Abstract
Modafinil is a central nervous system stimulant used to promote wakefulness, and it is being evaluated clinically as an agonist-based medication to treat stimulant abuse. This is the first report of the effects of modafinil on the abuse-related effects of cocaine in nonhuman primates. Three studies were conducted to examine the behavioral effects of modafinil. In the first study, the discriminative stimulus effects of modafinil were evaluated in monkeys trained to discriminate either low (0.18 mg/kg, IM) or high (0.4 mg/kg, IM) doses of cocaine from saline. Modafinil dose-dependently substituted for cocaine in 6/7 monkeys. In the second study, the effects of chronically administered modafinil (32-56 mg/kg/day, IV) on food- and cocaine-maintained operant responding were examined. Modafinil was administered 3 times/hr for 23 hr/day to ensure stable drug levels. Chronic treatment with 32 mg/kg/day modafinil selectively reduced responding maintained by intermediate (0.003 mg/kg/inj) and peak (0.01 mg/kg/inj) reinforcing doses of cocaine, but responding maintained by higher doses of cocaine was unaffected. Food-maintained behavior did not change during chronic treatment with modafinil. In a third study, after extinction of cocaine self-administration, modafinil (32 and 56 mg/kg/day, IV) significantly increased saline self-administration on the first day of treatment. These findings indicate that modafinil shares discriminative stimulus effects with cocaine and selectively reduces responding maintained by reinforcing doses of cocaine. These data are generally consistent with clinical findings and provide new evidence that these preclinical models may be useful for predicting the effectiveness of novel medications for drug abuse treatment.
Keywords: Modafinil, Cocaine, Self-administration, Drug discrimination, Rhesus monkey, Agonist therapy
INTRODUCTION
Modafinil is an FDA approved stimulant medication used to treat sleep-wakefulness disorders (Keating & Raffin, 2005; Schwartz, 2009). Modafinil affects several neurotransmitter systems, but its primary mechanism of action remains elusive. Recent evidence suggests that modafinil binds with weak affinity to the dopamine transporter (DAT) and inhibits dopamine uptake, thereby increasing extracellular dopamine at clinically relevant doses (Madras et al., 2006; Mignot, Nishino, Guilleminault, & Dement, 1994; Zolkowska et al., 2009). A recent PET imaging study in humans supports these preclinical findings (Volkow et al., 2009).
Despite its ability to increase extracellular dopamine levels, modafinil's abuse liability appears relatively low (Jasinski, 2000; Jasinski & Kovacevic-Ristanovic, 2000; Myrick, Malcolm, Taylor, & LaRowe, 2004). Modafinil produces some stimulant-like positive subjective effects (Jasinski, 2000; Makris, Rush, Frederich, Taylor, & Kelly, 2007; Rush, Kelly, Hays, Baker, & Wooten, 2002; Vansickel, Fillmorex, Hays, & Rush, 2008) and cocaine-like discriminative stimulus effects in some human subjects (Rush, Kelly, Hays, Baker et al., 2002). However, modafinil's effects were weak in comparison to cocaine and amphetamine. Studies of the positive reinforcing effects of clinically relevant doses of modafinil (200-400 mg) have been inconsistent. One recent clinical laboratory study found that modafinil (200-600 mg) was not self-administered by cocaine-experienced individuals (Vosburg, Hart, Haney, Rubin, & Foltin, 2010) but another study reported that modafinil (200-400 mg) produced reinforcing effects under certain experimental conditions (Stoops, Lile, Fillmore, Glaser, & Rush, 2005).
In preclinical models, modafinil partially substituted for the discriminative stimulus effects of cocaine in rats (Dopheide, Morgan, Rodvelt, Schachtman, & Miller, 2007; Gold & Balster, 1996), but data describing modafinil's reinforcing effects are inconsistent. In rats, doses as high as 1.7 mg/kg/inj modafinil failed to maintain self-administration (Deroche-Gamonet et al., 2002), whereas modafinil (0.03 to 0.32 mg/kg/inj) maintained responding at levels comparable to cocaine in rhesus monkeys (Gold & Balster, 1996). However, the cumulative doses of modafinil that were reinforcing were 3-6-fold greater than doses used clinically (Gold & Balster, 1996). These findings indicated that doses of modafinil outside the clinically relevant dose range are necessary to achieve reinforcing effects.
Modafinil's relatively low abuse liability and weak psychomotor stimulant effects suggest that it may be useful as an agonist-based medication for treating stimulant-dependence disorders (Haney & Spealman, 2008; Herin, Rush, & Grabowski, 2010; Martinez-Raga, Knecht, & Cepeda, 2008; Preti, 2007; Vocci & Elkashef, 2005). Agonist-based pharmacotherapies have proven useful in treating opioid and nicotine dependence and are being evaluated for treatment of stimulant abuse (Grabowski, Shearer, Merrill, & Negus, 2004; Herin et al., 2010). For example, putative medications such as amphetamine are effective in reducing cocaine abuse (Grabowski et al., 2001; Shearer, Wodak, van Beek, Mattick, & Lewis, 2003). Several clinical studies have reported that modafinil attenuated some abuse-related effects of cocaine. In clinical laboratory studies, modafinil reduced the positive subjective effects of cocaine (Dackis et al., 2003; Hart, Haney, Vosburg, Rubin, & Foltin, 2008; Malcolm et al., 2006), and the reinforcing effects of smoked cocaine (Hart et al., 2008). In outpatient studies, modafinil reduced cocaine use in subjects who also received cognitive behavioral therapy (Dackis, Kampman, Lynch, Pettinati, & O'Brien, 2005). Most recently, a multi-center, double-blind, placebo-controlled 12-week clinical trial of modafinil was conducted in 125 cocaine-dependent subjects (Anderson et al., 2009). The major finding was that modafinil decreased craving and cocaine use, but only in subjects without co-morbid alcohol dependence (Anderson et al., 2009). Several clinical studies have examined modafinil as a treatment for methamphetamine dependence, but the findings were less encouraging (De La Garza, Zorick, London, & Newton, 2009; Heinzerling et al., 2010; McElhiney, Rabkin, Rabkin, & Nunes, 2009; McGaugh et al., 2009).
This is the first evaluation of the effects of chronic modafinil treatment of cocaine administration by cocaine-experienced non-human primates. To date, only one preclinical study has examined the ability of modafinil to modulate the abuse-related effects of cocaine (Deroche-Gamonet et al., 2002). Acute administration of modafinil (32, 64 or 128 mg/kg, IP) failed to alter cocaine self-administration during repeated administration in rats (Deroche-Gamonet et al., 2002). The purpose of the present study was to evaluate the effects of chronic modafinil treatment on cocaine self-administration in rhesus monkeys and to characterize the stimulus effects of modafinil in both cocaine discrimination and cocaine reinstatement paradigms. Modafinil produced cocaine-like discriminative stimulus effects, and chronic treatment with 32 mg/kg/day modafinil selectively reduced responding maintained by some reinforcing doses of cocaine without altering food-maintained responding. In addition, after extinction of cocaine self-administration, modafinil administration reinstated saline responding. These findings are consistent with results from most clinical laboratory and outpatient trials described earlier. The concordance between clinical and preclinical evaluations of modafinil provide further support for the validity of these preclinical behavioral models (Mello, 2005). These data also encourage the further evaluation of modafinil as an agonist-based pharmacotherapy to aid in treatment of cocaine abuse disorders.
METHODS
Subjects
Three experiments were conducted in eleven adult rhesus monkeys (Macaca mulatta) that weighed 6 to 9 kg. The effects of acute modafinil administration on cocaine discrimination were studied in seven monkeys (four males and three females). Four other male monkeys were used to examine the effects of chronic modafinil administration on food and cocaine self-administration and reinstatement. These monkeys had self-administered cocaine under the same experimental conditions for over 30 months. Chow (Purina Jumbo Monkey Chow #503), fresh fruit daily and vitamins were given to maintain 95% free feeding weights. Water was freely available at all times. Animal maintenance and research were conducted in accordance with the guidelines provided by the Committee on Laboratory Animal Resources and the NIH Office of Laboratory Animal Welfare (OLAW).
Apparatus
Each monkey lived in a well ventilated, stainless steel chamber for drug discrimination (56 × 71 × 69 cm) or drug self-administration (60 × 100 × 76 cm) studies. An operant panel (28 × 28 cm) was mounted on the front wall. Each panel contained three square translucent response keys, arranged in a horizontal row, which could be transilluminated by red or green stimulus lights, and a set of three vertical stimulus lights (red, green, yellow) located beneath the center response key. A dispenser delivered 1-g banana-flavored food pellets to a receptacle below the operant panel. Reinforcement schedules were controlled by IBM-compatible computers and Med-Associates interface systems (St. Albans, VT) located in an adjacent room.
Drug Discrimination
Discrimination studies were conducted to determine if modafinil shared stimulus characteristics with cocaine and to assess the time course of discriminative stimulus effects. As in our previous studies (Mello, Negus, Knudson, Kelly, & Mendelson, 2008; Negus, Mello, Blough, Baumann, & Rothman, 2007), daily sessions consisted of 1 to 5 20-min cycles. A 15-min time-out period was followed by a 5-min response period. During the time-out, all stimulus lights remained off, and responding had no scheduled consequences. During the response period, the right and left response keys were transilluminated red or green, and monkeys could earn up to 10 food pellets by responding on a fixed-ratio 30 (FR30) schedule. For four monkeys, the left key was illuminated green, and the right key was illuminated red. The colors of the response keys were reversed for the other three monkeys. If all available food pellets were delivered before the end of the 5-min response period, the response key stimulus lights were turned off, and responding had no scheduled consequences for the remainder of the 5-min period.
Training Procedure
Monkeys were trained to discriminate either a low or a high dose of cocaine from saline. Three females were trained on a low dose of 0.18 mg/kg cocaine and four males were trained on a high dose of 0.4 mg/kg cocaine. On training days, monkeys were given an IM injection of cocaine or a similar volume of saline 5-min after the beginning of each time-out period. Following saline administration, responding on only the green, saline-appropriate key produced food, whereas following cocaine administration, only responding on the red, drug-appropriate key produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. Training days consisted of 0 to 5 saline cycles followed by 0 to 1 drug cycles. Cocaine was administered only during the last cycle.
Acquisition of cocaine discrimination was defined by meeting three criteria for 7 of 8 consecutive training sessions: 1) the percent injection-appropriate responding prior to delivery of the first reinforcer was greater than or equal to 80% for all cycles; 2) the percent injection-appropriate responding for each cycle was greater than or equal to 90%; and 3) response rates during saline training cycles were greater than 0.5 responses per second.
Testing Procedure
Test sessions were identical to training sessions except that 1) responding on either key produced food, and 2) a single injection of a test drug [saline, cocaine (0.18 or 0.4 mg/kg)], amphetamine (0.032-0.32 mg/kg) or modafinil (3.2-32 mg/kg) was administered IM at the beginning of the session, and 5-min response periods began after 10, 30, and 100 min. Additional response periods occurred at 60 min, 300 min and 24 h in some cases.
Data Analysis
The mean ± SEM percent cocaine-appropriate responding (for the entire response period) was plotted as a function of time after injection. Test drugs were considered to substitute for the training dose of cocaine if they produced ≥90% cocaine-appropriate responding. In addition, discrimination ED50 values were defined as the dose of modafinil that produced 50% cocaine-appropriate responding. ED50 values were calculated by linear interpolation in all monkeys in which modafinil dose-dependently produced >50% cocaine-appropriate responding. ED50 values were calculated using data collected at 100 min, because this was the time of peak modafinil effect for both groups of subjects. Data from individual subjects are also plotted. Response rate data for both groups are presented in tabular format.
Cocaine self-administration
To permit intravenous drug delivery, a double-lumen catheter was surgically implanted into each monkey under aseptic conditions as described previously (Mello et al., 2008). The intravenous catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical Inc., Malone, NY). Two syringe pumps were mounted above each cage for delivery of saline or drug solutions through the two lumens of the intravenous catheters. One syringe pump delivered self-administered cocaine injections through one catheter lumen, and the second syringe pump was used for non-contingent delivery of saline or modafinil through the second catheter lumen.
Procedures for evaluating the effects of modafinil on cocaine and food-maintained responding were similar to those used in our previous studies (Mello, Lukas, Kamien, Mendelson, & Cone, 1992; Mello & Negus, 1998; Negus & Mello, 2003; Negus et al., 2007). Each session contained four experimental components and sessions were conducted 7 d/week. Food was available for self-administration at 11am, 3pm, 7pm, and 6am the next morning, and the cocaine self-administration sessions began at 12pm, 4pm, 8pm, and 7am the next morning. Room lights were off during all experimental sessions.
Training Procedure
The monkeys were trained to respond under an FR2 [VR16:S] second-order schedule of reinforcement. At the beginning of each session, stimulus lights in the center response key were illuminated (red for food, green for drug). Completion of a variable ratio (VR) averaging 16 responses on the center key turned off the appropriate colored stimulus light (red for food, green for cocaine) and turned on the appropriate colored stimulus light located below the center response key for 1 s (VR16:S). Completion of two VR16s (FR2) turned off the stimulus light illuminating the center response key and turned on the appropriate colored stimulus light beneath it for 1 s and initiated delivery of a 1-g banana-flavored food pellet or a 0.1 ml injection given over 1 s. Reinforcer delivery was followed by a 10-s time-out period during which stimulus lights remained off and responding had no scheduled consequences. If 25 food pellets or 20 injections were delivered before expiration of the 1-hr session, then all stimulus lights were turned off, and responding had no scheduled consequences for the remainder of that session. Thus, a monkey could earn a maximum of 100 pellets/day and 80 injections/day. Only the center key was active.
During training, responding was maintained by 1-g food pellets and 0.01 mg/kg/inj cocaine, the lowest dose of cocaine that reliably maintained peak responding in all monkeys. Training continued until monkeys met the following criteria for stable food and cocaine self-administration under the FR2 [VR16:S] schedule of reinforcement: 1) three consecutive days during which the number of drug injections/day varied by no more than 20% of the three-day mean with no upward or downward trend in injection numbers, and 2) during the same 3 consecutive days, the mean number of both food pellets and injections delivered per day was greater than 60.
Chronic modafinil treatment
After responding stabilized according to the above criteria, cocaine dose-effect relationships were determined during saline treatment. Monkeys self-administered each unit dose of cocaine (0.001-0.1 mg/kg/inj) for at least 5 days and until stability criteria were met or 10 days had elapsed, whichever occurred first. Cocaine doses or saline were presented in an irregular order that differed for each monkey.
After determination of cocaine dose-effect curves during saline treatment, cocaine dose-effect curves were redetermined during chronic modafinil treatment. Monkeys typically remained at each cocaine dose for at least 7 days and until stability criteria were met or 10 days had elapsed, whichever occurred first. Modafinil was infused at a rate of 0.33 ml every 20 min from 10:30 am until 9:30 am the next day. Between 9:30 am and 10:30 am, monkeys were fed, their health monitored, and modafinil catheter lines were flushed. The effects of 32 mg/kg/day modafinil were evaluated in four monkeys and the effects of 56 mg/kg/day modafinil were evaluated in two monkeys. Tests with 56 mg/kg/day modafinil were not completed in the other two monkeys because of stereotypies and difficulties maintaining catheter patency during modafinil treatment. The doses of modafinil used for chronic studies were empirically determined in pilot studies. Initial tests were conducted with 10 mg/kg modafinil infused over one hour prior to the experimental session for 10 days. Neither cocaine- nor food-maintained behaviors were altered by this dose of modafinil under those conditions.
Reinstatement by modafinil (32 and 56 mg/kg/day) during extinction
After evaluation of chronic modafinil treatment on cocaine's self-administration was completed, cocaine (0.01 mg/kg/inj) was made available for at least 10 days until responding stabilized according to criteria stated above. Subsequently, saline was substituted for cocaine until responding extinguished. Once the number of self-administered saline injections was <10 per day for three consecutive days, treatment with modafinil (32 and 56 mg/kg/day) was begun in 3 monkeys and continued for 3 days. Prior to completing the reinstatement experiments, the fourth monkey (monkey 13541), died due to causes unrelated to the study.
Data Analysis
The primary dependent variables were the total injections per day and total pellets per day delivered during the last 3 days of each test period. ED50 values for cocaine self-administration were defined as the dose of cocaine that produced 50% of maximum possible numbers of injections per day (80 inj/day). For statistical analysis, the effects of modafinil on food- and cocaine-maintained responding were analyzed by two-factor analysis of variance (ANOVA) with treatment condition (saline vs modafinil) as one factor and cocaine dose as the other factor. A significant ANOVA was followed by a Bonferroni post hoc test when appropriate (P < 0.05). Repeated measures ANOVAs followed by Dunnetts post hoc tests were used to analyze the effects of modafinil on reinstatement responding (GraphPad Prism 4.0c for Macintosh; GraphPad Software Inc.).
Drugs
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile saline. Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water. Modafinil was synthesized as described previously (Prisinzano, Podobinski, Tidgewell, Luo, & Swenson, 2004). Initially, a vehicle of 1:1 ethanol:emulphor was used for the animals trained to discriminate 0.18 mg/kg cocaine from saline in the discrimination study. A vehicle of 40% beta-cyclodextrin was used for the group trained to discriminate 0.4 mg/kg cocaine and for intravenous infusion in the self-administration study (following dilution with sterile water). All drugs were sterile-filtered using a 0.22-μm syringe filter (Millipore, Billerica, MA).
RESULTS
Discriminative stimulus effects of cocaine, amphetamine, and modafinil in monkeys trained to discriminate 0.18 mg/kg cocaine from saline (Figures 1 and 2; Tables 1 and 3)
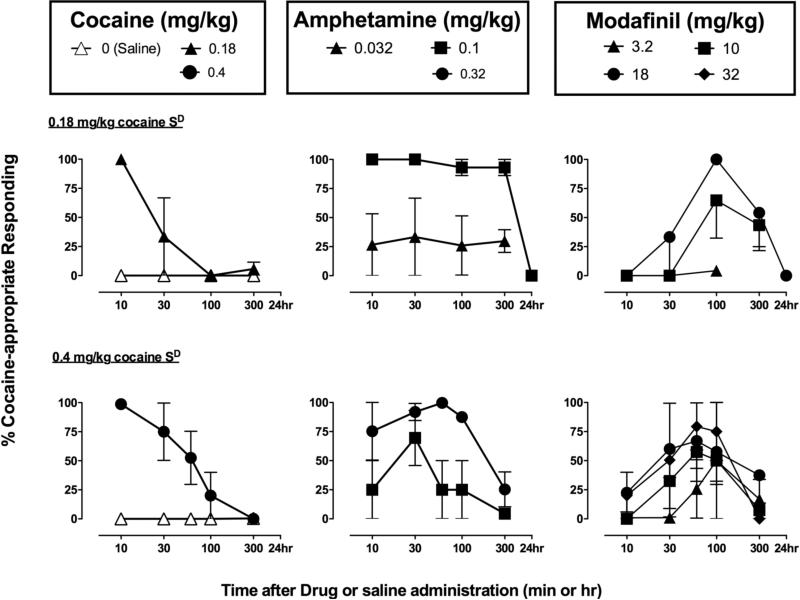
Figure 1.
Effects of saline, cocaine, amphetamine, and modafinil in three female rhesus monkeys trained to discriminate 0.18 mg/kg cocaine from saline (Upper panels) and four male rhesus monkeys trained to discriminate 0.4 mg/kg cocaine from saline (Lower panels). Each data point represents mean ± SEM data from a single determination. Abscissae: min or hr after drug or saline administration. Ordinates: Percent cocaine-appropriate responding.
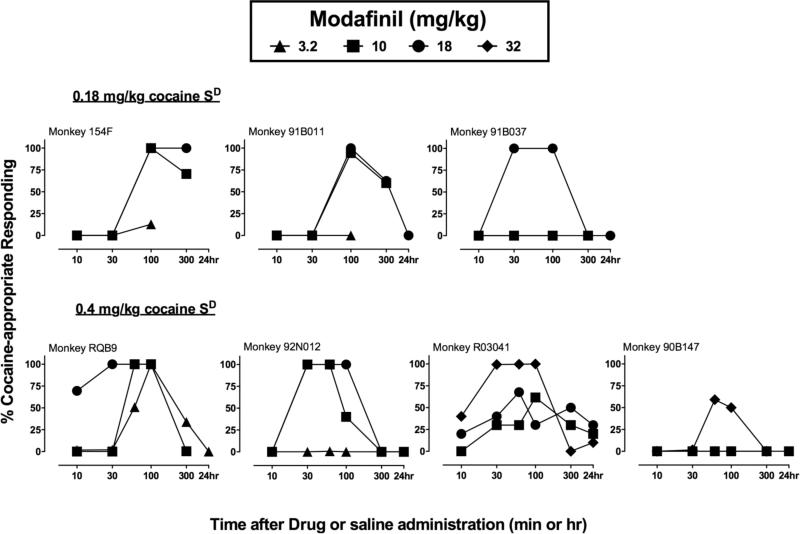
Figure 2.
Discriminative stimulus effects of modafinil in three individual monkeys trained to discriminate 0.18 mg/kg cocaine from saline (Upper panels) and in four individual monkeys trained to discriminate 0.4 mg/kg cocaine from saline (Lower panels). Abscissae: min or hr after drug administration. Ordinates: Percent cocaine-appropriate responding. All points show data from a single determination in each monkey. The identification number for each monkey is shown in the upper left of each panel.
Table 1.
Maximum percent Cocaine-Appropriate Responding (%CAR) Observed during Substitution Experiments with Cocaine, Amphetamine and Modafinil in a Group of Monkeys Trained to Discriminate 0.18 mg/kg Cocaine from Saline.
| Drug | Dose range tested (mg/kg; IM) | Maximum %CAR | # of monkeys substituting1 | N2 |
|---|---|---|---|---|
| Cocaine | 0.18 | 100 | 3 | 3 |
| Amphetamine | 0.032-0.1 | 100 | 3 | 3 |
| Modafinil3 | 3.2-18 | 100 | 3 | 3 |
Total number of monkeys in which the test drug produced ≥90% CAR.
Indicates total number of animals tested.
Data obtained 100 min following modafinil administration.
Table 3.
Mean (SEM) response rates in a group of three monkeys trained to discriminate 0.18 mg/kg cocaine.
| Drug | Dose (mg/kg) | 10 min | 30 min | 100 min | 300 min | 24 hr |
|---|---|---|---|---|---|---|
| Cocaine | 0 | 1.74 (0.18) | 1.53 (0.34) | 1.00 (0.27) | 1.42 (0.52) | -- |
| 0.18 | 2.15 (0.18) | 1.85 (0.13) | 0.96 (0.39) | 1.18 (0.43) | -- | |
| Amphetamine | 0.032 | 1.3 (0.19) | 1.85 (0.31) | 0.81 (0.13) | 1.03 (0.76) | -- |
| 0.1 | 1.54 (0.18) | 1.64 (0.32) | 1.95 (0.48) | 1.33 (0.48) | 0.85 (0.34) | |
| Modafinil | 3.2 | 1.94 (0.42) | 1.18 (0.54) | 1.07 (0.46) | -- | -- |
| 10 | 1.46 (0.36) | 1.0 (0.45) | 1.49 (0.25) | 1.61 (0.33) | -- | |
| 18 | 1.81 (0.50) | 1.62 (0.33) | 1.78 (0.25) | 1.27 (0.44) | 2.77 (0.39) | |
Control performance during training days
During the training days preceding test days, the monkeys responded almost exclusively on the saline key during saline cycles (mean % saline-appropriate responding = 99.91 ± 0.08) and almost exclusively on the cocaine key during cocaine training sessions (mean % cocaine-appropriate responding = 99.74 ± 0.22). Mean response rates (±SEM) were 1.65 (±0.30) and 1.91 (±0.08) responses/s during saline and drug training cycles, respectively.
Mean effects of cocaine, amphetamine and modafinil
Figure 1 shows group data and Figure 2 shows individual data for the time course of the cocaine-like discriminative stimulus effects of saline, cocaine, amphetamine, and modafinil in the group trained to discriminate 0.18 mg/kg cocaine from saline (Upper panels). Saline administration produced only saline-appropriate responding for the entire test period, whereas the training dose of 0.18 mg/kg cocaine produced peak levels of cocaine-appropriate responding after 10 min, the same time interval used for training (training data not shown). The discriminative stimulus effects of the cocaine-training dose decreased within 30 min and were no longer apparent after 100 min. Amphetamine (0.032-0.1 mg/kg) produced a dose- and time-dependent substitution for cocaine. Treatment with 0.032 mg/kg amphetamine produced a maximum of 33% cocaine-appropriate responding for the group of three monkeys. The higher dose of 0.1 mg/kg amphetamine produced full substitution for cocaine in all three monkeys 10-300 min after administration. The cocaine-like discriminative stimulus effects of 0.1 mg/kg amphetamine were no longer apparent after 24hr. Pretreatment with 10 mg/kg modafinil produced 100% cocaine-appropriate responding in 2 of 3 monkeys. Increasing the dose of modafinil to 18 mg/kg produced 100% cocaine-appropriate responding in all three monkeys. However, in contrast to cocaine and amphetamine, peak levels of substitution for the group were not observed until 100 min after modafinil administration. The cocaine-like discriminative stimulus effects of modafinil were still present at 300 min but were no longer apparent after 24hr. The mean (95%CL) ED50 value of modafinil determined at the time of peak effect (100 min) was 7.4 (4.2-13.3) mg/kg. Table 3 shows response rates for the time course tests with saline, cocaine, amphetamine, and modafinil. Generally, rates of responding were not affected by amphetamine, cocaine, or modafinil.
Discriminative stimulus effects of cocaine, amphetamine, and modafinil in monkeys trained to discriminate 0.4 mg/kg cocaine from saline (Figures 1 and 2; Tables 2 and 4)
Table 2.
Maximum percent Cocaine-Appropriate Responding (%CAR) Observed during Substitution Experiments with Cocaine, Amphetamine and Modafinil in a Group of Monkeys Trained to Discriminate 0.4 mg/kg Cocaine from Saline.
| Drug | Dose range tested (mg/kg; IM) | Maximum %CAR | # of monkeys substituting1 | N2 |
|---|---|---|---|---|
| Cocaine | 0.4 | 100 | 3 | 3 |
| Amphetamine | 0.032-0.1 | 100 | 3 | 3 |
| Modafinil3 | 3.2-18 | 100 | 2 | 4 |
| 32 | 75 | 1 | 2 |
Total number of monkeys in which the test drug produced ≥90% CAR.
Indicates total number of animals tested.
Data obtained 60 min following modafinil administration
Table 4.
Mean (SEM) response rates in a group of four monkeys trained to discriminate 0.4 mg/kg cocaine.
| Drug | Dose (mg/kg) | 10 min | 30 min | 60 min | 100 min | 300 min | 24 hr |
|---|---|---|---|---|---|---|---|
| Cocaine | 0 | 2.46 (0.48) | 2.54 (0.54) | 2.15 (0.47) | 2.31 (0.30) | 2.3 (0.43) | -- |
| 0.4 | 2.65 (0.71) | 2.92 (0.43) | 3.30 (0.27) | 3.05 (0.17) | 2.42 (0.38) | -- | |
| Amphetamine | 0.1 | 2.75 (0.29) | 2.95 (0.38) | 2.87 (0.47) | 3.01 (0.45) | 2.14 (0.29) | -- |
| 0.32 | 2.93 (0.70) | 2.80 (0.53) | 2.57 (0.52) | 2.79 (0.60) | 2.90 (0.38) | 2.13 (0.84) | |
| Modafinil | 3.21 | 2.97 (1.09) | 2.97 (1.13) | 3.54 (0.82) | 2.81 (1.41) | 2.65 (0.40) | 3.07 (1.46) |
| 10 | 2.6 (0.37) | 2.58 (0.49) | 2.79 (0.33) | 2.67 (0.55) | 2.79 (0.47) | 1.83 (0.18) | |
| 18 | 2.98 (0.23) | 3.34 (0.46) | 3.13 (0.45) | 2.35 (0.71) | 2.32 (0.81) | 2.01 (0.51) | |
| 322 | 2.63 (0.11) | 2.29 (0.46) | 2.21 (0.13) | 1.61 (1.31) | 1.79 (1.79) | -- | |
n=2
n=2
Control performance during training days
During the training days preceding test days, the monkeys responded almost exclusively on the saline key during saline cycles (mean % saline-appropriate responding = 99.92±0.07) and almost exclusively on the cocaine key during cocaine training sessions (mean % cocaine-appropriate responding = 99.95±0.04). Mean response rates (±SEM) were 2.19 (±0.22) and 2.46 (±0.29) responses/s during saline and drug training cycles, respectively.
Mean effects of cocaine, amphetamine and modafinil
Figure 1 shows group data and Figure 2 shows individual data for the time course of the cocaine-like discriminative stimulus effects of saline, cocaine, amphetamine and modafinil in monkeys trained to discriminate 0.4 mg/kg cocaine from saline (Lower panels). Saline administration produced only saline-appropriate responding for the entire test period, whereas the training dose of 0.4 mg/kg cocaine produced peak levels of cocaine-appropriate responding within 10 min, the same time interval used for training (training data not shown). The discriminative stimulus effects of the cocaine training dose then dissipated and were no longer apparent after 300 min. Amphetamine (0.1-0.32 mg/kg) produced a dose- and time-dependent substitution for cocaine. The higher dose of amphetamine produced full substitution for cocaine in all four monkeys with peak effects observed from 10 to 300 min after administration. Modafinil also produced a dose- and time-dependent substitution for cocaine. Treatment with 18 mg/kg modafinil produced full substitution for cocaine's discriminative stimulus effects in two of four monkeys, and partial substitution in a third monkey. Increasing the dose to 32 mg/kg produced complete substitution in this third monkey and partial substitution (67% cocaine-appropriate responding) in the fourth monkey. Higher doses were not tested in this monkey due to poor solubility of modafinil at higher concentrations. It is worth noting that the lower dose of modafinil (10 mg/kg) substituted in the two monkeys for which the lower dose of amphetamine, 0.1 mg/kg, also substituted for cocaine's discriminative stimulus. Conversely, higher doses of both modafinil and amphetamine were necessary to produce cocaine-appropriate responding in the other two monkeys. Peak levels of substitution for the group were observed 30-100 min after modafinil administration. In this group, the discriminative stimulus effects of the three drugs were evaluated at an additional time point (60 min), at which the peak effects generally occurred. The cocaine-like discriminative stimulus effects of modafinil then dissipated after 300 min. The mean (95% CL) ED50 value for the group determined at 100 min was 18.7 (8.4-41.8) mg/kg. Generally, none of the drugs tested altered response rates. However, in one monkey (RQB9), pretreatment with 18 mg/kg modafinil produced a decrease in response rates 100 and 300 min following its administration. Table 4 shows response rates for the time course tests with saline, cocaine, amphetamine, and modafinil.
Effects of modafinil on cocaine and food self-administration (Figures 3, 4, and 5)
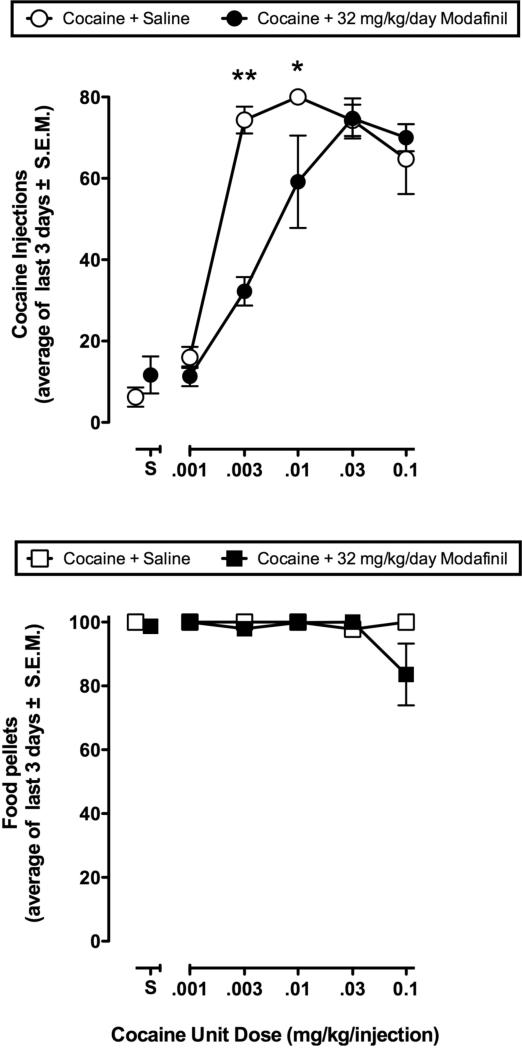
Figure 3.
Effects of modafinil on the cocaine self-administration dose-effect curve and concurrent food-maintained responding in four monkeys. Abscissae: Unit dose of cocaine (mg/kg/inj) available during daily drug components. Points above ‘S’ show data collected when saline was the solution available for self-administration. Ordinate (Upper panel): Mean ±SEM numbers of cocaine injections self-administered per day during saline treatment baseline conditions (open symbols) and during chronic modafinil treatment (filled symbols). Each data point is the average of the last three days of each cocaine dose condition. Ordinate (Lower panel): Mean ±SEM numbers of food pellets delivered per day during saline treatment baseline conditions (open symbols) and during chronic modafinil treatment (filled symbols). All data points show data from four monkeys, except for saline + modafinil which shows data from three monkeys. *P < 0.05 **P < 0.01, compared to baseline.
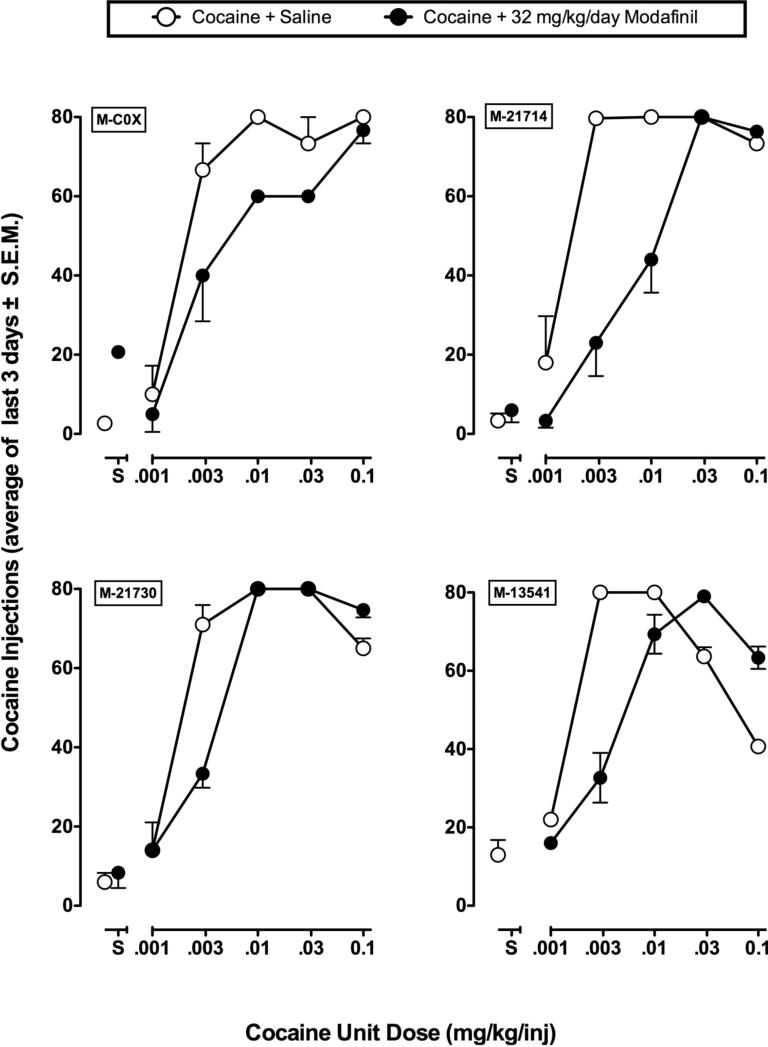
Figure 4.
Effects of modafinil on the cocaine self-administration dose-effect curve in individual monkeys. Ordinate: numbers of cocaine injections self-administered per day during baseline conditions (open symbols) and during chronic modafinil treatment (filled symbols). Points above ‘S’ show data collected when saline was the solution available for self-administration. Each data point shows the mean ±SEM from the last three days of each cocaine dose condition. The identification number for each monkey is shown in the upper left of each panel.
Figure 5.
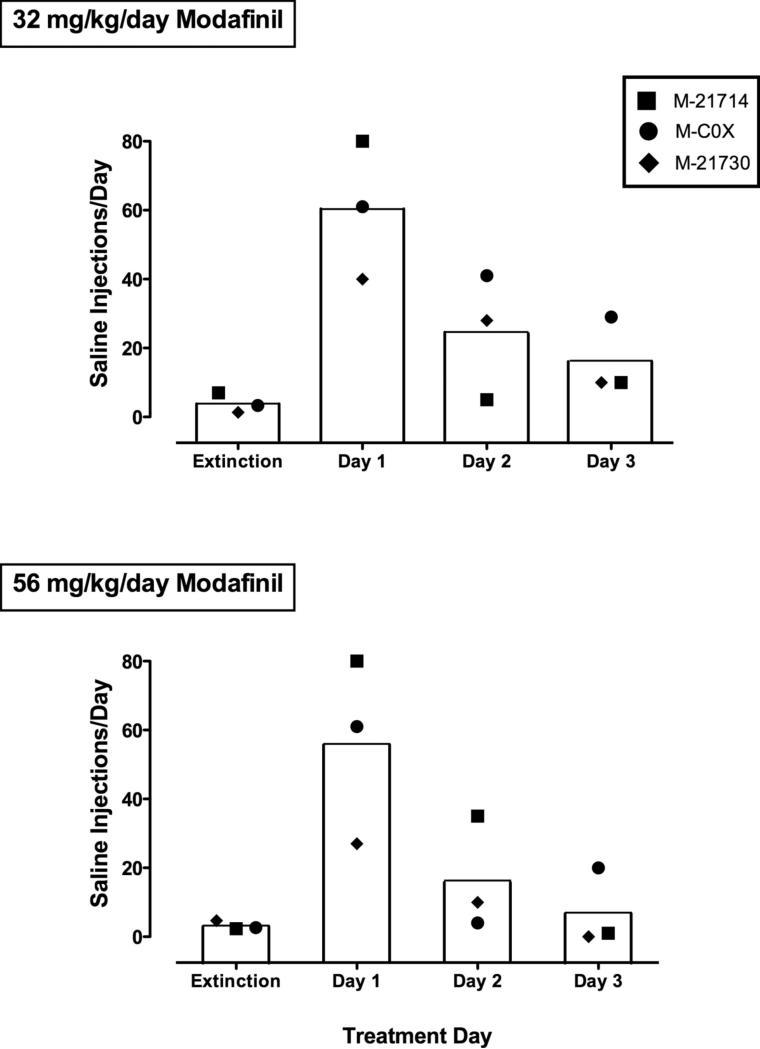
Effects of modafinil on extinguished responding. Ordinates: Numbers of saline injections self-administered during extinction and on three consecutive days of 32 mg/kg/day of modafinil treatment (Upper panel) and 56 mg/kg/day of modafinil treatment (Lower panel). Each bar represents the mean number of saline injections/day by a group of three monkeys, and the closed symbols show each individual monkey's data. Abscissae: Treatment day. The column above ‘Extinction’ show mean saline injections from the last three days of extinction prior to the introduction of modafinil. Treatment days 1, 2, and 3 are saline injections from the first, second, and third day of modafinil treatment, respectively.
Figure 3 shows data from the last three days of cocaine and food self-administration as a function of cocaine dose during saline treatment conditions, and during chronic treatment with modafinil in four monkeys. Cocaine self-administration during saline treatment was dose-dependent, and the cocaine dose-effect curve generally had an inverted-U shape. A one-way repeated measures ANOVA revealed a statistically significant effect of cocaine dose [F (5, 15) = 51.62, P < 0.001]. Monkeys responded at low levels for saline injections (points above ‘S’), and for the lowest dose of cocaine (0.001 mg/kg/inj). Cocaine (0.0032 – 0.1 mg/kg/inj) maintained significantly more responding than saline (P < 0.0001). Monkeys responded at high, stable levels for food across all cocaine doses.
During chronic treatment with 32 mg/kg/day modafinil, the ascending limb and peak of the cocaine dose-effect curve was shifted downward relative to the saline treatment condition. A two-way repeated measures ANOVA revealed a significant effect of cocaine dose [F (5, 30) = 68.56, P < 0.001], a significant treatment effect [F (1, 6) = 9.56, P < 0.05], and a significant interaction between cocaine dose and treatment [F (5, 30) = 7.03, P < 0.001]. Post hoc tests revealed a significant difference between treatment with saline and 32 mg/kg/day modafinil when 0.0032 mg/kg/inj cocaine was the available for self-administration (P < 0.001). Behavior maintained by the peak reinforcing dose of cocaine, 0.01 mg/kg/inj, was also significantly reduced during modafinil treatment (P < 0.05). Responding maintained by higher doses of cocaine (0.032 and 0.1 mg/kg/inj) was equivalent during the saline treatment baseline and modafinil treatment conditions. Chronic treatment with 32 mg/kg/day modafinil produced a 2.7-fold rightward shift in ED50 values calculated for the cocaine dose-effect curve. The ED50 (95% CL) values for cocaine during saline treatment and chronic modafinil treatment were 0.0020 mg/kg (0.0016-0.0024 mg/kg) and 0.0054 mg/kg (0.0025-0.0115), respectively. Food-maintained behavior during saline treatment conditions was stable across all doses of cocaine. No statistically significant difference in food-maintained behavior was observed during chronic modafinil treatment.
Group data shown in Figure 3 are representative of the individual data shown in Figure 4. Self-administration of 0.01 mg/kg/inj cocaine was reduced and the peak of the cocaine dose-effect curve was shifted rightward during chronic treatment with modafinil in three of the four monkeys. One exception to the group, monkey 21730, only showed decreased responding at 0.0032 mg/kg/inj cocaine. Chronic modafinil treatment did not markedly alter self-administration of 0.001 mg/kg/inj cocaine in any monkey. Self-administration of 0.0032 mg/kg/inj cocaine decreased in all monkeys during chronic treatment with modafinil. At higher cocaine doses (0.032 and 0.1 mg/kg/inj) cocaine self-administration was comparable to baseline conditions. All monkeys remained healthy and weights were stable during chronic modafinil treatment. As noted earlier, one monkey (13541) died due to causes unrelated to the study and was unable to complete his test with the saline + 32 mg/kg/day modafinil condition.
Two monkeys (monkeys 21730 and C0X) were tested at a higher dose of 56 mg/kg/day modafinil (data not shown). In monkey 21730, this dose of modafinil produced similar effects as treatment with 32 mg/kg/day, in that it reduced self-administration of 0.0032 mg/kg/inj, but not 0.01 mg/kg/inj cocaine. In monkey C0X, 56 mg/kg/day modafinil decreased cocaine self-administration (0.0032 mg/kg/inj) more than treatment with 32 mg/kg/day, but self-administration of 0.01 and 0.032 mg/kg/inj was not affected. Daily treatment with 56 mg/kg/day produced a reduction of food-maintained responding for the first seven days of treatment in both monkeys, and reduced food intake in monkey C0X. Both monkeys developed stereotypies characterized by focused grooming and prolonged downward gazing. Chronic testing with 56 mg/kg/day modafinil was not completed in the other two monkeys because of stereotypies and difficulties maintaining catheter patency during the 7-10 day modafinil treatment period.
Reinstatement of cocaine-seeking behavior by acute modafinil
After a drug-free period, three of the same monkeys were used to test the effects of modafinil on extinguished responding. Saline was substituted for the maintenance dose of cocaine, 0.01 mg/kg/inj, until responding extinguished (< 10 injections for three consecutive days), which occurred after a mean of 10.7 days. After extinction, modafinil treatment (32 or 56 mg/kg/day) was introduced and responding maintained by saline was observed for three days. Figure 5 shows numbers of saline injections for the group (bars) as well as individuals (symbols) for the mean of the last three days of extinction and on each of the three consecutive days of treatment with 32 mg/kg/day modafinil (Upper figure) and 56 mg/kg/day modafinil (Lower figure). During the first day of modafinil treatment (32 mg/kg/day), responding for saline increased significantly from the previous day's extinction levels [F (3, 11) = 7.97, P < 0.05]. Dunnett's post hoc tests revealed a significant increase on the first day of modafinil treatment (P < .01), but not days 2 and 3. Infusion of 56 mg/kg/day modafinil also produced a significant increase in saline self-administration [F (3, 11) = 4.70, P = 0.05], and again, the numbers of self-administered saline injections were significantly greater on the first treatment day only (P < 0.05).
DISCUSSION
The present studies were designed to evaluate modafinil as a candidate medication for treating cocaine abuse disorders. This is the first preclinical study to demonstrate that chronic modafinil treatment selectively reduced cocaine self-administration by rhesus monkeys. The rhesus monkey drug self-administration model is valuable for evaluation of potential drug abuse treatment medications, and shows high concordance with clinical treatment trials (Haney & Spealman, 2008; Mello, 2005; Mello & Negus, 1996). The major findings of the present study are consistent with clinical laboratory studies and outpatient trials of modafinil effects on the abuse-related effects of cocaine described below. Cross-validation of preclinical and clinical evaluations of drug abuse treatment medications is very important because novel medications that appear to reduce cocaine self-administration in preclinical models often are not FDA-approved for human testing (Mello, 2005).
Agonist-based pharmacotherapies are often more effective than antagonist-based pharmacotherapies and are more acceptable to patients (Grabowski et al., 2004; Mello & Mendelson, 1995; Mendelson & Mello, 1996). One important feature of agonist-based pharmacotherapies is that they retain behavioral and pharmacological features of the abused drug (Grabowski et al., 2004). To more fully characterize the behavioral interactions between modafinil and cocaine, the similarities between these drugs were assessed in a drug discrimination procedure and in a reinstatement procedure. We found that modafinil produced a dose- and time-dependent substitution for the discriminative stimulus effects of cocaine, and this is consistent with clinical reports (Rush, Kelly, Hays, & Wooten, 2002). Modafinil significantly reinstated responding previously reinforced by cocaine, further suggesting its similarity to cocaine. Finally, chronic administration of modafinil on cocaine- and food-maintained behavior was evaluated. We found that modafinil selectively attenuated the reinforcing effects of cocaine doses on the ascending limb of the cocaine dose-effect curve. Some implications of these findings and limitations of the study are discussed below.
Cocaine-like Discriminative Stimulus Effects of Modafinil
We compared the effects of modafinil in rhesus monkeys trained to discriminate low and high cocaine doses, in part because it has been suggested that use of a lower training dose may reduce the pharmacological selectivity of the training cue and increase the maximal effects of individual drugs and/or the overall range of drugs that produce substitution (Stolerman, 1993; Terry, Witkin, & Katz, 1994). Modafinil produced cocaine-like discriminative stimulus effects in monkeys trained to discriminate both low and high doses of cocaine from saline. These data in rhesus monkeys are generally consistent with previous reports in rats showing that modafinil partially substitutes for cocaine in a dose- (Gold & Balster, 1996) and time- dependent manner (Dopheide et al., 2007). Modafinil doses up to 100 mg/kg did not substitute for cocaine; however, a dose of 250 mg/kg produced full substitution, but markedly reduced responding in four of six rats (Gold & Balster, 1996). Orally administered modafinil also substituted for cocaine in two groups of rats trained to discriminate different doses of cocaine, in a dose- and time-dependent manner (Dopheide et al., 2007). Peak effects of oral modafinil occurred between 60-120 min, similar to onset of peak effects following IM administration in monkeys in the present study. These data indicate that modafinil's onset of action is slower than that of both cocaine and amphetamine, and its duration of action for producing cocaine-like discriminative stimulus effects is shorter than that of amphetamine. Taken together, these data indicate that modafinil engenders partial to full substitution for cocaine at doses 25-80 times greater than the training dose of cocaine. Similarly, a dose of modafinil 4 times greater than the training dose (600 mg modafinil) fully substituted for cocaine in three of six human subjects trained to discriminate effects of oral cocaine from placebo (Rush, Kelly, Hays, & Wooten, 2002). Limitations in modafinil solubility prevented administration of the higher doses in rat studies, and doses in human studies were presumably limited by ethical and safety considerations. However, authors of all these studies speculated that higher modafinil doses might have produced higher levels of cocaine-appropriate responding. The results of the present study support this conclusion and demonstrate good concordance between species for the cocaine-like discriminative stimulus effects of modafinil.
Interestingly, the ED50 value for modafinil in the lower dose training group (7.4 mg/kg) was similar to the modafinil dose that occupied 54% of striatal dopamine transporters in PET studies conducted in rhesus monkeys (8 mg/kg IV, Madras et al., 2006). The doses that produced cocaine-like discriminative stimulus effects in the present study are also similar to (a) doses used clinically to promote wakefulness in humans (100-600 mg, or roughly 1.5-10 mg/kg), and (b) doses that maintain self-administration and decrease cocaine consumption in human laboratory and outpatient studies (200-400 mg, or roughly 3-8 mg/kg) (Dackis et al., 2005; Hart et al., 2008; Stoops et al., 2005). In comparison to cocaine and amphetamine, modafinil had at least 100-fold lower potency and a slower onset of action. The low potency and slow onset of modafinil likely contribute to modafinil's relatively low abuse liability (Myrick et al., 2004)
The extent to which sex differences may have contributed to the apparent differences between the low and high dose training groups cannot be determined with certainty from these data. The low dose (0.18 mg/kg) cocaine training group consisted of only females, whereas the high dose (0.4 mg/kg) cocaine training group consisted only of males. Whether or not there are sex differences in cocaine's discriminative stimulus effects has not been evaluated in rhesus monkeys. However, studies using rodents (Anderson & van Haaren, 1999; Craft & Stratmann, 1996) reported no sex differences, and studies using humans reported no effect of sex or menstrual cycle phase on subjective effects produced by cocaine in humans (Collins, Evans, Foltin, & Haney, 2007; Mendelson et al., 1999; Singha, McCance-Katz, Petrakis, Kosten, & Oliveto, 2000). One exception to this general finding is that smoked cocaine appears to produce weaker subjective effects during luteal phase than during the follicular phase or in men (Evans, Haney, & Foltin, 2002; Sofuoglu, Dudish-Poulsen, Nelson, Pentel, & Hatsukami, 1999). Also, no effects of sex or menstrual cycle phase have been reported in clinical studies that examined the discriminative stimulus of modafinil (Rush, Kelly, Hays, & Wooten, 2002). However, one report suggested that females may be more sensitive to the stimulant effects of high doses (800 mg) of modafinil (referenced in Jasinski & Kovacevic-Ristanovic, 2000).
A second difference was that the two training groups were administered modafinil in different vehicles. In the 0.18 mg/kg training group, modafinil was administered in a 1:1 ethanol: emulphor vehicle, and in the 0.4 mg/kg training group, modafinil was administered in a 40% beta cyclodextrin vehicle. The vehicles could influence the rate of absorption, and potentially alter the onset of stimulus effects. However, the time course of modafinil's effects were comparable between the two training groups, suggesting that differences in potency for producing cocaine-like discriminative stimulus effects were not attributable to the use of different vehicles.
Effects of Chronic Modafinil Administration on Food- and Cocaine-Maintained Responding
Chronic IV treatment with 32 mg/kg/day modafinil produced a rightward shift in the ascending limb and peak of the cocaine self-administration dose-effect curve (0.0032 and 0.01 mg/kg/inj). Modafinil had no effect on self-administration of higher doses of cocaine, suggesting that effects of modafinil are surmountable. This is consistent with the common finding that behavior maintained by lower unit doses is more vulnerable to modification by pharmacological treatments (for a discussion see Mello & Negus, 1996). Importantly, chronic treatment with 32 mg/kg/day modafinil did not change food-maintained responding, indicating that the reduction of cocaine self-administration was not due to general suppression of responding. Chronic treatment with this dose also did not produce any overt behavioral effects, and did not reduce consumption of food given outside of experimental sessions.
This is the first evaluation of chronic modafinil treatment on cocaine self-administration in cocaine-experienced rhesus monkeys. The present results are consistent with the findings of a clinical laboratory study of the effects of modafinil maintenance on cocaine's reinforcing effects (Hart et al., 2008). In that study, cocaine-dependent subjects were maintained on placebo, 200 mg, or 400 mg modafinil over a 48-day period and given the opportunity to choose between cocaine and money. Both doses of modafinil significantly reduced the number of cocaine choices across a range of cocaine doses. Our findings are not consistent with the one previous study in rats in which modafinil failed to alter cocaine self-administration (Deroche-Gamonet et al., 2002). That study examined acute doses of 32-128 mg/kg modafinil administered 20 min before each 60 or 130 min session. Modafinil has a slow onset of action and peak effects usually occur within 60-100 min. Thus, modafinil may not have been maximally effective at time of testing in rats.
The effects of modafinil observed in the present study are consistent with results of previous studies showing that DAT inhibitors selectively reduce cocaine self-administration in monkeys. In particular, GBR12909, a high affinity DAT inhibitor, reduced rates of responding maintained by cocaine at doses that did not alter rates of responding maintained by food (Glowa et al., 1995). The effects of GBR12909 were also dependent upon the dose of cocaine available for self-administration. Specifically, GBR12909 produced a downward shift in the ascending limb and peak of the cocaine self-administration dose-effect curve, but rates maintained by higher cocaine doses were not affected. GBR12909 was under consideration as a candidate agonist-based pharmacotherapy, but reports of potential adverse cardiovascular effects during a phase I clinical trial halted further investigation (Vocci, Acri, & Elkashef, 2005). Modafinil has been extensively evaluated for safety, and appears to have no adverse toxicological consequences by itself or when combined with cocaine (Dackis et al., 2003; Donovan et al., 2005; Hart et al., 2008; Malcolm et al., 2006).
Previous work in our laboratory demonstrated that chronic treatment with selective monoamine releasers, d-amphetamine (Negus & Mello, 2003; Negus et al., 2007), and phenmetrazine (Negus, Baumann, Rothman, Mello, & Blough, 2009), produced selective reductions in cocaine-maintained responding. However, DAT inhibitors, indatraline, RTI-112 and RTI-113, were non-selective and reduced both cocaine and food-maintained responding (Negus, Brandt, & Mello, 1999; Negus, Mello, Kimmel, Howell, & Carroll, 2009), suggesting that high affinity and pharmacological selectivity for DAT is not sufficient to selectively reduce cocaine-maintained responding. Although medication-induced increases of extracellular dopamine appears to be a critical feature of agonist-based pharmacotherapies, the mechanism and influence of other monoamine transmitters remains to be determined.
Study Strengths and Limitations
One strength of the present study is that modafinil was administered every 20 min for 23 hr each day to ensure that stable levels were present during cocaine dose-effect curve determinations. One limitation of the present study is that plasma levels of modafinil were not measured. In clinical treatment trials, 200-400 mg modafinil is administered orally once or twice daily. The dose of modafinil that effectively reduced cocaine self-administration in the present study was greater than clinically relevant doses. Comparing plasma levels of modafinil obtained in the monkey to known effective values in humans would be useful in determining whether the dose of modafinil used in the present study produced levels comparable to those obtained clinically.
Implications for modafinil as a candidate agonist medication
Abuse Liability
The finding that modafinil both substituted for the discriminative stimulus effects of cocaine and renewed extinguished responding of saline self-administration could be interpreted to suggest that modafinil has a cocaine-like abuse liability. In agreement with this view, modafinil also maintained self-administration in rhesus monkeys (Gold & Balster, 1996) and under some (Stoops et al., 2005), but not all (Vosburg et al., 2010) conditions in humans. Three factors appear to mitigate the abuse liability of modafinil in comparison to other CNS stimulants. First, as described above, modafinil has extremely low potency, and as a result, very high doses are required to produce prominent stimulant effects. Even under the best circumstances, it would be logistically difficult to obtain and consume such high doses. For example, data from both monkeys and rats suggest that modafinil is approximately 100-fold less potent than cocaine in drug discrimination procedures. A second factor that may limit the abuse liability of modafinil is its relatively slow onset compared to stimulants such as cocaine or amphetamine. In the present study, peak effects of cocaine and amphetamine were generally observed at 10 min, whereas peak effects of modafinil were not observed until 60-100 minutes after administration. Rate of onset appears to be one factor that influences the reinforcing effects of drugs, and slower onsets have been associated with lower reinforcing effects (Balster & Schuster, 1973; Winger, Hursh, Casey, & Woods, 2002). Finally, the low solubility and heat sensitivity of modafinil function as physical barriers to the use of modafinil by parenteral routes. Modafinil is insoluble in water at significant concentrations, and potentially noxious vehicles are necessary to produce solutions that can be delivered parenterally (Dopheide et al., 2007; Gold & Balster, 1996). Moreover, even with optimal vehicles, solubility is limited to relatively low concentrations that require high injection volumes.
Concordance Between Clinical and Non-human Primate Studies
Clinical studies evaluating modafinil as a candidate agonist-based pharmacotherapy have provided some encouraging results. Findings from clinical laboratory studies showed that modafinil attenuated the positive subject-rated effects of IV cocaine (Dackis et al., 2003; Malcolm et al., 2006). As noted earlier, another clinical laboratory study found that modafinil (200 and 400 mg) reduced smoked cocaine self-administration and positive subjective-effect ratings (Hart et al., 2008). Results of an 8-week open-label, outpatient clinical trial demonstrated that subjects receiving modafinil achieved three weeks or more of abstinence and provided significantly fewer positive urine samples, however, the treatment group had fewer cocaine positive urine samples at baseline (Dackis et al., 2005). Most recently, a multi-site outpatient clinical trial demonstrated that, over a 12-week period, modafinil produced a nonsignificant trend in reduced cocaine use relative to placebo treatment (Anderson et al., 2009). Importantly, when a subgroup of subjects with co-morbid alcohol dependence was removed from the analyses, a reduction of cocaine use in subjects receiving modafinil achieved statistical significance. Overall, the positive results of clinical studies are consistent with the effects of modafinil observed in the current study.
The major findings of the present study reaffirm the usefulness of preclinical drug self-administration procedures as models of human drug abuse. A recent review by Haney and Spealman (2008) outlined the corresponding outcomes between preclinical self-administration studies and clinical measures. The present findings support the conclusion that preclinical drug self-administration models can predict the effectiveness of pharmacotherapies for drug abuse treatment. For example, studies evaluating buprenorphine (Mello, Kamien et al., 1993; Mello, Mendelson et al., 1993), d-amphetamine (Negus & Mello, 2003; Negus et al., 2007), and now modafinil, provide considerable evidence of the concordance between the results of clinical treatment trials and preclinical findings (Mello, 2005).
ACKNOWLEDGEMENTS
This research was supported by R01-DA02519 (NKM) and R01-DA018151S1 (TEP) from the National Institute on Drug Abuse, NIH. The authors wish to thank Olga Smirnova, Nicholas Taft, Meredith Mahnke, John Podobinski and Peter Fivel for their expert technical assistance. Preliminary data were presented at the annual meeting of the College on Problems of Drug Dependence in 2009.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1-2):133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, van Haaren F. Cocaine discrimination and time-course effects in male and female Wistar rats. Eur J Pharmacol. 1999;382(2):69–74. doi: 10.1016/s0014-2999(99)00597-x. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20(1):119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86(1):117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 1996;42(1):27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70(1):29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berl) 2002;161(4):387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Donovan JL, DeVane CL, Malcolm RJ, Mojsiak J, Chiang CN, Elkashef A, et al. Modafinil influences the pharmacokinetics of intravenous cocaine in healthy cocaine-dependent volunteers. Clin Pharmacokinet. 2005;44(7):753–765. doi: 10.2165/00003088-200544070-00006. [DOI] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568(1-3):112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159(4):397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojinicki FHE, Matecka D, Bacher JD, Mansbach RS, Balster RL, et al. Effects of Dopamine Reuptake Inhibitors on Food- and Cocaine-Maintained Responding: I. Dependence on Unit Dose of Cocaine. Exp Clin Psychopharmacol. 1995;3(3):219–231. [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126(4):286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21(5):522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29(7):1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199(3):403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2009.11.023. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Annals of the New York Academy of Sciences. 2010 doi: 10.1111/j.1749-6632.2009.05145.x. (In Press) [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14(1):53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Kovacevic-Ristanovic R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23(3):149–156. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Keating GM, Raffin MJ. Modafinil : a review of its use in excessive sleepiness associated with obstructive sleep apnoea/hypopnoea syndrome and shift work sleep disorder. CNS Drugs. 2005;19(9):785–803. doi: 10.2165/00023210-200519090-00005. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319(2):561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Makris AP, Rush CR, Frederich RC, Taylor AC, Kelly TH. Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp Clin Psychopharmacol. 2007;15(2):123–133. doi: 10.1037/1064-1297.15.2.123. [DOI] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N, et al. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32(4):577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- Martinez-Raga J, Knecht C, Cepeda S. Modafinil: a useful medication for cocaine addiction? Review of the evidence from neuropharmacological, experimental and clinical studies. Curr Drug Abuse Rev. 2008;1(2):213–221. doi: 10.2174/1874473710801020213. [DOI] [PubMed] [Google Scholar]
- McElhiney MC, Rabkin JG, Rabkin R, Nunes EV. Provigil (modafinil) plus cognitive behavioral therapy for methamphetamine use in HIV+ gay men: a pilot study. Am J Drug Alcohol Abuse. 2009;35(1):34–37. doi: 10.1080/00952990802342907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, et al. Open-label pilot study of modafinil for methamphetamine dependence. J Clin Psychopharmacol. 2009;29(5):488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. Marian W. Fischman Memorial Lecture (2004). Evaluation of drug abuse treatment medications: Concordance between clinical and preclinical studies. In: Dewey WL, editor. Problems of Drug Dependence 2004: Proceedings of othe 66th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. U.S. Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2005. pp. 82–104. [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Mendelson JH, Drieze JM, Sholar JW. Effects of intermittent buprenorphine administration on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1993;264(2):530–541. [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Kamien JB, Mendelson JH, Cone EJ. The effects of chronic buprenorphine treatment on cocaine and food self-administration by rhesus monkeys. J. Pharmacol. Exper. Ther. 1992;260:1185–1193. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine treatment of cocaine and heroin abuse. In: Cowan A, Lewis JW, editors. Buprenorphine:Combating Drug Abuse With A Unique Opioid. Wiley- Liss, Inc.; New York: 1995. pp. 241–287. [Google Scholar]
- Mello NK, Mendelson JH, Lukas SE, Gastfriend DR, Teoh SK, Holman BL. Buprenorphine treatment of opiate and cocaine abuse: clinical and preclinical studies. Harv Rev Psychiatry. 1993;1(3):168–183. doi: 10.3109/10673229309017075. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J. Pharmacol. Exp. Ther. 1998;285(2):444–456. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33(4):783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Drug Therapy: Management of cocaine abuse and dependence. N. Eng. J. Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, et al. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21(2):294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17(5):436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance--a review of abuse liability issues. Ann Clin Psychiatry. 2004;16(2):101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009;329(1):272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Mello NK. Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1999;291(1):60–69. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70(1):39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Kimmel HL, Howell LL, Carroll FI. Effects of the monoamine uptake inhibitors RTI-112 and RTI-113 on cocaine- and food-maintained responding in rhesus monkeys. Pharmacol Biochem Behav. 2009;91(3):333–338. doi: 10.1016/j.pbb.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12(2):133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, Podobinski J, Tidgewell K, Luo M, Swenson D. Synthesis and determination of the absolute configuration of the enantiomers of modafinil. Tetrahedron: Asymmetry. 2004;15(6):1053–1058. [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13(2):105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67(3):311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- Schwartz JR. Modafinil in the treatment of excessive sleepiness. Drug Des Devel Ther. 2009;2:71–85. doi: 10.2147/dddt.s2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98(8):1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Petrakis I, Kosten TR, Oliveto A. Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am J Drug Alcohol Abuse. 2000;26(4):643–657. doi: 10.1081/ada-100101900. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Stolerman I. Drug discrimination. In: Van Haaren F, editor. Techniques in the behavioral and neural sciences: Methods in behavioral pharmacology. Elsevier; Amsterdam: 1993. pp. 217–243. [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182(1):186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270(3):1041–1048. [PubMed] [Google Scholar]
- Vansickel AR, Fillmorex MT, Hays LR, Rush CR. Effects of potential agonist-replacement therapies for stimulant dependence on inhibitory control in cocaine abusers. Am J Drug Alcohol Abuse. 2008;34(3):293–305. doi: 10.1080/00952990802013565. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162(8):1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18(3):265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. Jama. 2009;301(11):1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010;106(2-3):233–236. doi: 10.1016/j.drugalcdep.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301(2):690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329(2):738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]