Abstract
Purpose
Dietary carotenoids lutein and zeaxanthin may play a protective role against visual loss from age-related macular degeneration (AMD) through antioxidant and light screening mechanisms. We used a novel noninvasive objective method to quantify lutein and zeaxanthin in the human macula using resonance Raman spectroscopy and compared macular pigment levels in AMD and normal subjects.
Design
Observational study of an ophthalmology clinic-based population.
Participants and Controls
Ninety-three AMD eyes from 63 patients and 220 normal eyes from 138 subjects.
Methods
Macular carotenoid levels were quantified by illuminating the macula with a low-power argon laser spot and measuring Raman backscattered light using a spectrograph. This technique is sensitive, specific, and repeatable even in subjects with significant macular pathologic features.
Main Outcome Measure
Raman signal intensity at 1525 cm−1 generated by the carbon–carbon double-bond vibrations of lutein and zeaxanthin.
Results
Carotenoid Raman signal intensity declined with age in normal eyes (P < 0.001). Average levels of lutein and zeaxanthin were 32% lower in AMD eyes versus normal elderly control eyes as long as the subjects were not consuming high-dose lutein supplements (P = 0.001). Patients who had begun to consume supplements containing high doses of lutein (≥4 mg/day) regularly after their initial diagnosis of AMD had average macular pigment levels that were in the normal range (P = 0.829) and that were significantly higher than in AMD patients not consuming these supplements (P = 0.038).
Conclusions
These findings are consistent with the hypothesis that low levels of lutein and zeaxanthin in the human macula may represent a pathogenic risk factor for the development of AMD. Resonance Raman measurement of macular carotenoid pigments could play an important role in facilitating large-scale prospective clinical studies of lutein and zeaxanthin protection against AMD, and this technology may someday prove useful in the early detection of individuals at risk for visual loss from AMD.
Age-related macular degeneration (AMD) is a devastating disease of the elderly that is the leading cause of irreversible blindness in the developed world. Because current treatments for both the wet and the dry forms are of limited efficacy, there is keen interest among clinicians and patients to identify modifiable risk factors so that interventions can be instituted at an early stage before significant irreversible damage has occurred. Among the dietary factors that may protect against AMD, the carotenoids lutein and zeaxanthin have received considerable attention in recent years.1–3 These compounds, derived from dietary intake of dark green leafy vegetables and orange or yellow fruits and vegetables, are specifically concentrated in the Henle fiber layer of the macula of the human eye,4,5 where they may act to protect against light-induced oxidative damage through their ability to absorb phototoxic blue light and to quench free radicals.6,7 Dietary supplementation with foods or supplements rich in lutein or zeaxanthin can raise macular pigment levels,8 –10 and nutritional supplements containing lutein are promoted actively by the health food and vitamin industry in the United States to individuals at risk for AMD. Zeaxanthin supplements, however, were not commercially available in the United States until recently except as a minor component of lutein prepared from marigold flowers, and it has been suggested that dietary lutein may serve as a precursor for the very high concentrations of zeaxanthin found in the primate fovea.11
A major epidemiologic study found that high dietary intakes and blood levels of these xanthophyll carotenoids are correlated with a significantly lower risk of AMD,12,13 but another study did not reach the same conclusion.14 These inconsistent findings derive in part from the fact that blood levels and dietary intakes of lutein and zeaxanthin are relatively poor markers of the actual amounts present in the macula.15 Clearly, it is of utmost importance to know the levels of lutein and zeaxanthin at their relevant site of action, the human macula. Recently, an autopsy study has reported that eyes from donors with a history of AMD had lower levels of macular carotenoids than eyes without a known history of AMD,16 but such studies relying on post mortem analysis and retrospective reviews of clinical records after death have substantial limitations.
Accurate noninvasive quantification of macular lutein and zeaxanthin in the elderly would be an enormously useful tool to facilitate the further study of the role of lutein and zeaxanthin in the prevention of AMD in cohort, cross-sectional, case-control, and prospective studies. Until now, the most commonly used test to measure macular pigment levels has been heterochromatic flicker photometry,9,17–19 a subjective psychophysical test requiring an attentive subject with good visual acuity. This type of test would be expected to yield unreliable results in the major population of interest, elderly patients with significant macular pathologic features.20 As an alternative to this subjective test, researchers have examined other objective methods to measure macular pigment, such as fundus reflectometry,21 lipofuscin autofluorescence attenuation,22 and multiwavelength scanning laser ophthalmoscopy,23 with variable success. We recently developed a novel objective in vivo carotenoid measurement system that uses resonance Raman spectroscopy.24–26 This test is rapid, sensitive, specific, and highly repeatable in patients with a wide variety of macular pathologic features as long as they retain central fixation (20/80 vision or better).27 This test has been validated against actual carotenoid levels measured by high-performance liquid chromatography in human donor eyes24 and in living monkey eyes.27 Here we report the first use of this technology in a large population of elderly individuals with and without AMD, and we present findings that support the hypothesis that low macular levels of lutein and zeaxanthin may represent a pathogenic risk factor for AMD.
Materials and Methods
The design, construction, and validation of the resonance Raman detector for macular pigment measurement in the living human eye have been described in detail elsewhere.24–27 A schematic diagram is shown in Figure 1. The subject aligns his or her gaze on a blue–green argon laser aiming beam superimposed on a fiberoptic collection bundle illuminated with a red light-emitting diode. The subject’s macula is then illuminated for 0.5 second with a 1-mm spot of 488-nm, 0.5-mW argon laser light. Backscattered light is routed via the fiberoptic collection bundle to a Raman spectrograph coupled with a Peltier-cooled charge-coupled device camera. The peak height at the carotenoid C = C stretch frequency of 1525 cm−1 is quantified after digital subtraction of background ocular fluorescence. Because subjects occasionally misalign or blink, the mean ±standard deviation of the best three of five measurements was used for data analysis. A two-way analysis of variance (ANOVA), chi-square test, two-sided unpaired t tests, and Fisher exact tests were performed using Sigma-Stat software (Jandel Scientific Software, San Rafael, CA). Unless otherwise indicated, statistical tests were based on individual eyes.
Figure 1.
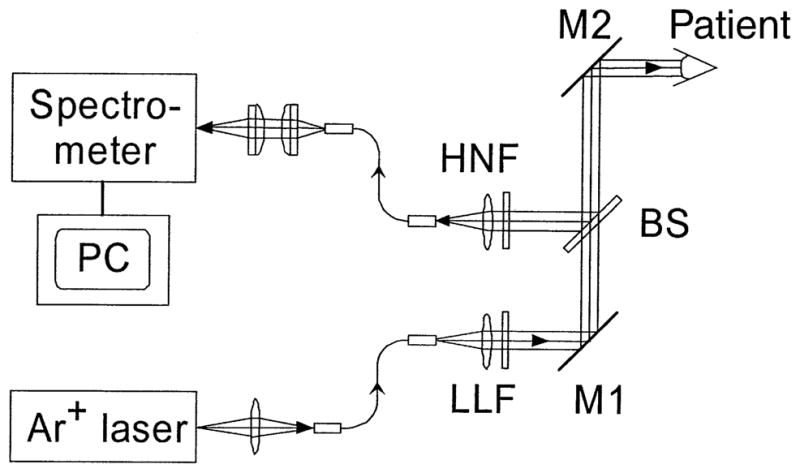
Schematic diagram of resonance Raman device to measure macular carotenoid levels in the living human eye. After pharmacologic dilation to at least 6 mm, the patient fixates on an aiming beam superimposed on a collection fiber array illuminated with a red light-emitting diode. Then, 0.5-mW 488-nm laser light from a low-power argon laser is routed through the multimode fiber, laser line filter (LLF), and mirrors (M1 and M2) into the human eye and is projected onto the foveal area as a 1-mm spot for 0.5 second. Raman shifted backscattered light is directed to the spectrometer through M2, beam splitter (BS), a holographic notch filter (HNF), and a fiberoptic bundle. The spectrometer is interfaced to a cooled charge-coupled device camera that is controlled by a Windows-based (Microsoft, Redmond, WA) custom software package.
Subjects were recruited from the clinic population of the Moran Eye Center of the University of Utah School of Medicine from October 2000 through May 2001. This study was approved by the University of Utah Institutional Review Board, and all subjects signed institutionally approved informed consent forms that complied with the tenets of the Declaration of Helsinki. The presence or absence of AMD was confirmed by a dilated retina examination according to a previously published grading scheme (choroidal neovascularization, pigment epithelial detachments, geographic atrophy, or extensive drusen with or without melanin pigment clumps).28 Patients with visually significant cataracts, other macular or retinal pathologic features (i.e., diabetic maculopathy, vascular occlusions, retinitis pigmentosa, etc.), inadequate pupil dilation (<6 mm), or poor visual acuity (worse than 20/80 best-corrected vision) were excluded. Goldmann applanation tonometry was contraindicated because topical fluorescein interferes with measurements for many hours after instillation in the eye. Likewise, fluorescein angiography was performed when necessary after completion of the Raman measurements.
Results
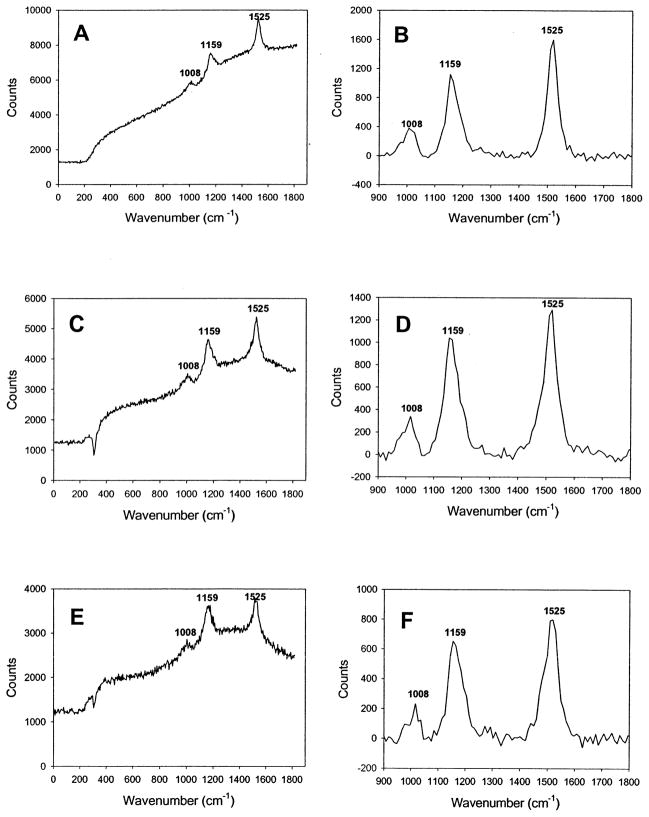
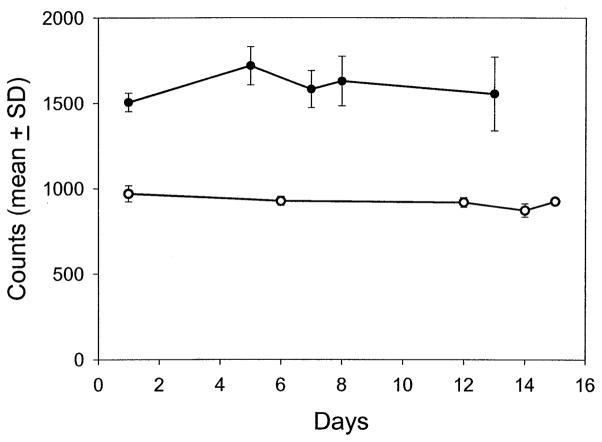
We initially measured carotenoid levels by resonance Raman spectroscopy in the central 1 mm of the macula of 220 eyes of 138 volunteers free of cataracts, AMD, and other ocular pathologic features. A typical subject’s resonance Raman spectrum is shown in Figure 2 before and after subtraction of background ocular fluorescence along with resonance Raman spectra of pure zeaxanthin and lutein dissolved in tetrahydrofuran. These spectra demonstrate that in vivo resonance Raman spectrum from the human macula is indistinguishable at the instrument’s resolution limit of 4 cm−1 from the spectra of either of the pure carotenoids after fluorescence subtraction is performed. Indeed, most commonly encountered carotenoids, such as β-carotene, canthaxanthin, and astaxanthin, have nearly identical resonance Raman spectra.29 Even without fluorescence subtraction, the spectra shown in Figure 2 are remarkably similar, indicating that much of the background fluorescence seen during in vivo measurements originates from the intrinsic low-level fluorescence of carotenoids themselves. Measurements with this system are highly repeatable. Two subjects had repeated measurement sessions five times over a 2-week period, and intrasession and intersession variabilities were generally less than ± 10% (Fig 3).
Figure 2.
Typical resonance Raman spectra collected by the instrument shown in Figure 1. The top two panels are spectra collected from the dilated eye of a 26-year-old male (A) before and (B) after background subtraction. The middle two panels are spectra collected from a solution of zeaxanthin (Hoffmann-La Roche, Basel, Switzerland) dissolved in tetrahydrofuran (THF) (C) before and (D) after background subtraction. The bottom two panels are spectra collected from a solution of lutein (Kemin Foods, Des Moines, IA) dissolved in THF (E) before and (F) after background subtraction. The lutein and zeaxanthin solutions were measured through the use of a “model eye” in which the solutions were placed in a 1-mm path-length quartz cuvette at the focal point of a 53-diopter lens generating a 1-mm laser spot size identical to the laser spot projected onto the macula in vivo. The number listed by each spectral peak denotes the wavenumber in cm−1 for the local maximum.
Figure 3.
Repeatability of ocular resonance Raman measurements. Two individuals had five repeated sessions of resonance Raman measurement of macular carotenoids over a 2-week period through the fully dilated pupil of the same eye in each patient. The mean ± standard deviation (SD) of peak height at 1525 cm−1 is given for the best three of five readings. The filled circles were obtained from a 26-year-old white male, and the open circles were obtained from a 37-year-old Asian male.
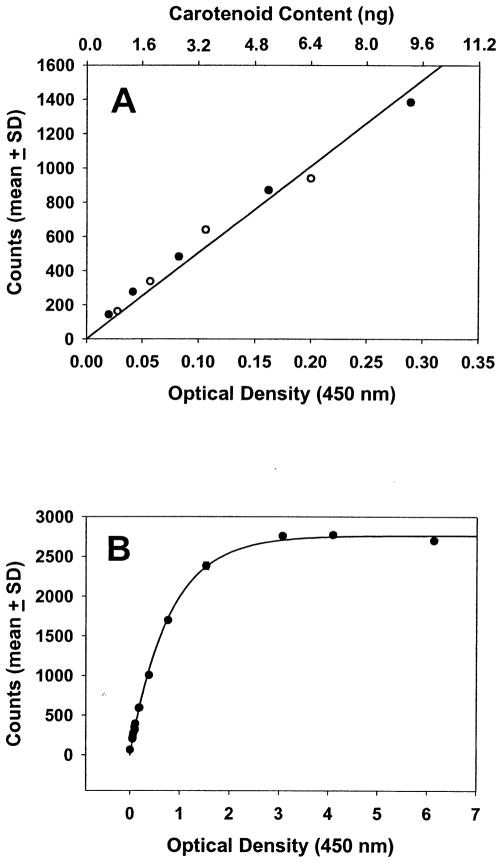
External calibration curves of the device versus standard solutions of lutein and zeaxanthin are shown in Figure 4. The instrument’s response to increasing carotenoid levels is linear at low optical density ranges likely to be encountered physiologically in the human macula, and lutein and zeaxanthin track along the same best-fit line (R = 1.0 for both compounds; Fig 4A). As expected, at extremely high concentrations of carotenoid, the Raman response becomes nonlinear because of the increasing inability of the 488-nm laser beam to penetrate optically denser solutions (Fig 4B). Macular carotenoid pigment readings obtained with the Raman method are not comparable directly with the readings obtained with psychophysical methods because the Raman technique measures total carotenoids in the entire region illuminated by the 1-mm spot, whereas psychophysics measures optical density at the edge of the illumination spot.17 Because the ocular Raman spectroscopic technique is based on a 180° backscattering collection geometry, it is not affected by reflections originating from outer retinal layers or by variations in fundus melanin pigmentation of the retinal pigment epithelium or choroid to any significant extent.27
Figure 4.
Calibration curves of the resonance Raman instrument. Optical density at 450 nm was measured on tetrahydrofuran solutions of lutein and zeaxanthin by an ultraviolet-visible spectrophotometer (Hitachi Instruments, San Jose, CA) in a 1-mm path length cuvette after appropriate dilution when necessary, and then a resonance Raman measurement was made using the model eye described in Figure 2. The mean ± standard deviation (SD) for five Raman signal intensity measurements at 1525 cm−1 at each concentration are shown. Note that the repeatability of measurement of this model system is so high that the error bars are not even visible. The top axis of the upper panel is the calculated amount of carotenoid in the illuminated volume (a cylinder 1 mm in diameter and 1 mm in height) using published extinction coefficient values for lutein and zeaxanthin.41 Filled circles are for lutein, and open circles are for zeaxanthin. A, The calibration curve is shown in the linear response range of the detector (R = 1.0 for both lutein and zeaxanthin along the best-fit line). B, Saturation of detector response is observed at very high carotenoid concentrations.
The demographic characteristics of the normal population are shown in Table 1. None of these normal subjects consumed lutein supplements in excess of 0.275 mg/day, a level much lower than the usual daily dietary intake of lutein and zeaxanthin by the American population (1–3 mg/day).3,13 Increasing subject age is associated significantly with declining carotenoid Raman intensity (P < 0.001, one-way ANOVA), and the population values are best fit with an exponential curve (R = 0.664; Fig 5). Only a small number of young subjects had macular pigment readings beyond the detector’s linear response range (>1500 counts). After subjects reached age 60 years, there was no further significant decline with age (P < 0.001 for ≤60 years and P = 0.921 for >60 years, one-way ANOVA tests). Elderly subjects more than 60 years of age with prosthetic intraocular lenses after cataract surgery had macular pigment levels that were not significantly different from age-matched patients who still had natural crystalline lenses (P = 0.059, two-sided t test). Subgroup analysis by two-way age-adjusted ANOVA did not show any statistically significant influence of gender (P = 0.827), eye color (P = 0.647), ethnicity (P = 0.510), or current smoker status (P = 0.276). It should be noted, however, that the number of current smokers and nonwhite persons in this study (<8% in all groups) was extremely low and therefore unlikely to have sufficient power to reach statistical significance.
Table 1.
Clinical Characteristics of Normal Patients
| No. Eyes (No. Patients) | |
|---|---|
| Age (yrs; mean ± SD) | 51 ± 17 |
| Age range (yrs) | 21–84 |
| No. males | 114 (71) |
| No. females | 106 (67) |
| No. white persons | 205 (128) |
| No. nonwhite persons | 15 (10) |
| Current smokers | 17 (12) |
| Past or nonsmokers | 203 (126) |
| Phakic eyes | 175 (112) |
| Pseudophakic eyes | 45 (29) |
| Eye color (blue, hazel/green, brown) | 97, 59, 64 (61, 36, 41) |
| Dilated pupil size (mean ± SD) | 8.0 ± 1.0 mm |
| Median visual acuity | 20/20 |
| Visual acuity range | 20/15–20/50 |
SD = standard deviation.
A few patients had unilateral pseudophakia and had both eyes enrolled, so the patient total adds to greater than 138.
Figure 5.
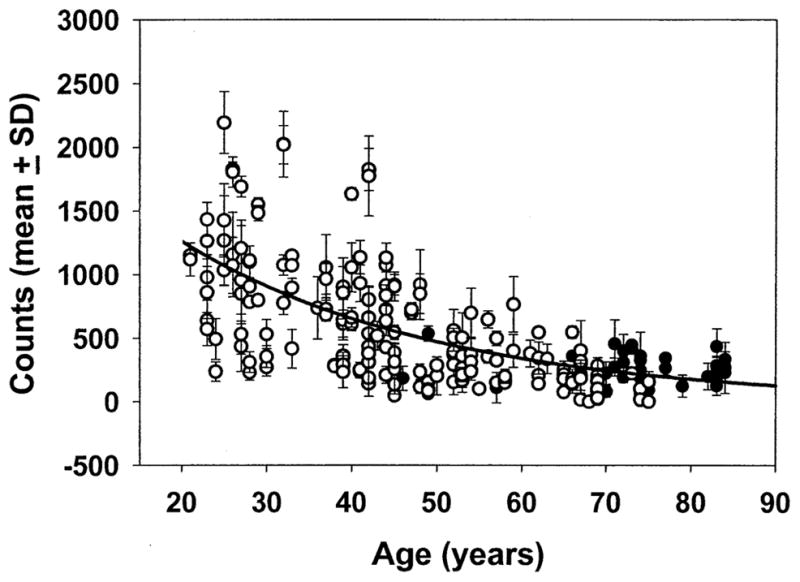
Resonance Raman measurement of macular carotenoid levels in normal eyes at 1525 cm−1. Two hundred twenty eyes from 138 normal subjects were measured by the ocular resonance Raman instrument using the protocol described in Methods. The best-fit curve is an exponential fit with R = 0.664. Open circles are phakic eyes, and filled circles are pseudophakic eyes. The mean ± standard deviation (SD) for the best three of five readings is shown.
We compared our normal population more than 60 years of age with a group of patients with wet and dry AMD of the same age who had visual acuity of 20/80 or better in the affected eye(s) (Table 2). Although usual dietary intakes of carotenoids were not assessed, detailed nutritional supplement histories were collected, and AMD subjects were divided into a group that regularly consumed supplements containing 4 mg/day or more of lutein for at least 2 months and a group that did not (Fig 6). The subjects consuming high-dose lutein supplements uniformly stated that they did not begin taking these supplements before their initial diagnosis of AMD but generally were unable to state exactly how long they had been taking any particular supplement. Many elderly subjects (7 of 52 normal subjects [13%] and 20 of 63 AMD patients [32%]) reported that they consumed supplements containing low doses of lutein (0.250– 0.275 mg/day), because this amount is so much lower than the usual American dietary intake of lutein and zeaxanthin (1–3 mg/day),3,13 they were included in the no lutein supplement groups for all data analyses. No subjects consumed lutein supplements containing between 0.275 mg and 4 mg of lutein daily because no such supplements were marketed in the United States at the time of this study.
Table 2.
Clinical Characteristics of Elderly Normal and Age-related Macular Degeneration Patients
| Normal | Age-related Macular Degeneration using High-dose (≥4 mg/day) Lutein Supplements | Age-related Macular Degeneration Using Low-dose (≤0.275 mg/day) or No Lutein Supplements | |
|---|---|---|---|
| Age (yrs; mean ± SD) | 71 ± 6 | 75 ± 8 | 76 ± 5 |
| Age range (yrs) | 61–84 | 63–91 | 65–88 |
| No. males | 38 (25) | 11 (6) | 33 (22) |
| No. females | 35 (27) | 14 (9) | 35 (26) |
| No. white persons | 68 (48) | 25 (15) | 67 (47) |
| No. Nonwhite persons | 5 (4) | 0 (0) | 1 (1) |
| Current smokers | 3 (3) | 1 (1) | 5 (3) |
| Past or nonsmokers | 70 (49) | 24 (14) | 63 (45) |
| Phakic eyes | 36 (28) | 10 (7) | 38 (22) |
| Pseudophakic eyes | 37 (24) | 15 (9) | 30 (27) |
| Eye color (blue, hazel/green, brown) | 33, 20, 20 (23, 14, 15) | 14, 6, 5 (8, 4, 3) | 35, 19, 14 (24, 13, 11) |
| Dilated pupil size (mean ± SD) | 8.0 ± 1.0 mm | 8.0 ± 1.0 mm | 8.0 ± 1.0 mm |
| Median visual acuity | 20/25 | 20/30 | 20/30 |
| Visual acuity range | 20/15–20/40 | 20/20–20/70 | 20/20–20/80 |
| Exudative AMD | 0 (0) | 2 (2) | 6 (4) |
| Nonexudative AMD | 0 (0) | 23 (15) | 62 (46) |
AMD = age-related macular degeneration; SD = standard deviation.
Data are expressed as number of eyes (number of patients).
A few patients had unilateral pseudophakia and had both eyes enrolled, so the patient totals may add to greater than the actual number enrolled.
Some patients had unilateral exudative AMD and had both eyes enrolled, so patient totals add to greater than the actual number enrolled.
Figure 6.
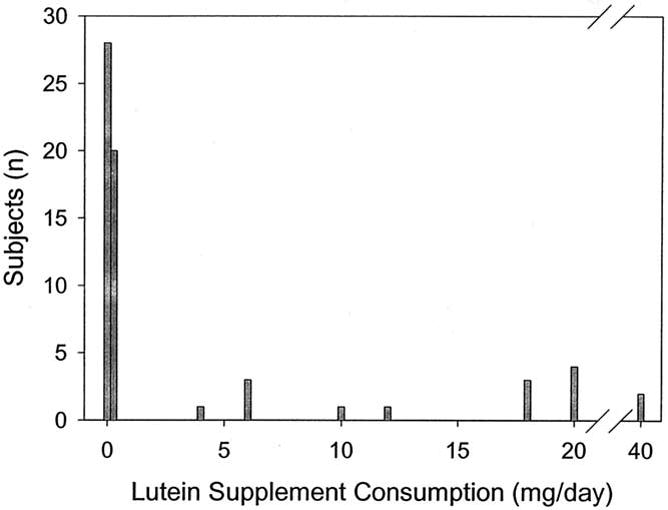
Daily lutein supplement consumption reported by age-related macular degeneration patients enrolled in this study.
The three groups were not imbalanced with respect to gender (P = 0.847, 2 × 3 chi-square test), eye color (P = 0.816, 3 × 3 chi-square test), current smoker status (P = 0.626–1.00, multiple Fisher exact tests), ethnicity (P = 0.326–1.00, multiple Fisher exact tests), exudative and nonexudative AMD (P = 1.00, Fisher’s exact test between AMD groups only), or presence of a prosthetic intraocular lens (P = 0.469, 2 × 3 chi-square test). Average dilated pupil sizes were identical in all groups, and median visual acuities were matched closely. A total of six subjects had one eye in the normal group and one eye in an AMD group. The average age of the elderly normal group was 4 to 5 years younger than that of the AMD groups (P = 0.001, two-sided t tests), but this difference would not be expected to be a confounding factor because average macular pigment levels do not decline with age after age 60 years (Fig 5).
As shown in Table 3, AMD patients not taking high-dose lutein supplements had macular carotenoid levels 32% lower than normal elderly eyes (P = 0.001, two-sided t test). The group of AMD patients regularly consuming high-dose lutein supplements had values that were significantly higher than their cohorts not using high-dose supplements (P = 0.038, two-sided t test) and that were indistinguishable from normal subjects (P = 0.829, two-sided t test). If the data are reanalyzed raising the minimum age of enrollment from 61 to 70 years, the normal control group and the no lutein supplement AMD group have improved age matching (76 ± 5 years vs. 77 ± 5 years, respectively; P = 0.377, two sided t test), and the difference in macular pigment levels remains highly significant (217 ± 139 counts for 38 eyes vs. 149 ± 148 counts for 62 eyes, respectively; P = 0.018, two-sided t test).
Table 3.
Resonance Raman Measurement of Macular Carotenoids in Elderly Normal and Age-related Macular Degeneration Patients
| No. Eyes (No. Patients) | Resonance Raman Carotenoid Signal Intensity at 1525 cm−1 (Mean Counts ± Standard Deviation) | |
|---|---|---|
| Normal | 73 (52) | 219 ± 134 |
| AMD using high-dose (≥4 mg/day) lutein supplements | 25 (15) | 212 ± 169 |
| AMD using low-dose (≤0.275 mg/day) or no lutein supplements | 68 (48) | 148 ± 147 |
AMD = age-related macular degeneration.
Discussion
When excited with monochromatic laser light, macular carotenoids exhibit characteristic wavelength shifts of backscattered light that are generated by vibrational modes in their chemical bonds, a phenomenon known as Raman scattering.30 Previous validation studies from our laboratory have shown that Raman scattering is a highly sensitive method to measure macular pigments because carotenoids exhibit a large resonance enhancement of their Raman signal when 488-nm laser excitation is used, a wavelength that overlaps with the visible absorption bands of these highly conjugated compounds.24 Specificity is easily confirmed because the characteristic carotenoid Raman spectral peaks at 1008, 1159, and 1525 cm−1 are discernible readily on in vivo spectra even without background subtraction of ocular fluorescence.24 Peak height at 1525 cm−1 scales linearly with carotenoid content in donor maculae,24 living monkey eyes,27 and model systems.27 The test is performed readily by patients, even in those with significant macular pathologic features, with high repeatability as long as they have central fixation, typically a visual acuity of 20/80 or better. The test is rapid (0.5 seconds), and light exposure levels to the macula are well within established laser safety standards.27,31,32
Our large sample of normal adult subjects demonstrates a wide variation in macular pigment levels in younger adults and a substantial drop in average carotenoid Raman levels with advancing age. The decline in macular pigment levels with age correlates well with some,19,33 but not all,18,22 recent psychophysical reports of macular pigment levels in normal populations. Some of this drop of Raman signal with age may be the result of increasing yellowing of the lens as subjects age,34–36 but this effect could account for only half of the more than 75% drop in average Raman signal seen between ages 20 and 60 years (see Ref. 27 for a detailed discussion of this issue). The minimal interference from natural lens yellowing is demonstrated clearly by the fact that elderly subjects without visually significant cataracts have Raman signals in the same range as elderly subjects with implanted prosthetic intraocular lenses that are designed for high clarity in the visible range, and even after cataract surgery, their measured readings never approach the average level for a 20- to 30-year-old (Fig 5). The physiologic basis for this decline with age remains to be elucidated. It may result from age-related changes in diet or in intestinal absorption of carotenoids, or there may be an age-related loss of specific xanthophyll-binding proteins37 in the macula.
Our finding that macular pigment levels in the eyes of AMD patients not consuming high-dose lutein supplements are 32% lower than elderly control eyes is striking and highly significant. Moreover, AMD patients who have begun taking high-dose lutein supplements after initial diagnosis of AMD have normal levels of macular carotenoid pigment. These results taken together are very supportive of a model in which low macular levels of lutein and zeaxanthin represent a pathogenic risk factor for development of AMD,1–3,6,7,19 presumably through decreased blue light screening or inadequate free radical quenching capacity. The apparent response to dietary supplementation implies that the carotenoid binding sites37 in the macula are not saturated with lutein and zeaxanthin in many AMD patients consuming an unsupplemented diet.
Because this was a clinic-based study of patients seeking routine eye examinations, we were unable to perform the extensive surveys required to assess usual dietary intake of lutein and zeaxanthin. Thus, we cannot determine whether the lower macular pigment levels in AMD patients are the result of diet or of other factors. Prospective lutein and zeaxanthin supplementation studies incorporating resonance Raman measurement of macular carotenoid pigment levels and comprehensive dietary surveys are in progress at our institution on young and elderly populations.
Should AMD be considered a manifestation of an ocular deficiency of lutein, zeaxanthin, or both, and should supplements containing these carotenoids be prescribed to normal and AMD individuals with low macular pigment levels to slow the progression of the disorder? The answer to these questions likely will require very large long-term prospective lutein and zeaxanthin interventional trials similar in scope to the recently completed Age-Related Eye Disease Study, which demonstrated a protective effect of high-dose supplementation with zinc, vitamin E, vitamin C, and β-carotene in subjects at high risk for progression to advanced AMD.38 The Age-Related Eye Disease Study was able to show for the first time that AMD is likely to be in part a disease related to oxidative stress and that antioxidant nutritional supplements could alter its course. The Age-Related Eye Disease Study did not include lutein or zeaxanthin in the study formulations because they were not readily available in supplement form at the time the study was initiated. However, there is general agreement that these two carotenoids eventually will need to be examined in a similar manner as an intervention against AMD,38,39 especially because β-carotene is essentially undetectable in the human macula, whereas lutein and zeaxanthin and their oxidation products are abundant.40
Resonance Raman spectroscopic measurement of macular pigment levels will be able to provide the objective quantitative information essential for the conduct of well-designed and well-executed clinical trials that should lead to rational, scientifically supported nutritional recommendations for those at risk for visual loss from AMD. With proper validation, this test may someday prove useful as an early diagnostic test to identify at a young age individuals with unusually low levels of macular pigment who may be at increased risk for development of AMD later in life. Dietary or nutritional supplement interventions then could be instituted, and macular carotenoid response to these interventions could be monitored with this instrument.
Acknowledgments
The authors thank Maia Ermakova, Stephen Alder, and Ninel Gregori for assistance in software development, statistical analysis, and patient measurements. Kemin Foods (Des Moines, IA) and Hoffmann-La Roche (Basel, Switzerland) generously provided, respectively, the lutein and zeaxanthin used to calibrate the instrument.
Supported by the National Eye Institute, Bethesda, Maryland (grant nos.: EY-11600 and EY-12324); Research to Prevent Blindness, Inc., New York, New York; and Spectrotek, LC, Salt Lake City, Utah. Dr. Bernstein is a Research to Prevent Blindness Sybil B. Harrington Scholar in macular degeneration research.
Footnotes
Three authors (PSB, RWM, and WG) and the University of Utah hold patent rights to the ocular Raman technology described in this article, and these authors and the university own significant equity interests in Spectrotek, LC, a company that has licensed the technology.
Presented in part as a poster at the annual meeting of the American Academy of Ophthalmology, New Orleans, Louisiana, November 2001.
References
- 1.Schalch W, Dayhaw-Barker P, Barker FM., II . The carotenoids of the human retina. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Boca Raton, FL: CRC Press; 1999. pp. 215–50. [Google Scholar]
- 2.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62(Suppl):1448S–61S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 3.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 4.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:660–73. [PubMed] [Google Scholar]
- 5.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–9. [PubMed] [Google Scholar]
- 6.Bernstein PS, Katz NB. The role of ocular free radicals in age-related macular degeneration. In: Fuchs J, Packer L, editors. Environmental Stressors in Health and Disease. New York: Marcel Dekker; 2001. pp. 423–56. [Google Scholar]
- 7.Beatty S, Koh HH, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 8.Hammond BR, Jr, Johnson EJ, Russell RM, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci. 1997;38:1795–801. [PubMed] [Google Scholar]
- 9.Landrum JT, Bone RA, Joa H, et al. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62. doi: 10.1006/exer.1997.0309. [DOI] [PubMed] [Google Scholar]
- 10.Berendschot TTJM, Goldbohm RA, Klöpping WAA, et al. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci. 2000;41:3322–6. [PubMed] [Google Scholar]
- 11.Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res. 1997;64:211–8. doi: 10.1006/exer.1996.0210. [DOI] [PubMed] [Google Scholar]
- 12.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–9. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 14.Mares-Perlman JA, Brady WE, Klein R, et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol. 1995;113:1518–23. doi: 10.1001/archopht.1995.01100120048007. [DOI] [PubMed] [Google Scholar]
- 15.Bone RA, Landrum JT, Dixon Z, et al. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res. 2000;71:239–45. doi: 10.1006/exer.2000.0870. [DOI] [PubMed] [Google Scholar]
- 16.Bone RA, Landrum JT, Mayne ST, et al. Macular pigment in donor eyes with and without AMD: a case-control study [published erratum appears in Invest Ophthalmol Vis Sci 2001;42:548] Invest Ophthalmol Vis Sci. 2001;42:235–40. [PubMed] [Google Scholar]
- 17.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A. 1997;14:1187–96. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- 18.Werner JS, Bieber ML, Schefrin BE. Senescence of foveal and parafoveal cone sensitivities and their relations to macular pigment density. J Opt Soc Am A. 2000;17:1918–32. doi: 10.1364/josaa.17.001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty S, Murray IJ, Henson DB, et al. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Invest Ophthalmol Vis Sci. 2001;42:439–46. [PubMed] [Google Scholar]
- 20.Snodderly DM, Hammond BR., Jr . In vivo psychophysical assessment of nutritional and environmental influences on human ocular tissues: lens and macular pigment. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Boca Raton, FL: CRC Press; 1999. pp. 251–73. [Google Scholar]
- 21.Kilbride PE, Alexander KR, Fishman M, Fishman GA. Human macular pigment assessed by imaging fundus reflectometry. Vision Res. 1989;29:663–74. doi: 10.1016/0042-6989(89)90028-x. [DOI] [PubMed] [Google Scholar]
- 22.Delori FC, Goger DG, Hammond BR, et al. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1212–30. doi: 10.1364/josaa.18.001212. [DOI] [PubMed] [Google Scholar]
- 23.Elsner AE, Burns SA, Beausencourt E, Weiter JJ. Foveal cone photopigment distribution: small alterations associated with macular pigment distribution. Invest Ophthalmol Vis Sci. 1998;39:2394–404. [PubMed] [Google Scholar]
- 24.Bernstein PS, Yoshida MD, Katz NB, et al. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci. 1998;39:2003–11. [PubMed] [Google Scholar]
- 25.Ermakov IV, McClane RW, Gellermann W, Bernstein PS. Resonant Raman detection of macular pigment levels in the living human retina. Opt Lett. 2001;26:202–4. doi: 10.1364/ol.26.000202. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein PS, Gellermann W, McClane RW, inventors. University of Utah Technology Transfer Office, assignee. Method and system for measurement of macular carotenoid levels. 5,873,831. United States patent. 1999 Feb 23;
- 27.Gellermann W, Ermakov IV, Ermakova MR, et al. In vivo resonant Raman measurement of macular carotenoid pigments in the young and the aging human retina. J Opt Soc Am A Opt Image Sci Vis. 2002;19:1172–86. doi: 10.1364/josaa.19.001172. [DOI] [PubMed] [Google Scholar]
- 28.De La Paz M, Pericak-Vance MA, Haines JL, Seddon JM. Phenotypic heterogeneity in families with age-related macular degeneration. Am J Ophthalmol. 1997;124:331–43. doi: 10.1016/s0002-9394(14)70825-6. [DOI] [PubMed] [Google Scholar]
- 29.Gellermann W, Yoshida MD, McClane RW, et al. Raman detection of pigments in the human retina. In: Alfano RR, editor. Optical Biopsy II; Proc SPIE Ser; Bellingham, WA: SPIE; 1998. pp. 8–17.pp. 3250 [Google Scholar]
- 30.Koyama Y, Takatsuka I, Nakata M, Tasumi M. Raman and infrared spectra of the all-trans, 7-cis, 9-cis, 13-cis, and 15-cis isomers of β-carotene: key bands distinguishing stretched or terminal-bent configurations from central-bent configurations. J Raman Spectrosc. 1988;19:37–49. [Google Scholar]
- 31.Delori FC, Parker JS, Mainster MA. Light levels in fundus photography and fluorescein angiography. Vision Res. 1980;20:1099–104. doi: 10.1016/0042-6989(80)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.American National Standards Institute. American National Standards for Safe Use of Lasers. Vol. 8.3. Orlando, FL: Laser Institute of America; 2000. ANSI Z136.1-2000. [Google Scholar]
- 33.Hammond BR, Jr, Caruso-Avery M. Macular pigment optical density in a southwestern sample. Invest Ophthalmol Vis Sci. 2000;41:1492–7. [PubMed] [Google Scholar]
- 34.Gaillard ER, Zheng L, Merriam JC, Dillon J. Age-related changes in the absorption characteristics of the primate lens. Invest Ophthalmol Vis Sci. 2000;41:1454–9. [PubMed] [Google Scholar]
- 35.Weale RA. Age and the transmittance of the human crystalline lens. J Physiol. 1988;395:577–87. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–40. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 37.Yemelyanov AY, Katz NB, Bernstein PS. Ligand-binding characterization of xanthophyll carotenoids to solubilized membrane proteins derived from human retina. Exp Eye Res. 2001;72:381–92. doi: 10.1006/exer.2000.0965. [DOI] [PubMed] [Google Scholar]
- 38.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jampol LM. Antioxidants, zinc, and age-related macular degeneration: results and recommendations [editorial] Arch Ophthalmol. 2001;119:1533–4. doi: 10.1001/archopht.119.10.1533. [DOI] [PubMed] [Google Scholar]
- 40.Bernstein PS, Khachik F, Carvalho LS, et al. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–23. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 41.Britton G. UV/visible spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids: Vol. 1B: Spectroscopy. Boston: Birkhäuser Verlag; 1995. pp. 13–62. [Google Scholar]