Abstract
Purpose
Recently, a complement component 1 inhibitor (SERPING1) gene polymorphism was identified as a novel risk factor for age-related macular degeneration (AMD) in Caucasians. We aimed to investigate whether variations in SERPING1 are associated with typical AMD or with polypoidal choroidal vasculopathy (PCV) in a Japanese population.
Methods
We performed a case-control study in a group of Japanese patients with typical AMD (n = 401) or PCV (n = 510) and in 2 independent control groups—336 cataract patients without age-related maculopathy and 1,194 healthy Japanese individuals. Differences in the observed genotypic distribution between the case and control groups were tested using chi-square test for trend. Age and gender were adjusted using logistic regression analysis.
Results
We targeted rs2511989 as the haplotype-tagging single nucleotide polymorphism (SNP) for the SERPING1 gene, which was reported to be associated with the risk of AMD in Caucasians. Although we compared the genotypic distributions of rs2511989 in typical AMD and PCV patients against 2 independent control groups (cataract patients and healthy Japanese individuals), SERPING1 rs2511989 was not significantly associated with typical AMD (P = 0.932 and 0.513, respectively) or PCV (P = 0.505 and 0.141, respectively). After correction for age and gender differences based on a logistic regression model, the difference in genotypic distributions remained insignificant (P>0.05). Our sample size had a statistical power of more than 90% to detect an association of a risk allele with an odds ratio reported in the original studies for rs2511989 for developing AMD.
Conclusions
In the present study, we could not replicate the reported association between SERPING1 and either neovascular AMD or PCV in a Japanese population; thus, the results suggest that SERPING1 does not play a significant role in the risk of developing AMD or PCV in Japanese.
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual loss in the developed world [1]. Several genes have been reported to be associated with this disease, including complement factor H [2]–[4] and the age-related maculopathy susceptibility 2/HtrA serine peptidase 1 (ARMS2/HTRA1) region [5], [6], and subsequent studies have replicated the association between susceptibility genes and the development of AMD using a different ethnic cohort [7]–[10].
Inner choroidal vascular networks that terminate in polypoidal lesions are defined as polypoidal choroidal vasculopathy (PCV), and are typically visualized by indocyanine green angiography [11]. Whether PCV represents a subtype of neovascular AMD remains controversial; moreover, whether this condition represents inner choroidal vascular abnormalities or is a variety of choroidal neovascularization remains unknown [12]. Previous studies identified several genes that contribute to the development of PCV; however, almost all reported genetic risk factors for PCV are the same as for AMD [13]–[15], and this suggests that AMD and PCV share, at least in part, the same genetic background.
Studies in cohorts from both the United Kingdom and the United States have shown that the complement component 1 inhibitor (SERPING1) gene is positively associated with AMD [16]. However, another study in a larger cohort (n = 7723 and 2327) which involved the same population could not replicate the finding of the previous study [17], [18]. Recently, Lee et al. have shown that SERPING1 is positively associated with AMD in Caucasians [19], but whether this gene is truly associated with AMD remains controversial.
Furthermore, the association of SERPING1 with AMD has been evaluated also in Asians. Lu et al. examined the association in 194 AMD patients and 285 controls and reported that SERPING1 is not associated with AMD in the Chinese population [20]. The association between PCV and SERPING1 has also been evaluated in a smaller Chinese cohort (118 patients and 115 controls), also with negative findings [21]. So far, all Asian studies for SERPING1 did use smaller cohorts than those of original studies and not consider their statistical power. For evaluating the true gene-disease association, it would be helpful to replicate the positive association reported in previous studies using a different ethnic cohort. The aim of this study, which involved a relatively large number of participants, was to investigate whether the SERPING1 gene variants are associated with typical AMD or PCV in a Japanese population.
Materials and Methods
All procedures in this study adhered to the tenets of the Declaration of Helsinki. This study was approved by the Ethics Committee of each institute involved (Kyoto University Graduate School and Faculty of Medicine, Ethics Committee, the Ethical Committee of Fukushima Medical University, the Ethical Committee of Kobe City Medical Center General Hospital, the Ethical Committee of Ozaki Eye Hospital, the Ethical Committee of the Otsu Red Cross Hospital, the Ethical Committee of Nagahama City Hospital, and the Ethical Committee at Aichi Cancer Center). All of the patients were fully informed about the purpose and procedures of this study, and written consent was obtained from each.
In this study, 401 patients with typical AMD and 510 patients with PCV were recruited from the Department of Ophthalmology at Kyoto University Hospital, Fukushima Medical University Hospital, and Kobe City Medical Center General Hospital. The control group included 2 populations: (1) 336 individuals who underwent cataract surgery and had no age-related maculopathy (ARM) (Control 1) were recruited from the Department of Ophthalmology, Kyoto University Hospital, Ozaki Eye Hospital, Japanese Red Cross Otsu Hospital, and Nagahama City Hospital; and (2) 1194 healthy individuals who were recruited from the Aichi Cancer Center Research Institute as the general population control (Control 2). AMD and ARM were defined according to the International Classification System for ARM and AMD [22]. The diagnosis of PCV was based on indocyanine green angiography, which showed a branching vascular network that terminated in polypoidal swelling. Typical AMD were late AMD which showed classic choroidal neovascularization (CNV), occult CNV, or both. All diagnoses were made by 3 retina specialists (K.Y., A.T., and A.O.); a fourth specialist (N.Y.) was consulted when the subtype classification could not be decided on by the initial 3 reviewers. All of the subjects were unrelated and were of the Japanese descent.
Genomic DNAs were isolated from the peripheral blood of the subjects by using a DNA extraction kit (QuickGene-610L, Fujifilm, Minato, Tokyo, Japan). The samples of all the patients with typical AMD and PCV and of cataract patients were genotyped using a Taqman single nucleotide polymorphism (SNP) assay with the ABI PRISM 7700 system (Applied Biosystems, Foster City, CA). The individuals recruited from the Aichi Cancer Center Research Institute were genotyped using Illumina Human-Hap 610 chips (Illumina Inc., San Diego, CA).
Linkage disequilibrium (LD) structures across the SERPING1 gene were compared between the Caucasian and Japanese populations, using genotype data retrieved from the HapMap CEU and JPT data sets [23]. The retrieved data were loaded into Haploview to estimate LD parameters and to identify haplotype blocks [24]. Deviations in genotype distributions from the Hardy–Weinberg equilibrium (HWE) were assessed using the HWE exact test. Statistical analyses for differences in the observed genotypic distribution were performed by the chi square test for trend; logistic regression analysis was performed for age and gender adjustments. The statistical power calculation was performed using QUANTO version 1.2 [25]. P values less than 0.05 were considered statistically significant.
Results
The demographic details of the study population are presented in Table 1. Because all SNPs of the SERPING1 gene are in the same haplotype block, rs2511989 was selected as the haplotype-tagging SNP; rs2511989 was reported to be associated with the risk of AMD in previous studies [16], [19] (Fig. 1). Details of allele and genotype counts and summary statistics for rs2511989 are shown in Table 2. The success rate of genotyping of rs2511989 was 98.1%, and the distributions of the genotypes for all study groups were in the Hardy–Weinberg equilibrium (P>0.05). Although we compared the genotype distributions of rs2511989 in typical AMD and PCV patients against 2 independent control groups (cataract patients without ARM and healthy Japanese individuals), SERPING1 rs2511989 was not significantly associated with typical AMD (P = 0.932 and 0.513, respectively); furthermore, it was not significantly associated with PCV (P = 0.505 and 0.141, respectively). After correction for age and gender differences based on a logistic regression model, the difference in the genotype distributions remained insignificant (P>0.05). Table 3 shows the odds ratios adjusted for age and gender under various genetic models. We could not find a significant association in any genetic model.
Table 1. Characteristics of the Study Population.
| Cases | Controls | ||||
| tAMD | PCV | Control 1* | Control 2† | ||
| No. of participants | 401 | 510 | 336 | 1194 | |
| Age | Mean ± SD | 77.38±8.39 | 74.98±7.77 | 74.16±8.42 | 50.34±15.9 |
| Gender | Men | 287 | 372 | 142 | 493 |
| Women | 114 | 138 | 194 | 701 | |
tAMD, typical age-related macular degeneration; PCV, polypoidal choroidal vasculopathy; SD, standard deviation.
*Cataract patients without age-related maculopathy.
†Healthy Japanese individuals.
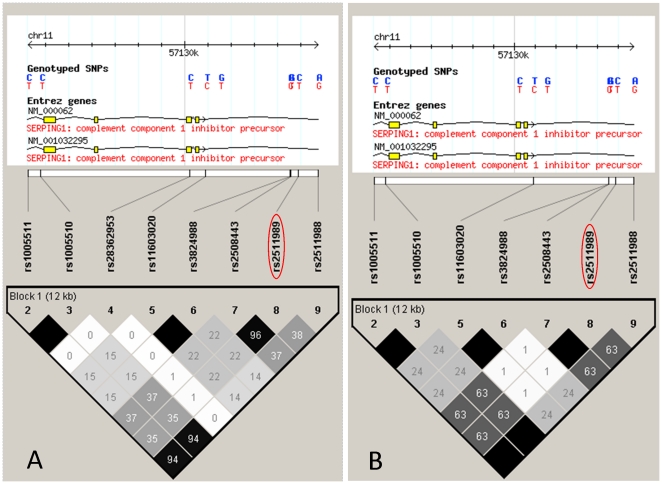
Figure 1. Linkage disequilibrium (LD) structure across the complement component 1 inhibitor (SERPING1) gene in Caucasian and Japanese populations.
Genotype data were retrieved from HapMap CEU (Utah residents with ancestry from northern and western Europe; A) and JPT (Japanese in Tokyo, Japan; B) data sets. Haplotype blocks were determined using the “four-gamete rule” option in Haploview; all HapMap single nucleotide polymorphisms on SERPING1 gene are in the same block in both populations. Each box provides estimated statistics of the coefficient of determination (r2), with darker shades representing stronger LD.
Table 2. SERPING1 rs2511989 Genotypic Distributions and Results of Association Tests and Power Analysis.
| vs Control 1 | vs Control 2 | ||||||||||
| GG | GA | AA | MAF | P Value | Adjusted P* | Power† | P Value | Adjusted P* | Power† | ||
| Cases | tAMD | 293 | 102 | 6 | 0.142 | 0.932 | 0.687 | 93.6% | 0.513 | 0.860 | 99.3% |
| PCV | 380 | 125 | 5 | 0.132 | 0.505 | 0.855 | 95.7% | 0.141 | 0.678 | 99.2% | |
| Controls | Control 1 | 248 | 76 | 10 | 0.144 | - | - | - | - | - | - |
| Control 2 | 859 | 308 | 27 | 0.152 | - | - | - | - | - | - | |
tAMD, typical age-related macular degeneration; PCV, polypoidal choroidal vasculopathy; MAF, minor allele frequency.
*Adjusted for age and gender.
†Statistical power for detecting the association reported in the previous study (odds ratio 0.63).
Table 3. Odds Ratios in Various Genetic Models.
| Adjusted Odds Ratio (95% Confidence Interval)* | |||
| Group | Model | vs tAMD | vs PCV |
| Control 1 | Additive | 0.938 (0.687–1.281) | 0.972 (0.72–1.312) |
| Dominant | 1.283 (0.746–2.204) | 0.598 (0.338–1.056) | |
| Recessive | 0.934 (0.783–1.114) | 1.283 (0.746–2.204) | |
| Control 2 | Additive | 1.034 (0.716–1.491) | 0.933 (0.673–1.294) |
| Dominant | 0.940 (0.470–1.879) | 0.709 (0.349–1.440) | |
| Recessive | 1.025 (0.839–1.254) | 0.983 (0.823–1.174) | |
*Adjusted for age and gender.
tAMD, typical age-related macular degeneration; PCV, polypoidal choroidal vasculopathy.
Next, we calculated our statistical power to detect an association of a risk allele with the odds ratio reported in the previous study that investigated the association of rs2511989 with developing AMD. When we targeted the original study reported by Ennis (odds ratio 0.63) [16], our sample size had more than 90% power to detect the association (Table 2). In addition, the statistical power calculation revealed that our sample size could detect the gene-disease association for an odds ratio of 0.797 by more than 80%.
Discussion
In the present study, we investigated whether SERPING1 gene variants are associated with typical AMD or with PCV in a Japanese population. We selected rs2511989 as the haplotype-tagging SNP, because this has been reported to be positively associated with the risk of AMD in Caucasians. The results of this study showed that SERPING1 rs2511989 was not associated with the risk for typical AMD in a Japanese population; thus, the results did not support the hypothesis that an association between the SERPING1 gene and AMD exists. Our sample size had more than 90% power to detect the association determined in the previous study in a Caucasian cohort (odds ratio 0.63) [16]. Furthermore, we found no evidence to support the role played by SERPING1 rs2511989 in the susceptibility to PCV, and this finding is in agreement with that of the previous study in a Chinese population [21].
The reported association between AMD and SERPING1 rs2511989 is shown in Table 4. The size of our Japanese cohort was similar to that of the original study [16]. Furthermore, the statistical power calculation revealed that our sample size could detect the gene-disease association for an odds ratio of 0.797 by more than 80%. Had there been a true protective effect of SERPING1 gene variants for developing AMD at the same level as was reported in previous studies [16], [19], the statistical power of our study would have detected such an association. Differences in the ethnicities of subjects might be 1 reason for the difference observed between the results of this study in a Japanese cohort and those of the previous study in a Caucasian cohort. Frequency of the minor allele of rs2511989 was reportedly greater in the earlier study in a Caucasian population than that of the present study in a Japanese population. In fact, in reference to the allele frequency data from the HapMap, all genetic variants across the SERPING1 gene showed smaller minor allele frequency in Japanese than in Caucasians.
Table 4. Comparison of Association Observed between AMD and SERPING1 rs2511989.
| Subject Group | Current Study (JP) | Mayo Subjects (US) | AREDS Subjects (US) | Ennis et al. (UK) | Ennis et al. (US) | Lee et al. (US) | Lu et al. (CH) | |||||||||
| Subjects | Case | Control 1 | Control 2 | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | |
| No. of participants | 401 | 336 | 1194 | 470 | 310 | 1221 | 295 | 479 | 479 | 248 | 252 | 556 | 256 | 194 | 285 | |
| Allele count | G | 688 | 572 | 2026 | 569 | 363 | 1435 | 357 | 597 | 500 | 322 | 282 | 669 | 283 | 336 | 493 |
| A | 114 | 96 | 362 | 371 | 257 | 1007 | 233 | 355 | 454 | 174 | 222 | 413 | 229 | 52 | 69 | |
| Genotype count | GG | 293 | 248 | 859 | 179 | 103 | 436 | 115 | 191 | 132 | 100 | 79 | 213 | 74 | 147 | 215 |
| GA | 102 | 76 | 308 | 211 | 157 | 563 | 127 | 215 | 236 | 122 | 124 | 273 | 135 | 42 | 63 | |
| AA | 6 | 10 | 27 | 80 | 50 | 222 | 53 | 70 | 109 | 26 | 49 | 70 | 47 | 5 | 3 | |
| MAF | 0.142 | 0.144 | 0.152 | 0.395 | 0.415 | 0.412 | 0.395 | 0.373 | 0.475 | 0.351 | 0.441 | 0.382 | 0.447 | 0.134 | 0.123 | |
| P values | - | 0.932 | 0.513 | - | 0.46 | - | 0.41 | - | 5.4×10−6 | - | 0.0037 | - | 0.011 | - | 0.61 | |
MAF, minor allele frequency.
Another possible explanation for the differences between our findings and those of other studies in different ethnic cohorts may include a difference in the phenotypes of AMD. Numerous studies have reported that distinguishing features of Asian AMD include male predominance, unilateral presentation, comparatively low incidence of soft drusen, and greater prevalence of neovascular AMD and PCV [26]–[29]. To address these concerns, we classified AMD patients into those with typical AMD and those with PCV, but the possible hidden differences in the phenotypes cannot be excluded. Alternatively, considering the fact that genetic variants that are associated with a particular disease in 1 population may not necessarily be associated in another population [30]–[32]; moreover, it is possible that gene-disease association of SERPING1 in populations from East Asia is very weak or absent as compared with Caucasian populations.
In this study, we used general population-based controls (Control 2). The possibility exists that some of the eyes in the control 2 group might have or develop AMD or PCV, and this might be a possible explanation for the negative results in this study. However, because the prevalence of AMD in the general population is estimated to be 0.5% in the Japanese population [33], the loss of the statistical power of association analysis must be negligible. In addition, we also performed a subset analysis on controls 2 with 55 years of age or older to minimize the possibility that some of the eyes in the control group might develop AMD or PCV. However, no new significant differences in the genotypic distributions were found in the current study (data not shown). Thus, we concluded that the result of the analysis using control 2 is valuable as reference data which supports a lack of association between SERPING1 and both typical AMD and PCV in a Japanese population. Another limitation is about geographical difference of Control 1, which may influence genetic background of the participants. However, because the Japanese population has been reported to have a rather small genetic diversity, according to data from the SNP discovery project in Japan [34], geographical difference should not be affect our statistical results.
In conclusion, this study showed a lack of association between SERPING1 and both typical AMD and PCV in a Japanese population; thus, the results suggest that SERPING1 does not play a significant role in the risk of developing AMD or PCV in Japanese.
Acknowledgments
We thank the patients and the controls who participated in this study, as well as Takahisa Kawaguchi at the Center for Genomic Medicine/Inserm U.852 for his assistance in data management. We also thank the following clinicians for their help in the recruitment of patients and controls for our study: Dr. Hiroshi Tamura and Dr. Sotaro Ooto, Kyoto University Hospital; Dr. Yasuo Kurimoto, Kobe City Medical Center General Hospital; Dr. Kuniharu Saito, Fukushima Medical University; Dr. Mineo Ozaki, Ozaki Eye Hospital; Dr. Shoji Kuriyama, Otsu Red-Cross Hospital; and Dr. Yoshiki Ueda, Nagahama City Hospital.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported in part by grants-in-aid for scientific research (Nos. 19390442, 22791706, and 27091294) from the Japan Society for the Promotion of Science, Tokyo, Japan, and by the Japanese National Society for the Prevention of Blindness. No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 2.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 4.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 6.Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 7.Seitsonen S, Lemmela S, Holopainen J, Tommila P, Ranta P, et al. Analysis of variants in the complement factor H, the elongation of very long chain fatty acids-like 4 and the hemicentin 1 genes of age-related macular degeneration in the Finnish population. Mol Vis. 2006;12:796–801. [PubMed] [Google Scholar]
- 8.Gotoh N, Nakanishi H, Hayashi H, Yamada R, Otani A, et al. ARMS2 (LOC387715) variants in Japanese patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;147:1037–1041. doi: 10.1016/j.ajo.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Yamashiro K, Gotoh N, Nakanishi H, Nakata I, et al. CFH and ARMS2 Variations in age-related macular degeneration, polypoidal choroidal vasculopathy, and retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2010;51:5914–5919. doi: 10.1167/iovs.10-5554. [DOI] [PubMed] [Google Scholar]
- 10.Simonelli F, Frisso G, Testa F, di Fiore R, Vitale DF, et al. Polymorphism p.402Y>H in the complement factor H protein is a risk factor for age related macular degeneration in an Italian population. Br J Ophthalmol. 2006;90:1142–1145. doi: 10.1136/bjo.2006.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–1396. doi: 10.1001/archopht.121.10.1392. [DOI] [PubMed] [Google Scholar]
- 12.Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002;86:321–327. doi: 10.1136/bjo.86.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo N, Honda S, Kuno S, Negi A. Coding variant I62V in the complement factor H gene is strongly associated with polypoidal choroidal vasculopathy. Ophthalmology. 2009;116:304–310. doi: 10.1016/j.ophtha.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh N, Yamada R, Nakanishi H, Saito M, Iida T, et al. Correlation between CFH Y402H and HTRA1 rs11200638 genotype to typical exudative age-related macular degeneration and polypoidal choroidal vasculopathy phenotype in the Japanese population. Clin Experiment Ophthalmol. 2008;36:437–442. [PubMed] [Google Scholar]
- 15.Lee KY, Vithana EN, Mathur R, Yong VH, Yeo IY, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008;49:2613–2619. doi: 10.1167/iovs.07-0860. [DOI] [PubMed] [Google Scholar]
- 16.Ennis S, Jomary C, Mullins R, Cree A, Chen X, et al. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008;372:1828–1834. doi: 10.1016/S0140-6736(08)61348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allikmets R, Dean M, Hageman GS, Baird PN, Klaver CC, et al. The SERPING1 gene and age-related macular degeneration. Lancet. 2009;374:875–876. doi: 10.1016/S0140-6736(09)61618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park KH, Ryu E, Tosakulwong N, Wu Y, Edwards AO. Common variation in the SERPING1 gene is not associated with age-related macular degeneration in two independent groups of subjects. Mol Vis. 2009;15:200–207. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AY, Kulkarni M, Fang AM, Edelstein S, Osborn MP, et al. The effect of genetic variants in SERPING1 on the risk of neovascular age-related macular degeneration. Br J Ophthalmol. 2010;94:915–917. doi: 10.1136/bjo.2009.172007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu F, Zhao P, Fan Y, Tang S, Hu J, et al. An association study of SERPING1 gene and age-related macular degeneration in a Han Chinese population. Mol Vis. 2010;16:1–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Wen F, Zuo C, Zhang X, Chen H, et al. SERPING1 polymorphisms in polypoidal choroidal vasculopathy. Mol Vis. 2010;16:231–239. [PMC free article] [PubMed] [Google Scholar]
- 22.Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 23.International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Statistics in Medicine. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- 26.Chang TS, Hay D, Courtright P. Age-related macular degeneration in Chinese-Canadians. Can J Ophthalmol. 1999;34:266–271. [PubMed] [Google Scholar]
- 27.Bird AC. The Bowman lecture. Towards an understanding of age-related macular disease. Eye (Lond) 2003;17:457–466. doi: 10.1038/sj.eye.6700562. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Horie-Inoue K, Gehlbach PL, Takita H, Kabasawa S, et al. Phenotype and genotype characteristics of age-related macular degeneration in a Japanese population. Ophthalmology. 2010;117:928–938. doi: 10.1016/j.ophtha.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22. doi: 10.1016/j.ajo.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 30.Helgason A, Pálsson S, Thorleifsson G, Grant SF, Emilsson V, et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet. 2007;39:218–225. doi: 10.1038/ng1960. [DOI] [PubMed] [Google Scholar]
- 31.Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, et al. Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia. 2007;50:63–67. doi: 10.1007/s00125-006-0502-2. [DOI] [PubMed] [Google Scholar]
- 32.Horikoshi M, Hara K, Ito C, Nagai R, Froguel P, et al. A genetic variation of the transcription factor 7-like 2 gene is associated with risk of type 2 diabetes in the Japanese population. Diabetologia. 2007;50:747–751. doi: 10.1007/s00125-006-0588-6. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki R, Wang JJ, Ji GJ, Taylor B, Oizumi T, et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology. 2008;115 doi: 10.1016/j.ophtha.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Haga H, Yamada R, Ohnishi Y, Nakamura Y, Tanaka T. Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet. 2002;47:605–610. doi: 10.1007/s100380200092. [DOI] [PubMed] [Google Scholar]