Abstract
With age, cardiac performance declines progressively and the risk of heart disease, a primary cause of mortality, rises dramatically. As the elderly population continues to increase, it is critical to gain a better understanding of the genetic influences and modulatory factors that impact cardiac aging. In an attempt to determine the relevance and utility of the Drosophila heart in unraveling the genetic mechanisms underlying cardiac aging, a variety of heart performance assays have recently been developed to quantify Drosophila heart performance that permit the use of the fruit fly to investigate the heart’s decline with age. As for the human heart, Drosophila heart function also deteriorates with age. Notably, with progressive age the incidence of cardiac arrhythmias, myofibrillar disorganization and susceptibility to heart dysfunction and failure all increase significantly. We review here the evidence for an involvement of the insulin-TOR pathway, the KATP channel subunit dSur, the KCNQ potassium channel, as well as Dystrophin and Myosin in fly cardiac aging, and discuss the utility of the Drosophila heart model for cardiac aging studies.
1-Introduction
Cardiac aging is regarded as the progressive and deleterious decline of heart function. In mammals, decline in cardiac function is accompanied by many age-related changes, such as decreases in cardiomyocyte number, left-ventricular hypertrophy, fibrosis and accumulation of collagen [1-3]. Cardiac aging contributes to increased cardiovascular mortality and morbidity in elderly humans, and because human lifespan is continuously rising, mortality associated with heart disease is dramatically increasing. In this regard, it is becoming increasingly critical to understand the mechanisms of cardiac aging. However, studies on fundamental mechanisms of cardiac aging in mammals are complicated by two main issues. First, heart function is exquisitely critical for mammalian viability. Second, the long life span of mammals makes them somewhat impractical for studying the genetics of aging.
Many genes controlling heart development have been shown to be conserved from flies to mammals, since the first identification of the Drosophila homeobox gene tinman that led to the cloning of the vertebrate homolog Nkx2-5, which has been shown to control cardiac development in mice [4-8]. More recently, Drosophila adult heart has been used to investigate cardiac function and aging [9, 10]. Fruit flies have an open circulatory system, with a linear heart tube located along the dorsal midline of the abdomen and an aorta that extends anteriorly into the head. Three sets of internal valves divide the abdominal heart into an anterior conical chamber and three posterior compartments. Each of the four heart compartments also contains a pair of valves to the exterior, called ostia that permit hemolymph to enter the heart during relaxation. Because Drosophila uses a tracheal system to transport oxygen directly to tissues, heart function is not as tightly coupled to survival as it is in vertebrates. Thus, more severe effects of genetic manipulation of heart physiology can be examined in flies than in mammals. Similar to larger vertebrates heart models, pharmacological manipulation can also be used to investigate cardiac aging in flies [11-13]. These advantages and the genetic power of Drosophila make this model very suitable to investigate the pathophysiology and genetics of cardiac aging.
2- Cardiac aging features of the fly
a - Decrease in stress resistance with age
Methods using electrical pacing, elevated temperature and hypoxia treatments were developed to investigate cardiac performance under stress conditions. The pacing protocol stresses the heart by roughly doubling its heart rate for a set time (eg. 30 sec) and heart function is then monitored to determine whether this treatment causes arrest or fibrillation of the fly’s heart (‘heart failure’). Strikingly, the percentage of flies that exhibit such heart failure increases progressively with age from 20-35 % in 1-2 week old flies to 65-85% in 5-7 week old flies, suggesting that cardiac performance also declines with age in flies [14]. This aspect of fly heart function is observed in both genders and in different genetic backgrounds considered wildtype. Interestingly, using this pacing protocol it has been determined that exercise-training in young flies reduces the age-related decline in cardiac performance [15], which is similar to the trend observed in mammals [16]. In a similar electrical pacing assay or when stressing the fly by elevating ambient temperature, it was found that the maximal beating rate a heart can achieve declined with age [17]. These observations are similar to the age-associated decrease in maximum heart rate observed in humans [18]. Another way of looking at heart activity under stress conditions is to analyze cardiac function under hypoxia treatment. Upon removal of oxygen, the hearts temporarily stop beating in both young and old flies but recover after a short period of time. This recovery of heart activity after hypoxia was much better in young flies, which begin beating within a minute, compared to older flies, where it takes much longer for the heart to recover [11].
b - Increase in arrhythmia with age
In order to precisely quantify heart contraction parameters, an optical heartbeat analysis methodology has been developed by K. Ocorr and co-workers [10]. In intact flies, heart beat frequencies alternate between faster and slower rates, probably because of neuronal and hormonal input [19-21]. To study the inherent myogenic contraction parameters without confounding influences from neuronal input, a semi-intact fly preparation was developed, in which the heart is surgically exposed and most neuronal inputs to the heart are disrupted [10]. A computerized analysis program was created to process these recordings, permitting quantification of a number of contraction parameters including arrhythmia [22]. It was found that 1-week-old hearts show rhythmic contractions at relatively constant rates. The highly rhythmic beating pattern deteriorates as flies age, and by 5–7 weeks, a majority of wildtype flies exhibit non-rhythmical heart contraction patterns, including frequent asystoles and fibrillations [10]. The observed changes in cardiac rhythmicity with age are reminiscent of the increased incidence of atrial fibrillation and heart failure in the aging human population [2].
c - Myofibrillar organization
Drosophila heart tubes have two types of muscle fibers, each with distinct myofibrillar structures [23, 24]: 1) Spirally or transversely oriented myofibrils that represent the contractile ‘working’ myocardium expressing the transcription factor tinman; 2) Longitudinally oriented myofibrils that are found along the ventral surface of the tube [25], whose origin and exact function have not yet been determined. Both types of myofibrils in young flies exhibit a tight and well-aligned arrangement that can be visualized with sarcomeric markers such as alpha-actinin [23]. At older ages, the spiral myofibrils become somewhat more disorganized, which is manifest in a less compact arrangement with spaces between myofibrils [24]. Similarly, it has been shown that in old canine atria the myocardial fibers are also less compacted [26], suggesting that the causes of structural changes in myofibrillar structure observed with age in fly hearts are relevant to mammalian cardiac aging. It will be interesting to see whether other aspects of structural remodeling seen during senescence of the mammalian heart, such as fibrosis and accumulation of collagen [27, 28], also occur and can be linked to dysfunction in old fly hearts.
3-Genes Involved in Drosophila cardiac aging
a - Insulin/TOR
The insulin-IGF receptor (InR) signaling plays a conserved role in regulating lifespan. Systemic inhibition of this pathway has been shown to extend lifespan in yeast, C. elegans, Drosophila and mice [29]. However, its involvement in the regulation of the aging process within individual organs is much less understood. In Drosophila, the lifespan extension observed in mutants for genes encoding the insulin receptor or its substrate (chico) is accompanied by a delay in cardiac aging [14], which is exemplified by the observation that cardiac performance of 7-week old flies is similar to 1-week control flies. Strikingly, this reduction in cardiac function decline is also observed when the insulin signaling pathway is reduced only in the cardiomyocytes, which is achieved by heart-specific overexpression of either dPTEN, a phosphatase that inhibits the PI3 kinase, or the transcription factor dFoxO, both negative modulators of insulin signal transduction. Remarkably, cardiac specific overexpression of dPTEN or dFoxO does not affect overall lifespan [14], which suggests that manipulating cardiac performance alone does not necessarily alter overall longevity and that the effect of the insulin pathway on cardiac aging can be uncoupled from the systemic effect of this signaling pathway on lifespan.
Systemic reduction of the nutrient sensing protein kinase Target of Rapamycin (TOR) has also been shown to extend lifespan and to delay cardiac aging [29]. The TOR and insulin pathways are interconnected at many levels, suggestive of the notion that both pathways may control cardiac aging via epistatic interactions and regulation of common targets. Experiments carried out by Wessells et al (2009) suggest that d4eBP acts in cardiac tissue downstream of these two pathways [30]. Indeed, the cardiac aging phenotypes caused by either cardiac manipulation of dFoxO or dTOR are suppressed by simultaneous d4eBP/eif4E manipulations [30]. Thus, the delay in cardiac aging observed when the insulin or the TOR pathways are downregulated may ultimately be due to an increase in d4eBP activity (Figure 1).
Figure 1.
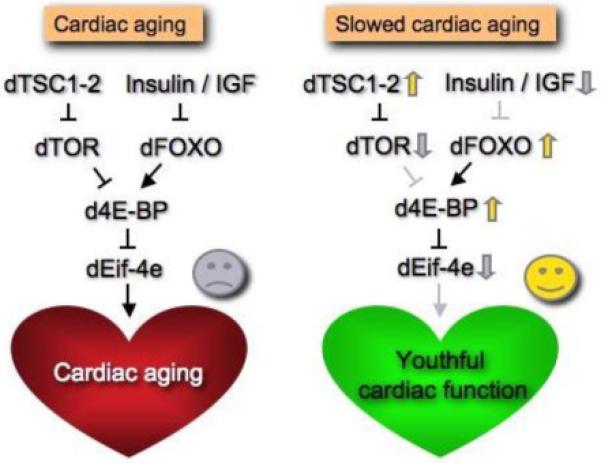
Model of the effects of insulin/TOR signaling on cardiac aging.
The insulin/TOR signaling is necessary for normal cardiac senescence to occur. A critical target of that pathway is d4eBP whose negative regulation in cardiac tissue is required for normal cardiac senescence to occur (red heart). In our model cardiac aging can be delayed when d4eBP function is maintained, either by increasing dFOXO expression or by reducing insulin-TOR pathway activation (green heart).
b -Ions channels
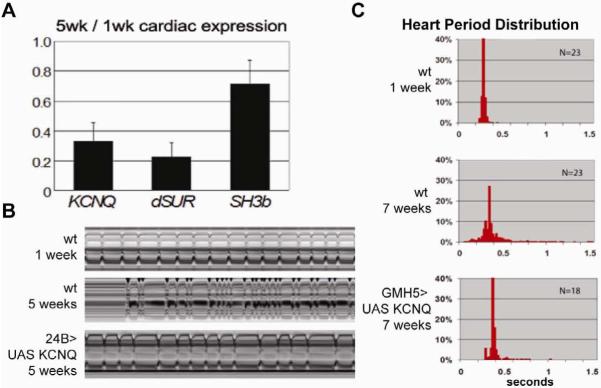
Despite its small size, the Drosophila heart is also well suited for analysis of gene expression changes in young versus old fly hearts. For example, the age-dependent decline in heart performance described earlier parallels the age-related decrease in cardiac RNA levels of the Drosophila homolog of human KCNQ1, encoding a potassium channel alpha subunit, and of a ATP-sensitive potassium channel (KATP) subunit dSur [10]. In humans, KCNQ1 is involved in myocardial repolarization and alterations in the function of this channel are associated with an increase risk for arrhythmia and sudden cardiac death [31]. In flies, cardiac KCNQ expression levels are reduced with age (Figure 2A), and KCNQ deficiency leads to compromised heart function characterized by an accelerated increase in the age-related incidence of arrhythmia and an increased susceptibility to pacing-induced cardiac dysfunction [10]. As in humans, these abnormalities can be explained by an insufficiency in repolarization capacity of the cardiac action potential due to KCNQ deficiency. Strikingly, heart specific KCNQ overexpression in old wildtype flies suppresses the elevated level of arrhythmias, suggesting that the normally occurring decrease in KCNQ expression within the aging heart contributes to the increased incidence of arrhythmias (Figure 2B & C) [10].
Figure 2.
Rescue of functional decline during cardiac aging by KCNQ expression
(A) KCNQ, dSUR expression decreases with age. RNA levels in isolated hearts were measured using qRT-PCR. KCNQ and dSUR RNA levels in hearts from 5-week old flies are decreased to 33% and 23%, respectively, of the levels in 1 week old flies (mean +/− independent experiments, a control gene SH3β was only reduced to 72% of 1-week levels. (B) Representative 10 sec M-modes made from wildtype flies and a fly in which a wildtype KCNQ cDNA is driven by the mesodermal/heart Gal4 driver 24B. Overexpression of KCNQ channels partially rescued the arrhythmic phenotype normally seen in 5 week old flies (bottom trace). (C) Heart-specific over-expression of KCNQ cDNA using the GMH5 driver13 rescues the age-related increases in arrhythmia. Hearts from 1 and 7 week old flies were filmed and the distribution of the heart beat lengths (heart periods) for each group of flies was plotted. Note that heart-specific over-expression of KCNQ reduces the variability of the heart period in (7week) old flies, to be similar to young flies. From ref.10.
Cardiac expression of dSur at 5 weeks of age is also dramatically decreased (Figure 2A). Heart-specific RNAi-mediated knockdown or pharmacological manipulation of dSur suggested that dSur protects cardiac functionality from pacing stress- and hypoxic stress-induced dysfunction [11], which is again reminiscent of the role of mammalian KATP channels [32]. For example, exposure of old flies to pinacidil, a KATP channel activator, reduces their sensitivity to pacing-induced dysfunction [11]. Together, this suggests that dSur activity contributes to youthful cardiac performance, which then declines with age. Thus, we speculate that the heart’s performance in old flies would be improved with cardiac overexpression of dSur, as with KCNQ described above.
c - Structural and contractile proteins
Mutations in Dystrophin (Dys) which codes for a component of the dystrophin-glycoprotein complex that links the extracellular matrix to the subsarcolemmal cytoskeleton are known to cause muscular dystrophy [33]. Flies deficient for Dys exhibit a dilated phenotype, as well as a premature disruption of cardiac myofibrillar organization that is evident already at young ages [24]. Furthermore, the myofibrillar disorganization becomes progressively more severe in older Dys mutant flies, showing large gaps between myocardial myofibrils. This suggests that Dys is required to maintain the physical integrity of the heart muscle and that Dys deficiency may also accelerate cardiac structural senescence. In addition, while heart rate typically is decreased in old wildtype flies, the rate in Dys mutants is faster and does not decrease with age. In sum, the observed age-dependent changes in Dys deficiency flies indicate that the mammalian dystrophic heart may experience a similar decline with age, and these changes are suggestive of an acceleration of at least some aspects of cardiac senescence,
In contrast to Dys mutants, a mutation in muscle myosin that depresses myosin activity in vitro (D45) shows a slowing of the heart rate, which is more severe in old flies compared to the wild type [34]. These mutants also exhibit more arrhythmic beating patterns than wildtype flies as early as middle age (3 week old flies), and the normal age-related increase in arrhythmia appears to be accelerated in these flies. Most strikingly, the heart tubes from D45 mutants are considerably dilated compared to wildtype hearts at all ages, although the myofibrillar structure appears normal, at least in young flies. This mutation also results in a decrease in contractility of the heart, which is measured as the fractional shortening (FS). Interestingly, although FS normally declines with age in wildtype flies, as it does in humans, there is no age-related decline in this parameter in these hypoactive myosin mutants.
A characteristic of human cardiac aging is a reduction in the number of cardiomyocytes as well as a moderate left-ventricular hypertrophy [3], which has not (yet) been observed in hearts of wildtype flies. It may be that loss of cardiomycytes in the fly heart, which contains only 50 pairs of post-mitotic cells along the heart tube, would be expected to be immediately and severely detrimental. Indeed, this phenotype has only been observed in severe mutant situations (eg. adult tinman deficiency [35]. In another hand, it is important to consider that flies have an exoskeleton (external cuticle) that makes the adult fly refractory to additional whole body and tissue growth following eclosion. Consequently, growth-related cardiac hypertrophy (in the classical sense) at advanced ages may not easily be inducible or evident in insects. It remains to be determined what the equivalent of ‘cardiac hypertrophy’ is in the aging fly heart.
4-Conclusion and Future Perspectives
Drosophila is emerging as an excellent genetic model for studying cardiac aging. The discovery of new genes regulating cardiac aging is facilitated by the fly’s short lifespan and the new tools developed recently to study cardiac performance.
The aging Drosophila heart will be a useful model to determine how genes that have a demonstrated role in modulating organismal aging specifically influence heart function, how they interact, and how different aspects of cardiac function might be linked (eg. myofibrillar organization and ion channel expression). For example, is the expression of dSur and KCNQ maintained when cardiac aging is delayed by reducing activity in insulin or TOR pathways? Does KCNQ overexpression, which improves cardiac performance in old flies (see Fig. 2) [9], also ameliorate age-dependent myofibrillar disorganization?
Furthermore, the Drosophila heart is also ideally suited for testing the capacity of new drug candidates to delay or rescue – in the context of a whole organism –the decrease in heart performance that characterizes cardiac aging. The potential cardioactive effects of drugs on the heart can be easily assessed in the fly either by adding them to the food and monitoring the effect on heart performance throughout life using intact and semi-intact heart preparations , or by directly adding the drug to the artificial hemolymph that perfusates the exposed fly heart preparation during the heart beat recording [10, 11, 22, 36]. These methods of drug administration, combined with the new analysis tools that have been developed to study cardiac function and aging are promising ways to quantitatively assess drug effects on heart performance. The conserved aspects of heart function between Drosophila and mammals indicates that the fly heart preparation could be used for primary screening of the arrhythmogenic potential of drugs in the context of a functional organ/organism; in addition the ability to insert human genes into the fly should extend this capability significantly.
The Drosophila heart model provides an excellent system in which it will be possible to elucidate the (cardiac-autonomous) role of insulin-TOR signaling in cardiac aging [13, 28]. Similar studies in vertebrate models are complicated by the additional ‘beneficial’ role IGF signaling plays in post-natal growth and damage repair. The post-natal role of insulin/IGF signaling in the fly heart appears to be confined to cardiac aging, since there is no evidence that increasing adult levels of cardiac insulin signaling has any effect on heart size. In future studies of cardiac aging in the fly model it will be interesting to compare heart-specific expression profiles between young and old flies under different genetic conditions. Such mRNAs arrays have already been successfully used to identify genes involved in whole organism aging and might be expected to provide gene candidates relevant for cardiac-specific aging [37].
Acknowledgments
R.B. is supported by grants from NHLBI (NIH), Glenn/AFAR, MDA and the Ellison Foundation. K.O. is supported by a Scientist Development Grant from AHA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan AS, et al. Growth hormone, insulin–like growth factor–1 and the aging cardiovascular system. Cardiovasc Res. 2002;54(1):25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 3.Olivetti G, et al. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 4.Bodmer R, Jan LY, Jan YN. A new homeobox–containing gene, msh–2, is transiently expressed early during mesoderm formation of Drosophila. Development. 1990;110(3):661–9. doi: 10.1242/dev.110.3.661. [DOI] [PubMed] [Google Scholar]
- 5.Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev Genet. 1998;22(3):181–6. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Komuro I, Izumo S. Csx: a murine homeobox–containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993;90(17):8145–9. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lints TJ, et al. Nkx–2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119(2):419–31. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- 8.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313(5795):1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocorr K, et al. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc Med. 2007;17(5):177–82. doi: 10.1016/j.tcm.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ocorr K, et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci U S A. 2007;104(10):3943–8. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akasaka T, et al. The ATP–sensitive potassium (KATP) channel–encoded dSUR gene is required for Drosophila heart function and is regulated by tinman. Proc Natl Acad Sci U S A. 2006;103(32):11999–2004. doi: 10.1073/pnas.0603098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson E, et al. Genetic and pharmacological identification of ion channels central to the Drosophila cardiac pacemaker. J Neurogenet. 1998;12(1):1–24. doi: 10.3109/01677069809108552. [DOI] [PubMed] [Google Scholar]
- 13.Johnson E, Ringo J, Dowse H. Modulation of Drosophila heartbeat by neurotransmitters. J Comp Physiol B. 1997;167(2):89–97. doi: 10.1007/s003600050051. [DOI] [PubMed] [Google Scholar]
- 14.Wessels A, Perez–Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276(1):43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 15.Piazza N, et al. Exercise–training in young Drosophila melanogaster reduces age–related decline in mobility and cardiac performance. PLoS One. 2009;4(6):e5886. doi: 10.1371/journal.pone.0005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascensao A, Ferreira R, Magalhaes J. Exercise–induced cardioprotection--biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol. 2007;117(1):16–30. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 17.Paternostro G, et al. Age–associated cardiac dysfunction in Drosophila melanogaster. Circ Res. 2001;88(10):1053–8. doi: 10.1161/hh1001.090857. [DOI] [PubMed] [Google Scholar]
- 18.Fleg JL, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78(3):890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 19.Dulcis D, Levine RB. Glutamatergic innervation of the heart initiates retrograde contractions in adult Drosophila melanogaster. J Neurosci. 2005;25(2):271–80. doi: 10.1523/JNEUROSCI.2906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulcis D, Levine RB, Ewer J. Role of the neuropeptide CCAP in Drosophila cardiac function. J Neurobiol. 2005;64(3):259–74. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- 21.Johnson E, Ringo J, Dowse H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J Insect Physiol. 2000;46(8):1229–1236. doi: 10.1016/s0022-1910(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 22.Fink M, et al. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46(2):101–13. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mery A, et al. The Drosophila muscle LIM protein, Mlp84B, is essential for cardiac function. J Exp Biol. 2008;211(Pt 1):15–23. doi: 10.1242/jeb.012435. [DOI] [PubMed] [Google Scholar]
- 24.Taghli–Lamallem O, et al. Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell. 2008;7(2):237–49. doi: 10.1111/j.1474-9726.2008.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina MR, Cripps RM. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev. 2001;109(1):51–9. doi: 10.1016/s0925-4773(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 26.Anyukhovsky EP, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54(2):462–9. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 27.Everett T.H.t., Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S24–7. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellman J, Lyon RC, Sheikh F. Extracellular matrix remodeling in atrial fibrosis: mechanisms and implications in atrial fibrillation. J Mol Cell Cardiol. 2010;48(3):461–7. doi: 10.1016/j.yjmcc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 30.Wessells R, et al. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8(5):542–52. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109(4):509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 34.Cammarato A, et al. Myosin transducer mutations differentially affect motor function, myofibril structure, and the performance of skeletal and cardiac muscles. Mol Biol Cell. 2008;19(2):553–62. doi: 10.1091/mbc.E07-09-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaffran S, et al. Cardioblast-intrinsic Tinman activity controls proper diversification and differentiation of myocardial cells in Drosophila. Development. 2006;133(20):4073–83. doi: 10.1242/dev.02586. [DOI] [PubMed] [Google Scholar]
- 36.Akasaka T, Ocorr K. Drug discovery through functional screening in the Drosophila heart. Methods Mol Biol. 2009;577:235–49. doi: 10.1007/978-1-60761-232-2_18. [DOI] [PubMed] [Google Scholar]
- 37.Murphy CT, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–83. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]