Abstract
The molecular basis for psychosocial-distress mediated immune-dysregulation is not well understood. The purpose of this study was to determine whether peripheral blood mononuclear cell (PBMC) epigenetic pattern associates with this form of immune dysregulation. Women newly diagnosed with early stage breast cancer were enrolled into the study and psychosocial, immunological and epigenetic assessments were made at diagnosis and four months later, after completion of cancer treatment. At diagnosis women reported increased perceived stress, anxiety, and mood disturbance and the PBMC of these women exhibited reduced natural killer cell activity and reduced production of interferon gamma, which contrasted with results, obtained after completion of treatment. At the epigenetic level, a PBMC subset derived from women at diagnosis exhibited a distinct epigenetic pattern, with reduced nuclear acetylation of histone residues H4-K8 and H4-K12, as well as reduced phosphorylation of H3-S10, when compared to similar cells derived after the completion of treatment. Natural killer cell activity and interferon-gamma production were associated with nuclear acetylation and phosphorylation status of these histone residues. These findings demonstrate associations among nuclear epigenetic pattern and the immune dysregulation that accompanies psychosocial distress.
Keywords: NK Cell Activity, Epigenetic, Histone Acetylation and Phosphorylation, Interferon gamma
1. Introduction
A diagnosis of breast cancer and the challenge of cancer treatment can be emotionally devastating and are often accompanied by elevations in perceived stress, anxiety and mood disturbance (National Cancer Institute, 1997; Witek-Janusek et al., 2007; Shapiro et al., 2001). Ample evidence demonstrates that psychosocial distress negatively impacts immune function (Witek-Janusek and Mathews, 2000; Kemeny and Schedlowski, 2007; Glaser and Kiecolt-Glaser, 2005; Segerstrom and Miller, 2004). The impact on immune function has been demonstrated to include reductions in natural killer cell activity (NKCA) (Biondi, 2001; Kiecolt-Glaser et al., 1987; Witek-Janusek et al., 2007; Witek-Janusek et al., 2008), alteration of cytokine production (Maes et al., 1999; Marshall et al., 1998; Witek-Janusek et al., 2007; Esterling et al., 1994; Witek-Janusek et al., 2008), decreased PBMC proliferative response to mitogens (Kiecolt-Glaser et al., 1991; Andersen et al., 1998; Andersen et al., 2004), reductions in specific B- and T-lymphocyte mediated immune responses (Kiecolt-Glaser et al., 1996), decreased production of interferon (IFN) gamma (Witek-Janusek et al., 2007; Glaser et al., 1986; Larson et al., 2000), and diminished NK cell response to cytokines (Andersen et al., 1998; Fawzy et al., 1990).
Stress-related impairment of immune function is evident in women with breast cancer. For example, we have previously reported that women undergoing breast cancer diagnosis and treatment exhibit increased anxiety and mood disturbance, accompanied by reduced NKCA and reduced production of IFN gamma (Witek-Janusek et al., 2007; Witek-Janusek et al., 2008). Others have shown that among women with breast cancer, those who report greater subjective distress after their breast cancer surgery have lower basal and IFN augmented NKCA and reduced T cell proliferative responses to mitogens (Andersen et al., 1998; Andersen et al., 2004). Because NK cells and IFN gamma could potentially contribute to surveillance and destruction of tumor cells (Dighe et al., 1994; Kagi et al., 1994; Kaplan et al., 1998; Smyth et al., 1998; Smyth et al., 1999; Street et al., 2001; Van Den Broek et al., 1996; Seki et al., 2003; Smyth et al., 2005; Wallace and Smyth, 2005), stress-induced immune dysregulation may jeopardize cancer control, especially during vulnerable period such as during and immediately post-treatment (Lutgendorf et al., 2007; Avraham and Ben-Eliyahu, 2007).
The molecular basis for the effects of psychosocial distress on immune function, relevant to cancer control, is unclear. For early stage breast cancer patients, a tumor is removed from the breast and the primary site and adjacent tissues are irradiated. These procedures are thought to eliminate any nascent tumor cells. Prognosis is good, but the disease can recur after apparent successful eradication of the tumor. Recurrence occurs in some women but not in others. The reasons for this disparity in recurrence are not known and are certainly multiple. However, it is possible that psychosocial distress mediated immune-dysregulation is a contributing factor. Therefore, explicating the molecular basis for this immune-dysregulation is a significant objective. One unexplored possibility is that psychosocial distress induces epigenetic modifications that alter transcription of relevant immune effector genes. Post translational modifications, regulate chromatin accessibility through modification of histone residues, which can limit production of immune effector molecules necessary to optimal immune function (Krukowski et al., 2010,Krukowski et al., in press). A series of studies have demonstrated epigenetic modifications to be responsive to environmental stimuli (Zhang and Meaney, 2010) and evidence demonstrates that psychological stressors result in epigenetic modification, which modify or impact glucocorticoid response pathways (Bilang-Bleuel et al., 2005; Chandramohan et al., 2007; Chandramohan et al., 2008). Psychosocial distress activates the hypothalamic-pituitary-adrenocortical (HPA) axis resulting in elevations in circulating levels of glucocorticoids that have strong immuno-modulatory effect. It is possible that stress-induced elevations in glucocorticoids (Chrousos and Gold, 1992; Chrousos, 2000) may mediate the immune dysregulation observed in women with breast cancer through epigenetic modifications.
Indeed, we have shown that a diagnosis of breast cancer is accompanied by elevations in plasma cortisol that persist over several months (Witek-Janusek et al., 2007; Witek-Janusek et al., 2008). Glucocorticoid can produce deacetylation of histones with reduced expression of immune response genes (Barnes, 1998; Kagoshima et al., 2003). This effect has been demonstrated with deacetylation of lysine residues of histone H4K-8 and K-12, resulting in suppressed gene transcription, including reduced NKCA and IFN gamma production (Krukowski et al., 2010,Krukowski et al., in press). Such deacetylation results in epigenetic repression of the associated genes (Kagoshima et al., 2001; Mishra et al., 2001). It is possible that similar epigenetic effects may mediate the effects of psychosocial distress on immune function. Hence, this study evaluated the epigenetic pattern of the PBMC of women experiencing psychosocial distress, as a consequence of breast cancer diagnosis, and compared that to the epigenetic pattern of the PBMC of women at a time after treatment, when the psychosocial response to cancer had dissipated and immune function had been improved. As such, the purpose of this study was to evaluate associations among epigenetic patterns and immune function during a period of psychosocial distress. In this study that period was designated P1.
2. Materials and Methods
2.1. Subjects
Thirty-three women (35–75 years of age) diagnosed with early stage breast cancer, who had breast conserving surgery and did not receive systemic chemotherapy, but whose treatment did include radiotherapy, participated in this study. These women were all diagnosed with stage 0 ductal carcinoma in situ (DCIS) and all had small lesions (< 1 cm) and uninvolved axillary lymph nodes. Women were excluded if they had an immune-based disease, were incapable of reading and writing English, were diagnosed with immune-based disorders, psychoses, anxiety disorders or cognitive impairments, were substance abusers, had a history of acute infection or were taking corticosteroids. The demographic characteristics of these women are described in Table 1. This study was approved by the Loyola University Medical Center Institutional Review Board for the Study of Human Subjects. All procedures were carried out with the adequate understanding and written consent of the subjects.
Table 1.
Demographics of Subjects
| Characteristics | Women N=33 | |
|---|---|---|
| Mean | SE | |
| Age (years) | 55.6 | 1.6 |
| Weight (lbs.) | 174.6 | 6.8 |
| Body Mass Index (Kg/m2) | 29.2 | 1.0 |
| Education (Total Years) | 14.8 | 0.5 |
| Marital Status | ||
| Married | 56.8% | |
| Single | 43.2% | |
| Employment | ||
| Employed | 70.3% | |
| Unemployed | 29.7% | |
| Race | ||
| Caucasian | 83.8% | |
| African-American | 10.8% | |
| Hispanic | 5.4% | |
| Familial Cancer | ||
| History | ||
| Yes | 81.1% | |
| No | 18.1% | |
2.2.1. Recruitment
Eligible women were identified in association with clinic physicians after completion of their breast surgery and when their full surgical pathology report was available and diagnosis and treatment plans were complete. Interested women were met by research clinical associates at a scheduled clinic visit. The purpose and nature of the study was further described and those willing to participate were consented. Data were collected at 3–4 weeks after the final cancer diagnosis was made (referred to as P1), which was 10–12 days post breast surgery and before any adjuvant therapy (i.e., radiotherapy) was initiated. This time period has been shown previously to a period of elevated anxiety, mood disturbance and perceived stress as well as immune dysregulation (Witek-Janusek et al., 2007). Data were collected as well approximately 4 months after P1, which was 2 months after the completion of radiotherapy (referred to as P2). This time period has been shown previously to be a period of reduced psychosocial distress and normalized immune function, similar to that of cancer free women (Witek-Janusek et al., 2007).
2.2 Psychosocial measures
For the purposes of this study the psychosocial distress associated with breast cancer was conceptualized as a state evoked by an event appraised as a threat to the individual’s well-being, which, in turn, led to feelings of anxiety and mood disturbance. With this in mind, the psychosocial assessment of these women included measurement of perceived stress (Perceived Stress Scale), mood state (Profile of Mood States) and anxiety (State-Trait Anxiety Inventory).
2.2.1. Perceived Stress Scale (PSS)
The PSS is a 10-item scale that provides a general appraisal index of stress (Monroe and Kelley, 1995). It measures the degree to which experiences are appraised as uncontrollable. For the PSS women were instructed to rate their degree of perceived stress over the past month. Internal consistency is good with coefficient alphas ranging from 0.75–0.86. Test-retest reliability has been reported to be 0.85 (Cohen and Williamson, 1988).
2.2.2. Profile of Mood States (POMS)
The POMS is a 65-item measure designed to identify and assess general distress/mood. A total mood score was obtained. Women were asked to indicate the extent to which each of the scale’s 65 adjectives of mood describe the way they had been feeling during the past week, on a scale ranging from 0 (not at all) to 4 (extremely). A total mood disturbance score is derived by summing each of the six subscales, with vigor weighted negatively. The possible range of scores is −40 through 192. Internal consistency alphas range from 0.87–0.95, and stability coefficients (test-retest) are 0.65–0.74 (Mcnair et al., 1992).
2.2.3. Spielberger State-Trait Anxiety Inventory (Anxiety)
The State-Trait Anxiety Inventory is a 40-item self-report measure of state and trait anxiety. Only the state anxiety inventory was used in this study. The State-Trait Anxiety Inventory has alpha reliability coefficients of 0.83–0.92 and convergent validity with other anxiety tools (alpha =0.75–0.80) (Spielberger et al., 1970). The State-Trait Anxiety Inventory has been used to discriminate anxiety in women with benign versus malignant breast biopsy findings (Sachs et al., 1995).
2.3. Immune measures
2.3.1. Isolation of peripheral blood mononuclear cells
Blood was collected in sterile heparinized tubes and processed immediately. Heparinized peripheral blood was overlaid onto Ficoll/Hypaque and centrifuged at 1,000 x g for 20 min. The peripheral blood mononuclear cells (PBMC) at the interface were washed twice with Hank’s Balanced Salt Solution prior to assessment of NKCA, cytokine production, or phenotypic analysis. Phenotypic analysis was as described previously (Nagabhushan et al., 2001). Briefly, isolated PBMCs were analyzed with specific fluorochrome conjugated antibodies (BD Biosciences, San Jose, CA) in order to identify specific subsets of PBMCs including: CD3 for T lymphocytes, CD56 for NK cells; CD3 and CD56 for NKT cells; CD4 for helper lymphocytes; CD8 for cytotoxic lymphocytes, and CD14 single positive cells were identified as circulating monocytes. Semi-quantitative analysis of the phenotypic expression of relevant leukocyte surface molecules was determined by immunofluorescence using a FACS Star Plus System. Interassay variability for PBMC laboratory values ranged from 1.5 – 7.9%. Essentially all women completed these psychosocial assessments, but not all immune assays were completed for all women due to limitations with regard to total volume of blood obtained for a particular assessment period.
2.3.2. Natural killer cell activity
K562 tumor cells, obtained from the American Type Culture Collection, Rockville, MD, were radioactively labeled with 100 uCi of [51Cr] (New England Nuclear, Boston, MA). Radiolabled K562 cells were incubated for 4 hr with PBMC. Following incubation the supernatants were removed using a Skatron harvesting press (Skatron Inc., Sterling, VA) and the associated radioactivity was determined. E:T ratios for NKCA were 50, 25, 12 and 6:1.
Results are expressed as % cytotoxicity and calculated by the formula:
All experimental means were calculated from triplicate values. Lytic units (LU) were calculated by a program written by David Coggins, FCRC, Frederick, MD and represents the number of cells per 107 effectors required to achieve 20% lysis of the targets. *DPM=disintegrations per minute.
2.3.3. Evaluation of PBMC for cytokine production
Interferon (IFN) gamma was measured under optimal conditions in bulk PBMC culture supernatant fluids as described previously (Witek-Janusek and Mathews, 1999). Briefly, PBMC (1 × 106 cells/ml) were cultured with and without PMA/PHA (PMA @ 20 ng/well; PHA @ 0.05%/well) in 24 well plates for 48 hr at 37° C. Aliquots of the culture supernatants were stored at –80° C for subsequent cytokine analysis.
2.3.4. Cytokine measurement (ELISA)
IFN gamma was measured using quantitative sandwich enzyme immunoassay (Quantikine kits, R & D Systems). Sensitivity for IFN gamma was <3 pg/ml. Coefficient of variation was 2.6%.
2.3.5. Immunofluorescent Flow Cytometric Analysis of Intra-Nuclear Epigenetic Pattern and Cytoplasmic IFN gamma
PBMC (1.0 × 105/assessment) were fixed and permeabilized with Cytofix/Cytoperm solution (BD Pharmingen, San Jose, CA) for 20 min at 4°C. The cells were then washed twice with Perm/Wash Buffer (BD Biosciences, San Jose, CA) and then probed with antibodies specific for molecules of interest. The antibodies used were anti-acetyl-histone H4-K8 (H4-K8 Ac) (Millipore, Temecula, CA) (unconjugated); anti-acetyl-histone H4-K12 (H4-K12 Ac) (Millipore, Temecula, CA) (unconjugated); anti-phospho-histone H3-S10 (H3-S10 PO4) (Alexa Fluor 488 conjugated) (Cell Signaling, Danvers, MA). Isotype matched control antibody (mouse IgG1, IgG2b or rabbit IgG; BD Bioscience, San Jose, CA & Millipore, Temecula, CA) were used to ensure specificity. The cells were then washed twice with Perm/Wash Buffer (BD Biosciences, San Jose, CA), after which, secondary anti-IgG (FITC conjugated) (Millipore, Temecula, CA) was added for 1 hr at 4°C as appropriate. The cells were then washed twice with Perm/Wash Buffer (BD Biosciences, San Jose, CA) resuspended in 0.1% BSA (Sigma Aldrich, St. Louis, MO) in PBS (Gibco, Grand Island, NY). For intracellular cytokine analysis of IFN gamma, cells were incubated in leukocyte activation cocktail (BD Bioscience, San Jose, CA) at 37°C for 3 hrs prior to permeabilization and antibody staining. Cells were then permeabilized and incubated with PE conjugated antibodies specific for IFN gamma (BD Bioscience, San Jose, CA) for 1 hr at 4°C as well as with antibodies specific for individual surface molecules. Cells were then washed twice with 0.1% BSA (Sigma Aldrich, St. Louis, MO) in PBS (Gibco, Grand Island, NY) and resuspended in 1% paraformaldehyde (PFA) (Sigma Aldrich, St. Louis, MO). Samples were analyzed with a FACSCanto Fluorescence-Activated Cell Sorter equipped with a 15mW argon-ion laser and a red diode laser using FACSDiva software for data acquisition (Beno et al., 1995; Yamamura et al., 1995; Nagabhushan et al., 2001). After staining, cells were analyzed by flow cytometry. 10,000–30,000 events were recorded and analyzed with FlowJo v8.4.1. Fluorescent activated cell sorting was used to isolate individual cell populations in order to confirm flow cytometric analysis by microscopy. Individual cell populations were analyzed by immunofluorescent microscopy and/or by confocal immunofluorescent microscopy. Nuclei were identified by staining with 4 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 10 min.
2.4. Statistical methods
Data are expressed as means with the standard error of the mean (SEM). Missing data were treated as such and no imputation techniques were used. A two-sided alpha of 0.05 was set for statistical significance. Comparison of data (P1) to 4 months later (P2) was by paired t test, two tailed. Associations were examined by Pearson’s correlation coefficient and an alpha of p < 0.05 was applied. The Statistical Package for Social Sciences (SPSS: version 15.0) was used for data analysis.
3. Results
Psychosocial Assessments
Table 2 illustrates the mean scores for anxiety, mood disturbance and perceived stress of women 3–4 weeks after cancer diagnosis (referred to as P1) and 2 months after the completion of radiotherapy and approximately 4 months after enrollment (referred to as P2). Total mood disturbance (POMS-TMD), state anxiety (STAI) and perceived stress (PSS) were significantly elevated at P1 for women diagnosed with breast cancer, when compared to P2.
Table 2.
Mood Disturbance, Perceived Stress, and Anxiety at Diagnosis (P1) and After Completion of Cancer Treatment (P2)
| Psychological Measure | Psychological Score |
||||
|---|---|---|---|---|---|
| P1 | P2 | t | df | p | |
| (mean ± SEM) [95% CI] |
|||||
| Total Mood Disturbance | 34.4 ± 5.9 | 18.9 ± 4.2 | −2.089 | 31 | 0.031 |
| [1.4–29.7 ] | |||||
| Perceived Stress | 20.4 ± 1.2 | 16.9 ± 1.1 | −2.059 | 31 | 0.042 |
| [0.1–6.8 ] | |||||
| Anxiety | 49.7 ± 2.2 | 38.2 ± 1.7 | −2.660 | 31 | 0.012 |
| [2.6–20.3 ] | |||||
P1= after surgery but before radiation therapy, P2 = 2 months after the completion of radiation therapy. N = 33.
Immnological Assessments
Natural killer cell activity (NKCA) is presented in Table 3 along with PBMC production of IFN gamma. At P1 NKCA and IFN gamma production were significantly reduced when compared to P2. Analysis of circulating PBMC subset number and percentage showed no difference between P1 and P2. Furthermore, there was no difference in the numbers of circulating CD56 bright or dim NK cells.
Table 3.
NK Cell Activity and IFN-gamma Production by PBMCs at Diagnosis (P1) and After Completion of Cancer Treatment (P2)
| Immunological Measure | Lytic Units or ng per ml |
||||
|---|---|---|---|---|---|
| P1 | P2 | t | df | p | |
| (mean ± SEM) [95% CI] |
|||||
| NKCA | 43.6 ± 2.6 | 98.9 ±5.3 | −10.809 | 26 | <0.001 |
| [44.7 – 65.8] | |||||
| IFN-gamma | 4.5 ± 0.4 | 12.7± 0.5 | −10.556 | 25 | <0.001 |
| [6.6 – 9.8] | |||||
NKCA, natural killer cell activity as lytic units at 20% lysis, N= 28.
IFN-gamma, as ng/ml, N=27.
P1= after surgery but before radiation therapy, P2 = 2 months after the completion of radiation therapy.
Epigenetic Assessments
The epigenetic pattern, as global mean fluorescence intensity (MFI) for CD56+ lymphocytes derived from these women, is presented in Table 4. A reduction in the acetylated forms of H4-K8 and H4-K12 was demonstrated at P1 compared to P2. A numerical reduction in phosphorylated H3-S10 was observed at P1 compared to P2.
Table 4.
Mean Fluorescent Intensity of CD56+ Lymphocytes at Diagnosis (P1) and After Completion of Cancer Treatment (P2)
| Histone Residue | Mean Fluorescent Intensity (MFI) |
||||
|---|---|---|---|---|---|
| P1 | P2 | t | df | p | |
| CD56+ | (mean ± SEM) [95% CI] |
||||
| H4-K8 Ac | 2,871 ±343 | 4,662 ± 579 | 2.660 | 28 | 0.013 |
| [1.4–29.7] | |||||
| H4-K12 Ac | 2,286 ± 325 | 4,054 ± 576 | 2.673 | 29 | 0.012 |
| [412–3,124] | |||||
| H3-S10 PO4 | 1,485 ± 360 | 1,885 ± 597 | 1.788 | 30 | 0.084 |
| [−86–1,305] | |||||
Isotype control antibody exhibited a mean fluorescent intensity (MFI) < 150.
P1= after surgery but before radiation therapy, P2 = 2 months after the completion of radiation therapy.
H4-K8 Ac, N=30, H4-K12 Ac, N= 31, H3-S10 P04, N=32.
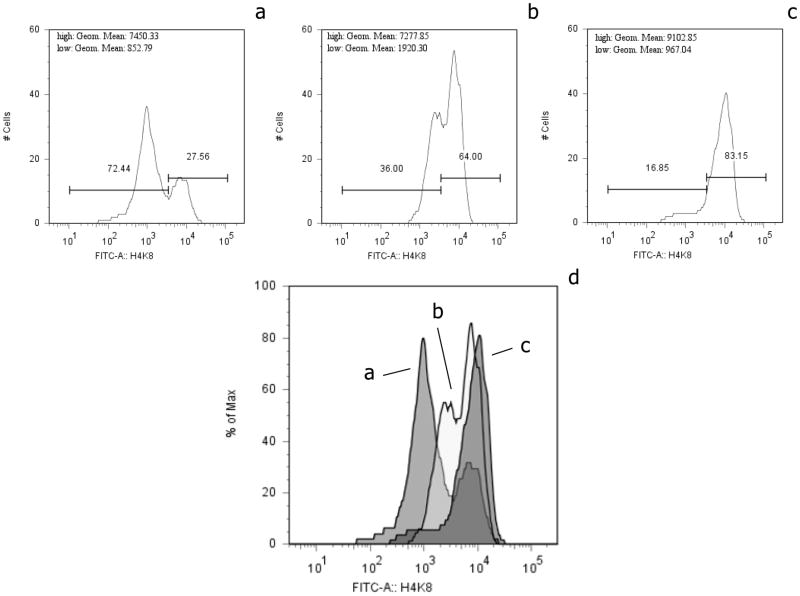
No correlation was found among epigenetic pattern and psychosocial measures. However, correlations were found to be significant for the PSS and immune values at P1. Scores for the PSS demonstrated correlation with NKCA ( r = −0.344, p = 0.043) and with IFN gamma production ( r = −0.478, p = 0.021). (No other psychosocial measure demonstrated significant correlation with immune values.) However, based on these observations, examples of flow cytometric patterns of PBMC derived from women with a spectrum of perceived stress scores were selected. In Figure 1, individual examples of flow cytometric epigenetic patterns for nuclear fluorescence of H4-K8 Ac of CD56+ lymphocytes are depicted. In panel (a) the histogram of cellular fluorescence is depicted for a woman with a high perceived stress score (global MFI = 1,549). In panel (b) the histogram of cellular fluorescence is depicted for a woman with an intermediate level of perceived stress (global MFI = 4,504) and in panel (c) the histogram of cellular fluorescence is depicted for a woman with a low perceived stress score (global MFI = 6,328). Panel (d) superimposes the three histograms.
Figure 1.
Examples of flow cytometric immunofluorescence with fluorochrome conjugated anti-H4-K8 Ac for CD56+ lymphocytes. Panel (a) is a histogram of cellular fluorescence depicted for a woman with a high perceived stress score (global MFI = 1,549). In panel (b) the histogram of cellular fluorescence is depicted for a woman with an intermediate level of perceived stress (global MFI = 4,504) and in panel (c) the histogram of cellular fluorescence is depicted for a woman with a low perceived stress score (global MFI = 6,328). Data are presented as the # of cells X 102. The geometric mean for each peak of immunofluorescence is presented for each panel as well as the percentage of the total cells within each of the selected cell populations. High: Geom. (geometric) mean refers to the cell peak with the greatest degree of fluorescence and Low: Geom. mean refers to the cell peak with the least degree of fluorescence. The MFI is indicated for each of these peaks, high and low. Panel (d) is a composite of panels (a-c) and depicts global MFIs. Isotype control antibody exhibited an MFI < 150.
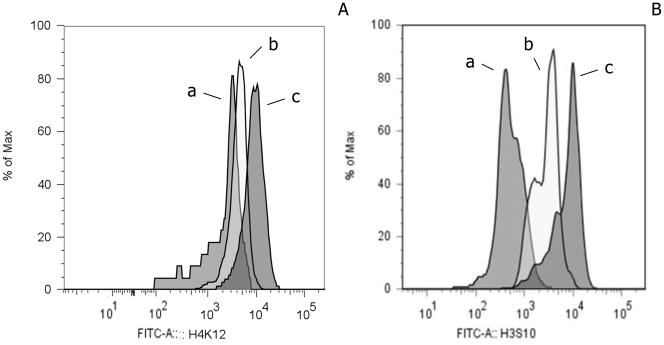
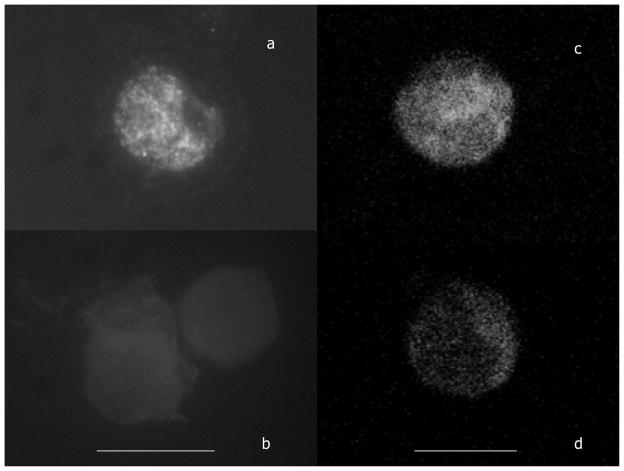
It is apparent that these histograms have two peaks of immunofluorescence, with a shift from the higher intensity peak to the lower intensity peaks with increased perceived stress. Global H4-K12 Ac and H3-S10 P04 flow cytometric epigenetic analysis of the same examples of CD56+ lymphocytes is presented in Figure 2. In each case the least MFI was demonstrable with the greatest degree of perceived stress. For global H4-K12 Ac (Figure 2A) the pattern for women with the greatest degree of perceived stress is represented by pattern (a) (global MFI = 1,499), an intermediate level of perceived stress by pattern (b) (global MFI = 4,043) and the least perceived stress by pattern (c) (global MFI = 8,245). For global H3-S10 PO4 (Figure 2B) the pattern for women with the greatest degree of perceived stress is represented by pattern (a) (global MFI = 280), an intermediate level of perceived stress by pattern (b) (global MFI = 2112) and the least perceived stress by pattern (c) (global MFI = 7,416). A comparison of fluorescently stained nuclei of these PBMCs is depicted in Figure 3. Figure 3a and c depict the fluorescence microscopic appearance of CD56+ lymphocyte nuclei derived from a woman with low perceived stress and high MFI. Figure 3b and d depict CD56+ lymphocyte nuclei from a woman with high perceived stress and a low MFI. The immunofluorescent microscopic images demonstrate the fluorescence to be primarily within the nuclei of the cell in Figure 3a, with little or no fluorescence detected in Figure 3b. The confocal microscopic images demonstrate that the fluorescence of the nuclei is distributed throughout the central z plane of the nuclei, Figure 3c compared to Figure 3d. Similar results were obtained with anti- H4K8 AC and anti-H3S10 PO4. Data are not shown. Other histone residues as well have been evaluated for the CD56+ lymphocytes including; acetylated H3-K9, H3-K14, H4-K5, H4-K16 and no differential effect was found when comparing the nuclear staining of PBMCs derived from women with and without perceived stress. In some cases nuclear staining was demonstrable for both groups of women at an equivalent level (i.e. H4-K5 and H4-K16). In other cases no staining of the nuclei was observed (i.e.. H3-K9 and H3-K14). In all cases, similar primary, secondary and isotype control antibodies were employed ensuring that the capacity to stain the nuclei was equivalent and specific.
Figure 2.
Examples of flow cytometric immunofluorescence with fluorochrome conjugated anti-H4-K12 Ac or anti-H3-S10 P04 for CD56+ lymphocytes. Panel (A) global H4-K12 Ac pattern for lymphocytes derived from a woman with of the greatest perceived stress score is represented by pattern (a) (global MFI = 1,499), an intermediate level by pattern (b) (global MFI = 4,043) and the least perceived stress score by pattern (c) (global MFI = 8,245). Data are presented as the percentage of maximum cell number. (B) Global H3-S10 PO4 pattern for lymphocytes from a woman with the greatest perceived stress score is represented by pattern (a) (global MFI = 280), an intermediate level by pattern (b) (global MFI = 2112) and the least perceived stress score by pattern (c) (global MFI = 7416). Isotype control antibody exhibited an MFI < 150.
Figure 3.
Nuclear immunofluorescence of CD56+ lymphocytes stained with fluorochrome conjugated anti H4-K12 Ac. Figure 3a and c depict the fluorescence microscopic appearance of CD56+ lymphocyte nuclei derived from a woman with a high MFI and Figure 3b and d depict the fluorescence microscopic images of CD56+ lymphocyte nuclei derived from a woman with low MFI. (a and b) are microscopic images of entire cells stained as well with DAPI to identify nuclear DNA, (c and d) are z patterns through the center of nuclei. These images are provided in color as supplemental material online. The line for the microscopic images is 10 microns and for the confocal images is 7 microns.
NKCA is carried out exclusively by CD56+ lymphocytes while IFN gamma production can be by CD4+ and CD8+ T lymphocytes as well as CD56+ lymphocytes. Comparison of the percentage of CD56+ lymphocytes producing intracellular IFN gamma showed a difference P1 to (9.6 + 5.2 % CD56+ IFN gamma+) to P2 (17.5 + 9.4 % CD56+ IFN gamma+), t = −3.298, df = 21, p = 0.002, while no difference was observed P1 to P2 for intracellular IFN gamma for either the CD4+ or CD8+ lymphocyte populations (p > 0.05).
Correlations were explored among the immunological variables and the histone epigenetic marks at P1 as judged by global MFI. Three epigenetic marks were evaluated for NKCA and the same three were evaluated for IFN gamma production. See Table 5. Phosphorylation of H3-S10 and acetylation of H4 residues K8 and K12 were correlated with NKCA and with IFN gamma production. No correlations among these variables were observed at P2 when psychosocial distress was reduced and immune function was increased.
Table 5.
Correlations Among Mean Fluorescent Intensity of Histone Residues and Immune Function At Cancer Diagnosis (P1)
| Histone Residue | IFN gamma | NKCA | ||
|---|---|---|---|---|
| r | p | r | p | |
| CD56+ | ||||
| H4-K8 Ac | 0.786 | 0.001 | 0.493 | 0.027 |
| H4-K12 Ac | 0.633 | 0.003 | 0.556 | 0.025 |
| H3-S10 P04 | 0.476 | 0.040 | 0.576 | 0.006 |
4. Discussion
Given the pervasiveness of psychosocial distress in society, and the importance of the immune system to health, explicating the molecular mechanisms by which distress disrupts immune function may have implications for cancer patients. For the women assessed by this study, total mood disturbance, state anxiety and perceived stress were significantly elevated at P1 when compared to P2. The elevated anxiety, mood disturbance and perceived stress at P1 were similar to that of a group of women who completed similar analysis at the time of breast cancer diagnosis (Witek-Janusek et al., 2007). In that study and in this, NKCA and IFN gamma production by PBMC (at P1) were significantly reduced when compared to a time period 2 months after the completion of radiotherapy (P2). Analysis of circulating PBMC subset number and percentage showed no difference between P1 and P2 and suggests that the reduction in NKCA and in IFN gamma was an actual reduction in functional activity and not in circulating PBMC subset numbers. Significantly, this immune dysregulation was accompanied by reductions in nuclear acetylated H4-K8 and H4-K12 derived at P1. For the PBMC subset analyzed, significant correlations were found among immune functional activity and the acetylation of H4 residues K8 and K12 and the phosphorylation of H3-S10. In comparison to P1, increased immune functional activity and significantly increased nuclear acetylation of H4-K8 and H4-K12 and increased phosphorylation of H3-S10 was observed at P2. These data suggest associations among nuclear epigenetic pattern and the immune effects that accompany psychosocial distress.
Epigenetics refers to a variety of processes that affect gene expression independent of actual DNA sequence. Most importantly, recent evidence demonstrates that epigenetic information is susceptible to change in response to environmental stimuli, including behavior and stressful experiences. For example, a variety of animal models of psychosocial stress, such as chronic social defeat (Tsankova et al., 2006; Wilkinson et al., 2009), social isolation (Wilkinson et al., 2009), forced swimming and rodent exposure to predators (Bilang-Bleuel et al., 2005) document that epigenetic modification are dynamic and responsive to adverse environmental events and, furthermore, that such modification relate to anxiety-like behaviors and depression (Tsankova et al., 2006; Wilkinson et al., 2009). Differential effects have also been demonstrated for acute versus chronic restraint stress (Hunter et al., 2009), which substantiate that stress-elicited epigenetic modifications can occur rapidly, and are sensitive to stressor duration (Hunter et al., 2009). Although the vast majority of stress-induced chromatin remodeling has been demonstrated in the brain, those studies are relevant to this in that collectively the data reported in those studies indicate that epigenetic modification contributes to the overall adaptive response to stress. However, there is scant evidence that psychosocial distress, maladaptive behaviors or emotions, produce epigenetic modifications that impact immune function; even though significant literature links each of these. However, evidence does exist that epigenetic pattern influences differentiation of T and B-lymphocytes as well as the fate and function of individual immune cell populations (Cuddapah et al., 2010; Martino and Prescott, 2010). Further, PBMC derived from individuals with post traumatic stress disorder (PTSD) have been demonstrated to exhibit differential epigenetic modification of genes that encode for immune effector function, when compared to PBMC derived from individuals without PTSD. The affected genes were significantly and negatively correlated with traumatic burden (i.e., number of traumatic event exposure). Moreover, the unique modification was also associated with differences in immune responsiveness to cytomegalovirus, a latent herpes virus. These results imply that immune dysfunction observed in those with PTSD is related to epigenetic pattern (Wrona, 2006). We have shown previously that the psychosocial distress of breast cancer diagnosis is associated with reduced immune function (Witek-Janusek et al., 2007; Witek-Janusek et al., 2008) and the data presented herein show an association with epigenetic modification of circulating immune cells.
It is important to note that the underlying cell and molecular mechanisms by which psychosocial distress adversely affects the immune system are unclear. However, functional genomic analyses have demonstrated alterations in the expression of genes regulated by the glucocorticoid receptor (GR) and NFkB in the PBMC of individuals experiencing prolonged chronic stressors or social isolation, with significant modification of glucocorticoid response element (GRE) mediated transcriptional activity (Miller et al., 2008; Cole et al., 2007). The modification of GRE mediated transcriptional activity was not explained by GR mRNA levels and the mechanistic basis for those observations remains unclear. However, one possible explanation is that the reduction relates to the transactional effects of GR recruitment of enzymes that modify acetylation and/or phosphorylation of histone residues, essential to promoter region transcriptional activity. The data reported herein suggest that epigenetic modification of immune cells may underlie immune effects observed in women responding to the stressful experience of breast cancer diagnosis.
Women enrolled into this study had data collected 3–4 weeks after cancer diagnosis and 10–12 days post breast surgery (referred to as P1). An interval of 10–12 days after surgery provides a time period in which ample evidence shows that the effects of surgery, anesthesia and analgesia, on immune function, dissipate (Whelan et al., 2003; Wichmann et al., 2003; Mokart et al., 2002; Decker et al., 1996; Koda et al., 1997; Kutza et al., 1997; Pollock et al., 1991; Koltun et al., 1996; Shakhar and Ben-Eliyahu, 2003; Spies et al., 2004; Homburger and Meiler, 2006). These women were not confounded by the effect of metastatic or advanced breast cancer nor did they receive adjuvant therapy. As such, this subject sample was well suited for the analysis of the molecular mechanisms whereby psychosocial distress affects immune function. Data were collected as well 4 months after P1, which was 2 months after the completion of radiotherapy (referred to as P2). At this time period the women, as a group, showed a quantitative reduction in psychosocial distress and increased immune function.
For non-housekeeping genes, transcriptional activation through sequence-specific DNA-binding proteins can localize histone acetyltransferases or phosphorylases (Henikoff and Ahmad, 2005; Lieb and Clarke, 2005; Reinke and Horz, 2003). These enzymes modify histone residues, which then adapt the promoter regions for transcription. These histone acetyl and phosphate groups are present while transcription is active and removed when transcription is no longer active. These acetylated histone residues act as epigenetic marks for transcription (Govind et al., 2007). Such transient epigenetic change has been described for the T lymphocyte IL-2 locus (Thomas et al., 2005). Genomic DNA spanning the 700-bp region upstream of the transcriptional start site for the IL-2 gene is completely inaccessible in naïve T lymphocytes, indicating that the IL-2 locus in these cells exists in a highly compact and condensed chromatin structure. Following cellular activation, the entire upstream region became accessible, suggesting that the chromatin encompassing the entire IL-2 promoter/enhancer in these effector cells was de-condensed and wrapped loosely around free nucleosomes. The genomic region remained accessible to DNA binding proteins during IL-2 transcription. When these T lymphocytes were allowed to come to rest, that is when the activation signal was interrupted, the entire probed region upstream of the IL-2 gene returned to an inaccessible configuration similar to that in naive T lymphocytes. These observations demonstrate the dynamic nature of chromatin structure at the IL-2 promoter/enhancer and upstream regions, as well as their susceptibility to kinetic variation and effects of histone acetylation and DNA demethylation (Thomas et al., 2005). That study was accomplished in vitro and this study was accomplished with cells derived from peripheral blood over a 4-month period. The differences between the two studies are obvious, but the data reported herein are consistent with that previously published in vitro work. The results of this study do indicate relationships among epigenetic pattern and immune functional activity. However, the means by which epigenetic pattern is modified during psychosocial distress has not been established. However, it is well established that cortisol levels are dysregulated in women diagnosed with breast cancer, who exhibit psychosocial-distress, mood disturbance, and immune-dysregulation (Witek-Janusek et al., 2008). Cortisol acts by binding to cytosolic GR, which can suppress gene transcription by direct interaction with the coactivator, cyclic AMP response-element-binding protein-binding protein (CBP). This interaction inhibits CBP-associated histone acetyltransferase activity resulting in the active recruitment of histone deacetylase (Ito et al., 2000; Matthews et al., 2004; Tsaprouni et al., 2002; Espada and Esteller, 2007; Ito et al., 2006) and dephosphorylation complexes (Parra et al., 2007; Kim et al., 2008). Deacetylation and dephosphorylation lead to chromatin modification, resulting in immune dysregulation (Kagoshima et al., 2001; Mishra et al., 2001; Plesko et al., 1983). Epigenetic patterns have been shown to regulate IFN gamma production (Avni et al., 2002; Muegge et al., 2003; Mishra et al., 2001; Parra et al., 2007; Kim et al., 2008) as well as molecules that contribute to NKCA (e.g. perforin) (Krukowski et al., 2010,Krukowski et al., in press; Lu et al., 2003; Babichuk et al., 1996) and glucocorticoids are known to decrease PBMC NKCA and IFN gamma production (Mishra et al., 2001; Barnes, 1995; Witek-Janusek and Mathews, 2000; Biondi, 2001; Cippitelli et al., 1995). Reductions in PBMC NKCA and IFN gamma production may therefore be a direct consequence of epigenetic patterns influenced by exposure to cortisol, as a consequence of HPA activation (resultant from psychosocial distress). If so, then the distinctive epigenetic pattern detected in the PBMC of women who experience psychosocial distress may be a consequence of these altered cortisol levels. Most women do report emotional distress at diagnosis and most women exhibit recovery from the initial distress of breast cancer diagnosis. Reductions in perceived stress, anxiety and mood disturbance were demonstrated at P2 compared to P1 in this study and have been demonstrated in other similar investigations during such time periods (Thornton et al., 2007; Edgar et al., 1992; Ganz et al., 1996). When psychosocial distress is reduced, cortisol levels would normalize and restore immune function (Thornton et al., 2007). Although not proven in this investigation it is possible that the observed PBMC epigenetic patterns are subsequent to changes in circulating cortisol levels. It has been shown that a synthetic glucocorticoid, dexamethasone, does induce similar immune and epigenetic effects in immune cells (Krukowski et al., 2010,Krukowski et al., in press), and it may be that the distinct PBMC epigenetic patterns observed during psychosocial distress may be similarly modified by cortisol levels in response to stress-induced activation of the HPA axis.
It is acknowledged that other histone post-translational modifications exist. The number of these is quite large. For example, acetylation of H3-K9, H3-K14, H4-K5 and H4-K16 as well as methylation modifications of H3-K4 and/or H3-K9 (mono-, di- and tri-methylation) have been described and may contribute to chromatin accessibility (Schotta et al., 2004; Bjornsson et al., 2004; Shahbazian and Grunstein, 2007). Many of these histone modifications have been evaluated in the context of cell types other than human PBMC. It was not the purpose of this study to survey all such modifications, but it is worth nothing that H4-K8 and H4-K12 have been demonstrated to be hypo-acetylated in glucocorticoid treated cell populations, including PBMC (Ito et al., 2000; Matthews et al., 2004; Tsaprouni et al., 2002) and that H3-S10 phosphorylation has been associated with opening of condensed and transcriptionally active chromatin (Saccani et al., 2002; Arbibe et al., 2007). Those results are fully consistent with the observed correlations among immune function, acetylation and phosphorylation of histone residues described herein and the fact that accessible promoter regions for genes encoding immune effector molecules are necessary for optimal immune response (Krukowski et al., 2010,Krukowski et al., in press).
There are limitations to this study. Women were enrolled into the study after the diagnosis of breast cancer. As such there is no baseline data for these women. In addition, the design of the study is temporal and over a 4 month period. This design permits a comparison of the women at two separate time periods but does not permit a comparison to actual baseline values or to a non-cancer group of women. Further, the women were treated for breast cancer by radiotherapy and that treatment may have effect upon P2 outcomes, even though the women received localized radiotherapy, which was completed 2 months prior to P2. Another limitation of this study is that analysis was accomplished with whole PBMC. That is, batch preparations of PBMC were analyzed for nuclear epigenetic pattern and immune function. This approach allowed for the identification of associations among the measured aspects of epigenetic pattern and immune function, but did not allow for a correlation of epigenetic pattern and reduced immune function at the level of the individual cell. That is, no direct evidence indicates that an individual cell with a particular demonstrable epigenetic pattern also had a demonstrable modification in its immune functional capacity (e.g. a change in levels of intracellular IFN gamma). Epigenetic pattern and immune function were correlated for a PBMC subset as a whole, but the observed changes may represent a summation of the entire PBMC subset and may not be a consequence of a unique change within an individual cell.
Highlights.
The effect of psychosocial distress on epigenetic pattern and functional activity of human peripheral blood cells is demonstrated.
Individual epigenetic histone marks that are affected by psychosocial distress are identified.
Associations among individual epigenetic histone marks and functional immune activity are demonstrated.
Supplementary Material
Acknowledgments
The study was supported in part by the National Cancer Institute R21-CA–117261, R01-CA-134736 and the Research Committee of the Council, Stritch School of Medicine. The authors gratefully acknowledge the expertise of Patricia Simms Loyola University Health Systems Flow Cytometry Facility and the assistance of Valerie Bednar, RN, MSN and Jennie Johnson, RN for recruitment of subjects and collection of clinical and psychological data. Importantly, the authors express sincere gratitude to those women with breast cancer who volunteered to participate in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, Maccallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE., 3rd Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, Parsot C, Sansonetti PJ. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- Avraham R, Ben-Eliyahu S. Neuroendocrine regulation of cancer progression: II. Immunological mechanisms, clinical relevance, and prophylactic measures. In: Ader R, editor. Psychoneuroimmunology. 4. Elsevier Academic Press; Burlington, MA: 2007. pp. 251–265. [Google Scholar]
- Babichuk CK, Duggan BL, Bleackley RC. In vivo regulation of murine granzyme B gene transcription in activated primary T cells. J Biol Chem. 1996;271:16485–16493. doi: 10.1074/jbc.271.28.16485. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory mechanisms of glucocorticoids. Biochem Soc Trans. 1995;23:940–945. doi: 10.1042/bst0230940. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Beno DW, Stover AG, Mathews HL. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J Immunol. 1995;154:5273–5281. [PubMed] [Google Scholar]
- Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur J Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Biondi M. Effects of stress on immune functions: An overview. In: Ader R, Cohen N, editors. Psychoneuroimmunology. 3. Academic Press; New York: 2001. pp. 189–226. [Google Scholar]
- Bjornsson HT, Daniele Fallin M, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Chan HW, Miller JS, Moore MB, Lutz CT. Epigenetic control of highly homologous killer Ig-like receptor gene alleles. J Immunol. 2005;175:5966–5974. doi: 10.4049/jimmunol.175.9.5966. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H Spector Lecture. Ann N Y Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cippitelli M, Sica A, Viggiano V, Ye J, Ghosh P, Birrer MJ, Young HA. Negative transcriptional regulation of the interferon-gamma promoter by glucocorticoids and dominant negative mutants of c-Jun. J Biol Chem. 1995;270:12548–12556. doi: 10.1074/jbc.270.21.12548. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. The Social Psychology of Health. 1988:31–67. [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker D, Schondorf M, Bidlingmaier F, Hirner A, Von Ruecker AA. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119:316–325. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Edgar L, Rosberger Z, Nowlis D. Coping with cancer during the first year after diagnosis. Assessment and intervention. Cancer. 1992;69:817–828. doi: 10.1002/1097-0142(19920201)69:3<817::aid-cncr2820690334>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Espada J, Esteller M. Epigenetic control of nuclear architecture. Cell Mol Life Sci. 2007;64:449–457. doi: 10.1007/s00018-007-6358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterling BA, Kiecolt-Glaser JK, Bodnar JC, Glaser R. Chronic stress, social support, and persistent alterations in the natural killer cell response to cytokines in older adults. Health Psychol. 1994;13:291–298. doi: 10.1037//0278-6133.13.4.291. [DOI] [PubMed] [Google Scholar]
- Fawzy FI, Kemeny ME, Fawzy NW, Elashoff R, Morton D, Cousins N, Fahey JL. A structured psychiatric intervention for cancer patients. II. Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behav Neurosci. 1986;100:675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- Govind CK, Zhang F, Qiu H, Hofmeyer K, Hinnebusch AG. Gcn5 promotes acetylation, eviction, and methylation of nucleosomes in transcribed coding regions. Mol Cell. 2007;25:31–42. doi: 10.1016/j.molcel.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K. Assembly of variant histones into chromatin. Annu Rev Cell Dev Biol. 2005;21:133–153. doi: 10.1146/annurev.cellbio.21.012704.133518. [DOI] [PubMed] [Google Scholar]
- Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol. 2006;19:423–428. doi: 10.1097/01.aco.0000236143.61593.14. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Mccarthy KJ, Milne TA, Pfaff DW, Mcewen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kagoshima M, Ito K, Cosio B, Adcock IM. Glucocorticoid suppression of nuclear factor-kappa B: a role for histone modifications. Biochem Soc Trans. 2003;31:60–65. doi: 10.1042/bst0310060. [DOI] [PubMed] [Google Scholar]
- Kagoshima M, Wilcke T, Ito K, Tsaprouni L, Barnes PJ, Punchard N, Adcock IM. Glucocorticoid-mediated transrepression is regulated by histone acetylation and DNA methylation. Eur J Pharmacol. 2001;429:327–334. doi: 10.1016/s0014-2999(01)01332-2. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21:1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Shuttleworth EC, Dyer CS, Ogrocki P, Speicher CE. Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosom Med. 1987;49:523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kim HG, Lee KW, Cho YY, Kang NJ, Oh SM, Bode AM, Dong Z. Mitogen- and stress-activated kinase 1-mediated histone H3 phosphorylation is crucial for cell transformation. Cancer Res. 2008;68:2538–2547. doi: 10.1158/0008-5472.CAN-07-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda K, Saito N, Takiguchi N, Oda K, Nunomura M, Nakajima N. Preoperative natural killer cell activity: correlation with distant metastases in curatively research colorectal carcinomas. Int Surg. 1997;82:190–193. [PubMed] [Google Scholar]
- Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, Gifford RM, Kauffman GL., Jr Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am J Surg. 1996;171:68–72. doi: 10.1016/S0002-9610(99)80076-2. discussion 72–63. [DOI] [PubMed] [Google Scholar]
- Krukowski K, Eddy J, Kosik KL, Konley T, Janusek LW, Mathews HL. Glucocorticoid Dysregulation of Natural Killer Cell Function through Epigenetic Modification. Brain Behavior and Immunity. 2010 doi: 10.1016/j.bbi.2010.07.244. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutza J, Gratz I, Afshar M, Murasko DM. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85:918–923. doi: 10.1097/00000539-199710000-00037. [DOI] [PubMed] [Google Scholar]
- Larson MR, Duberstein PR, Talbot NL, Caldwell C, Moynihan JA. A presurgical psychosocial intervention for breast cancer patients. psychological distress and the immune response. J Psychosom Res. 2000;48:187–194. doi: 10.1016/s0022-3999(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Lieb JD, Clarke ND. Control of transcription through intragenic patterns of nucleosome composition. Cell. 2005;123:1187–1190. doi: 10.1016/j.cell.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Lu Q, Wu A, Ray D, Deng C, Attwood J, Hanash S, Pipkin M, Lichtenheld M, Richardson B. DNA methylation and chromatin structure regulate T cell perforin gene expression. J Immunol. 2003;170:5124–5132. doi: 10.4049/jimmunol.170.10.5124. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Costanzo E, Siegel S. Psychosocial influences in oncology: An expanded model of biobehavioral mechanisms. In: Ader R, editor. Psychoneuroimmunology. 4. Elsevier Academic Press; Burlington, MA: 2007. pp. 869–895. [Google Scholar]
- Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–839. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. 2010;65:7–15. doi: 10.1111/j.1398-9995.2009.02186.x. [DOI] [PubMed] [Google Scholar]
- Matthews JG, Ito K, Barnes PJ, Adcock IM. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–1108. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Mcnair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. 1992. [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-kappaB Signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N, Brown DR, Olorenshaw IM, Kammer GM. Trichostatin A reverses skewed expression of CD154, interleukin-10, and interferon-gamma gene and protein expression in lupus T cells. Proc Natl Acad Sci U S A. 2001;98:2628–2633. doi: 10.1073/pnas.051507098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokart D, Capo C, Blache JL, Delpero JR, Houvenaeghel G, Martin C, Mege JL. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br J Surg. 2002;89:1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- Monroe S, Kelley J. Measurement of stress appraisal. Measuring stress. A guide for health and social scientists. 1995:122–147. [Google Scholar]
- Muegge K, Young H, Ruscetti F, Mikovits J. Epigenetic control during lymphoid development and immune responses: aberrant regulation, viruses, and cancer. Ann N Y Acad Sci. 2003;983:55–70. doi: 10.1111/j.1749-6632.2003.tb05962.x. [DOI] [PubMed] [Google Scholar]
- Nagabhushan M, Mathews HL, Witek-Janusek L. Aberrant nuclear expression of ap-1 and nfkb in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav Immun. 2001;15:78–84. doi: 10.1006/brbi.2000.0589. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Cancer facts: 1996. Washington D.C.: 1997. [Google Scholar]
- Parra M, Mahmoudi T, Verdin E. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 2007;21:638–643. doi: 10.1101/gad.1513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesko MM, Hargrove JL, Granner DK, Chalkley R. Inhibition by sodium butyrate of enzyme induction by glucocorticoids and dibutyryl cyclic AMP. A role for the rapid form of histone acetylation. J Biol Chem. 1983;258:13738–13744. [PubMed] [Google Scholar]
- Pollock RE, Lotzova E, Stanford SD. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch Surg. 1991;126:338–342. doi: 10.1001/archsurg.1991.01410270082013. [DOI] [PubMed] [Google Scholar]
- Reinke H, Horz W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol Cell. 2003;11:1599–1607. doi: 10.1016/s1097-2765(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Sachs G, Rasoul-Rockenschaub S, Aschauer H, Spiess K, Gober I, Staffen A, Zielinski C. Lytic effector cell activity and major depressive disorder in patients with breast cancer: a prospective study. J Neuroimmunol. 1995;59:83–89. doi: 10.1016/0165-5728(95)00029-2. [DOI] [PubMed] [Google Scholar]
- Schotta G, Lachner M, Peters AH, Jenuwein T. The indexing potential of histone lysine methylation. Novartis Found Symp. 2004;259:22–37. discussion 37–47, 163–169. [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayers TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–213. [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Lopez AM, Schwartz GE, Bootzin R, Figueredo AJ, Braden CJ, Kurker SF. Quality of life and breast cancer: relationship to psychosocial variables. J Clin Psychol. 2001;57:501–519. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RC, Lushene RE. Manual for the State-Trait Anxiety Inventory. 1970. p. 457. [Google Scholar]
- Spies CD, Von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schroder T, Hoflich C, Sinha P, Paschen C, Mirsalim P, Brunsch R, Hopf J, Marks C, Wernecke KD, Pragst F, Ehrenreich H, Muller C, Tonnesen H, Oelkers W, Rohde W, Stein C, Kox WJ. Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology. 2004;100:1088–1100. doi: 10.1097/00000542-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsaprouni LG, Ito K, Punchard N, Adcock IM. Triamcinolone acetonide and dexamethasome suppress TNF-alpha-induced histone H4 acetylation on lysine residues 8 and 12 in mononuclear cells. Ann N Y Acad Sci. 2002;973:481–483. doi: 10.1111/j.1749-6632.2002.tb04688.x. [DOI] [PubMed] [Google Scholar]
- Van Den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ME, Smyth MJ. The role of natural killer cells in tumor control--effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, Doorman J, Balli JE, Glass J, Gonzalez JJ, Bessler M, Xie H, Treat M. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972–978. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Meyer G, Adam M, Hochtlen-Vollmar W, Angele MK, Schalhorn A, Wilkowski R, Muller C, Schildberg FW. Detrimental immunologic effects of preoperative chemoradiotherapy in advanced rectal cancer. Dis Colon Rectum. 2003;46:875–887. doi: 10.1007/s10350-004-6677-z. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, Laplant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced NK cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology. 2007;32:22–35. doi: 10.1016/j.psyneuen.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witek-Janusek L, Mathews HL. Differential effects of glucocorticoids on colony stimulating factors produced by neonatal mononuclear cells. Pediatr Res. 1999;45:224–229. doi: 10.1203/00006450-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Mathews HL. Stress, Immunity, and Health Outcomes. In: Rice V, editor. Handbook of Stress, Coping, and Health: Implications for Nursing Research, Theory, and Practice. 1. Sage Publications; Thousand Oaks: 2000. pp. 47–67. [Google Scholar]
- Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58. doi: 10.1016/j.jneuroim.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Yamamura Y, Rodriguez N, Schwartz A, Eylar E, Bagwell B, Yano N. A new flow cytometric method for quantitative assessment of lymphocyte mitogenic potentials. Cell Mol Biol. 1995;41:S121–132. [PubMed] [Google Scholar]
- Zhang TY, Meaney MJ. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61:439–466. C431–433. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.