the intervillus region until postnatal life in the mouse. Thus, while active Wnt/β-catenin signaling is required for adult ISC maintenance, it appears that there is a repressive role for Wnt signaling in maintenance of the intervillus progenitor zone during development. In addition to clarifying a role for Wnt/β-catenin in regulating intervillus progenitors during development, identification of additional regulators of progenitor establishment and maintenance are critical to further our understanding of intestine development. Many questions remain surrounding the transition from an embryonic progenitor to an adult ISC. That is, it is unknown if all intervillus progenitors are capable of becoming an ISC or if a small pool of cells is set-aside during development, which will give rise to ISCs in the crypt.
Lastly, one of the final hurdles is to translate the mechanisms controlling intestinal development into directing differentiation of pluripotent stem cells (PSCs; induced and/or embryonic) into intestinal tissue. It seems that this lofty goal will require a synthesis of the information reviewed here to piggyback on work that has led to differentiation of other endodermal organs such as liver and pancreas from PSCs.
Acknowledgments
Grant Support: NIH R01 CA142826 (NFS), R03 DK084167 (NFS), F32 DK83202-01 (JRS), and T32 HD07463 (JRS)
References
- Allan D, Houle M, Bouchard N, Meyer BI, Gruss P, Lohnes D. RARgamma and Cdx1 interactions in vertebral patterning. Dev Biol. 2001;240:46–60. doi: 10.1006/dbio.2001.0455. [DOI] [PubMed] [Google Scholar]
- Ameri J, Stahlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- Aoki TO, David NB, Minchiotti G, Saint-Etienne L, Dickmeis T, Persico GM, Strahle U, Mourrain P, Rosa FM. Molecular integration of casanova in the Nodal signalling pathway controlling endoderm formation. Development. 2002;129:275–286. doi: 10.1242/dev.129.2.275. [DOI] [PubMed] [Google Scholar]
- Aubin J, Lemieux M, Tremblay M, Berard J, Jeannotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Alvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Battle MA, Bondow BJ, Iverson MA, Adams SJ, Jandacek RJ, Tso P, Duncan SA. GATA4 is essential for jejunal function in mice. Gastroenterology. 2008;135:1676–1686. doi: 10.1053/j.gastro.2008.07.074. e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- Bayha E, Jorgensen MC, Serup P, Grapin-Botton A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JF, Menard D, Calvert R. Influence of epidermal growth factor on the maturation of the fetal mouse duodenum in organ culture. J Pediatr Gastroenterol Nutr. 1985;4:476–481. doi: 10.1097/00005176-198506000-00026. [DOI] [PubMed] [Google Scholar]
- Beland M, Lohnes D. Chicken ovalbumin upstream promoter-transcription factor members repress retinoic acid-induced Cdx1 expression. J Biol Chem. 2005;280:13858–13862. doi: 10.1074/jbc.M412981200. [DOI] [PubMed] [Google Scholar]
- Beland M, Pilon N, Houle M, Oh K, Sylvestre JR, Prinos P, Lohnes D. Cdx1 autoregulation is governed by a novel Cdx1-LEF1 transcription complex. Mol Cell Biol. 2004;24:5028–5038. doi: 10.1128/MCB.24.11.5028-5038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L, Williams L. A scanning and transmission electron microscopical study of the morphogenesis of human colonic villi. Anat Embryol (Berl) 1982;165:437–455. doi: 10.1007/BF00305579. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S, Trumpp A. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol. 2005;25:7868–7878. doi: 10.1128/MCB.25.17.7868-7878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Cell Lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev Biol. 2010;345:49–63. doi: 10.1016/j.ydbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet AM, Capecchi MR. Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol. 1996;177:232–249. doi: 10.1006/dbio.1996.0159. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Brolen G, Sivertsson L, Bjorquist P, Eriksson G, Ek M, Semb H, Johansson I, Andersson TB, Ingelman-Sundberg M, Heins N. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Burns RC, Fairbanks TJ, Sala F, De Langhe S, Mailleux A, Thiery JP, Dickson C, Itoh N, Warburton D, Anderson KD, Bellusci S. Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev Biol. 2004;265:61–74. doi: 10.1016/j.ydbio.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Calvert R, Beaulieu JF, Menard D. Epidermal growth factor (EGF) accelerates the maturation of fetal mouse intestinal mucosa in utero. Experientia. 1982;38:1096–1097. doi: 10.1007/BF01955387. [DOI] [PubMed] [Google Scholar]
- Calvert R, Pothier P. Migration of fetal intestinal intervillous cells in neonatal mice. Anat Rec. 1990;227:199–206. doi: 10.1002/ar.1092270208. [DOI] [PubMed] [Google Scholar]
- Cattin AL, Le Beyec J, Barreau F, Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pincon-Raymond M, Cardot P, Lacasa M, Ribeiro A. Hepatocyte nuclear factor 4alpha, a key factor for homeostasis, cell architecture, and barrier function of the adult intestinal epithelium. Mol Cell Biol. 2009;29:6294–6308. doi: 10.1128/MCB.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers AD, Slack JM. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development. 2000;127:381–392. doi: 10.1242/dev.127.2.381. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MY, Romer AI, Hu M, Lepourcelet M, Mechoor A, Yesilaltay A, Krieger M, Gray PA, Shivdasani RA. A dynamic expression survey identifies transcription factors relevant in mouse digestive tract development. Development. 2006;133:4119–4129. doi: 10.1242/dev.02537. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. Notochord patterning of the endoderm. Dev Biol. 2001;234:1–12. doi: 10.1006/dbio.2001.0214. [DOI] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126:4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- Clements D, Woodland HR. Changes in embryonic cell fate produced by expression of an endodermal transcription factor, Xsox17. Mech Dev. 2000;99:65–70. doi: 10.1016/s0925-4773(00)00476-7. [DOI] [PubMed] [Google Scholar]
- Coant N, Ben Mkaddem S, Pedruzzi E, Guichard C, Treton X, Ducroc R, Freund JN, Cazals-Hatem D, Bouhnik Y, Woerther PL, Skurnik D, Grodet A, Fay M, Biard D, Lesuffleur T, Deffert C, Moreau R, Groyer A, Krause KH, Daniel F, Ogier-Denis E. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colony PC, Kois JM, Peiffer LP. Structural and enzymatic changes during colonic maturation in the fetal and suckling rat. Gastroenterology. 1989;97:338–347. doi: 10.1016/0016-5085(89)90069-3. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Darsigny M, Babeu JP, Dupuis AA, Furth EE, Seidman EG, Levy E, Verdu EF, Gendron FP, Boudreau F. Loss of hepatocyte-nuclear-factor-4alpha affects colonic ion transport and causes chronic inflammation resembling inflammatory bowel disease in mice. PLoS One. 2009;4:e7609. doi: 10.1371/journal.pone.0007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development. 2002;129:4457–4468. doi: 10.1242/dev.129.19.4457. [DOI] [PubMed] [Google Scholar]
- Davies PS, Dismuke AD, Powell AE, Carroll KH, Wong MH. Wnt-reporter expression pattern in the mouse intestine during homeostasis. BMC Gastroenterol. 2008;8:57. doi: 10.1186/1471-230X-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa Barbara P, Williams J, Goldstein AM, Doyle AM, Nielsen C, Winfield S, Faure S, Roberts DJ. Bone morphogenetic protein signaling pathway plays multiple roles during gastrointestinal tract development. Dev Dyn. 2005;234:312–322. doi: 10.1002/dvdy.20554. [DOI] [PubMed] [Google Scholar]
- Deschamps J, van den Akker E, Forlani S, De Graaff W, Oosterveen T, Roelen B, Roelfsema J. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Duboc V, Rottinger E, Besnardeau L, Lepage T. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell. 2004;6:397–410. doi: 10.1016/s1534-5807(04)00056-5. [DOI] [PubMed] [Google Scholar]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–3025. doi: 10.1242/dev.125.16.3015. [DOI] [PubMed] [Google Scholar]
- Duh G, Mouri N, Warburton D, Thomas DW. EGF regulates early embryonic mouse gut development in chemically defined organ culture. Pediatr Res. 2000;48:794–802. doi: 10.1203/00006450-200012000-00016. [DOI] [PubMed] [Google Scholar]
- Duluc I, Freund JN, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JS. The fine structure of the absorptive epithelial cells of the developing small intestine of the rat. J Anat. 1967;101:57–68. [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Sugimura H, Kino I. Monoclonality of normal human colonic crypts. Pathol Int. 1995;45:602–604. doi: 10.1111/j.1440-1827.1995.tb03509.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks TJ, Kanard RC, De Langhe SP, Sala FG, Del Moral PM, Warburton D, Anderson KD, Bellusci S, Burns RC. A genetic mechanism for cecal atresia: the role of the Fgf10 signaling pathway. J Surg Res. 2004;120:201–209. doi: 10.1016/j.jss.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Flandez M, Guilmeau S, Blache P, Augenlicht LH. KLF4 regulation in intestinal epithelial cell maturation. Exp Cell Res. 2008;314:3712–3723. doi: 10.1016/j.yexcr.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flentjar N, Chu PY, Ng AY, Johnstone CN, Heath JK, Ernst M, Hertzog PJ, Pritchard MA. TGF-betaRII rescues development of small intestinal epithelial cells in Elf3-deficient mice. Gastroenterology. 2007;132:1410–1419. doi: 10.1053/j.gastro.2007.02.054. [DOI] [PubMed] [Google Scholar]
- Franklin V, Khoo PL, Bildsoe H, Wong N, Lewis S, Tam PP. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech Dev. 2008;125:587–600. doi: 10.1016/j.mod.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Swietlicki EA, Lefebvre O, Kedinger M, Iordanov H, Levin MS, Rubin DC. Epimorphin expression in intestinal myofibroblasts induces epithelial morphogenesis. J Clin Invest. 2002;110:1629–1641. doi: 10.1172/JCI13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CE, Davies RP, Williams GT, Williams ED. Crypt restricted heterogeneity of goblet cell mucus glycoprotein in histologically normal human colonic mucosa: a potential marker of somatic mutation. Br J Cancer. 1990;61:382–384. doi: 10.1038/bjc.1990.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer LW, Wright CV. Autonomous endodermal determination in Xenopus: regulation of expression of the pancreatic gene XlHbox 8. Dev Biol. 1995;171:240–251. doi: 10.1006/dbio.1995.1275. [DOI] [PubMed] [Google Scholar]
- Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol. 2009;331:58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Shibata M, Anderson KV. Chato, a KRAB zinc-finger protein, regulates convergent extension in the mouse embryo. Development. 2008;135:3053–3062. doi: 10.1242/dev.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison AP, Helmrath MA, Dekaney CM. Intestinal stem cells. J Pediatr Gastroenterol Nutr. 2009;49:2–7. doi: 10.1097/MPG.0b013e3181ad3021. [DOI] [PubMed] [Google Scholar]
- Garrison WD, Battle MA, Yang C, Kaestner KH, Sladek FM, Duncan SA. Hepatocyte nuclear factor 4alpha is essential for embryonic development of the mouse colon. Gastroenterology. 2006;130:1207–1220. doi: 10.1053/j.gastro.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geske MJ, Zhang X, Patel KK, Ornitz DM, Stappenbeck TS. Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development. 2008;135:2959–2968. doi: 10.1242/dev.020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, Clevers H, Moon RT, Zon LI. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Goke M, Podolsky DK. Regulation of the mucosal epithelial barrier. Baillieres Clin Gastroenterol. 1996;10:393–405. doi: 10.1016/s0950-3528(96)90049-4. [DOI] [PubMed] [Google Scholar]
- Grainger S, Savory JG, Lohnes D. Cdx2 regulates patterning of the intestinal epithelium. Dev Biol. 2010;339:155–165. doi: 10.1016/j.ydbio.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Grand RJ, Watkins JB, Torti FM. Development of the human gastrointestinal tract. A review. Gastroenterology. 1976;70:790–810. [PubMed] [Google Scholar]
- Grapin-Botton A. Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin's PhD thesis. Int J Dev Biol. 2005;49:335–347. doi: 10.1387/ijdb.041946ag. [DOI] [PubMed] [Google Scholar]
- Grapin-Botton A, Melton DA. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. doi: 10.1016/s0168-9525(99)01957-5. [DOI] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W. Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci U S A. 2003;100:5193–5198. doi: 10.1073/pnas.0531290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, Leedham SJ, Deheragoda M, Sasieni P, Novelli MR, Jankowski JA, Turnbull DM, Wright NA, McDonald SA. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci U S A. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastrointestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. e1331–e1333. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Griffiths DF, Davies SJ, Williams D, Williams GT, Williams ED. Demonstration of somatic mutation and colonic crypt clonality by X-linked enzyme histochemistry. Nature. 1988;333:461–463. doi: 10.1038/333461a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Gonzalez L, Deheragoda M, Elia G, Leedham SJ, Shankar A, Imber C, Jankowski JA, Turnbull DM, Novelli M, Wright NA, McDonald SA. Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations. J Pathol. 2009;217:489–496. doi: 10.1002/path.2502. [DOI] [PubMed] [Google Scholar]
- Hagos EG, Dougan ST. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev Biol. 2007;7:22. doi: 10.1186/1471-213X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PP, Elefanty AG, Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Helander HF. Morphological studies on the development of the rat colonic mucosa. Acta Anat (Basel) 1973;85:155–176. [PubMed] [Google Scholar]
- Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981;241:G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. doi: 10.1242/dev.122.3.1007. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Takebe K, Takashina M, Kobayashi S, Takeichi M. Epimorphin: a mesenchymal protein essential for epithelial morphogenesis. Cell. 1992;69:471–481. doi: 10.1016/0092-8674(92)90448-l. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Hou SX. Intestinal stem cell asymmetric division in the Drosophila posterior midgut. J Cell Physiol. 2010;224:581–584. doi: 10.1002/jcp.22194. [DOI] [PubMed] [Google Scholar]
- Houle M, Prinos P, Iulianella A, Bouchard N, Lohnes D. Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol. 2000;20:6579–6586. doi: 10.1128/mcb.20.17.6579-6586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth GS. Insulin-like growth factor-I and the gastrointestinal system: therapeutic indications and safety implications. J Nutr. 2003;133:2109–2112. doi: 10.1093/jn/133.7.2109. [DOI] [PubMed] [Google Scholar]
- Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, Vogelstein B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Ido A, Numata M, Kodama M, Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol. 2005;40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Johannesson M, Stahlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One. 2009;4:e4794. doi: 10.1371/journal.pone.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. The development of the mucous membrane of the esophagus, stomach and small intestine in the human embryo. The American Journal of Anatomy. 1910;10:521–575. [Google Scholar]
- Joo JH, Taxter TJ, Munguba GC, Kim YH, Dhaduvai K, Dunn NW, Degan WJ, Oh SP, Sugrue SP. Pinin modulates expression of an intestinal homeobox gene, Cdx2, and plays an essential role for small intestinal morphogenesis. Dev Biol. 2010;345:191–203. doi: 10.1016/j.ydbio.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Silberg DG, Traber PG, Schutz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanjian A, Noah T, Brown D, Burkart J, Shroyer NF. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139:918–928. doi: 10.1053/j.gastro.2010.05.081. 928 e911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan ID, Sharrard RM, Isaacs HV. FGF signal transduction and the regulation of Cdx gene expression. Dev Biol. 2006;299:478–488. doi: 10.1016/j.ydbio.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Griffin KJ. Vertebrate mesendoderm induction and patterning. Curr Opin Genet Dev. 2000;10:350–356. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol. 2006;289:283–295. doi: 10.1016/j.ydbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kinkel MD, Eames SC, Alonzo MR, Prince VE. Cdx4 is required in the endoderm to localize the pancreas and limit beta-cell number. Development. 2008;135:919–929. doi: 10.1242/dev.010660. [DOI] [PubMed] [Google Scholar]
- Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, Dlugosz AA, Kottmann AH, Gumucio DL. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Chang HJ, Yoon MJ, Choi Y, Kong MP, Kim CH, Kim JM, Park JG, Kong YY. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology. 2009;137:145–155. doi: 10.1053/j.gastro.2009.03.046. 155 e141–143. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MC, Koo BK, Kim YY, Lee SH, Kim NS, Kim JH, Kong YY. Essential role of CR6-interacting factor 1 (Crif1) in E74-like factor 3 (ELF3)-mediated intestinal development. J Biol Chem. 2009;284:33634–33641. doi: 10.1074/jbc.M109.059840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson A, Schoenwolf GC. Epiblast and primitive-streak origins of the endoderm in the gastrulating chick embryo. Development. 2003;130:3491–3501. doi: 10.1242/dev.00579. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev Biol. 1986;115:325–339. doi: 10.1016/0012-1606(86)90253-8. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- Lebenthal E. Human gastrointestinal development. New York: Raven Press; 1989. xvii, 824 p. pp. [Google Scholar]
- Lengyel JA, Iwaki DD. It takes guts: the Drosophila hindgut as a model system for organogenesis. Dev Biol. 2002;243:1–19. doi: 10.1006/dbio.2002.0577. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Tou L, Cai L, Sawada J, Lazar AJ, Glickman JN, Williamson JA, Everett AD, Redston M, Fox EA, Nakatani Y, Shivdasani RA. Insights into developmental mechanisms and cancers in the mammalian intestine derived from serial analysis of gene expression and study of the hepatoma-derived growth factor (HDGF) Development. 2005;132:415–427. doi: 10.1242/dev.01579. [DOI] [PubMed] [Google Scholar]
- Lev R, Orlic D. Histochemical and radioautographic studies of normal human fetal colon. Histochemistry. 1974;39:301–311. doi: 10.1007/BF00495681. [DOI] [PubMed] [Google Scholar]
- Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev Dyn. 2006;235:2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Festing M, Thompson JC, Hester M, Rankin S, El-Hodiri HM, Zorn AM, Weinstein M. Smad2 and Smad3 coordinately regulate craniofacial and endodermal development. Dev Biol. 2004;270:411–426. doi: 10.1016/j.ydbio.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Lopez-Diaz L, Jain RN, Keeley TM, VanDussen KL, Brunkan CS, Gumucio DL, Samuelson LC. Intestinal Neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev Biol. 2007;309:298–305. doi: 10.1016/j.ydbio.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- Lu CC, Robertson EJ. Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol. 2004;273:149–159. doi: 10.1016/j.ydbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- MacDonald RS. The role of insulin-like growth factors in small intestinal cell growth and development. Horm Metab Res. 1999;31:103–113. doi: 10.1055/s-2007-978706. [DOI] [PubMed] [Google Scholar]
- Madara JL, Neutra MR, Trier JS. Junctional complexes in fetal rat small intestine during morphogenesis. Dev Biol. 1981;86:170–178. doi: 10.1016/0012-1606(81)90327-4. [DOI] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF, Rothman JH. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol. 2002;246:68–85. doi: 10.1006/dbio.2002.0655. [DOI] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y. Wnt/beta-catenin signaling and body plan formation in mouse embryos. Semin Cell Dev Biol. 2006;17:175–184. doi: 10.1016/j.semcdb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Mathan M, Moxey PC, Trier JS. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976;146:73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Hashimoto K, Yoshioka T, Otani H. Occlusion and subsequent re-canalization in early duodenal development of human embryos: integrated organogenesis and histogenesis through a possible epithelial-mesenchymal interaction. Anat Embryol (Berl) 2002;205:53–65. doi: 10.1007/s00429-001-0226-5. [DOI] [PubMed] [Google Scholar]
- Matsuyama M, Aizawa S, Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009;69:4125–4133. doi: 10.1158/0008-5472.CAN-08-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD. The C. elegans intestine. WormBook. 2007:1–36. doi: 10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- McKnight KD, Hou J, Hoodless PA. Foxh1 and Foxa2 are not required for formation of the midgut and hindgut definitive endoderm. Dev Biol. 2010;337:471–481. doi: 10.1016/j.ydbio.2009.10.040. [DOI] [PubMed] [Google Scholar]
- McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Menard D, Arsenault P, Pothier P. Biologic effects of epidermal growth factor in human fetal jejunum. Gastroenterology. 1988;94:656–663. doi: 10.1016/0016-5085(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Menard D, Corriveau L, Arsenault P. Differential effects of epidermal growth factor and hydrocortisone in human fetal colon. J Pediatr Gastroenterol Nutr. 1990;10:13–20. doi: 10.1097/00005176-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology. 2010;138:2233–2245. doi: 10.1053/j.gastro.2010.02.056. 2245 e2231–2214. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog F. The functional differentiation of the small intestine. III. The influence of the pituitary-adrenal system on the differentiation of phosphatase in the duodenum of the suckling mouse. Journal of Experimental Zoology. 1953;124:329–346. [Google Scholar]
- Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Muncan V, Faro A, Haramis AP, Hurlstone AF, Wienholds E, van Es J, Korving J, Begthel H, Zivkovic D, Clevers H. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007;8:966–973. doi: 10.1038/sj.embor.7401071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncan V, Sansom OJ, Tertoolen L, Phesse TJ, Begthel H, Sancho E, Cole AM, Gregorieff A, de Alboran IM, Clevers H, Clarke AR. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol Cell Biol. 2006;26:8418–8426. doi: 10.1128/MCB.00821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997;189:270–274. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Ng AY, Waring P, Ristevski S, Wang C, Wilson T, Pritchard M, Hertzog P, Kola I. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology. 2002;122:1455–1466. doi: 10.1053/gast.2002.32990. [DOI] [PubMed] [Google Scholar]
- Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli MR, Williamson JA, Tomlinson IP, Elia G, Hodgson SV, Talbot IC, Bodmer WF, Wright NA. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science. 1996;272:1187–1190. doi: 10.1126/science.272.5265.1187. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Pabst O, Zweigerdt R, Arnold HH. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126:2215–2225. doi: 10.1242/dev.126.10.2215. [DOI] [PubMed] [Google Scholar]
- Pacha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Bayha E, Pieler T. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–531. doi: 10.1016/j.mod.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Patten BM. Human Embryology. Philladelphia: Blakiston Co.; 1948. [Google Scholar]
- Peignon G DA, Cacheux W, Ayrault O, Terris B, Laurent-Puig P, Shroyer NF, Seuningen IV, Honjo T, Perret C, Romagnolo B. Complex interplay between β-catenin signaling and Notch effectors in intestinal tumorigenesis. Gut. 2010 doi: 10.1136/gut.2009.204719. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak-Charcon S, Shoham J, Ben-Shaul Y. Tight junctions in epithelial cells of human fetal hindgut, normal colon, and colon adenocarcinoma. J Natl Cancer Inst. 1980;65:53–62. [PubMed] [Google Scholar]
- Ponder BA, Schmidt GH, Wilkinson MM, Wood MJ, Monk M, Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Ratineau C, Duluc I, Pourreyron C, Kedinger M, Freund JN, Roche C. Endoderm- and mesenchyme-dependent commitment of the differentiated epithelial cell types in the developing intestine of rat. Differentiation. 2003;71:163–169. doi: 10.1046/j.1432-0436.2003.t01-1-710203.x. [DOI] [PubMed] [Google Scholar]
- Reed RA, Womble MA, Dush MK, Tull RR, Bloom SK, Morckel AR, Devlin EW, Nascone-Yoder NM. Morphogenesis of the primitive gut tube is generated by Rho/ROCK/myosin II-mediated endoderm rearrangements. Dev Dyn. 2009;238:3111–3125. doi: 10.1002/dvdy.22157. [DOI] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–3174. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Norris DP, Brennan J, Bikoff EK. Control of early anterior-posterior patterning in the mouse embryo by TGF-beta signalling. Philos Trans R Soc Lond B Biol Sci. 2003;358:1351–1357. doi: 10.1098/rstb.2003.1332. discussion 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A, Patient R. Mesendoderm. an ancient germ layer? Cell. 2001;105:169–172. doi: 10.1016/s0092-8674(01)00307-5. [DOI] [PubMed] [Google Scholar]
- Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development. 1999;126:3067–3078. doi: 10.1242/dev.126.14.3067. [DOI] [PubMed] [Google Scholar]
- Rosenquist GC. The location of the pregut endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev Biol. 1971;26:323–335. doi: 10.1016/0012-1606(71)90131-x. [DOI] [PubMed] [Google Scholar]
- Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004;15:573–581. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala FG, Curtis JL, Veltmaat JM, Del Moral PM, Le LT, Fairbanks TJ, Warburton D, Ford H, Wang K, Burns RC, Bellusci S. Fibroblast growth factor 10 is required for survival and proliferation but not differentiation of intestinal epithelial progenitor cells during murine colon development. Dev Biol. 2006;299:373–385. doi: 10.1016/j.ydbio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Schmidt GH, Winton DJ, Ponder BA. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988;103:785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008;318:143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest. 2010;120:2081–2093. doi: 10.1172/JCI40676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Chen TY, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama N, Lutz W, Kretzschmar R, Sauter N, Roth JF, Marino S, Wittwer J, Scheidweiler A, Eckner R. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J. 2003;22:5175–5185. doi: 10.1093/emboj/cdg502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojiri N, Mori M. Mosaic analysis of small intestinal development using the spf(ash)-heterozygous female mouse. Histochem Cell Biol. 2003;119:199–210. doi: 10.1007/s00418-003-0505-8. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer NF, Wong MH. BMP signaling in the intestine: cross-talk is key. Gastroenterology. 2007;133:1035–1038. doi: 10.1053/j.gastro.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JRMC, Rankin SA, Kuhar MK, Tolle K, Vallance JE, Hoskins EE, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2010 doi: 10.1038/nature09691. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Wells JM. Translational embryology: using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev Dyn. 2007;236:3218–3227. doi: 10.1002/dvdy.21366. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Markwald RR. Endodermal growth factors promote endocardial precursor cell formation from precardiac mesoderm. Dev Biol. 2003;263:35–49. doi: 10.1016/s0012-1606(03)00433-0. [DOI] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tam PP, Beddington RS. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Found Symp. 1992;165:27–41. doi: 10.1002/9780470514221.ch3. discussion 42-29. [DOI] [PubMed] [Google Scholar]
- Tam PP, Kanai-Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev. 2003;13:393–400. doi: 10.1016/s0959-437x(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Tam PP, Khoo PL, Lewis SL, Bildsoe H, Wong N, Tsang TE, Gad JM, Robb L. Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development. 2007;134:251–260. doi: 10.1242/dev.02724. [DOI] [PubMed] [Google Scholar]
- Tam PP, Khoo PL, Wong N, Tsang TE, Behringer RR. Regionalization of cell fates and cell movement in the endoderm of the mouse gastrula and the impact of loss of Lhx1(Lim1) function. Dev Biol. 2004;274:171–187. doi: 10.1016/j.ydbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. doi: 10.1016/j.gde.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Technau U, Scholz CB. Origin and evolution of endoderm and mesoderm. Int J Dev Biol. 2003;47:531–539. [PubMed] [Google Scholar]
- Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton DA. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-3-3zeta. Cell Cycle. 2005;4:215–216. [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Tou L, Liu Q, Shivdasani RA. Regulation of mammalian epithelial differentiation and intestine development by class I histone deacetylases. Mol Cell Biol. 2004;24:3132–3139. doi: 10.1128/MCB.24.8.3132-3139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota T, Yamamoto M, Kataoka K. Light and electron microscope study on developing intestinal mucosa in rat fetuses with special reference to the obliteration of the intestinal lumen. Arch Histol Cytol. 1989;52:51–60. doi: 10.1679/aohc.52.51. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Hoodless PA, Bikoff EK, Robertson EJ. Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development. 2000;127:3079–3090. doi: 10.1242/dev.127.14.3079. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Trier JS, Moxey PC. Morphogenesis of the small intestine during fetal development. Ciba Found Symp. 1979:3–29. doi: 10.1002/9780470720530.ch2. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Nakamura T, Okamoto R, Kanai T, Watanabe M. Reciprocal targeting of Hath1 and beta-catenin by Wnt glycogen synthase kinase 3beta in human colon cancer. Gastroenterology. 2007;132:208–220. doi: 10.1053/j.gastro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van Es JH, de Geest N, van de Born M, Clevers H, Hassan BA. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1:1–5. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005a;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005b;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- van Nes J, de Graaff W, Lebrin F, Gerhard M, Beck F, Deschamps J. The Cdx4 mutation affects axial development and reveals an essential role of Cdx genes in the ontogenesis of the placental labyrinth in mice. Development. 2006;133:419–428. doi: 10.1242/dev.02216. [DOI] [PubMed] [Google Scholar]
- Vandussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010 doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnat F, Heggeler BB, Grisel P, Boucard N, Corthesy-Theulaz I, Wahli W, Desvergne B. PPARbeta/delta regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006;131:538–553. doi: 10.1053/j.gastro.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Vidrich A, Buzan JM, Brodrick B, Ilo C, Bradley L, Fendig KS, Sturgill T, Cohn SM. Fibroblast growth factor receptor-3 regulates Paneth cell lineage allocation and accrual of epithelial stem cells during murine intestinal development. Am J Physiol Gastrointest Liver Physiol. 2009;297:G168–G178. doi: 10.1152/ajpgi.90589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–482. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Wen J, Chiang YJ, Gao C, Xue H, Xu J, Ning Y, Hodes RJ, Gao X, Chen YG. Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J Biol Chem. 2010;285:11023–11030. doi: 10.1074/jbc.M109.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng W, Stemple DL. Nodal signaling and vertebrate germ layer formation. Birth Defects Res C Embryo Today. 2003;69:325–332. doi: 10.1002/bdrc.10027. [DOI] [PubMed] [Google Scholar]
- Wessel GM, Wikramanayake A. How to grow a gut: ontogeny of the endoderm in the sea urchin embryo. Bioessays. 1999;21:459–471. doi: 10.1002/(SICI)1521-1878(199906)21:6<459::AID-BIES3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Wills A, Dickinson K, Khokha M, Baker JC. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn. 2008;237:2177–2186. doi: 10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington S, Beddington R, Cooke J. Foregut endoderm is required at head process stages for anteriormost neural patterning in chick. Development. 2001;128:309–320. doi: 10.1242/dev.128.3.309. [DOI] [PubMed] [Google Scholar]
- Wong MH, Huelsken J, Birchmeier W, Gordon JI. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/beta-catenin signaling. J Biol Chem. 2002;277:15843–15850. doi: 10.1074/jbc.M200184200. [DOI] [PubMed] [Google Scholar]
- Xian CJ. Roles of growth factors in chemotherapy-induced intestinal mucosal damage repair. Curr Pharm Biotechnol. 2003;4:260–269. doi: 10.2174/1389201033489793. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Meno C, Sakai Y, Shiratori H, Mochida K, Ikawa Y, Saijoh Y, Hamada H. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 2001;15:1242–1256. doi: 10.1101/gad.883901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Tavare S, Shibata D. Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci U S A. 2001;98:10839–10844. doi: 10.1073/pnas.191225998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchetti G, Duboule D, Zakany J. Hox gene function in vertebrate gut morphogenesis: the case of the caecum. Development. 2007;134:3967–3973. doi: 10.1242/dev.010991. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M. Patterning of the embryo: the first spatial decisions in the life of a mouse. Development. 2002;129:815–829. doi: 10.1242/dev.129.4.815. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stappenbeck TS, White AC, Lavine KJ, Gordon JI, Ornitz DM. Reciprocal epithelial-mesenchymal FGF signaling is required for cecal development. Development. 2006;133:173–180. doi: 10.1242/dev.02175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Pritchard DM, Yang X, Bennett E, Liu G, Liu C, Ai W. KLF4 gene expression is inhibited by the notch signaling pathway that controls goblet cell differentiation in mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G490–G498. doi: 10.1152/ajpgi.90393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int Rev Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]