Abstract
Natural killer (NK) cells are found in lymphoid and non-lymphoid organs. In addition to important roles in immune surveillance, some NK cells contribute to angiogenesis and circulatory regulation. The uterus of early pregnancy is a non-lymphoid organ enriched in NK cells that are specifically recruited to placental attachment sites. In species with invasive hemochorial placentation, these uterine natural killer (uNK) cells, via secretion of cytokines, chemokines, mucins, enzymes and angiogenic growth factors, contribute to the physiological change of mesometrial endometrium into the unique stromal environment called decidua basalis. In humans, uNK cells have the phenotype CD56brightCD16dim and they appear in great abundance in the late secretory phase of the menstrual cycle and early pregnancy. Gene expression studies indicate that CD56brightCD16dim uterine and circulating cells are functionally distinct. In humans but not mice or other species with post-implantation decidualization, uNK cells may contribute to blastocyst implantation and are of interest as therapeutic targets in female infertility. Histological and genetic studies in mice first identified triggering of the process of gestation spiral arterial modification as a major uNK cell function, achieved via interferon (IFN)-γ secretion. During spiral arterial modification, branches from the uterine artery that traverse the endometrium/decidua transiently lose their muscular coat and ability to vasoconstrict. The expression of vascular markers changes from arterial to venous as these vessels dilate and become low-resistance, high-volume channels. Full understanding of the vascular interactions of human uNK cells is difficult to obtain because endometrial time-course studies are not possible in pregnant women. Here we briefly review key information concerning uNK cell functions from studies in rodents, summarize highlights concerning human uNK cells and describe our preliminary studies on development of a humanized, pregnant mouse model for in vivo investigations of human uNK cell functions.
Keywords: decidua, humanized mice, pregnancy, uterine natural killer cell
Introduction
Natural killer (NK) cells are classically viewed as innate lymphocytes with high cytolytic potential against virus-infected and tumor-transformed cells. More recently, NK cells were found to share traits with the adaptive immune system such as memory, repertoire and dynamic trafficking.1, 2 NK cells are now known to have important physiological roles in mucosal tissue including lymphoid tissue induction. Uterus is a mucosal tissue that undergoes massive steroid hormone-promoted restructuring during pregnancy to support conceptus development (Figure 1). These changes are accompanied by the differentiation and proliferation of a unique, transient NK cell lineage, uterine natural killer (uNK) cells. UNK cells are terminally differentiated cells of limited life-span that decline in number after mid-gestation in humans, rats and mice. This has made uNK cells refractory to most in vitro study approaches. UNK cells reach 70% of all decidual leukocytes in early human gestation suggesting that they have important roles. Because investigative manipulations of pregnant patients to fully define these roles is not possible, the understanding of human uNK cell functions has been extrapolated from alternative approaches (Table 1) with rodent uNK cell studies (i.e. mouse, rat and others) providing important information.
Figure 1.
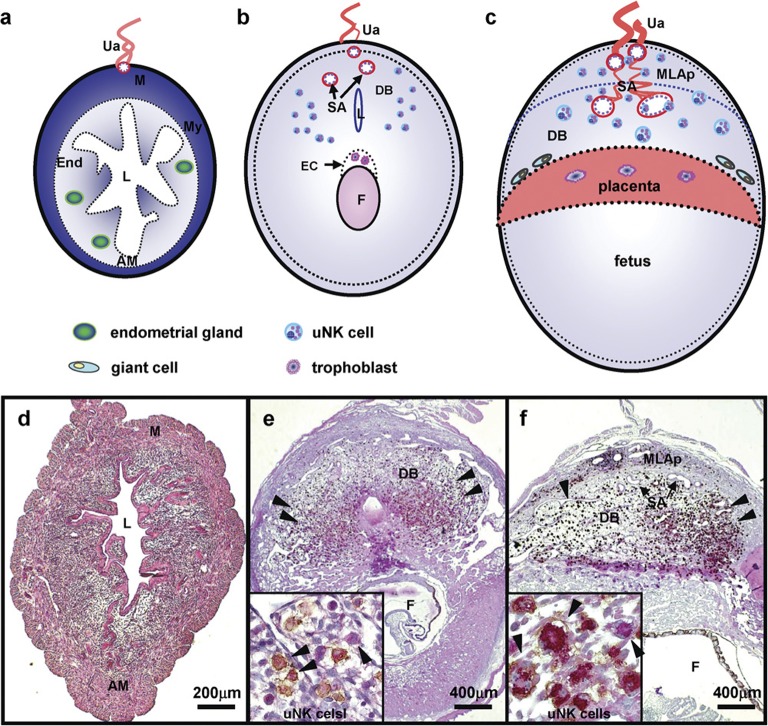
Structure of mouse implantation sites in cross-section. The upper panel drawings illustrate regions of the mouse uterus as virgin (a), gd6–8 (b) and gd10–12 (c). The lower panel presents matched photomicrographs of midsaggital sections of C57BL/6J uterus dually stained with PAS and DBA lectin to detect uNK cells in virgin (d), gd8 (e) and gd10 (f). PAS+DBA+ and PAS+DBA− uNK cells, shown in high power insets, appear in mouse DB after embryonic implantation has occurred (arrowheads indicated). Briefly, the uNK cell population's life history in mouse endometrium is summarized as undetectable in virgin uterus (a, d), enriched by the end of first trimester (b, e), proliferating and functional in the second trimester (c, f) and dying in the third trimester (not shown). DBA lectin is useful for uNK cell identification in mice but not in all species. AM, antimesometrial side; DB, decidua basalis; DBA, Dolichos biflorus agglutinin; EC, ectoplacental cone; End, endometrium; F, fetus; gd, gestation day; L, uterine lumen; M, mesometrial side; MLAp, the transient mesometrial lymphoid aggregate of pregnancy; My, myometrium; P, placenta; PAS, periodic acid Schiff; SA, spiral artery; Ua, uterine artery; uNK cell, uterine natural killer cells.
Table 1. Approaches for the study of human uNK cell functions.
| Approach | Samples available | Methods of analysis | References |
|---|---|---|---|
| Studies in pregnant animal models especially normal mice and rats | Full time course of pregnancy in the reproductive tract including ovaries, genetically repeatable | Histology and morphometry, immunohistochemistry, cell suspensions, tissue explants, intravital microscopy, RNA analyses as homogenate or in situ, cell collection by laser capture microdissection | 16, 24, 90–95 |
| Studies of pregnant genetically, surgically or treatment modified animals (i.e., inhibitors, adoptive cell transplantation, etc.). | Full time course of pregnancy in the reproductive tract including ovaries, genetically repeatable, assessment of site of the origin of the lineage, homing mechanisms | Histology and morphometry, immunohistochemistry, cell suspensions, tissue explants, intravital microscopy | 11, 13, 22, 96, 97 |
| Early human gestational materials from elective terminations | Dissociated embryo, placenta and some decidua. Spiral arteries are not present | Immunohistochemistry, cell suspensions, tissue explants, RNA analyses as homogenate or in situ collections by laser capture microdissection | 38, 65, 67, 98, 99 |
| Term human placenta, with or without labor. The latter may include an endometrial biopsy marginal to the delivery incision that would reflect the placental bed | Surgical waste with cord. This is the most readily available specimen. It is critically important to know if placenta was obtained by Cesarean section or experienced labor. With the former, the placental bed where spiral arteries are found is usually available. uNK cells are relatively depleted at this time point but not absent | Histology and morphometry, immunohistochemistry, cell suspensions, tissue explants, RNA analyses as homogenate or in situ collections by laser capture microdissection, perfusion experiments | 100–104 |
| Menstrual cycle endometrial biopsies (non-pregnant women) | This is a surgical intervention associated with diagnosis and treatment of infertility. Very small core samples are obtained that are precisely timed within the cycle and may be collected serially from a single woman. They represent only a tiny region of the uterus and are random | Immunohistochemistry, cell suspensions, RNA isolation | 4, 105 |
| Humanized mice | Tissues or cells can be engrafted into immune deficient mice to assess human cell functions in vivo | Analyses anticipated from studies of non-pregnant humanized mice are histology, immunohistochemistry , biophotonics, dissection for RNA or protein expression, manipulations such as post-immune challenge, chemokine or cytokine challenge, blocking of functions, migration | 46, 76–79, 106–108 |
The rodent cells now called uNK cells have had a variety of names which readers should remember in order to obtain full bibliographies of previous work. For mice and rats, the term granulated metrial gland cell was in use from the 1930s to 1990s. It still appears occasionally today. The most comprehensive monograph on earlier terminologies and on the first century of histological study of these cells was published by S. Peel in 1989.3 Human uNK cells also have a number of synonyms. These include decidual or dNK cell and endometrial or eNK cell to distinguish gestational from preconception intervals.4 A common antecedent term was endometrial granulocyte.5
uNK cells and their functions in mice
Life history, origins and subsets of uNK cells
Cells of the NK lineage are first detected in mouse uterus by immunohistochemistry in infancy (∼2 weeks of age).6 This precedes the appearance of uterine T cells by ∼1 week.7 Puberty with 4-day to 5-day estrous cycles onsets over the next 2 weeks but brings no changes in the location or relative numbers of NK cells. The NK cells detected in non-pregnant cycling mice are randomly distributed, small, agranular lymphocytes that might be more appropriately called pre-uNK cells.
In naturally mated mice, conception occurs in the uterine tube and early blastocysts, still enclosed within the zona pellucida, arrive in the uterus 3.5 days later. These embryos expand, hatch and implant by gestation day (gd) 4, triggering the primary decidual response. Initial attachment and decidualization occur on the antimesometrial side of the uterus. This positions polar trophoblast which forms the placental primordium called the ectoplacental cone, for growth towards the mesometrial side of the uterus where the mesentery delivers the uterine blood supply. As the endometrium undergoes secondary decidualization around the ectoplacental cone, differentiation of uNK cells is induced within a region of secondary decidua called the decidua basalis (DB).
It is important to realize that models of artificial decidualization, induced, for example, by small beads in hormone-primed mice,8 create a microenvironment that promotes uNK cell differentiation. Establishing this microenvironment requires progesterone but mouse uNK cells do not express the progesterone receptor.9 Studies using artificial decidualization clearly show that uNK cell differentiation and the primary functions of these cells are independent of a conceptus and thus of trophoblast.3, 8, 10 During a pregnancy, mouse uNK cells first appear in a region of DB with the unique addressin expression of VCAM-1 (vascular cell adhesion molecule-1) alone.11 Discrete microdomains for recruitment of other hematopoietic cell lineages are also present in early decidua and, over the next few days, the domains blend in this constantly changing tissue. Stromal cell markers that are abundantly expressed in lymphoid organs, particularly the thymus, are strongly expressed by early post-implantation decidual cells. These include thymopoientin (TSLP), ERTR7 and gp38/podoplanin (Zhang JH, Fritz J, Rochman Y, Leonard WJ, Gommerman J, Croy BA, MS “revised”). IL-15 is expressed in mouse uterus from the onset of decidualization until gd11.5.12
It is unclear where the progenitors of uNK cells reside. In situ proliferation and recruitment from the circulation are the hypotheses usually considered, both mechanisms may participate. At gd5.5, uNK cells are difficult to identify histologically and it has been suggested from 3H-TdR incorporation estimates of the high rate of early uNK cell proliferation that only five progenitor cells would be needed to give the abundant uNK cell numbers present at mid-pregnancy3 (Figure 1b, c, e and f). We orthotopically transplanted uterine segments from normal (+/+) mice into mice genetically deficient in T and NK cells using end-to-end anastomoses, then bred the recipients. Neither decidualized grafts nor decidualized host uterus differentiated uNK cells, strongly suggesting that most uNK cell progenitors are recruited from the circulation.13 In that study and most studies reported prior to 1995, mouse uNK cells were recognized by their lymphoid shape and the reactivity of their cytoplasmic granules with periodic acid Schiff's (PAS) reagent, a histochemical stain for glycoproteins, especially mucin.14 Over the past decade, most investigators of mouse uNK cells have switched to use of the lectin Dolichos biflorus agglutinin (DBA) which reacts with terminal N-acetyl-𝒹-galactosamine, for uNK cell identification. This lectin reactivity is not applicable for uNK cells across species (for example, rat uNK cells lack reactivity) and must be tested in each species of interest. Mouse uNK cells display DBA lectin reactivity over their plasma membranes and the membranes covering their cytoplasmic granules. This dual reactivity is not seen in lymphocytes in any other organ of unmated or gd0.5–7.5 mice.15 The use of DBA lectin was widely adopted because it is more visually distinct than PAS in histological sections and is compatible with RNA isolation after cell collection from tissues by laser capture microdissection while PAS is not.16 Additionally, DBA lectin can be magnetically tagged for isolation of uNK cells from decidual cell suspensions for fluorescence-activated cell sorting, short-term culture or RNA isolation.16
We asked if PAS and DBA lectin were fully coincident stains and documented that they were not. Two subtypes of uNK cells were identified: PAS+DBA− and PAS+DBA+ (Figure 1). At gd6.5, these subsets were equally abundant. As gestation continued and uNK cells increased, the percentage of PAS+DBA+ uNK cells also increased. UNK cells achieve peak numbers gd10.5–12.5 and, at these times, ∼90% of uNK cells were PAS+DBA+. When alymphoid recipients (genotype Rag2−/−Il2rg−/−; NK−T−B−) were engrafted by congenic +/+ marrow however, PAS+DBA− cells are difficult to find (<1%), even at gd6.5.17 This clearly shows that PAS+DBA+ uNK cells arise from circulating progenitors and suggests that PAS+DBA− uNK cells may be endogenous progenitors. Colucci and his colleagues also identified two distinct subsets of uNK cells in mid-gestation mouse decidual cell suspensions using flow cytometry.18 Their smaller diameter CD3−CD122+ uNK cells had the phenotype of peripheral NK cells (NK1.1+ or DX5+) while cells of larger size were NKp46+Ly49+NK1.1− (in C57BL/6) or NKp46+Ly49+DX5− (in BALB/c).18 Using their marker strategy, we flow sorted uNK cells from mid-gestation random-bred mice, isolated RNA from each uNK cell subset and conducted real-time PCR analyses. This has revealed that DBA+ and DBA− uNK cell subsets differ functionally in their production of cytokines and angiogenic factors (Chen ZL et al., MS in preparation).
Between gd6.5 and 11.5, proliferating uNK cells occur in DB and, from morphological criteria, four stages of uNK cell maturation have been proposed. These are: (i) non-granulated; (ii) a few cytoplasmic granules; (iii) numerous cytoplasmic granules and greatly expanded radius; and (iv) senescent which is a large, heavily granulated cell with nuclear changes. Senescent cells then break apart and scatter their granules. Active granule secretion by uNK cells has not been documented and it is thought that less mature uNK cells store their granule cargos of perforin and other cytolytic compounds. From gd8.5, all four uNK cell subtypes are found, no doubt complicating attempts to culture freshly isolated uNK cells. Also between gd8.5 and late pregnancy, a dense lymphoid structure full of uNK cells is found in the uterine wall at each implantation site. It separates the two smooth muscle layers of the uterus and rings the branches of the uterine artery entering to each implantation site. This donut-like ring, referred to as the mesometrial lymphoid aggregate of pregnancy (MLAp), becomes the site sustaining proliferative uNK cells while larger, post-mitotic uNK cells dominate in the mid-gestation DB.
Functions of mouse uNK cells
The original histological studies of implantation sites in mice genetically deficient in NK cells made several key observations. There were no uNK cells, decidual and mid-gestational myometrial structures were abnormal and the spiral arterial branches of the uterine arteries were not modified. These features have been consistently found across a number of different immune-deficient mouse strains and their correction by transplantation of NK+T−B− marrow has confirmed that normal uNK cell functions include decidual stromal and vascular remodeling. Reciprocal congenic transfers of IL-15−/− and +/+ marrows between mice who were subsequently mated showed that the interactions between uNK cells and stromal cells are cross-regulatory. Absence of IL-15 in decidual stroma-blocked uNK cell differentiation from +/+ marrow while +/+ decidua promoted uNK cell differentiation from IL-15−/− marrow. Under barrier husbandry conditions, most strains of mice lacking NK cells breed successfully.19
Absence of structural change to the maternal arteries feeding into implantation sites becomes apparent when uNK cell-sufficient and -insufficient mice are compared. This led us to examine more closely the relationship between uNK cells and the implantation site vasculature. Morphometry showed that normal vascular remodeling occurred after gd8.5 and was usually completed by gd10.5. This is a physiologically important time interval during which placental structure is completed and placental blood flow begins. At this time, ∼10% of the very large heavily granulated uNK cells are within lumens of decidual vessels, particularly small capillaries. About 25% of uNK cells are embedded within arterial walls and the remainder associate with decidual cells. It is very unusual to find significant numbers of uNK cells in the placenta but rare cells occur that may kill individual trophoblast cells.20, 21
Mouse uNK cells produce most (∼90%) of the interferon (IFN)-γ in the mesometrial decidua and MLAp. Systemic administration of IFN-γ to NK cell-deficient mice fully replaces uNK cell contributions to spiral arterial modification.22, 23 Because IFN-γ acts on hundreds of genes, we suspect that it influences the transcription of different molecules in endothelial, vascular smooth muscle, myometrial and decidual cells to orchestrate highly regulated arterial changes. Eomes regulates Ifng transcription in uNK cells while Tbet regulates Ifng transcription in peripheral NK cells.16 UNK cells also differ by acquiring IL-7Rα (CD127) after reaching maturity. This receptor appears to participate in maintaining IFN-γ production by uNK cells (Zhang JH, et al. submitted). Not all mid-gestation mouse uNK cells synthesize IFN-γ (Figure 2a).
Figure 2.
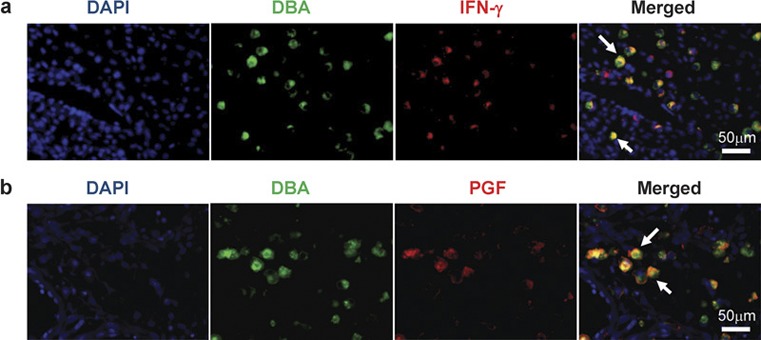
Fluorescence photomicrographs show colocalization of IFN-γ (a) or PGF (b) to uNK cells in gd10 C57BL/6 DB. Mouse uNK cells in 4% PFA fixed, paraffin-embedded tissue were tagged by FITC-conjugated DBA lectin then stained with anti-PGF (Abcam, Cambridge, MA, USA) or anti-IFN-γ (Mebtech, Mariemont, OH, USA), which was visualized by Alexa 594 goat-antirabbit antibody or Alexa Fluor 594-conjugated Streptavidin (Molecular Probes, Eugene, OR, USA). DNA was labeled with DAPI (blue). DBA+PGF+ and DBA+IFN-γ+ uNK cells are indicated (arrows). DAPI, 4,6-diamidino-2-phenylindole; DBA, Dolichos biflorus agglutinin; gd, gestation day; IFN-γ, interferon-γ PFA, paraformaldehyde; PGF, placenta growth factor; uNK cells, uterine natural killer cells.
UNK cells associate only with the arterial side of the vasculature. Typically, nucleated cells enter and exit tissue via the venous side. One explanation for the special positioning of uNK cells, in addition to the addressin profiles expressed by endothelial cells of DB, comes from studies of the ephrin family. EphrinB2 (EFNB2) is a signaling tyrosine kinase associated with arteries. Cells expressing EFNB2 associate together during arterial development. EphrinB4 (EPHB4) ligates EFNB2 and is characteristically expressed by veins. EPHB4 is also a tyrosine kinase and cells that express it dissociate from BFNB2+ cells in a ‘push–pull' interaction seen in differentiating capillaries. We found the expected expression of EFNB2 in implantation-site spiral arteries at gd8.5 when these vessels have an arterial appearance but expression was lost over the next 4 days and by gd12.5, spiral arteries did not express this marked but had acquired EPHB4, suggesting that they were now functioning as veins. Unexpectedly, uNK cells showed dynamic expression of both markers and this occurred in a time-course manner that preceded the vascular changes. At gd6.5–12.5, uNK cells expressed EFNB2 which would prevent their association with veins and promote arterial associations. At gd6.5 and 8.5, uNK cells were EPHB4-negative but they coexpressed this ligand with its receptor at gd10.5 and 12.5. At these times, the lymphocytes were the most strongly stained cells for both molecules in normal implantation sites. These studies highlight significant functional changes in the lymphocytes as spiral arteries transform and the placental circulation opens.24
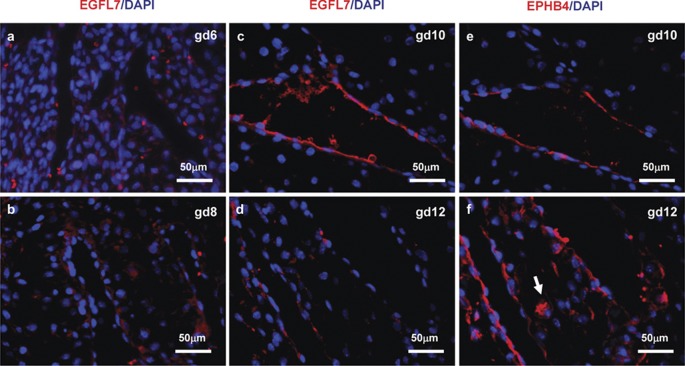
Mouse uNK cells express angiogenic molecules. This was first recognized by Wang et al., who showed immunoreactive vascular endothelial growth factor (VEGF) in uNK cells. By costaining for endothelial cells, they deduced that the peak of neo-angiogenesis in mouse implantation sites was at gd8.5 and that the vasculature was maximal at gd13.5.25, 26 uNK cells are one of several cell types in implantation sites that make placenta growth factor (PGF; Figure 2b). This marker has higher affinity than VEGF for VEGFR1 and accelerates angiogenesis by displacing VEGF from this receptor making it more bioavailable.27 The highest numbers of Pgf transcripts are found in immature uNK cells with few granules.28 We hypothesized that the role of angiogenic uNK cells is to locate the site of blastocyst implantation and to move towards it, thereby creating a ‘custom made' guidance system for maternal vascular growth into an implantation site. We examined decidual expression of epidermal growth factor-like domain 7 (EGFL7), a key molecule in endothelial progenitor cell guidance and movement during blood vessel formation.29, 30 Between gd6.5 and 12.5, EGLF7 was expressed by venous (EPHB4+) endothelium with peak expression at gd10, but it was not expressed by uNK cells (Figure 3).
Figure 3.
Fluorescence photomicrographs showing EGFL7 expression at gd6 (a), gd8 (b), gd10 (c) and gd12 (d) in C57BL/6J implantation sites. PFA-fixed paraffin-embedded implantation sites were sectioned, stained with anti-EGFL7 (a kind gift from Dr Huilian Ye, Tumor Biology and Angiogenesis Department, Genentech Inc., South San Francisco, CA, USA), then visualized by PE-conjugated goat anti-Armenian Hamster IgG (red). DNA was labeled with DAPI (blue). EGFL7 had a restricted pattern of expression at the maternal–fetal interface. EGFL7 was transiently expressed at gd10 DB in vein ECs and downregulated by gd12. No uNK cell with detectable EGFL7 was seen. Modified spiral arteries that become vein-like EPHB4+ vessels are shown in gd10–12 implantation sites (e, f). UNK cells express the arterial marker EFNB2 at gd6 and 8 but lose this marker and become EPHB4+ (arrow; f) prior to the switch in expression of these molecules by the spiral arteries.24 DAPI, 4,6-diamidino-2-phenylindole; EGFL7, epidermal growth factor-like domain 7; gd, gestation day; IgG, immunoglobulin G; PE, phycoerythrin; PFA, paraformaldehyde; uNK cell, uterine natural killer cell.
In humans, NK and T cells express all components of the renin-angiotensin system. Mouse T cells also express the renin-angiotensin system and mouse T-cell deletion reduces drug-induced vasoconstriction. We asked if uNK cells express vasoactive molecules that could contribute to blood pressure control during pregnancy. We identified uNK and splenic NK cell subsets immunoreactive with antibodies against type 1 and type 2 receptors for angiotensin II (Hatta et al., submitted). Nitric oxide synthase, the enzyme-producing NO, a potent vasodilating gas, is also synthesized by uNK cells.31 Recently, we reported a detailed examination of hemodynamic outcomes in pregnancy in normal and alymphoid (Rag2−/−Il2rg−/−) mice.32 The former have mid-gestational spiral arterial modification; the latter do not. Unexpectedly, there were no differences between the strains in mean arterial pressure patterns, in hypoxia of the placenta or fetus Leno-Durán E, et al., Placenta (2010), doi:10.1016/j.placenta.2010.06.002 or in placental growth.32 These studies do not give insight into the potential hemodynamic roles for either NK or T cells because opposing, lymphocyte-based regulatory interactions cannot be assessed. Repetition of these studies in mice lacking only NK or only T cells is warranted as are drug challenges of the pregnancy NK33, 34 and/or T cell-deficient mice to evaluate if pregnancy has modified normal pathways of peripheral vasoreactivity. Short-term adoptive transfer of B versus T cells in non-pregnancy Rag1−/− mice has shown that B cells do not participate in hemodynamic alterations.35
Human uNK cells and their functions
Life history and origins of human uNK cells
Human uNK cells are not found in infants or children36 but appear in every post-pubertal menstrual cycle after ovulation and are sustained by pregnancy in the endometrial decidua, even if the pregnancy is ectopic.5, 37 The presence of NK cells in the cycling uterus even if the implantation site is outside of the uterus clearly indicates that human uNK cells, like those in rats and mice, are induced by endocrine-regulated stromal signals and not by the presence of trophoblast or of a conceptus. During the progesterone-dominant phase of the menstrual cycle, uNK cells associate with elongating spiral arteries38, 39, 40 and with basal components of uterine glands. During this interval, human uNK cells may have actions not seen in mice due to the lack of mouse uNK cells prior to conception.
Human uNK cells are highly proliferative in late secretory phase endometrium and in early decidua41 and reach 70% of all nucleated decidual leukocytes.37 Their phenotype, CD3−CD56brightCD16−, is displayed by only a very small proportion of blood leukocytes but is associated with mucosal NK cells.42, 43, 44 Human uNK cells are much less frequent in term decidua but it is difficult to evaluate their pattern of decline due to the need to study pregnancies continuing beyond times typical for elective terminations. In one study with a small number of across pregnancy samples, peak human uNK cell numbers were found between 8 and 13 weeks of gestation.45
While uNK cells in mice are considered to be activated cells because of their secretion of IFN-γ, human uNK cells are considered activated because they constitutively express killer cell immunoglobulin-like receptors (KIR). In both species, uNK cells are considered to be cytokine producing cells armed for but not effecting cytotoxicity.46, 47, 48 In humans, extravillous trophoblast cells that invade the decidua and maternal vasculature express human leukocyte antigen (HLA)-C, -E and -G. Detailed characterization of KIR–HLA-C relationships in couples with gestational complications such as recurrent miscarriage or acute onset hypertension with proteinuria (pre-eclampsia), suggests that activation of uNK cells is a component of normal pregnancy and that a genetic deficit in activation elevates risk for pregnancy complications.
Human uNK cell origins are not defined; a number of sites are postulated. These include endometrium, where CD34+CD45+ hematopoietic stem cells as well as pluripotent mesenchymal and epithelial stem cells are found,49 thymus50, peripheral lymph nodes51 and blood.52 Differentiation of uNK cells from thymus and peripheral lymph nodes had been demonstrated in mice.13 Interestingly, lymph nodes draining a non-pregnant but not pregnant (gd3.5–7.5 were tested) mouse uNK progenitor cells, suggesting that the gestational uterus retains mobilized uNK progenitor cells. Pregnancy induces changes in lymphoid organs, inducing, for example, thymic depletion53, 54 and blockade of dendritic cell movement to uterine-draining lymph nodes.55 Several investigators suggest that in women, as in mice, uNK cell progenitors are of mixed endometrial and peripheral origins.4, 56
Functions of human uNK cells
Studies of timed endometrial biopsies support the hypothesis that human uNK cells have a significant angiogenic role. Over the progesterone-dominated phase of the menstrual cycle, uNK cells show changes in abundance of transcripts for VEGFC (a molecule that promotes lymphatic vessel development), PGF and angiopoietin 2 (ANG2). Protein array studies of CD56+ uNK cells collected at 8–10 weeks of gestational age show that uNK cells are major producers of angiogenic growth factors. This is not true of uNK cells collected at 12- to 14-week gestation. The later cells (12–14 weeks) are major producers of cytokines.5 Matrigel-supported cocultures that contain a trophoblast cell line or umbilical cord endothelial cells plus isolated or cloned human blood or uNK cells reveal that VEGFC-producing uNK cells induce transporter associated with antigen processing (TAP)-1 expression and major histocompatibility complex (MHC) class I assembly in trophoblast and endothelial cells and facilitate capillary tube formation.46 Peripheral blood NK cells do not produce VEGFC or have these properties but do display cytotoxicity.57 An ex vivo chorionic plate artery model has been developed to investigate the role of human first trimester uNK cells and angiogenic growth factors in spiral artery remodeling.58 In this system, uNK cell culture supernatants stimulated the separation of vascular smooth muscle cells from each other, disrupted their organization and initiated their dedifferentiation as assessed by decreased immunoreactivity to vascular smooth muscle cell markers.5 Similarly uNK cell-conditioned medium promoted vessel-like assembly of the extravillous cytotrophoblast cell line HTR8/SVneo.59, 60 This was associated with increased expression of the adhesion molecule intercellular adhesion molecule-1 (ICAM-1) which was identified as a major molecule participating the migration and network formation of the trophoblast cell line.60 Human uNK cell supernatants also promote angiogenesis and tube formation in human umbilical vein endothelial cells and in aortic ring assays.46 These in vitro data support the conclusions from in vivo xenogeneic engraftment of the human trophoblast tumor cell line (JEG-3) into nude (Foxn1) mice in matrigel plugs. Surrounding the plug with uterine but not with peripheral blood CD56+ NK cells promoted a fivefold denser vasculature in the resulting tumor. Expression of matrix metalloproteinases (MMP) 7 and MMP9 by uNK cells and by the macrophages that coinfiltrate into spiral arterial vascular smooth muscle is also considered important for early initiation of trophoblast-independent spiral arterial remodeling.61
In humans, CD56bright NK cells are the NK cell subset associated with the synthesis of immunoregulatory cytokines, particularly IFN-γ.62 IFN-γ significantly upregulates chemokines (CXCL9, CXCL10, CCL8 and CCL5), enzymes (GBP5, TAP1, CYP27B1, SOD2, MX1, CASP1 and PTGES) and transcription factors (TFAP2C, IRF1 and NFE2L3) but downregulates cytokine genes such as CSF2, IL1R2, SPP1, WISP2 and IGFBP3.63 These actions, combined with uNK cell production of the chemokines CXCL10 and CXCR2, direct migration and invasion of CXCR1+, CXCR3+, CXCR4+ and CCR3+ trophoblast,46 and promote physiological angiogenesis in the placental bed.37, 64 Insufficient uNK cell activation would reduce these processes, and contribute to poor arterial remodeling in decidua, a frequent histopathology associated with pre-eclampsia and with intrauterine fetal growth restriction.65 Because NK cell functions reflect the sum of signals from their multiple activating and inhibitory receptors,66 the complex pathways that modulate uNK cell activation in the pregnant uterus are not yet fully understood. Additionally, outcomes from inappropriately activated uNK cells may not be immunologically predictable. For example, recent studies of women categorized as superfertile but who have repeated early pregnancy losses suggest that defective early decidualization extends the window of endometrial receptivity for a blastocyst. This permits karyotypically abnormal or developmentally delayed embryos to implant; they subsequently die, often before 6-week gestation. Endometrial biopsies collected from these women during the progesterone-dominated phase of their menstrual cycle, typically reveal elevated uNK cells. These data and the strong two-way interactions defined between mouse decidual and uNK cells suggest that human uNK cells will be shown to be an in vivo factor contributing to pathological elongation of the window of endometrial receptivity.
In women with recurrent spontaneous abortion, high numbers of uNK cells in endometrial biopsies taken in the late secretory (i.e., progesterone dominant) phase of the menstrual cycle, correlated positively with the formation of blood and lymphatic vessels, spiral arteriole smooth muscle differentiation and extent of endometrial edema. It is postulated that this exposes implanting blastocysts to excessive oxidative stress, leading to embryonic loss.67 Opposite clinical thinking is also reported. For example, some infertility patients planning to undergo embryo transfer, use a clinical protocol developed to stimulate uNK cells. This involves the collection of two serial endometrial biopsies in cycles prior to the embryo transfer.68, 69, 70 This procedure, reported to double the rate of ‘take home baby', is designed to enhance the inflammatory milieu of the uterus and to increase numbers of peri-implantation uNK cells. It must be remembered that female infertility is a heterogeneous problem. Not all infertile women will have an NK cell-related problem and, of those who do, a variety of NK cell-related problems are possible as outlined above.
Many questions remain regarding the biology, origins, functions and regulation of human uNK cells, because detailed, gestational time-course studies are not feasible and because endometrial sampling (small biopsies or hysterectomy specimens) occurs after pathology is recognized and does not represent normal uterus.71 We hypothesized that answers to some of the questions regarding human uNK cells would be provided by studying implantation sites in humanized mice and therefore embarked on experiments to develop an appropriate model.
Humanized mouse modeling of human uNK cell function
Since T cell-deficient ‘nude' (Foxn1 mutation) mice were identified in 1970, xenogeneic grafting of normal and pathologic human cells and tissues to immune-deficient mice has been embraced as an approach to move in vitro models closer to the more complex in vivo situation.72 Success in humanizing mice with normal tissues moved forward significantly when the severe combined immunodeficient T–B mice (Scid, Prkdc mutation) were identified and found to support human hematopoietic cells and lymphoid organs.73, 74 Sequential improvements have given several relatively simple and reproducible mouse models for generation of ‘human immune system' (HIS) mice. Strains now commonly used to prepare HIS mice are NOD-SCID-Il2rg−/− (NOG) and BALB/c-Rag2−/−Il2rg−/−,75, 76 and protocol refinements continue.75, 77 HIS mice have been useful for studying pathogens such as HIV that directly target the human lymphohematopoietic cells, including cells found in the female reproductive tract.78 However, engraftment of the different human lymphocyte lineages in HIS mice varies markedly. B-cell reconstitution is robust and T-cell reconstitution is reasonable, but NK cell and myeloid lineage reconstitutions are generally poor to undetectable.79 Since IL-15 transpresentation regulates endogenous human NK cell homeostasis, the use of IL-15 receptor agonists has been recommended to improve xenogeneic NK cell engraftment.77 No current model is suitable for addressing the question of whether xenogeneically-engrafted human NK cells home to the gestational uterus and/or effect spiral arterial modification. This is due to preconditioning of recipients by irradiation (between 320 and 375 cGy) rendering them reproductively sterile. We turned to 5-fluorouracil (FU), a thymidylate synthase inhibitor, as a preconditioning agent for 6-week-old female BALB/c-Rag2−/−Il2rg−/− mice, at a dose of 150 mg/kg.80 After 24 h, human CD34+ cord blood cells that were enriched by negative selection were inoculated. After 6 weeks, the females were bred and euthanized for study.
The choice of xenograft recipient is important. Because our research question is focused on promotion of decidual angiogenesis and spiral arterial modification, we are not able to use recipients with a NOD background because the decidual arteries in NOD mice are abnormal.81 Our syngeneic mouse to mouse grafting of Rag2−/−Il2rg−/− mice on either the C57BL/6 or BALB/c is consistently successful in establishing fully functional, graft-derived uNK cells82, 83 that affect quantifiable spiral arterial modification. Thus, we selected preconditioned BALB/c-Rag2−/−Il2rg−/− females for study and used 16 as recipients for 1×105 human CD34+ cord blood cells. Variables compared were:
administration of freshly isolated cells versus cells expanded in culture (24 h in StemSpan SFEM medium with CC100 Cytokine Cocktail);84, 85
with or without human IL-15/IL-15Rα complex treatment at 6 and 7 weeks of age and at day 6.5 after mating.77 These four females were paired with males immediately after their second injection and bred within a few days.
Two additional variables are present in these studies. The first is placenta donor variability—three placentae were used. The second is whether the prepared females successfully mated and subsequently conceived.
All mice were killed at gd12 with sera and organs collected for quantification of human immunoglobulin G (IgG) by ELISA, PCR for human chromosome 17-specific α-satellite DNA and histology. Table 2 summarizes our progress. Of the 16 females used in this study, 14 became pregnant and carried a viable litter to gd12.5. There were no differences in implantation site numbers between unmanipulated BALB/c-Rag2−/−Il2rg−/− and hu-CD34+ cell inoculated BALB/c-Rag2−/−Il2rg−/− (Figure 4a). Of the 14 pregnant females, three were identified as chimeric in their implantation sites (decidua and MLAp), peripheral organs (maternal liver and spleen) and serum (hu-IgG+; Figure 4 and Table 2). One of the two mice who mated but were not pregnant was also chimeric in spleen and liver and had circulating hu-IgG. Cells prepared from one of the three donor placentae gave no reconstitution.
Table 2. Summary of BALB/c-Rag2−/−Il2rg−/− mice engrafted with human cord blood CD34+ cells at gestation day 12.
| Donors | Recipients | Hu-CD34+ cells | IL15/IL15Rα | Pregnancy | Human DNA | Hu-IgG | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| i.v. | i.f. | L | S | D | M | |||||
| A | 1–4 | Fresh | + | − | − | − | − | − | ||
| 5–7 | Cultured | + | − | − | − | − | − | |||
| 8 | Cultured | + | + | + | Ha | Ha | + | |||
| B | 9 | Fresh | Yes | − | − | − | na | na | − | |
| 10 | Fresh | Yes | + | − | − | − | − | − | ||
| 11–12 | Fresh | + | − | − | − | − | − | |||
| C | 13 | Fresh | Yes | + | + | + | + | + | + | |
| 14 | Fresh | Yes | − | + | + | na | na | + | ||
| 15 | Fresh | + | + | + | + | + | + | |||
| 16 | Fresh | + | − | − | − | − | − | |||
All animals were pretreated with 5-FU 24 h before transplantation. Either freshly isolated or cultured human CD34+ cells were given by intravenous or intrafemeral injection. Some recipients were treated thrice with IL-15/IL-15Rα complex (−gd7, gd0 and gd7). PCR for human DNA was undertaken using liver, spleen, decidua basalis and MLAp. Hu-IgG was analyzed in serum collected at euthanasia by ELISA.
Only one conceptus in the litter. It was used for paraffin embedding and not PCR analysis.
Abbreviations: D, decidua basalis; gd, gestation day; i.f., intrafemoral injection; IgG, immunoglobulin G; i.v., intravenous injection; L, liver; M, MLAp (mesometrial lymphoid aggregate of pregnancy); na, not applicable because a mid-gestation pregnancy did not result following mating of these mice; S, spleen; 5-FU, 5-fluorouracil.
Figure 4.
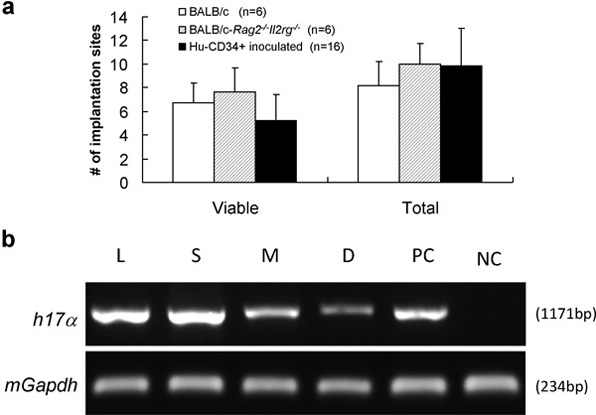
Implantation sites outcomes after human CD34+ cell inoculation. A total of 16 BALB/c-Rag2−/−Il2rg−/− mice were pretreated with 5-FU (150 mg/kg) and inoculated with 1×105 human CD34+ cord blood cells. Six weeks later, they were paired with syngeneic males and 14 pregnancies were obtained and studied at gd12. (a) The reproductive performance in our colony at gd12 for wild-type BALB/c, BALB/c-Rag2−/−Il2rg−/− and human CD34+ cell-inoculated BALB/c-Rag2−/−Il2rg−/− mice. There was no significant difference for either viable or total numbers of implantation site between these groups. (b) Human genomic DNA detection by PCR. Genomic DNA was extracted from maternal liver, spleen, and individual MLAps and deciduas of gd12 BALB/c-Rag2−/−Il2rg−/− females using Qiagen QIAamp DNA Blood Mini Kit. The resulting genomic DNA was used as the PCR template. Primer sequences were: human chromosome 17-specific α-satellite (h17α) sequences, 5'-ACACTCTTTTTGCAGGATCTA-3' (forward) and 5'-AGCAATGTGAAACTCTGGGA-3' (reverse); mouse glyceraldehyde-3-phosphate dehydrogenase (mGapdh), 5'-GGTCGGTGTGAACGGATTTGGC-3' (forward) and 5'-GTGGGGTCTCGCTCCTGGAAGA-3' (reverse). PCR was performed under the following conditions: 94°C for 3 min (1 cycle); 94°C for 30 s, 55°C for 30 s, 72°C for 1 min (33 cycles); and 72°C for 10 min (1 cycle). PCR products were separated on 1.0% agarose gels and visualized by ethidium bromide staining. Detection of human chromosome 17-specific α-satellite sequences from an individual pregnancy is shown and verifies that human graft derived cells are present within the implantation site. D, decidual basalis; gd, gestation day; L, liver; M, MLAp (mesometrial lymphoid aggregate of pregnancy); NC, negative control (non-transplanted mouse liver DNA); PC, positive control human placenta; S, spleen; 5-FU, 5-fluorouracil.
Implantation site histology in the pregnant, chimeric mice was quite variable between littermates, which we have never seen in unmanipulated or syngeneically-transplanted females. The histology also differed to that anticipated (Figure 5). Cells with a lymphoid appearance were not present in the DB and there was an increase in spiral arterial pathology. In the most severely altered sites (Figure 5), a greatly enlarged vascular wall surrounded the spiral arteries. This region showed a localized loss of reactivity with many histochemical stains and appeared to have lost all of its collagen fibers (eosin-reactive in normal BALB/c and BALB/c-Rag2−/−Il2rg−/− mice (Figure 5A, B, a and b). This region is not an artifact and has been seen multiple times in another series of intrahepatically inoculated neonatal recipients (Bilinski M, Croy BA, data not shown). A littermate implantation site (Figure 5c2 and c3) had much milder changes. In it, individual, large, irregularly shaped, pale staining mononuclear cells with low nuclear to cytoplasmic ratios were seen that appeared to have specifically homed to the spiral arterial walls. The unusual cells could not be stained by PAS, Alcian blue or Masson's trichrome indicating lack of glycoprotein, collagen and mucopolysaccharides (not shown). We anticipate but as yet have no evidence that the unusual cells and tissue are of human origin because 5-FU treatment followed by syngeneic grafting does not induce these changes. Neither does administration of three doses of the IL-15/IL-15Rα complex alone without cells (not shown). The unusual cells we report are not reactive with antibodies to human CD45 and may represent progeny of circulating human mesenchymal or other stem cells that were not removed by the CD34 cord blood negative selection procedure. It is of interest that these proven chimeric implantation sites do not reveal an immune graft versus placenta pathology and that if the fetus imaged in Figure 5C and c1– c3 was doomed to die in late gestation, its compromise would have been effected through the maternal vasculature with particular involvement at the spiral arteries. That components of human decidual tissue beyond immune cells and circulating endothelial progenitor cells might arise from circulating progenitor cells is a novel hypothesis suggested by these preliminary studies. Might it also be possible that the unusual, spiral artery-homed cells we detected are the early progenitors of uNK cells? This could suggest that uNK cells do not arise from committed lymphoid cells or, in humans, from circulating CD56bright cells. Readers will realize that we have not yet shown that the unusual cells depicted in Figure 5 are human in origin and that we remain far from our goal of a humanized pregnant mouse model that will enable in vivo studies of the interactions between human uNK cells and the stromal cells comprising the spiral arteries.
Figure 5.
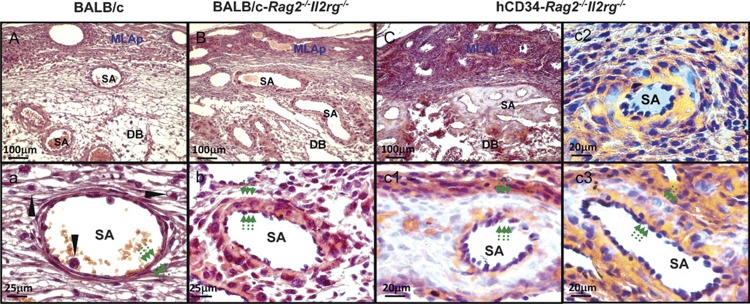
Photomicrographs comparing gd12 implantation sites from wild-type BALB/c (A, a), BALB/c-Rag2−/−Il2rg−/− (alymphoid; B, b) and human CD34+ cell-engrafted BALB/c-Rag2−/−Il2rg−/− mice (i.v. injection, fresh cells, 3× IL15/IL15Ra treatment) (C, c1–c3). All samples were fixed with 4% PFA and wax embedded using standard histological methods,89 sectioned at 7 µm then H&E stained. (A–C) Low power images of the fetal maternal interface. These lower panels provide high power images of SAs from the same implantation sites. Wild-type SAs are post-modification by gd12 and have widely diluted lumens and very thin walls (a), relative to the lumens and walls of unmodified SA of alymphoid mice (b). Six weeks after inoculation of human CD34+ cord blood cells, pregnant alymphoid mice had abnormal SAs but the extent of abnormality varied greatly between littermates, (C, c1) are from the same implantation site while (c2, c3) are a littermate implantation site. In (C, c1), SA walls were hugely thickened, lacked eosinophilic collagen fibers and contained small pools of red blood cells that appeared to be developing vasculature. In some vessels, individual cells with the same unusual properties were found localized to SA walls (c2, c3). Mononuclear lymphoid cells were not seen (c1–c3). In all genotypes, the thickness of the SA wall is indicated by pairs of arrows. UNK cells (arrow head; a) were only identified in wild-type BALB/c where they associated with blood vessels in the DB. DB, decidua basalis; H&E, hematoxylin and eosin; MLAp, mesometrial lymphoid aggregate of pregnancy; DB, decidua basalis, gd, gestation day; iv, intravenous; PFA, paraformaldehyde; SA, spiral artery; uNK cell, uterine natural killer cell.
Significance
An individual's life-long health is determined by the quality of his or her environment during pregnancy.88 Rapid and massive changes are induced in the maternal cardiovascular system early in pregnancy to support the conceptus and its nutritional welfare. These changes occur systemically, including functional changes in the heart and kidneys and locally, within the reproductive tract. Evolution of mammalian pregnancy, a much more recent event than evolution of the immune system, appears to have shaped at least some elements of the immune system into specialized tools to promote and optimize the success of pregnancy. uNK cells are an example of these specialized niche cells. They are transient cells endowed with angiogenic and, potentially, circulatory regulatory activities that participate in the early optimization of maternal care of the fetus before birth. This role of lymphocytes appears to be conserved across species that have forms of placentation other than the hemochorial placentae discussed in this review.28 Understanding of the potential for immune cells to function in angiogenesis and in circulatory regulation and the mechanisms that physiologically link the immune and cardiovascular systems is an important new horizon in health research.
Acknowledgments
We thank Ms Valérie Barrette and Mr Michael Bilinski (Queen's University) for technical assistance and helpful discussions, Dr Huilian Ye (Genentech Inc., South San Francisco, CA, USA) for providing anti-EGFL7 antibody, Dr Aureo T. Yamada (UNICAMP, Campinas, Brazil) for histological consultations and Mr Richard C. Casselman and Ms Heather Ramshaw (Kingston General Hospital) for human sample collection. These studies were supported by awards from the Natural Sciences and Engineering Research Council, Canada, the Canadian Institutes of Health Research and the Canada Research Chairs Program to BAC and a Province of Ontario/Queen's Postdoctoral Fellowship award to JHZ.
References
- Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16− natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181:1869–1876. doi: 10.4049/jimmunol.181.3.1869. [DOI] [PubMed] [Google Scholar]
- Lash GE, Robson SC, Bulmer JN. Review: functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31:S87–S92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Kiso Y, Yamashiro S, McBey BA, Croy BA. Tissue-specific differentiation of a natural killer cell subset in ectopically grafted murine uterine tissue. Transplantation. 1992;54:185–187. [PubMed] [Google Scholar]
- Kiso Y, McBey BA, Mason L, Croy BA. Histological assessment of the mouse uterus from birth to puberty for the appearance of LGL-1+ natural killer cells. Biol Reprod. 1992;47:227–232. doi: 10.1095/biolreprod47.2.227. [DOI] [PubMed] [Google Scholar]
- Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294:445–456. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Oh MJ, Croy BA. A map of relationships between uterine natural killer cells and progesterone receptor expressing cells during mouse pregnancy. Placenta. 2008;29:317–323. doi: 10.1016/j.placenta.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Herington JL, Underwood T, McConaha M, Bany BM. Paracrine signals from the mouse conceptus are not required for the normal progression of decidualization. Endocrinology. 2009;150:4404–4413. doi: 10.1210/en.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol Reprod. 2002;66:333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- Ye W, Zheng LM, Young J, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med. 1996;184:2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, et al. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168:22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- Croy BA, Zhang J, Tayade C, Colucci F, Yadi H, Yamada AT.Analysis of uterine natural killer cells in miceIn: Campbell KSNatural Killer Cell Protocols, 2nd edn Totowa NJ: Humana Press; 2010465–503. [Google Scholar]
- Bianco J, Stephenson K, Yamada AT, Croy BA. Time-course analyses addressing the acquisition of DBA lectin reactivity in mouse lymphoid organs and uterus during the first week of pregnancy. Placenta. 2008;29:1009–1015. doi: 10.1016/j.placenta.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Tayade C, Fang Y, Black GP, V A, Jr, Erlebacher A, Croy BA. Differential transcription of Eomes and T-bet during maturation of mouse uterine natural killer cells. J Leukoc Biol. 2005;78:1347–1355. doi: 10.1189/jlb.0305142. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Yamada AT, Croy BA. DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta. 2009;30:968–973. doi: 10.1016/j.placenta.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- Luross JA, Yamashiro S, Croy BA. A study on the relationship between parity and differentiation of granulated metrial gland cells. Placenta. 1996;17:521–525. doi: 10.1016/s0143-4004(96)90035-1. [DOI] [PubMed] [Google Scholar]
- Stewart I, Peel S. Granulated metrial gland cells at implantation sites of the pregnant mouse uterus. Anat Embryol (Berl) 1980;160:227–238. doi: 10.1007/BF00301863. [DOI] [PubMed] [Google Scholar]
- Stewart IJ. Granulated metrial gland cells in ‘minor' species. J Reprod Immunol. 1998;40:129–146. doi: 10.1016/s0165-0378(98)00038-2. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dong H, Wang B, Zhu S, Croy BA. Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice. Biol Reprod. 2008;79:450–458. doi: 10.1095/biolreprod.108.067975. [DOI] [PubMed] [Google Scholar]
- Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, et al. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, et al. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. 2000;300:285–293. doi: 10.1007/s004410000198. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Tayade C, Fang Y, Hilchie D, Croy BA. Lymphocyte contributions to altered endometrial angiogenesis during early and midgestation fetal loss. J Leukoc Biol. 2007;82:877–886. doi: 10.1189/jlb.0507330. [DOI] [PubMed] [Google Scholar]
- Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, et al. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- Hunt JS, Miller L, Vassmer D, Croy BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biol Reprod. 1997;57:827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- Burke SD, Barrette VF, Bianco J, Thorne JG, Yamada AT, Pang SC, et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension. 2010;55:729–737. doi: 10.1161/HYPERTENSIONAHA.109.144253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. . J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162:887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- Moffett A, Regan L, Braude P. Natural killer cells, miscarriage, and infertility. BMJ. 2004;329:1283–1285. doi: 10.1136/bmj.329.7477.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Wooding P, Gardner L, Loke YW. Expression of perforin, granzyme A and TIA-1 by human uterine CD56+ NK cells implies they are activated and capable of effector functions. Hum Reprod. 1993;8:2061–2067. doi: 10.1093/oxfordjournals.humrep.a137982. [DOI] [PubMed] [Google Scholar]
- Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, et al. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6:28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G, Buret A, Batey RT, Chen QY, Couch L, Cripps A, et al. Morphological, phenotypic and functional characteristics of a pure population of CD56+CD16−CD3− large granular lymphocytes generated from human duodenal mucosa. Immunology. 1993;79:498–505. [PMC free article] [PubMed] [Google Scholar]
- Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42:511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Strominger JL. Human decidual lymphocytes and the immunobiology of pregnancy. J Reprod Immunol. 2004;62:17–18. doi: 10.1016/j.jri.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Goff OR, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Peralta C, Bashar S, Taylor S, Horrocks J, Croy BA. Trafficking of peripheral blood CD56bright cells to the decidualizing uterus—new tricks for old dogmas. J Reprod Immunol. 2005;67:21–34. doi: 10.1016/j.jri.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall MD, Clarke AG. The thymus in the mouse changes its activity during pregnancy: a study of the microenvironment. J Anat. 2000;197:393–411. doi: 10.1046/j.1469-7580.2000.19730393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc Natl Acad Sci USA. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaya K. Accumulation of uterine CD16− natural killer (NK) cells: friends, foes, or Jekyll-and-Hyde relationship for the conceptus. Immunol Invest. 2008;37:467–481. doi: 10.1080/08820130802191292. [DOI] [PubMed] [Google Scholar]
- Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol. 2006;80:572–580. doi: 10.1189/jlb.0406250. [DOI] [PubMed] [Google Scholar]
- Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol. 2006;177:8522–8530. doi: 10.4049/jimmunol.177.12.8522. [DOI] [PubMed] [Google Scholar]
- Hu Y, Eastabrook G, Tan R, MacCalman CD, Dutz JP, von Dadelszen P. Decidual NK cell-derived conditioned medium enhances capillary tube and network organization in an extravillous cytotrophoblast cell line. Placenta. 2010;31:213–221. doi: 10.1016/j.placenta.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, et al. Cytokine production by CD16−CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993;5:559–563. doi: 10.1093/intimm/5.5.559. [DOI] [PubMed] [Google Scholar]
- Kitaya K, Yasuo T, Yamaguchi T, Fushiki S, Honjo H. Genes regulated by interferon-gamma in human uterine microvascular endothelial cells. Int J Mol Med. 2007;20:689–697. [PubMed] [Google Scholar]
- Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology. 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, et al. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod. 2009;24:45–54. doi: 10.1093/humrep/den348. [DOI] [PubMed] [Google Scholar]
- Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–1322. doi: 10.1016/s0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Lothar H, Martin S, Thomas H. CD3−CD56+CD16+ natural killer cells and improvement of pregnancy outcome in IVF/ICSI failure after additional IVIG-treatment. Am J Reprod Immunol. 2010;63:263–265. doi: 10.1111/j.1600-0897.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Almog B, Shalom-Paz E, Dufort D, Tulandi T.Promoting implantation by local injury to the endometrium. Fertil Steril 2010. in press. [DOI] [PubMed]
- Kitaya K, Yasuo T. Leukocyte density and composition in human cycling endometrium with uterine fibroids. Hum Immunol. 2010;71:158–163. doi: 10.1016/j.humimm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2:S134–S139. [PubMed] [Google Scholar]
- Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin Immunol. 2010;135:84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10:1039–1042. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Shultz LD, Greiner DL. Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. . J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton P, Garcia J. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13–19. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hollander GA, Nichoglannopoulou A, Simpson SJ, Orange JS, Gutierrez-Ramos JC, et al. Natural killer cell development is blocked in the context of aberrant T lymphocyte oxtogeny. Int Immunol. 1996;8:939–951. doi: 10.1093/intimm/8.6.939. [DOI] [PubMed] [Google Scholar]
- Burke SD, Dong H, Hazan AD, Croy A. Aberrant endometrial features of pregnancy in diabetic NOD mice. Diabetes. 2007;56:2919–2926. doi: 10.2337/db07-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantakru S, Kuziel WA, Maeda N, Croy BA. A study on the density and distribution of uterine natural killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR5 and the CCR5 receptor ligand, MIP-1 alpha. J Reprod Immunol. 2001;49:33–47. doi: 10.1016/s0165-0378(00)00076-0. [DOI] [PubMed] [Google Scholar]
- Xie X, He H, Colonna M, Seya T, Takai T, Croy BA. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod. 2005;73:510–518. doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- Lewis ID, Almeida-Porada G, Du J, Lemischka IR, Moore KA, Zanjani ED, et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 2001;97:3441–3449. doi: 10.1182/blood.v97.11.3441. [DOI] [PubMed] [Google Scholar]
- Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T, Greiner DL, Shultz LD. Humanized SCID mouse models for biomedical research. Curr Top Microbiol Immunol. 2008;324:25–51. doi: 10.1007/978-3-540-75647-7_2. [DOI] [PubMed] [Google Scholar]
- Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Nutrition in the Womb: How to Reduce Chronic Disease in the Next Generation. Vols. 1–174. Portland: The Barker Foundation; 2008. [Google Scholar]
- Croy BA, Zhang J, Tayade C, Colucci F, Yadi H, Yamada AT. Analysis of uterine natural killer cells in mice. Methods Mol Biol. 2010;61:465–503. doi: 10.1007/978-1-60761-362-6_31. [DOI] [PubMed] [Google Scholar]
- Peel S. Granulated Metrial Gland Cells. New York: Springer-Verlag; 1989. pp. 1–112. [DOI] [PubMed] [Google Scholar]
- Croy BA, Xie X. In vivo models for studying homing and function of murine uterine natural killer cells. Methods Mol Med. 2006;122:77–92. doi: 10.1385/1-59259-989-3:75. [DOI] [PubMed] [Google Scholar]
- Leonard S, Murrant C, Tayade C, van den Heuvel M, Watering R, Croy BA. Mechanisms regulating immune cell contributions to spiral artery modification—facts and hypotheses—a review. Placenta. 2006;122 (Suppl A:S40–S46. doi: 10.1016/j.placenta.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mukhtar DD, Stewart I. Migration of granulated metrial gland cells from cultured explants of mouse metrial gland tissue. Cell Tissue Res. 1988;253:413–417. doi: 10.1007/BF00222298. [DOI] [PubMed] [Google Scholar]
- Hatta K, Chen Z, Carter AL, Leno-Duran E, Zhang J, Ruiz-Ruiz C, et al. Orphan receptor kinase ROR2 is expressed in the mouse uterus. Placenta. 2010;31:327–333. doi: 10.1016/j.placenta.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun R, Wei H, Wu D, Tian Z. Toll-like receptor 3 agonist enhances IFN-gamma and TNF-alpha production by murine uterine NK cells. Int Immunopharmacol. 2007;7:588–596. doi: 10.1016/j.intimp.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, et al. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol. 2007;178:4267–4275. doi: 10.4049/jimmunol.178.7.4267. [DOI] [PubMed] [Google Scholar]
- Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82:24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar S, Dinda AK, Luthra K. Differential expression of CXCR4 receptor in early and term human placenta. Placenta. 2004;25:347–351. doi: 10.1016/j.placenta.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Schneider Henning, Miller RK. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int J Dev Biol. 2010;54:367–375. doi: 10.1387/ijdb.082773hs. [DOI] [PubMed] [Google Scholar]
- von Versen-Höynck F, Rajakumar A, Bainbridge SA, Gallaher MJ, Roberts JM, Powers RW. Human placental adenosine receptor expression is elevated in preeclampsia and hypoxia increases expression of the A2A receptor. Placenta. 2009;30:434–442. doi: 10.1016/j.placenta.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman G, Murthi P, Pathirage N, Brennecke SP, Kalionis B. Downstream targets of homeobox gene HLX show altered expression in human idiopathic fetal growth restriction. Am J Pathol. 2010;176:278–287. doi: 10.2353/ajpath.2010.090187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, Huppertz B. Human placental explants in culture: approaches and assessments. Placenta. 2005;26:439–448. doi: 10.1016/j.placenta.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta. 2008;29:60–66. doi: 10.1016/j.placenta.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Mastaglio S, Bondanza A, Ponzoni M, Sanvito F, Aldrighetti L, et al. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood. 2009;113:1006–1015. doi: 10.1182/blood-2008-05-156059. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Rivera J, Schanz A, Zhou Y, Winn V, Kapidzic M, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J Clin Invest. 2006;116:2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal–fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]